Sex Differences in Metabolic Indices and Chronic Neuroinflammation in Response to Prolonged High-Fat Diet in ApoE4 Knock-In Mice
Abstract
:1. Introduction
2. Results
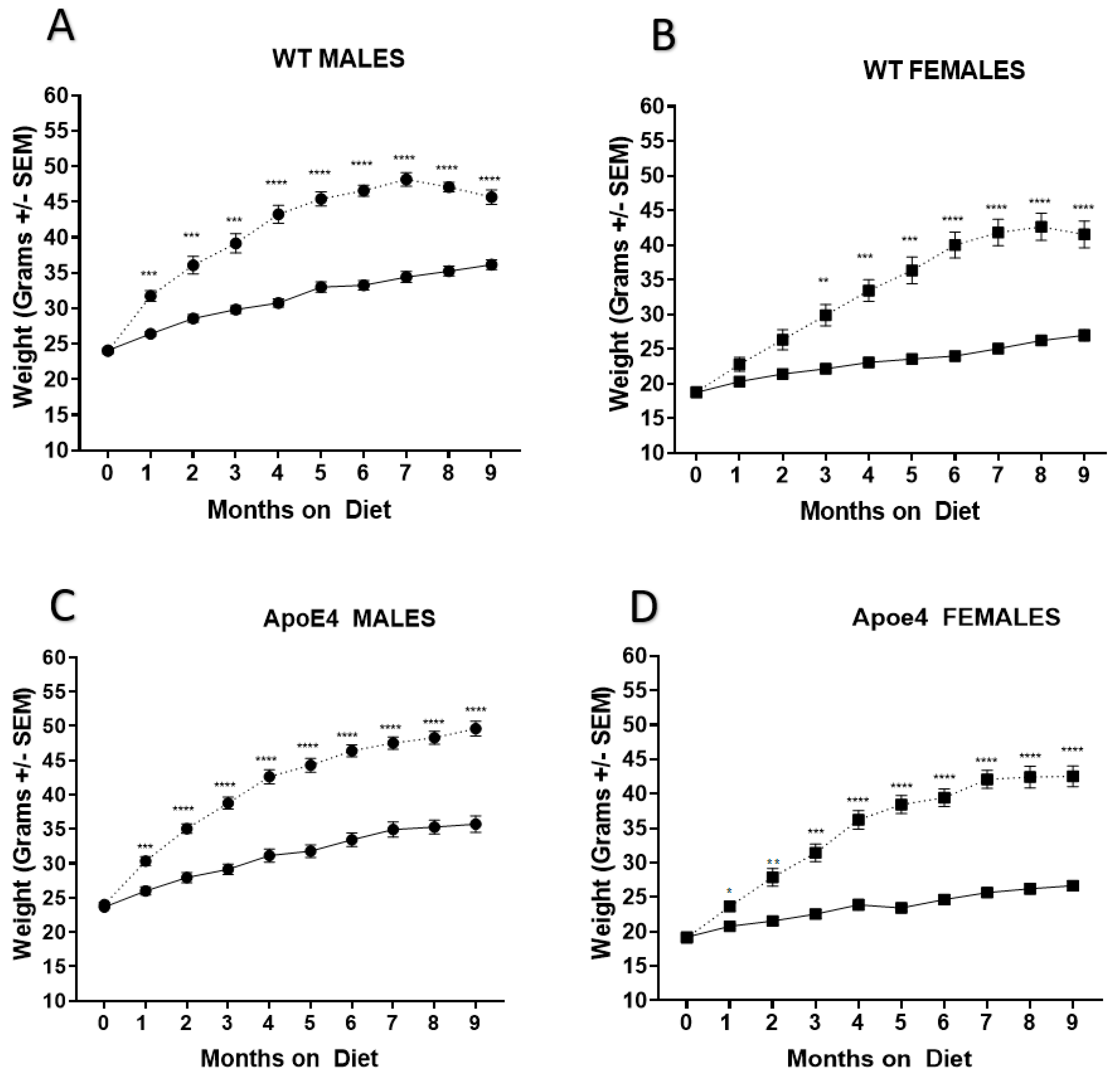
2.1. Genotype Does Not Affect Weight Gain
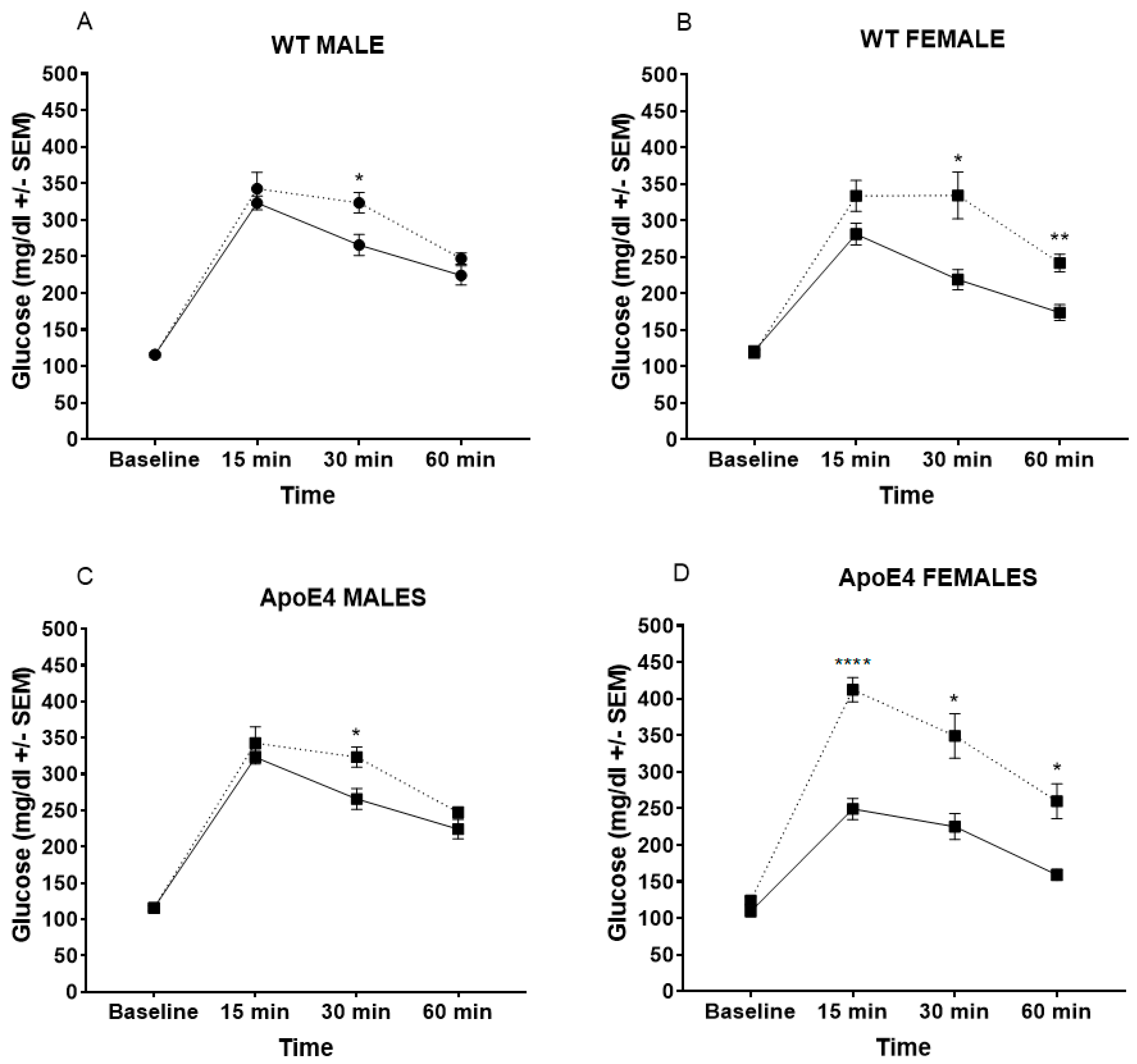
2.2. WD Alters Glucose Metabolism, Especially in Female Mice
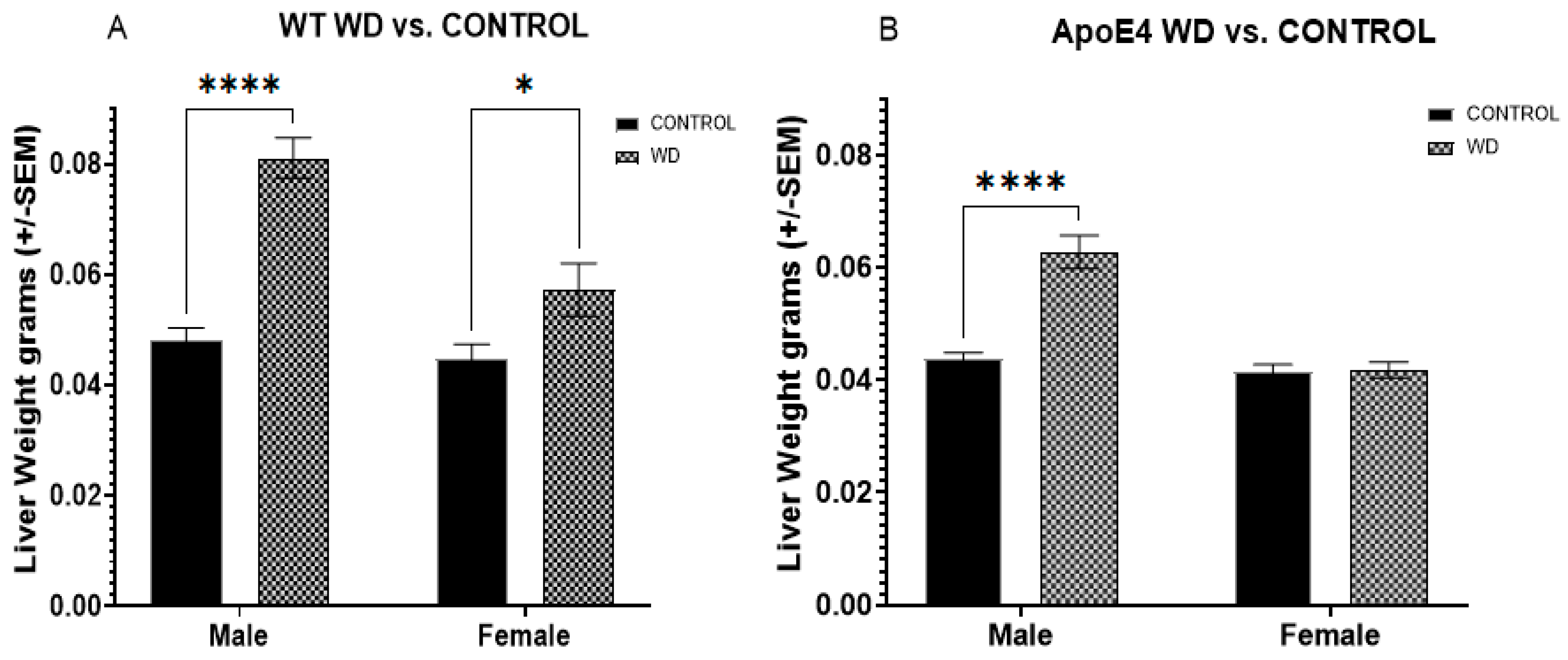
2.3. Selective Effects of WD on Liver Weights in ApoE4 Males and WT Animals
2.4. Reduced Glutathione Levels in WD Male ApoE4 Mice
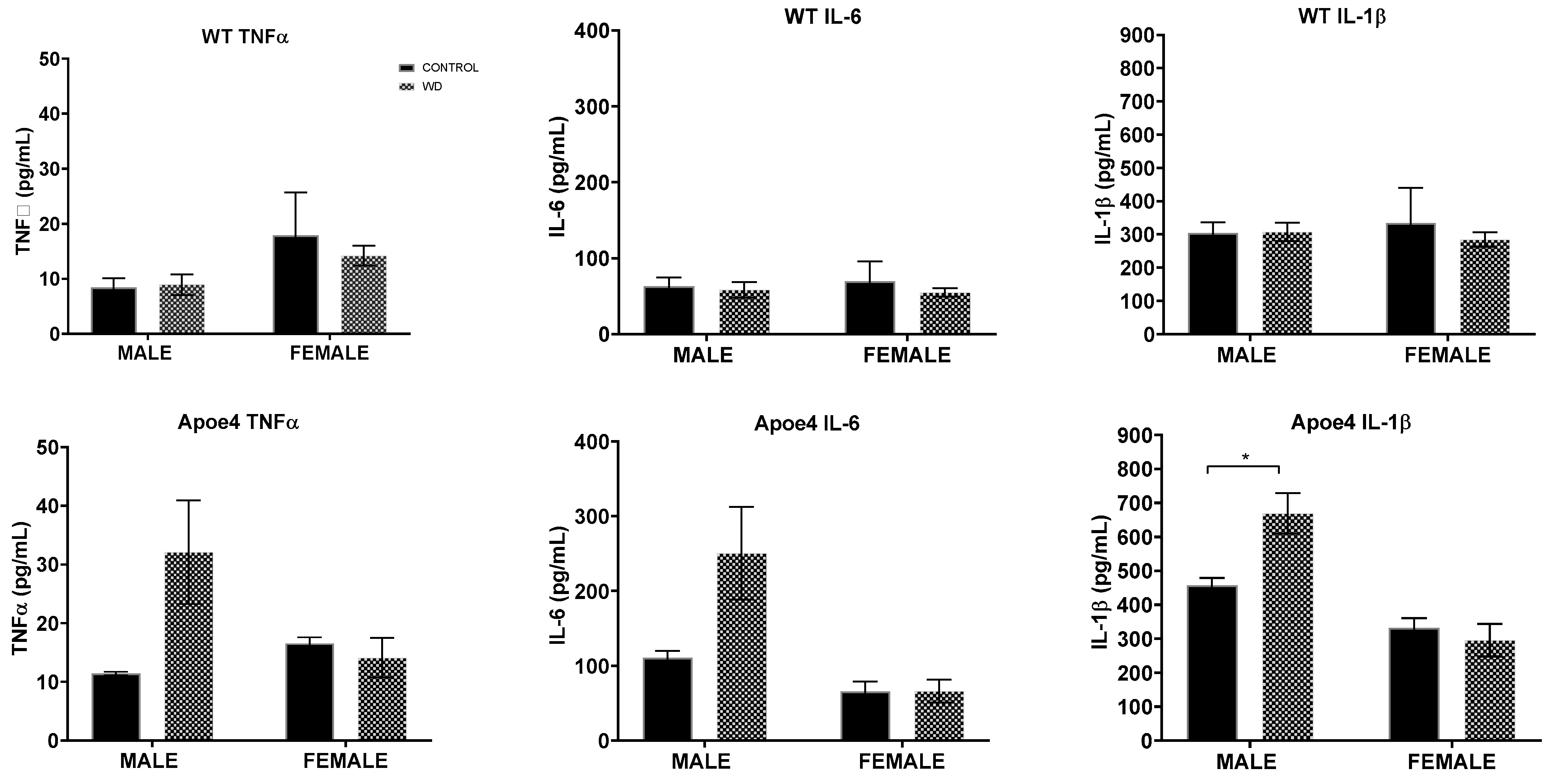
2.5. Interaction Effects of WD with Genotype and Sex on Measures of Neuroinflammation
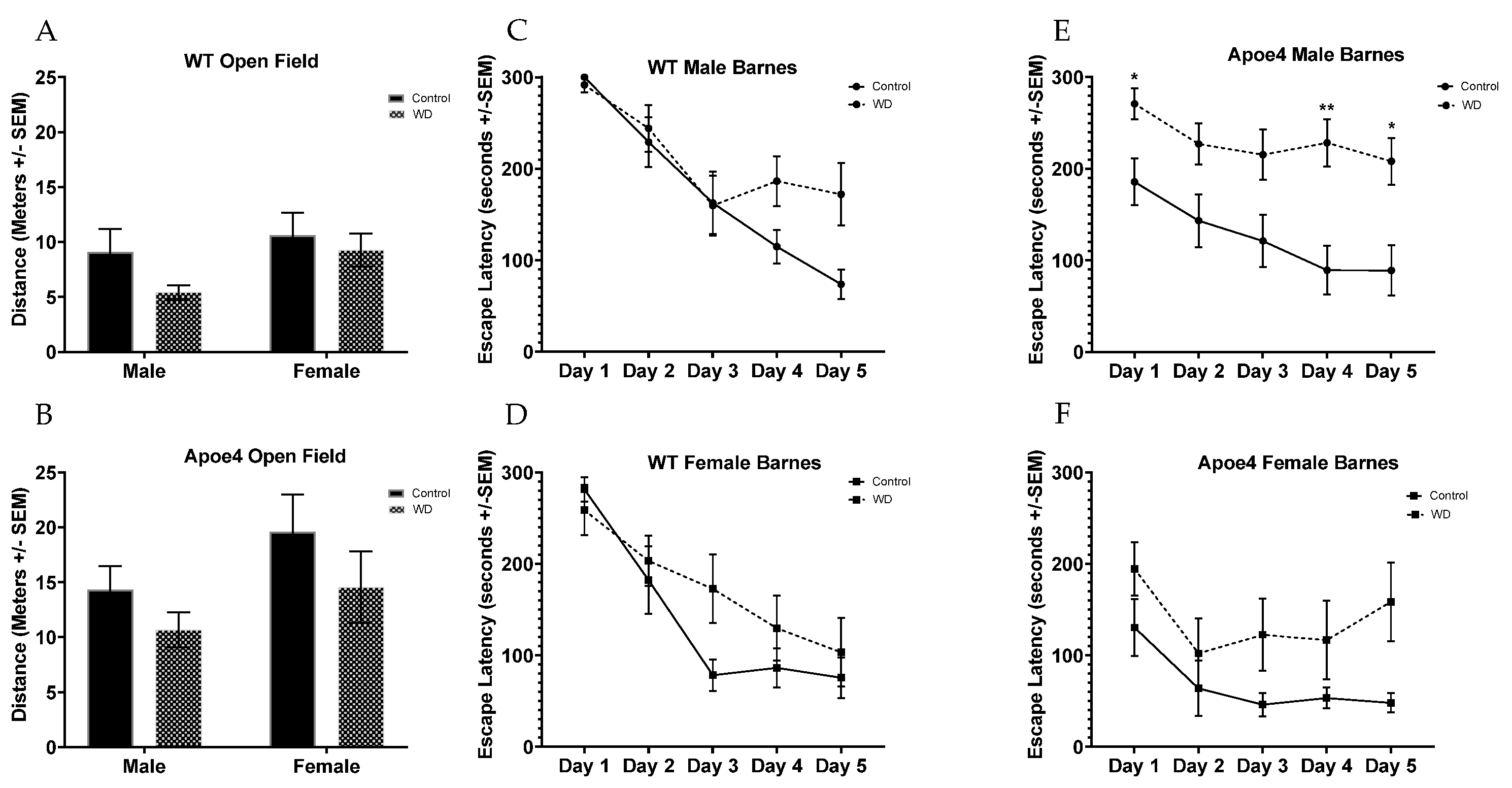
2.6. Behavioral Testing Reveals Impairments from WD in Genotype Are Moderated by Sex
3. Discussion
4. Limitations
5. Materials and Methods
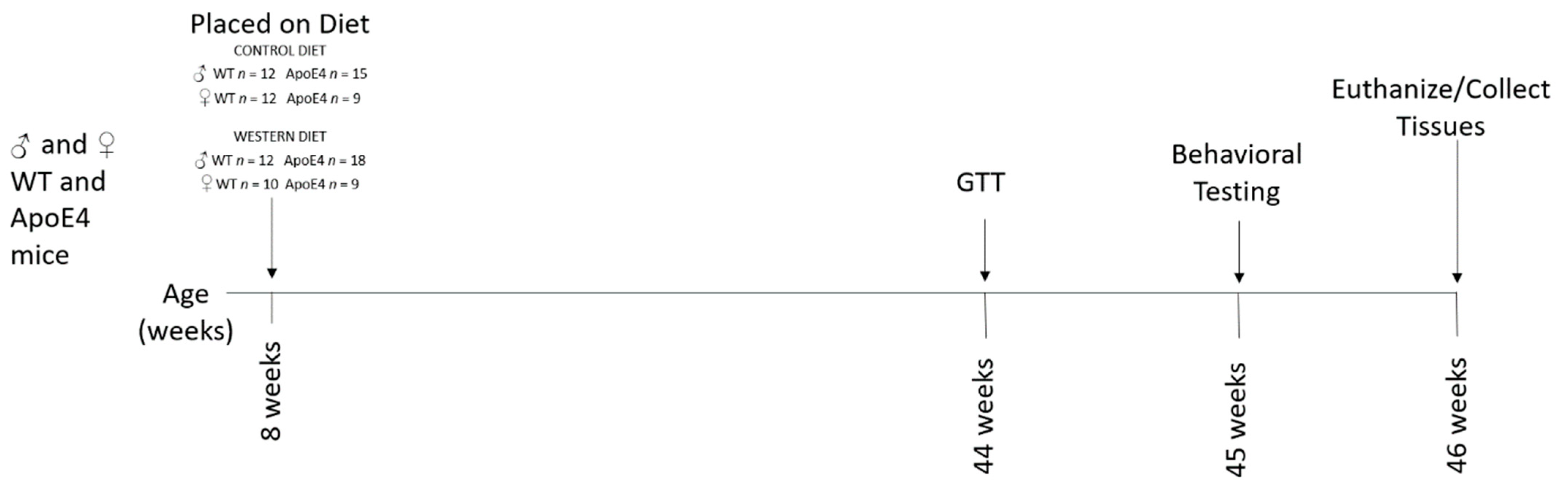
5.1. Animals
5.2. Glucose Tolerance Testing (GTT)
5.3. Behavioral Assays
5.3.1. Open Field
5.3.2. Barnes Maze
5.4. Enzyme-Linked Immunosorbent Assay (ELISA)
5.5. GSH ELISA
5.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Aβ | amyloid β-protein |
| ANOVA | analysis of variance |
| AD | Alzheimer’s disease |
| CNS | central nervous system |
| ELISA | enzyme-Linked immunosorbent assay |
| GSH | glutathione |
| GTT | glucose tolerance test |
| LOAD | Late-onset Alzheimer’s disease |
| MetS | metabolic syndrome |
| NDDs | neurodegenerative diseases |
| OF | open field |
| SAT | subcutaneous adipose tissue |
| VAT | visceral adipose tissue |
| WD | Western-pattern Diet |
| WT | wild-type |
References
- Farrer, L.A.; Cupples, L.A.; Haines, J.L.; Hyman, B.; Kukull, W.A.; Mayeux, R.; Myers, R.H.; Pericak-Vance, M.A.; Risch, N.; van Duijn, C.M. Effects of Age, Sex, and Ethnicity on the Association between Apolipoprotein E Genotype and Alzheimer Disease. A Meta-Analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA 1997, 278, 1349–1356. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Toldi, J.; Vécsei, L. Exploring the Etiological Links behind Neurodegenerative Diseases: Inflammatory Cytokines and Bioactive Kynurenines. IJMS 2020, 21, 2431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guzman-Martinez, L.; Maccioni, R.B.; Andrade, V.; Navarrete, L.P.; Pastor, M.G.; Ramos-Escobar, N. Neuroinflammation as a Common Feature of Neurodegenerative Disorders. Front. Pharm. 2019, 10, 1008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caldeira, C.; Cunha, C.; Vaz, A.R.; Falcão, A.S.; Barateiro, A.; Seixas, E.; Fernandes, A.; Brites, D. Key Aging-Associated Alterations in Primary Microglia Response to Beta-Amyloid Stimulation. Front. Aging Neurosci. 2017, 9, 277. [Google Scholar] [CrossRef]
- Frontiers|Key Aging-Associated Alterations in Primary Microglia Response to Beta-Amyloid Stimulation|Aging Neuroscience. Available online: https://www.frontiersin.org/articles/10.3389/fnagi.2017.00277/full (accessed on 12 March 2022).
- Baik, S.H.; Kang, S.; Son, S.M.; Mook-Jung, I. Microglia Contributes to Plaque Growth by Cell Death Due to Uptake of Amyloid β in the Brain of Alzheimer’s Disease Mouse Model: Aβ Plaque Formation and Microglial Activation. Glia 2016, 64, 2274–2290. [Google Scholar] [CrossRef]
- Perea, J.R.; Llorens-Martín, M.; Ávila, J.; Bolós, M. The Role of Microglia in the Spread of Tau: Relevance for Tauopathies. Front. Cell. Neurosci. 2018, 12, 172. [Google Scholar] [CrossRef] [Green Version]
- Riedel, B.C.; Thompson, P.M.; Brinton, R.D. Age, APOE and Sex: Triad of Risk of Alzheimer’s Disease. J. Steroid Biochem. Mol. Biol. 2016, 160, 134–147. [Google Scholar] [CrossRef] [Green Version]
- Aono, M.; Lee, Y.; Grant, E.R.; Zivin, R.A.; Pearlstein, R.D.; Warner, D.S.; Bennett, E.R.; Laskowitz, D.T. Apolipoprotein E Protects against NMDA Excitotoxicity. Neurobiol. Dis. 2002, 11, 214–220. [Google Scholar] [CrossRef] [Green Version]
- Altmann, A.; Tian, L.; Henderson, V.W.; Greicius, M.D.; Alzheimer’s Disease Neuroimaging Initiative Investigators. Sex Modifies the APOE-Related Risk of Developing Alzheimer Disease. Ann. Neurol. 2014, 75, 563–573. [Google Scholar] [CrossRef] [Green Version]
- Available online: Alzheimers-Facts-and-Figures-2019-r.Pdf (accessed on 10 January 2022).
- Ungar, L.; Altmann, A.; Greicius, M.D. Apolipoprotein E, Gender, and Alzheimer’s Disease: An Overlooked, but Potent and Promising Interaction. Brain Imaging Behav. 2014, 8, 262–273. [Google Scholar] [CrossRef] [Green Version]
- Yaffe, K.; Haan, M.; Byers, A.; Tangen, C.; Kuller, L. Estrogen Use, APOE, and Cognitive Decline: Evidence of Gene-Environment Interaction. Neurology 2000, 54, 1949–1954. [Google Scholar] [CrossRef] [PubMed]
- Obesity Accelerates Alzheimer-Related Pathology in APOE4 but Not APOE3 Mice|eNeuro. Available online: https://www.eneuro.org/content/4/3/ENEURO.0077-17.2017 (accessed on 10 January 2022).
- Pizzorno, J. Glutathione! Integr. Med. 2014, 13, 8–12. [Google Scholar]
- Hales, C.M.; Carroll, M.D.; Fryar, C.D.; Ogden, C.L. Prevalence of Obesity Among Adults and Youth: United States, 2015–2016. NCHS Data Brief. 2017, 288, 1–8. [Google Scholar]
- Neth, B.J.; Craft, S. Insulin Resistance and Alzheimer’s Disease: Bioenergetic Linkages. Front. Aging Neurosci. 2017, 9, 345. [Google Scholar] [CrossRef] [PubMed]
- Tucsek, Z.; Toth, P.; Sosnowska, D.; Gautam, T.; Mitschelen, M.; Koller, A.; Szalai, G.; Sonntag, W.E.; Ungvari, Z.; Csiszar, A. Obesity in Aging Exacerbates Blood-Brain Barrier Disruption, Neuroinflammation, and Oxidative Stress in the Mouse Hippocampus: Effects on Expression of Genes Involved in Beta-Amyloid Generation and Alzheimer’s Disease. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, 1212–1226. [Google Scholar] [CrossRef]
- Fernández-Matarrubia, M.; Goni, L.; Rognoni, T.; Razquin, C.; Fernández-Lázaro, C.I.; Bes-Rastrollo, M.; Martínez-González, M.Á.; Toledo, E. An Active Lifestyle Is Associated with Better Cognitive Function Over Time in APOE µ4 Non-Carriers. JAD 2021, 79, 1257–1268. [Google Scholar] [CrossRef]
- Xu, H.; Barnes, G.T.; Yang, Q.; Tan, G.; Yang, D.; Chou, C.J.; Sole, J.; Nichols, A.; Ross, J.S.; Tartaglia, L.A.; et al. Chronic Inflammation in Fat Plays a Crucial Role in the Development of Obesity-Related Insulin Resistance. J. Clin. Investig. 2003, 112, 1821–1830. [Google Scholar] [CrossRef]
- Mattson, M.P. Late-Onset Dementia: A Mosaic of Prototypical Pathologies Modifiable by Diet and Lifestyle. NPJ Aging Mech. Dis. 2015, 1, 15003. [Google Scholar] [CrossRef] [Green Version]
- Freeman, L.R.; Granholm, A.-C.E. Vascular Changes in Rat Hippocampus Following a High Saturated Fat and Cholesterol Diet. J. Cereb. Blood Flow Metab. 2012, 32, 643–653. [Google Scholar] [CrossRef] [Green Version]
- Filomeni, G.; Aquilano, K.; Rotilio, G.; Ciriolo, M.R. Reactive Oxygen Species-Dependent c-Jun NH2-Terminal Kinase/c-Jun Signaling Cascade Mediates Neuroblastoma Cell Death Induced by Diallyl Disulfide. Cancer Res. 2003, 63, 5940–5949. [Google Scholar]
- Arbones-Mainar, J.M.; Johnson, L.A.; Altenburg, M.K.; Kim, H.-S.; Maeda, N. Impaired Adipogenic Response to Thiazolidinediones in Mice Expressing Human ApolipoproteinE4. FASEB J. 2010, 24, 3809–3818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arbones-Mainar, J.M.; Johnson, L.A.; Altenburg, M.K.; Maeda, N. Differential Modulation of Diet-Induced Obesity and Adipocyte Functionality by Human Apolipoprotein E3 and E4 in Mice. Int. J. Obes. 2008, 32, 1595–1605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ho, L.; Qin, W.; Pompl, P.N.; Xiang, Z.; Wang, J.; Zhao, Z.; Peng, Y.; Cambareri, G.; Rocher, A.; Mobbs, C.V.; et al. Diet-Induced Insulin Resistance Promotes Amyloidosis in a Transgenic Mouse Model of Alzheimer’s Disease. FASEB J. 2004, 18, 902–904. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.B.; Mattson, M.P. The Neuropathology of Obesity: Insights from Human Disease. Acta Neuropathol. 2014, 127, 3–28. [Google Scholar] [CrossRef]
- Stranahan, A.M.; Mattson, M.P. Recruiting Adaptive Cellular Stress Responses for Successful Brain Ageing. Nat. Rev. Neurosci. 2012, 13, 209–216. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Basak, J.M.; Holtzman, D.M. The Role of Apolipoprotein E in Alzheimer’s Disease. Neuron 2009, 63, 287–303. [Google Scholar] [CrossRef] [Green Version]
- Fouesnard, M.; Zoppi, J.; Petera, M.; Le Gleau, L.; Migné, C.; Devime, F.; Durand, S.; Benani, A.; Chaffron, S.; Douard, V.; et al. Dietary Switch to Western Diet Induces Hypothalamic Adaptation Associated with Gut Microbiota Dysbiosis in Rats. Int. J. Obes. 2021, 45, 1271–1283. [Google Scholar] [CrossRef]
- Crescenzo, R.; Spagnuolo, M.S.; Cancelliere, R.; Iannotta, L.; Mazzoli, A.; Gatto, C.; Iossa, S.; Cigliano, L. Effect of Initial Aging and High-Fat/High-Fructose Diet on Mitochondrial Bioenergetics and Oxidative Status in Rat Brain. Mol. Neurobiol. 2019, 56, 7651–7663. [Google Scholar] [CrossRef]
- Hwang, L.-L.; Wang, C.-H.; Li, T.-L.; Chang, S.-D.; Lin, L.-C.; Chen, C.-P.; Chen, C.-T.; Liang, K.-C.; Ho, I.-K.; Yang, W.-S.; et al. Sex Differences in High-Fat Diet-Induced Obesity, Metabolic Alterations and Learning, and Synaptic Plasticity Deficits in Mice. Obesity 2010, 18, 463–469. [Google Scholar] [CrossRef]
- Hohman, T.J.; Kaczorowski, C.C. Modifiable Lifestyle Factors in Alzheimer Disease: An Opportunity to Transform the Therapeutic Landscape Through Transdisciplinary Collaboration. JAMA Neurol. 2020, 77, 1207–1209. [Google Scholar] [CrossRef]
- Samieri, C.; Sun, Q.; Townsend, M.K.; Chiuve, S.E.; Okereke, O.I.; Willett, W.C.; Stampfer, M.; Grodstein, F. The Association Between Dietary Patterns at Midlife and Health in Aging. Ann. Intern. Med. 2013, 159, 584–591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiȩckowska-Gacek, A.; Mietelska-Porowska, A.; Chutorański, D.; Wydrych, M.; Długosz, J.; Wojda, U. Western Diet Induces Impairment of Liver-Brain Axis Accelerating Neuroinflammation and Amyloid Pathology in Alzheimer’s Disease. Front. Aging Neurosci. 2021, 13, 136. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-C.; Kanekiyo, T.; Xu, H.; Bu, G. Apolipoprotein E and Alzheimer Disease: Risk, Mechanisms and Therapy. Nat. Rev. Neurol. 2013, 9, 106–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holtzman, D.M.; Herz, J.; Bu, G. Apolipoprotein E and Apolipoprotein E Receptors: Normal Biology and Roles in Alzheimer Disease. Cold Spring Harb. Perspect. Med. 2012, 2, a006312. [Google Scholar] [CrossRef] [Green Version]
- Denver, P.; Gault, V.A.; McClean, P.L. Sustained High-Fat Diet Modulates Inflammation, Insulin Signalling and Cognition in Mice and a Modified Xenin Peptide Ameliorates Neuropathology in a Chronic High-Fat Model. Diabetes Obes. Metab. 2018, 20, 1166–1175. [Google Scholar] [CrossRef]
- Prasad, K.N.; Bondy, S.C. Inhibition of Early Upstream Events in Prodromal Alzheimer’s Disease by Use of Targeted Antioxidants. Curr. Aging Sci. 2014, 7, 77–90. [Google Scholar] [CrossRef] [Green Version]
- Mandal, P.K.; Tripathi, M.; Sugunan, S. Brain Oxidative Stress: Detection and Mapping of Anti-Oxidant Marker ‘Glutathione’ in Different Brain Regions of Healthy Male/Female, MCI and Alzheimer Patients Using Non-Invasive Magnetic Resonance Spectroscopy. Biochem. Biophys. Res. Commun. 2012, 417, 43–48. [Google Scholar] [CrossRef]
- Yassine, H.N.; Finch, C.E. APOE Alleles and Diet in Brain Aging and Alzheimer’s Disease. Front. Aging Neurosci. 2020, 12, 150. [Google Scholar] [CrossRef]
- Udeh-Momoh, C.; Watermeyer, T. Female Specific Risk Factors for the Development of Alzheimer’s Disease Neuropathology and Cognitive Impairment: Call for a Precision Medicine Approach. Ageing Res. Rev. 2021, 71, 101459. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H.; Rinna, A. Glutathione: Overview of Its Protective Roles, Measurement, and Biosynthesis. Mol. Asp. Med. 2009, 30, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Genin, E.; Hannequin, D.; Wallon, D.; Sleegers, K.; Hiltunen, M.; Combarros, O.; Bullido, M.J.; Engelborghs, S.; De Deyn, P.; Berr, C.; et al. APOE and Alzheimer Disease: A Major Gene with Semi-Dominant Inheritance. Mol. Psychiatry 2011, 16, 903–907. [Google Scholar] [CrossRef]
- Absolute 10-Year Risk of Dementia by Age, Sex and APOE Genotype: A Population-Based Cohort Study|CMAJ. Available online: https://www.cmaj.ca/content/190/35/E1033 (accessed on 10 January 2022).
- Eskelinen, M.H.; Ngandu, T.; Tuomilehto, J.; Soininen, H.; Kivipelto, M. Midlife Healthy-Diet Index and Late-Life Dementia and Alzheimer’s Disease. DEE 2011, 1, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Forman, H.J. Glutathione Synthesis and Its Role in Redox Signaling. Semin. Cell Dev. Biol. 2012, 23, 722–728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walker, J.M.; Dixit, S.; Saulsberry, A.C.; May, J.M.; Harrison, F.E. Reversal of High Fat Diet-Induced Obesity Improves Glucose Tolerance, Inflammatory Response, β-Amyloid Accumulation and Cognitive Decline in the APP/PSEN1 Mouse Model of Alzheimer’s Disease. Neurobiol. Dis. 2017, 100, 87–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Metabolic Disturbances of a High-Fat Diet Are Dependent on APOE Genotype and Sex|eNeuro. Available online: https://www.eneuro.org/content/6/5/ENEURO.0267-19.2019 (accessed on 10 January 2022).
- Effects of Apolipoprotein E isoform, Sex, and Diet on Insulin BBB Pharmacokinetics in Mice|Scientific Reports. Available online: https://www.nature.com/articles/s41598-021-98061-1 (accessed on 10 January 2022).
- Więckowska-Gacek, A.; Mietelska-Porowska, A.; Wydrych, M.; Wojda, U. Western Diet as a Trigger of Alzheimer’s Disease: From Metabolic Syndrome and Systemic Inflammation to Neuroinflammation and Neurodegeneration. Ageing Res. Rev. 2021, 70, 101397. [Google Scholar] [CrossRef]
- Hadaegh, F.; Hasheminia, M.; Lotfaliany, M.; Mohebi, R.; Azizi, F.; Tohidi, M. Incidence of Metabolic Syndrome over 9 Years Follow-Up; the Importance of Sex Differences in the Role of Insulin Resistance and Other Risk Factors. PLoS ONE 2013, 8, e76304. [Google Scholar] [CrossRef]
- Pradhan, A.D. Sex Differences in the Metabolic Syndrome: Implications for Cardiovascular Health in Women. Clin. Chem. 2014, 60, 44–52. [Google Scholar] [CrossRef] [Green Version]
- Torres-Perez, E.; Ledesma, M.; Garcia-Sobreviela, M.P.; Leon-Latre, M.; Arbones-Mainar, J.M. Apolipoprotein E4 Association with Metabolic Syndrome Depends on Body Fatness. Atherosclerosis 2016, 245, 35–42. [Google Scholar] [CrossRef]
- Prasad, K.N. Oxidative Stress and Pro-Inflammatory Cytokines May Act as One of the Signals for Regulating MicroRNAs Expression in Alzheimer’s Disease. Mech. Ageing Dev. 2017, 162, 63–71. [Google Scholar] [CrossRef]
- Barron, A.M.; Rosario, E.R.; Elteriefi, R.; Pike, C.J. Sex-Specific Effects of High Fat Diet on Indices of Metabolic Syndrome in 3xTg-AD Mice: Implications for Alzheimer’s Disease. PLoS ONE 2013, 8, e78554. [Google Scholar] [CrossRef]
- Christensen, A.; Pike, C.J. APOE Genotype Affects Metabolic and Alzheimer-Related Outcomes Induced by Western Diet in Female EFAD Mice. FASEB J. 2019, 33, 4054–4066. [Google Scholar] [CrossRef] [PubMed]
- Porter, S.A.; Massaro, J.M.; Hoffmann, U.; Vasan, R.S.; O’Donnel, C.J.; Fox, C.S. Abdominal Subcutaneous Adipose Tissue: A Protective Fat Depot? Diabetes Care 2009, 32, 1068–1075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Writing Committee; Unwin, N.; Shaw, J.; Zimmet, P.; Alberti, K.G.M.M. Impaired Glucose Tolerance and Impaired Fasting Glycaemia: The Current Status on Definition and Intervention. Diabet. Med. 2002, 19, 708–723. [Google Scholar] [CrossRef]
- Pond, C.M. An Evolutionary and Functional View of Mammalian Adipose Tissue. Proc. Nutr. Soc. 1992, 51, 367–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fox, C.S.; Massaro, J.M.; Hoffmann, U.; Pou, K.M.; Maurovich-Horvat, P.; Liu, C.-Y.; Vasan, R.S.; Murabito, J.M.; Meigs, J.B.; Cupples, L.A.; et al. Abdominal Visceral and Subcutaneous Adipose Tissue Compartments: Association with Metabolic Risk Factors in the Framingham Heart Study. Circulation 2007, 116, 39–48. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Fox, C.S.; Hickson, D.A.; May, W.D.; Hairston, K.G.; Carr, J.J.; Taylor, H.A. Impact of Abdominal Visceral and Subcutaneous Adipose Tissue on Cardiometabolic Risk Factors: The Jackson Heart Study. J. Clin. Endocrinol. Metab. 2010, 95, 5419–5426. [Google Scholar] [CrossRef] [Green Version]
- Tran, T.T.; Yamamoto, Y.; Gesta, S.; Kahn, C.R. Beneficial Effects of Subcutaneous Fat Transplantation on Metabolism. Cell Metab. 2008, 7, 410–420. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Yegla, B.; Foster, T.C. Redox Signaling in Neurotransmission and Cognition During Aging. Antioxid Redox Signal. 2018, 28, 1724–1745. [Google Scholar] [CrossRef] [Green Version]
- When Are Mice Considered Old? Available online: https://www.jax.org/news-and-insights/jax-blog/2017/november/when-are-mice-considered-old# (accessed on 16 February 2022).
- Lemieux, S.; Prud’homme, D.; Bouchard, C.; Tremblay, A.; Després, J.P. Sex Differences in the Relation of Visceral Adipose Tissue Accumulation to Total Body Fatness. Am. J. Clin. Nutr. 1993, 58, 463–467. [Google Scholar] [CrossRef]
- Lovejoy, J.C.; Champagne, C.M.; de Jonge, L.; Xie, H.; Smith, S.R. Increased Visceral Fat and Decreased Energy Expenditure during the Menopausal Transition. Int. J. Obes. 2008, 32, 949–958. [Google Scholar] [CrossRef] [Green Version]
- Jones, N.; Rebeck, G. The Synergistic Effects of APOE Genotype and Obesity on Alzheimer’s Disease Risk. IJMS 2018, 20, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pontifex, M.G.; Martinsen, A.; Saleh, R.N.M.; Harden, G.; Tejera, N.; Müller, M.; Fox, C.; Vauzour, D.; Minihane, A. APOE4 Genotype Exacerbates the Impact of Menopause on Cognition and Synaptic Plasticity in APOE-TR Mice. FASEB J. 2021, 35, e21583. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mattar, J.M.; Majchrzak, M.; Iannucci, J.; Bartman, S.; Robinson, J.K.; Grammas, P. Sex Differences in Metabolic Indices and Chronic Neuroinflammation in Response to Prolonged High-Fat Diet in ApoE4 Knock-In Mice. Int. J. Mol. Sci. 2022, 23, 3921. https://doi.org/10.3390/ijms23073921
Mattar JM, Majchrzak M, Iannucci J, Bartman S, Robinson JK, Grammas P. Sex Differences in Metabolic Indices and Chronic Neuroinflammation in Response to Prolonged High-Fat Diet in ApoE4 Knock-In Mice. International Journal of Molecular Sciences. 2022; 23(7):3921. https://doi.org/10.3390/ijms23073921
Chicago/Turabian StyleMattar, Jennifer M., Mark Majchrzak, Jaclyn Iannucci, Sydney Bartman, John K. Robinson, and Paula Grammas. 2022. "Sex Differences in Metabolic Indices and Chronic Neuroinflammation in Response to Prolonged High-Fat Diet in ApoE4 Knock-In Mice" International Journal of Molecular Sciences 23, no. 7: 3921. https://doi.org/10.3390/ijms23073921






