Gestational Exercise Increases Male Offspring’s Maximal Workload Capacity Early in Life
Abstract
1. Introduction
2. Results
3. Discussion
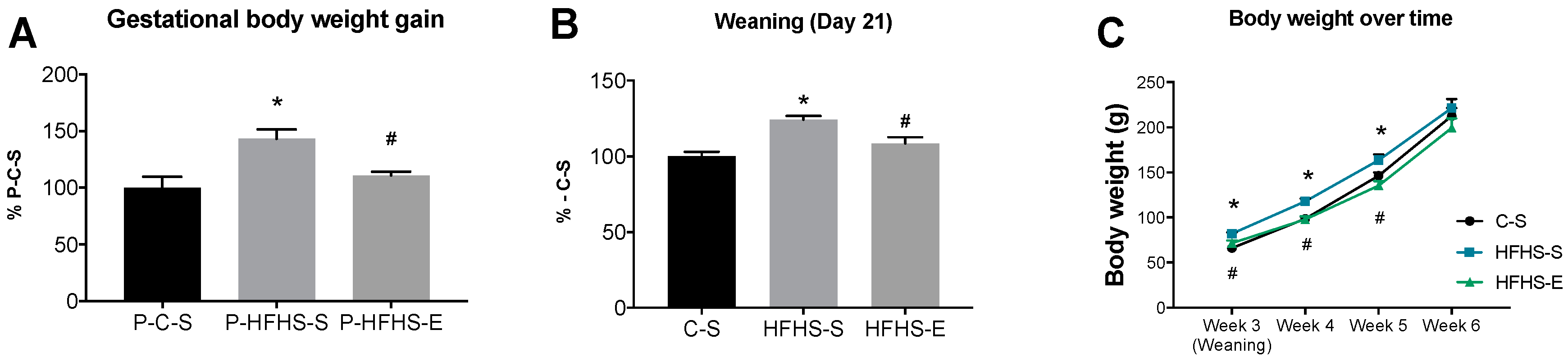
3.1. Offspring Body Composition
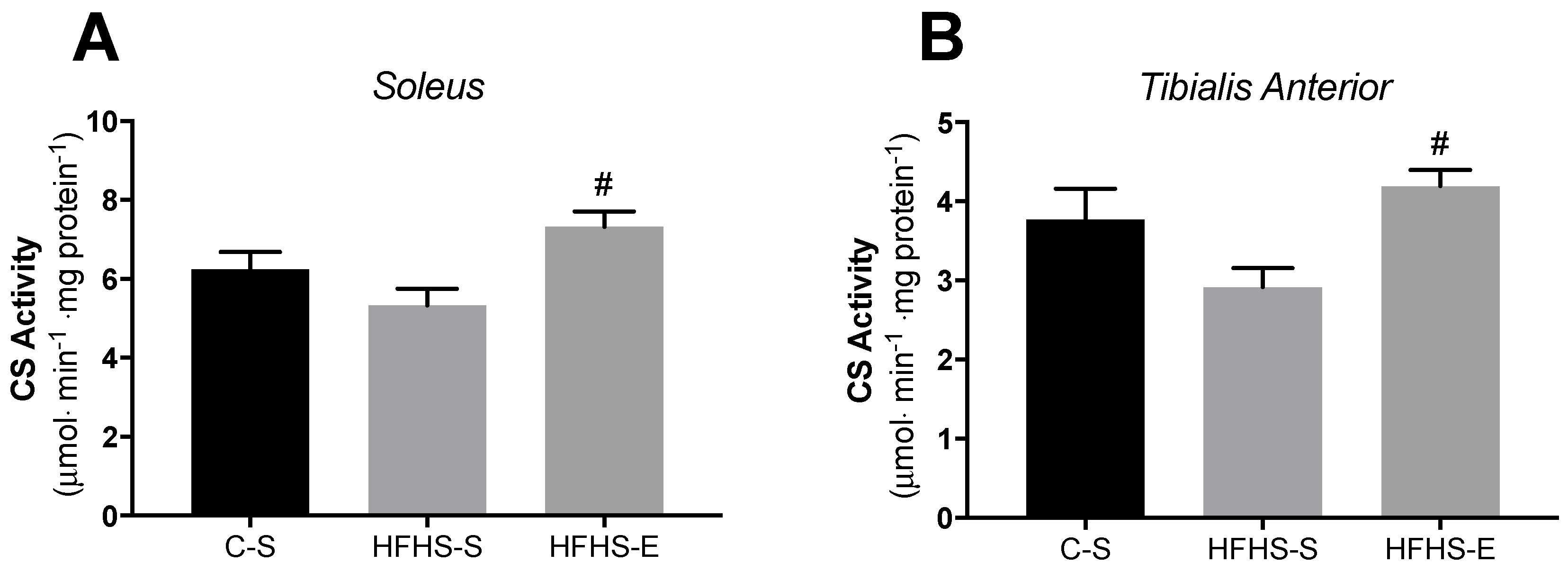
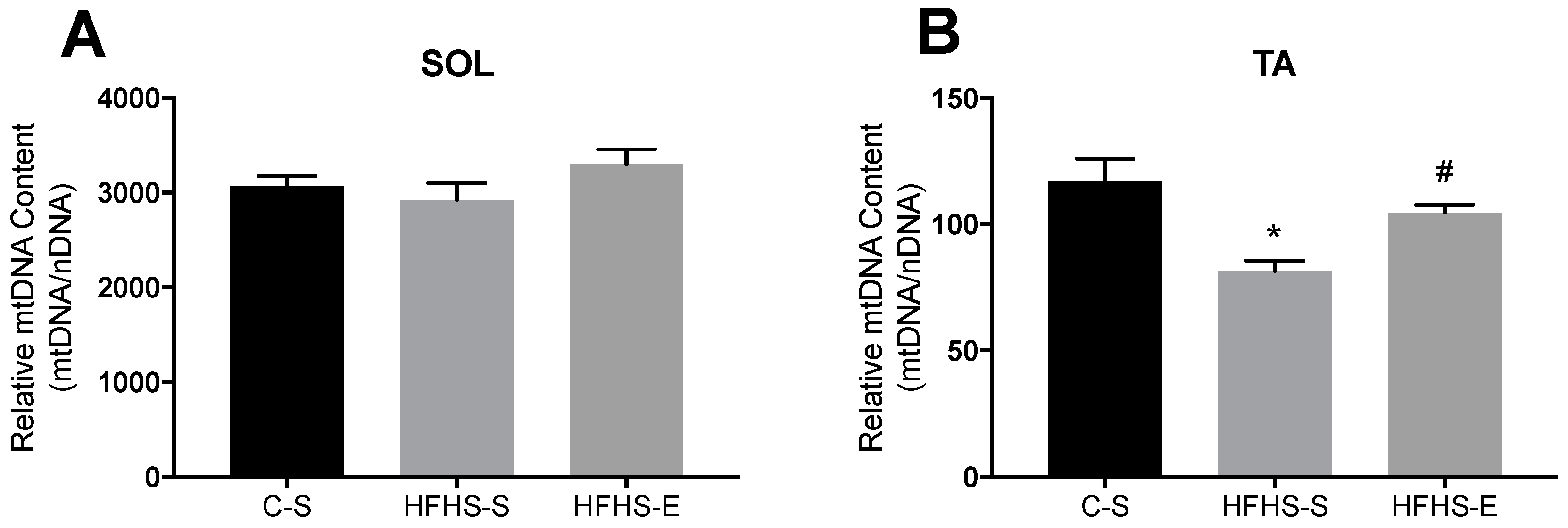
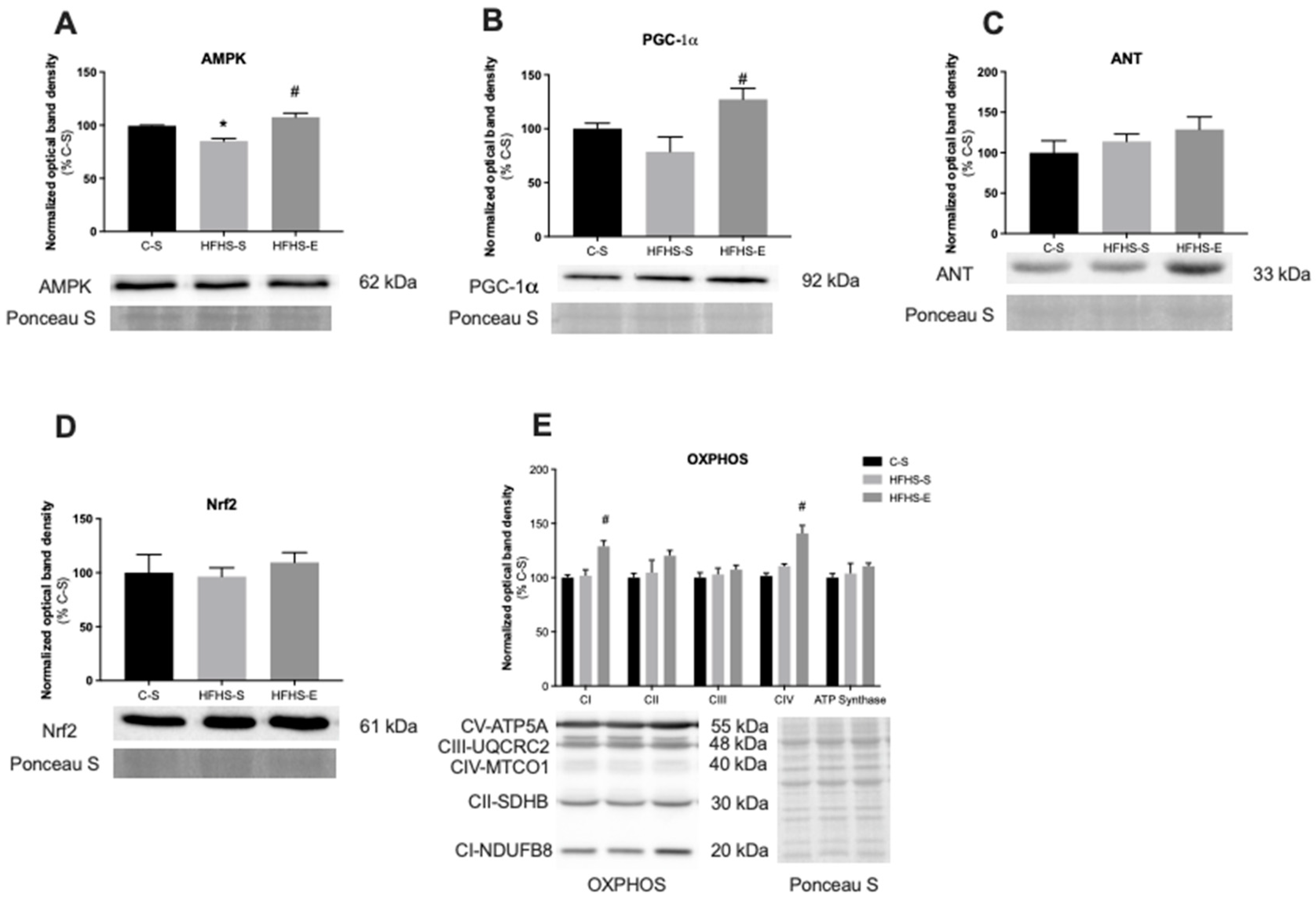
3.2. Skeletal Muscle Mitochondrial Network Physiology
3.3. Offspring Maximal Workload Capacity
4. Materials and Methods
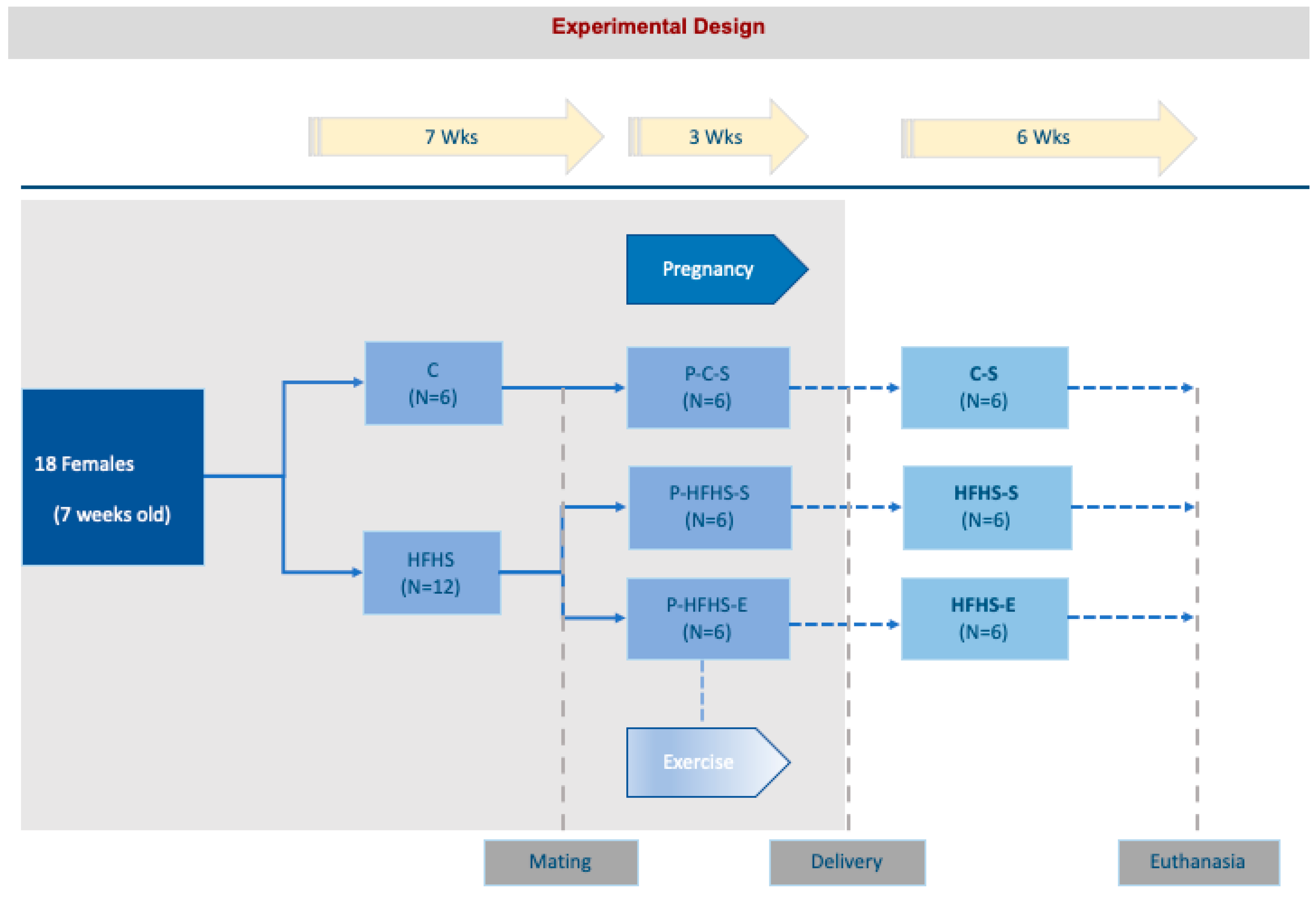
4.1. Animals
4.2. Physical Exercise Protocol
4.3. Maximal Workload Test
4.4. Animal Euthanasia and Muscle Harvesting
4.5. Citrate Synthase Activity
4.6. Muscle Acylcarnitine Levels
4.7. Mitochondrial DNA Content
4.8. Protein Semiquantification by Western Blotting
4.9. Real-Time Polymerase Chain Reaction (RT-qPCR)
4.10. Statistical Analysis
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Armitage, J.A.; Poston, L.; Taylor, P.D. Developmental origins of obesity and the metabolic syndrome: The role of maternal obesity. Front. Horm. Res. 2008, 36, 73–84. [Google Scholar] [PubMed]
- Falcão-Tebas, F.; Bento-Santos, A.; Fidalgo, M.A.; de Almeida, M.B.; dos Santos, J.A.; Lopes de Souza, S.; Manhães-de-Castro, R.; Leandro, C. Maternal low-protein diet-induced delayed reflex ontogeny is attenuated by moderate physical training during gestation in rats. Br. J. Nutr. 2012, 107, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Falcão-Tebas, F.; Kuang, J.; Arceri, C.; Kerris, J.P.; Andrikopoulos, S.; Marin, E.C.; McConell, G. Four weeks of exercise early in life reprograms adult skeletal muscle insulin resistance caused by a paternal high-fat diet. J. Physiol. 2019, 597, 121–136. [Google Scholar] [CrossRef] [PubMed]
- Gluckman, P.D.; Hanson, M.A.; Cooper, C.; Thornburg, K.L. Effect of in utero and early-life conditions on adult health and disease. N. Engl. J. Med. 2008, 359, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Bayol, S.A.; Simbi, B.H.; Stickland, N. A maternal cafeteria diet during gestation and lactation promotes adiposity and impairs skeletal muscle development and metabolism in rat offspring at weaning. J. Physiol. 2005, 567, 951–961. [Google Scholar] [CrossRef] [PubMed]
- Masuyama, H.; Hiramatsu, Y. Effects of a high-fat diet exposure in utero on the metabolic syndrome-like phenomenon in mouse offspring through epigenetic changes in adipocytokine gene expression. Endocrinology 2012, 153, 2823–2830. [Google Scholar] [CrossRef]
- Stanford, K.I.; Lee, M.Y.; Getchell, K.M.; So, K.; Hirshman, M.F.; Goodyear, L.J. Exercise before and during pregnancy prevents the deleterious effects of maternal high-fat feeding on metabolic health of male offspring. Diabetes 2015, 64, 427–433. [Google Scholar] [CrossRef]
- Pileggi, C.A.; Hedges, C.P.; Segovia, S.A.; Markworth, J.F.; Durainayagam, B.R.; Gray, C.; Zhang, X.D.; Barnett, M.P.; Vickers, M.H.; Hickey, A.J.; et al. Maternal High Fat Diet Alters Skeletal Muscle Mitochondrial Catalytic Activity in Adult Male Rat Offspring. Front. Physiol. 2016, 7, 546. [Google Scholar] [CrossRef]
- Simar, D.; Chen, H.; Lambert, K.; Mercier, J.; Morris, M. Interaction between maternal obesity and post-natal over-nutrition on skeletal muscle metabolism. Nutr. Metab. Cardiovasc. Dis. NMCD 2012, 22, 269–276. [Google Scholar] [CrossRef]
- Son, J.S.; Zhao, L.; Chen, Y.; Chen, K.; Chae, S.A.; de Avila, J.M.; Wang, H.; Zhu, M.J.; Jiang, Z.; Du, M. Maternal exercise via exerkine apelin enhances brown adipogenesis and prevents metabolic dysfunction in offspring mice. Sci. Adv. 2020, 6, eaaz0359. [Google Scholar] [CrossRef]
- Beleza, J.; Rizo-Roca, D.; Ascensão, A.; Magalhães, J. Targeting Mitochondria with Sweat: Improving Mitochondrial Function with Physical Activity. In Mitochondrial Biology and Experimental Therapeutics; Springer: Cham, Switzerland, 2018; pp. 379–406. [Google Scholar]
- Eclarinal, J.D.; Zhu, S.; Baker, M.S.; Piyarathna, D.B.; Coarfa, C.; Fiorotto, M.L.; Waterland, R. Maternal exercise during pregnancy promotes physical activity in adult offspring. FASEB J. 2016, 30, 2541–2548. [Google Scholar] [CrossRef] [PubMed]
- Walter, I.; Klaus, S. Maternal high-fat diet consumption impairs exercise performance in offspring. J. Nutr. Sci. 2014, 3, e61. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fiuza-Luces, C.; Garatachea, N.; Berger, N.A. Exercise is the real polypill. Physiology 2013, 28, 330–358. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.K. Saltin, Exercise as medicine—Evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand. J. Med. Sci. Sports 2015, 25, 1–72. [Google Scholar] [CrossRef]
- Rocha-Rodrigues, S.; Goncalves, I.O.; Beleza, J.; Ascensao, A.; Magalhaes, J. Physical exercise mitigates high-fat diet-induced adiposopathy and related endocrine alterations in an animal model of obesity. J. Physiol. Biochem. 2018, 74, 235–246. [Google Scholar] [CrossRef]
- Gilbert, J.S.; Banek, C.T.; Bauer, A.J.; Gingery, A.; Dreyer, H. Placental and vascular adaptations to exercise training before and during pregnancy in the rat. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012, 303, R520–R526. [Google Scholar] [CrossRef]
- Mottola, M.F.; Artal, R. Fetal and maternal metabolic responses to exercise during pregnancy. Early Hum. Dev. 2016, 94, 33–41. [Google Scholar] [CrossRef]
- Beleza, J.; Stevanović Silva, J.; Coxito, P.; Costa, R.C.; Ascensão, A.; Torrella, J.R. Magalhães, Building-up fit muscles for the future. Eur. J. Clin. Investig. 2021, 51, e13515. [Google Scholar]
- Stevanović-Silva, J.; Beleza, J.; Coxito, P.; Pereira, S.; Rocha, H.; Gaspar, T.B.; Gärtner, F.; Correia, R.; Martins, M.J.; Guimarães, T.; et al. Maternal high-fat high-sucrose diet and gestational exercise modulate hepatic fat accumulation and liver mitochondrial respiratory capacity in mothers and male offspring. Metabolism 2021, 116, 154704. [Google Scholar] [CrossRef]
- Carter, L.G.; Lewis, K.N.; Wilkerson, D.C.; Tobia, C.M.; Ngo Tenlep, S.Y.; Shridas, P.; Garcia-Cazarin, M.L.; Wolff, G.; Andrade, F.H.; Charnigo, R.J.; et al. Perinatal exercise improves glucose homeostasis in adult offspring. Am. J. Physiol. Endocrinol. Metab. 2012, 303, E1061–E1068. [Google Scholar] [CrossRef]
- Ferrari, N.; Bae-Gartz, I.; Bauer, C.; Janoschek, R.; Koxholt, I.; Mahabir, E.; Appel, S.; Alejandre Alcazar, M.A.; Grossmann, N.; Vohlen, C.; et al. Exercise during pregnancy and its impact on mothers and offspring in humans and mice. J. Dev. Orig. Health Dis. 2018, 9, 63–76. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.E.; Baer, L.A.; Stanford, K. Maternal Exercise Improves the Metabolic Health of Adult Offspring. Trends Endocrinol. Metab. 2018, 29, 164–177. [Google Scholar] [CrossRef] [PubMed]
- Mottola, M. Physical activity and maternal obesity: Cardiovascular adaptations, exercise recommendations, and pregnancy outcomes. Nutr. Rev. 2013, 71, S31–S36. [Google Scholar] [CrossRef]
- Raipuria, M.; Bahari, H.; Morris, M. Effects of maternal diet and exercise during pregnancy on glucose metabolism in skeletal muscle and fat of weanling rats. PLoS ONE 2015, 10, e0120980. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO Guidelines on Physical Activity and Sedentary Behaviour: At a Glance; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- Falcão-Tebas, F.; Marin, E.C.; Kuang, J.; Bishop, D.J.; McConell, G. Maternal exercise attenuates the lower skeletal muscle glucose uptake and insulin secretion caused by paternal obesity in female adult rat offspring. J. Physiol. 2020, 598, 4251–4270. [Google Scholar] [CrossRef]
- Beleza, J.; Albuquerque, J.; Santos-Alves, E.; Fonseca, P.; Santocildes, G.; Stevanovic, J.; Rocha-Rodrigues, S.; Rizo-Roca, D.; Ascensão, A.; Torrella, J.R.; et al. Self-Paced Free-Running Wheel Mimics High-Intensity Interval Training Impact on Rats’ Functional, Physiological, Biochemical, and Morphological Features. Front. Physiol. 2019, 10, 593. [Google Scholar] [CrossRef] [PubMed]
- Power, M.L.; Schulkin, J. Maternal regulation of offspring development in mammals is an ancient adaptation tied to lactation. Appl. Transl. Genom. 2013, 2, 55–63. [Google Scholar] [CrossRef]
- Kruse, M.; Seki, Y.; Vuguin, P.M.; Du, X.Q.; Fiallo, A.; Glenn, A.S.; Singer, S.; Breuhahn, K.; Katz, E.B.; Charron, M.J. High-fat intake during pregnancy and lactation exacerbates high-fat diet-induced complications in male offspring in mice. Endocrinology 2013, 154, 3565–3576. [Google Scholar] [CrossRef]
- Shankar, K.; Harrell, A.; Liu, X.; Gilchrist, J.M.; Ronis, M.J.; Badger, T.M. Maternal obesity at conception programs obesity in the offspring. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 294, R528–R538. [Google Scholar] [CrossRef]
- Sheldon, R.D.; Nicole Blaize, A.; Fletcher, J.A.; Pearson, K.J.; Donkin, S.S.; Newcomer, S.C.; Rector, R.S. Gestational exercise protects adult male offspring from high-fat diet-induced hepatic steatosis. J. Hepatol. 2016, 64, 171–178. [Google Scholar] [CrossRef]
- Pileggi, C.A.; Segovia, S.A.; Markworth, J.F.; Gray, C.; Zhang, X.D.; Milan, A.M.; Mitchell, C.J.; Barnett, M.P.; Roy, N.C.; Vickers, M.H.; et al. Maternal conjugated linoleic acid supplementation reverses high-fat diet-induced skeletal muscle atrophy and inflammation in adult male rat offspring. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016, 310, R432–R439. [Google Scholar] [CrossRef] [PubMed]
- Bayol, S.A.; Macharia, R.; Farrington, S.J.; Simbi, B.H.; Stickland, N. Evidence that a maternal "junk food" diet during pregnancy and lactation can reduce muscle force in offspring. Eur. J. Nutr. 2009, 48, 62–65. [Google Scholar] [CrossRef] [PubMed]
- Son, J.S.; Chae, S.A.; Wang, H.; Chen, Y.; Bravo Iniguez, A.; de Avila, J.M.; Jiang, Z.; Zhu, M.J.; Du, M. Maternal Inactivity Programs Skeletal Muscle Dysfunction in Offspring Mice by Attenuating Apelin Signaling and Mitochondrial Biogenesis. Cell Rep. 2020, 33, 108461. [Google Scholar] [CrossRef] [PubMed]
- Latouche, C.; Heywood, S.E.; Henry, S.L.; Ziemann, M.; Lazarus, R.; El-Osta, A.; Armitage, J.A.; Kingwell, B.A. Maternal overnutrition programs changes in the expression of skeletal muscle genes that are associated with insulin resistance and defects of oxidative phosphorylation in adult male rat offspring. J. Nutr. 2014, 144, 237–244. [Google Scholar] [CrossRef]
- Pereira, T.J.; Fonseca, M.A.; Campbell, K.E.; Moyce, B.L.; Cole, L.K.; Hatch, G.M.; Doucette, C.A.; Klein, J.; Aliani, M.; Dolinsky, V.W. Maternal obesity characterized by gestational diabetes increases the susceptibility of rat offspring to hepatic steatosis via a disrupted liver metabolome. J. Physiol. 2015, 593, 3181–3197. [Google Scholar] [CrossRef]
- Aschrafi, A.; Schwechter, A.D.; Mameza, M.G.; Natera-Naranjo, O.; Gioio, A.E.; Kaplan, B.B. MicroRNA-338 regulates local cytochrome c oxidase IV mRNA levels and oxidative phosphorylation in the axons of sympathetic neurons. J. Neurosci. 2008, 28, 12581–12590. [Google Scholar] [CrossRef]
- Simão, A.L.; Afonso, M.B.; Rodrigues, P.M.; Gama-Carvalho, M.; Machado, M.V.; Cortez-Pinto, H.; Rodrigues, C.M.P.; Castro, R.E. Skeletal muscle miR-34a/SIRT1:AMPK axis is activated in experimental and human non-alcoholic steatohepatitis. J. Mol. Med. 2019, 97, 1113–1126. [Google Scholar] [CrossRef]
- Lee, J.; Padhye, A.; Sharma, A.; Song, G.; Miao, J.; Mo, Y.Y.; Wang, L.; Kemper, J.K. A pathway involving farnesoid X receptor and small heterodimer partner positively regulates hepatic sirtuin 1 levels via microRNA-34a inhibition. J. Biol. Chem. 2010, 285, 12604–12611. [Google Scholar] [CrossRef]
- Carrer, M.; Liu, N.; Grueter, C.E.; Williams, A.H.; Frisard, M.I.; Hulver, M.W.; Bassel-Duby, R.; Olson, E.N. Control of mitochondrial metabolism and systemic energy homeostasis by microRNAs 378 and 378*. Proc. Natl. Acad. Sci. USA 2012, 109, 15330–15335. [Google Scholar] [CrossRef]
- Fulzele, S.; Mendhe, B.; Khayrullin, A.; Johnson, M.; Kaiser, H.; Liu, Y.; Isales, C.M.; Hamrick, M.W. Muscle-derived miR-34a increases with age in circulating extracellular vesicles and induces senescence of bone marrow stem cells. Aging 2019, 11, 1791–1803. [Google Scholar] [CrossRef]
- Yamamoto, H.; Morino, K.; Nishio, Y.; Ugi, S.; Yoshizaki, T.; Kashiwagi, A.; Maegawa, H. MicroRNA-494 regulates mitochondrial biogenesis in skeletal muscle through mitochondrial transcription factor A and Forkhead box j3. Am. J. Physiol. Endocrinol. Metab. 2012, 303, E1419–E1427. [Google Scholar] [CrossRef]
- Granata, C.; Oliveira, R.S.; Little, J.P.; Renner, K.; Bishop, D.J. Mitochondrial adaptations to high-volume exercise training are rapidly reversed after a reduction in training volume in human skeletal muscle. FASEB J. 2016, 30, 3413–3423. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, R.A.; Flück, D.; Bonne, T.C.; Bürgi, S.; Christensen, P.M.; Toigo, M.; Lundby, C. Improvements in exercise performance with high-intensity interval training coincide with an increase in skeletal muscle mitochondrial content and function. J. Appl. Physiol. 2013, 115, 785–793. [Google Scholar] [CrossRef] [PubMed]
- Montero, D.; Cathomen, A.; Jacobs, R.A.; Flück, D.; de Leur, J.; Keiser, S.; Bonne, T.; Kirk, N.; Lundby, A.K. Lundby, Haematological rather than skeletal muscle adaptations contribute to the increase in peak oxygen uptake induced by moderate endurance training. J. Physiol. 2015, 593, 4677–4688. [Google Scholar] [CrossRef]
- Podkalicka, P.; Mucha, O.; Bronisz-Budzyńska, I.; Kozakowska, M.; Pietraszek-Gremplewicz, K.; Cetnarowska, A.; Głowniak-Kwitek, U.; Bukowska-Strakova, K.; Cieśla, M.; Kulecka, M.; et al. Lack of miR-378 attenuates muscular dystrophy in mdx mice. JCI Insight 2020, 5, e135576. [Google Scholar] [CrossRef] [PubMed]
- Petucci, C.; Rojas-Betancourt, S.; Gardell, S.J. Comparison of tissue harvest protocols for the quantitation of acylcarnitines in mouse heart and liver by mass spectrometry. Metabolomics 2012, 8, 784–792. [Google Scholar] [CrossRef]
- Carabelli, J.; Burgueño, A.L.; Rosselli, M.S.; Gianotti, T.F.; Lago, N.R.; Pirola, C.J.; Sookoian, S. High fat diet-induced liver steatosis promotes an increase in liver mitochondrial biogenesis in response to hypoxia. J. Cell. Mol. Med. 2011, 15, 1329–1338. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Locke, M.; Noble, E.G.; Atkinson, B.G. Exercising mammals synthesize stress proteins. Am. J. Physiol. Cell Physiol. 1990, 258, C723–C729. [Google Scholar] [CrossRef]
- Romero-Calvo, I.; Ocón, B.; Martínez-Moya, P.; Suárez, M.D.; Zarzuelo, A.; Martínez-Augustin, O.; de Medina, F.S. Reversible Ponceau staining as a loading control alternative to actin in Western blots. Anal. Biochem. 2010, 401, 318–320. [Google Scholar] [CrossRef]
- Coheh, J. Statistical Power Analysis for the Behavior Sciences; Routledge: New York, NY, USA, 1988. [Google Scholar]

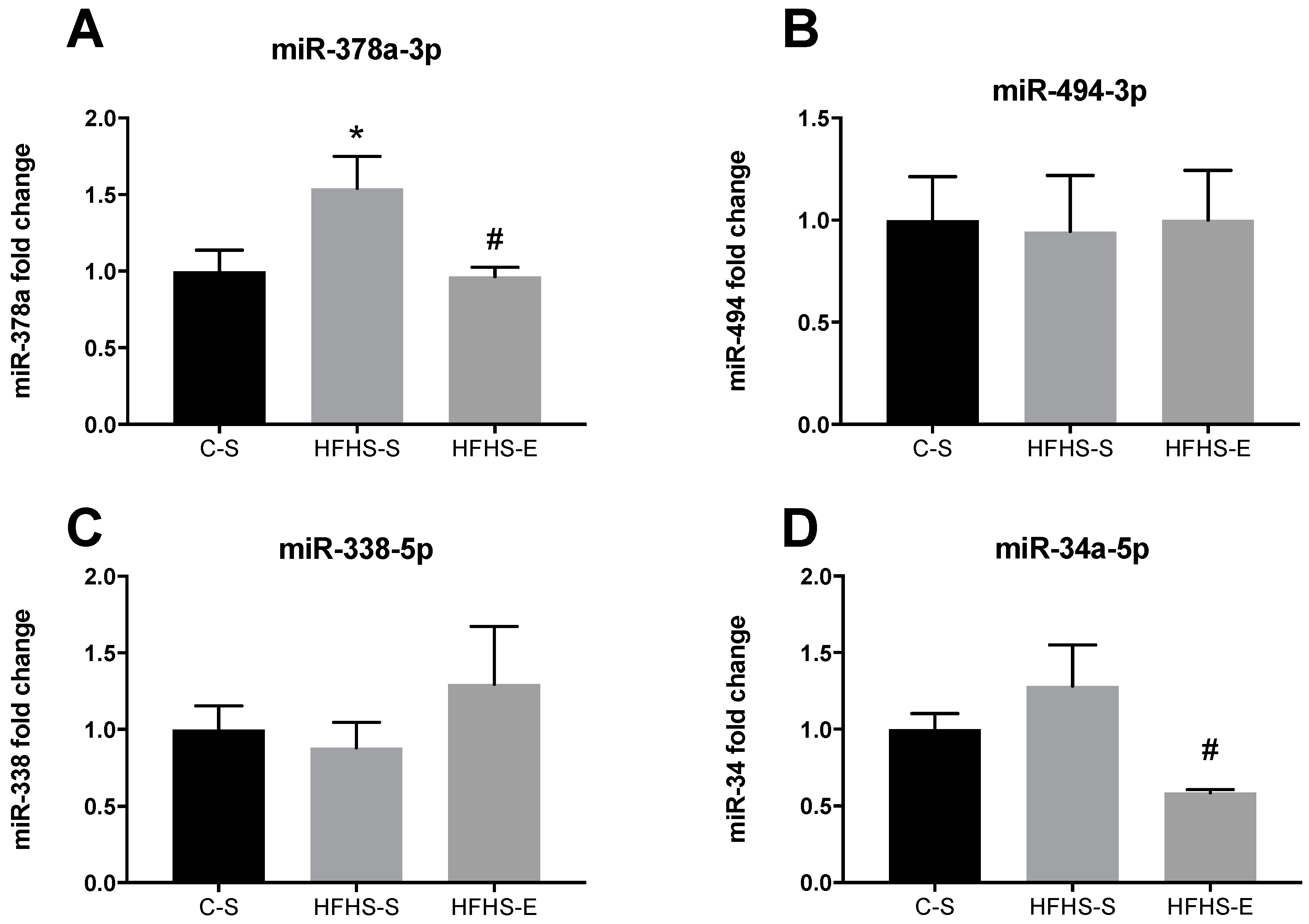
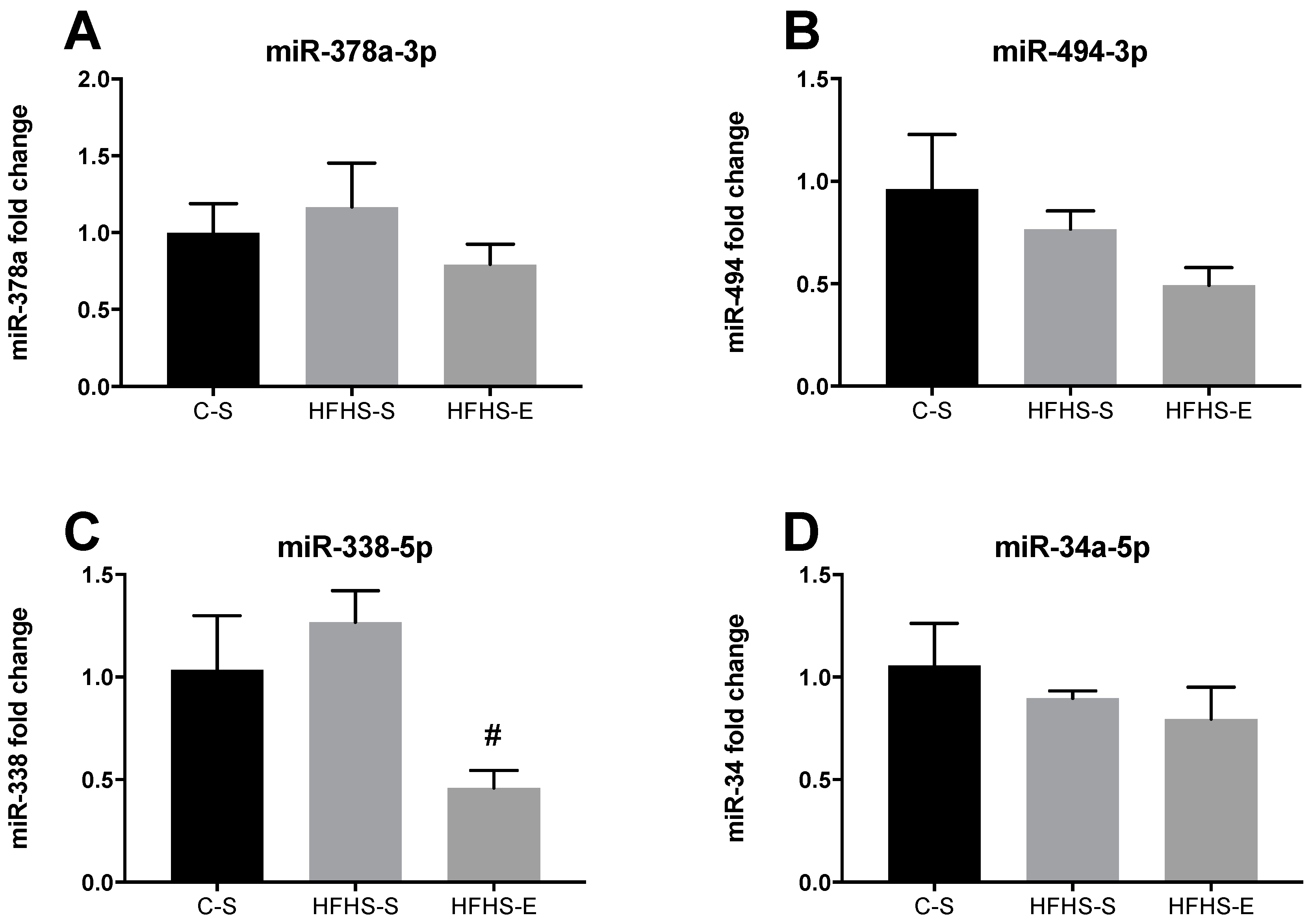
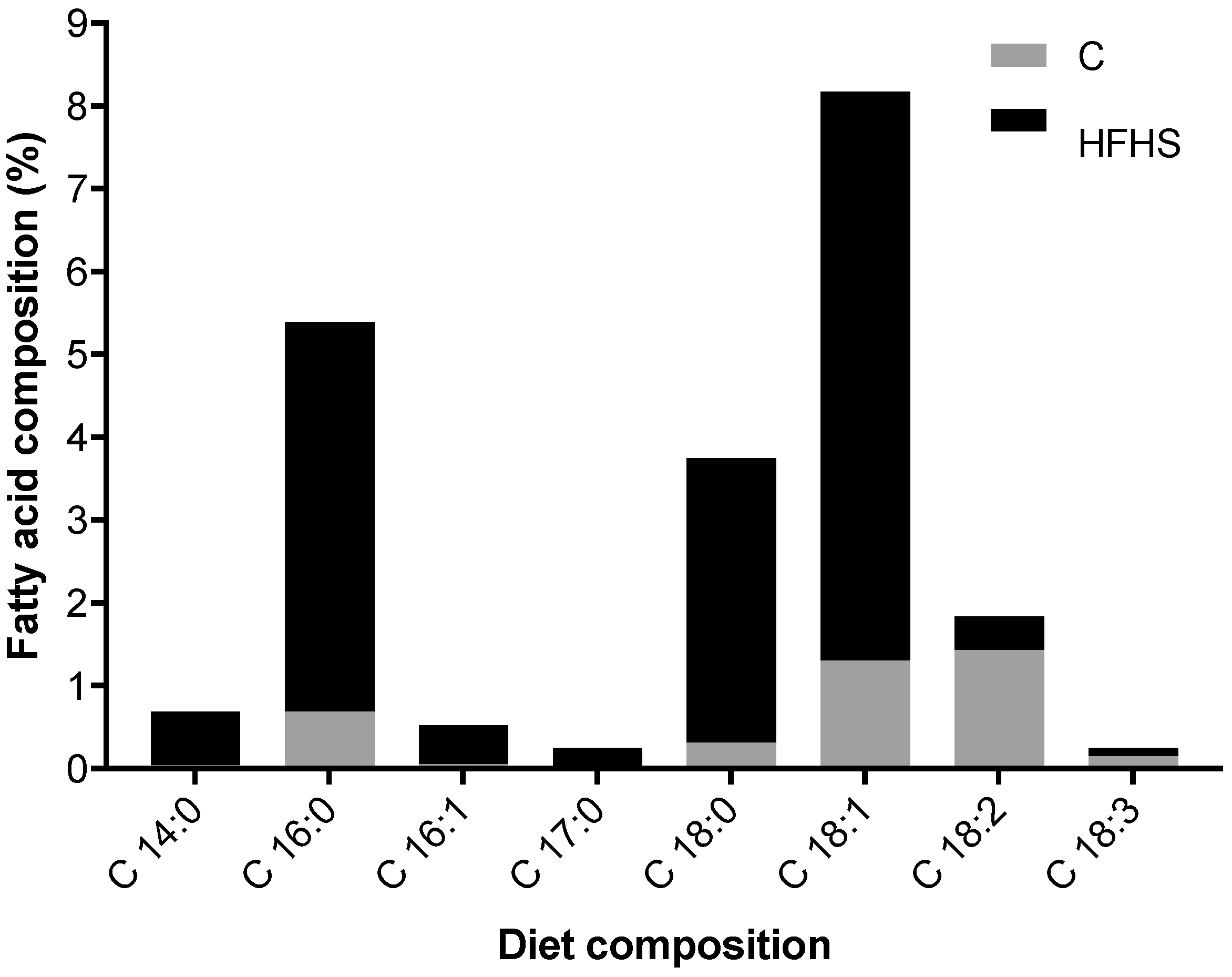
| C–S | HFHS–S | HFHS–E | |
|---|---|---|---|
| Body weight (g) | 212.40 ± 8.74 | 221.20 ± 10.02 | 199.30 ± 10.23 |
| Soleus/Body Weight (mg/g) | 0.47 ± 0.03 | 0.38 ± 0.02 * | 0.45 ± 0.01 # |
| Tibialis Anterior/Body Weight (mg/g) | 1.60 ± 0.03 | 1.52 ± 0.04 | 1.74 ± 0.03 # |
| Glucose (mg/dL) | 193.43 ± 11.60 | 180.20 ± 3.44 | 211.83 ± 5.78 # |
| Triglycerides (mg/dL) | 64.00 ± 4.78 | 79.00 ± 0.63 * | 51.50 ± 2.14 # |
| Max Speed (cm·s−1) | TExhaustion (min) | LactateBasal (mmol/L) | LactatePostEx (mmol/L) | GlucoseBasal (mg·min/dL) | GlucosePostEx (mg·min/dL) | |
|---|---|---|---|---|---|---|
| C–S | 48.57 ± 1.52 | 15.31 ± 2.86 | 1.81 ± 0.12 | 7.66 ± 0.24 | 132.21 ± 3.93 | 134.00 ± 6.47 |
| HFHS–S | 47.50 ± 2.36 | 15.53 ± 1.33 | 1.96 ± 0.90 | 7.50 ± 0.34 | 135.33 ± 3.00 | 136.00 ± 6.59 |
| HFHS–E | 56.25 ± 2.19 # | 20.67 ± 1.50 # | 1.94 ± 0.90 | 7.33 ± 0.80 | 128.30 ± 3.09 | 135.40 ± 5.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beleza, J.; Stevanović-Silva, J.; Coxito, P.; Rocha, H.; Santos, P.; Ascensão, A.; Ramon Torrella, J.; Magalhães, J. Gestational Exercise Increases Male Offspring’s Maximal Workload Capacity Early in Life. Int. J. Mol. Sci. 2022, 23, 3916. https://doi.org/10.3390/ijms23073916
Beleza J, Stevanović-Silva J, Coxito P, Rocha H, Santos P, Ascensão A, Ramon Torrella J, Magalhães J. Gestational Exercise Increases Male Offspring’s Maximal Workload Capacity Early in Life. International Journal of Molecular Sciences. 2022; 23(7):3916. https://doi.org/10.3390/ijms23073916
Chicago/Turabian StyleBeleza, Jorge, Jelena Stevanović-Silva, Pedro Coxito, Hugo Rocha, Paulo Santos, António Ascensão, Joan Ramon Torrella, and José Magalhães. 2022. "Gestational Exercise Increases Male Offspring’s Maximal Workload Capacity Early in Life" International Journal of Molecular Sciences 23, no. 7: 3916. https://doi.org/10.3390/ijms23073916
APA StyleBeleza, J., Stevanović-Silva, J., Coxito, P., Rocha, H., Santos, P., Ascensão, A., Ramon Torrella, J., & Magalhães, J. (2022). Gestational Exercise Increases Male Offspring’s Maximal Workload Capacity Early in Life. International Journal of Molecular Sciences, 23(7), 3916. https://doi.org/10.3390/ijms23073916







