Site-Directed Immobilization of an Engineered Bone Morphogenetic Protein 2 (BMP2) Variant to Collagen-Based Microspheres Induces Bone Formation In Vivo
Abstract
:1. Introduction
2. Results
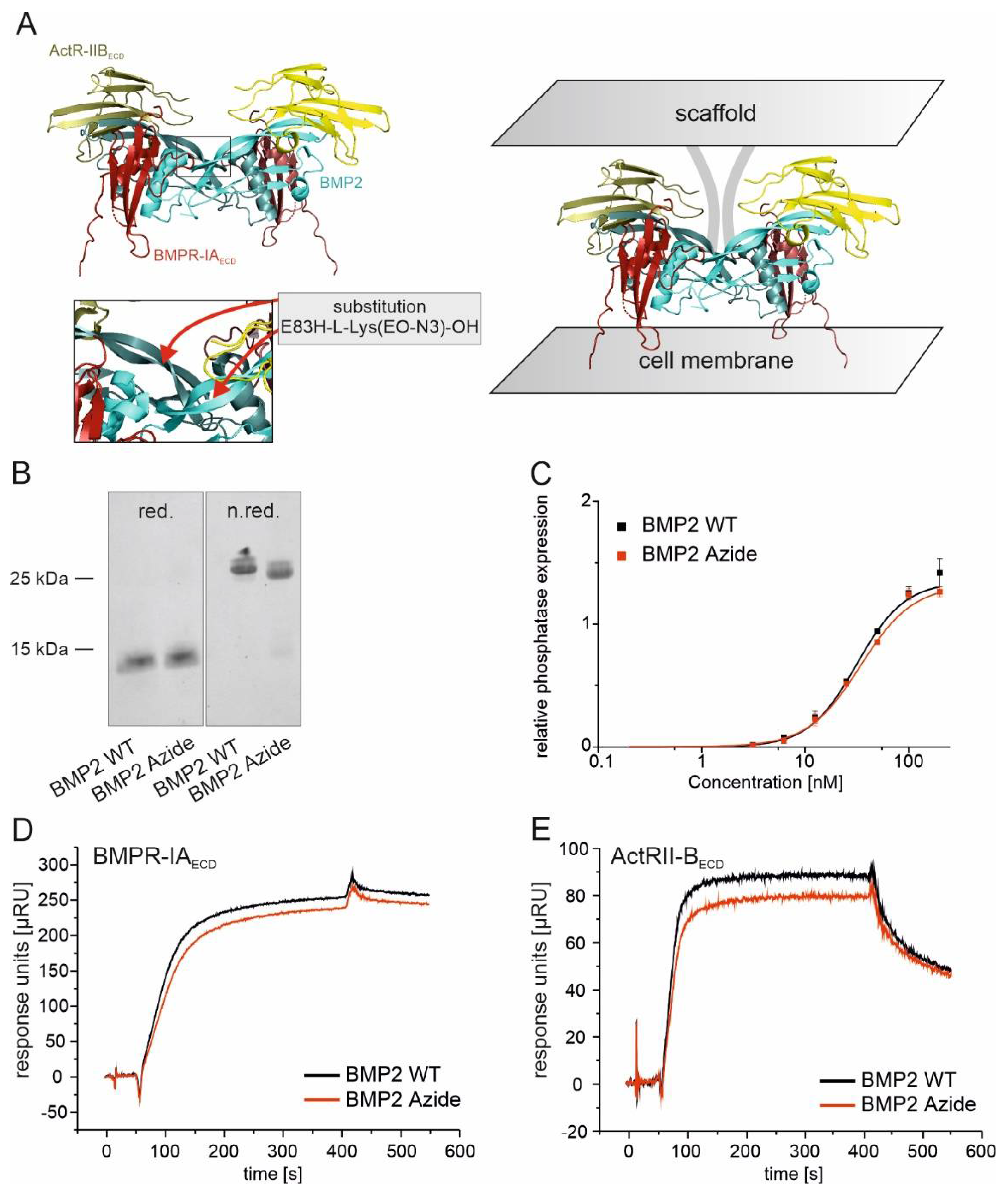
2.1. BMP2 WT and BMP2 Azide Bind to BMP Receptors with Comparable Binding Affinities In Vitro
2.2. BMP2 Azide Can Be Efficiently and Specifically Coupled to a DBCO Functionalized Fluorophore by a Copper-Free SPAAC Reaction
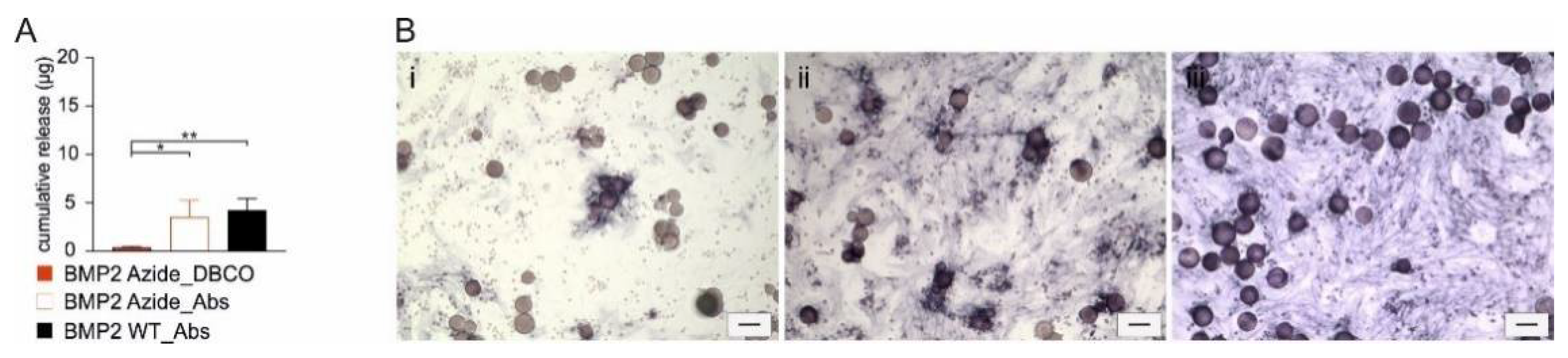
2.3. BMP2 Azide Can Be Coupled to Collagen-Based Microspheres with High Efficiency
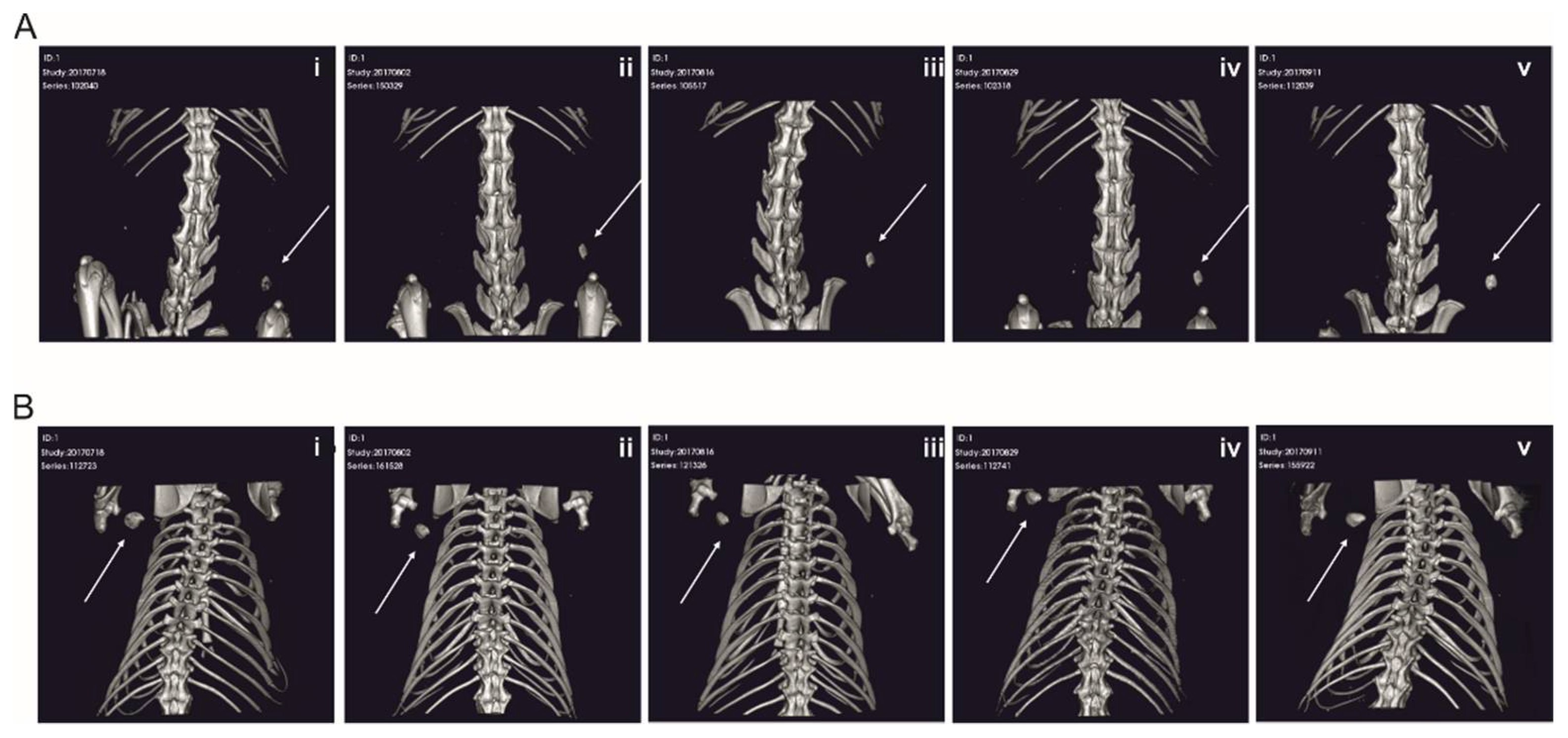
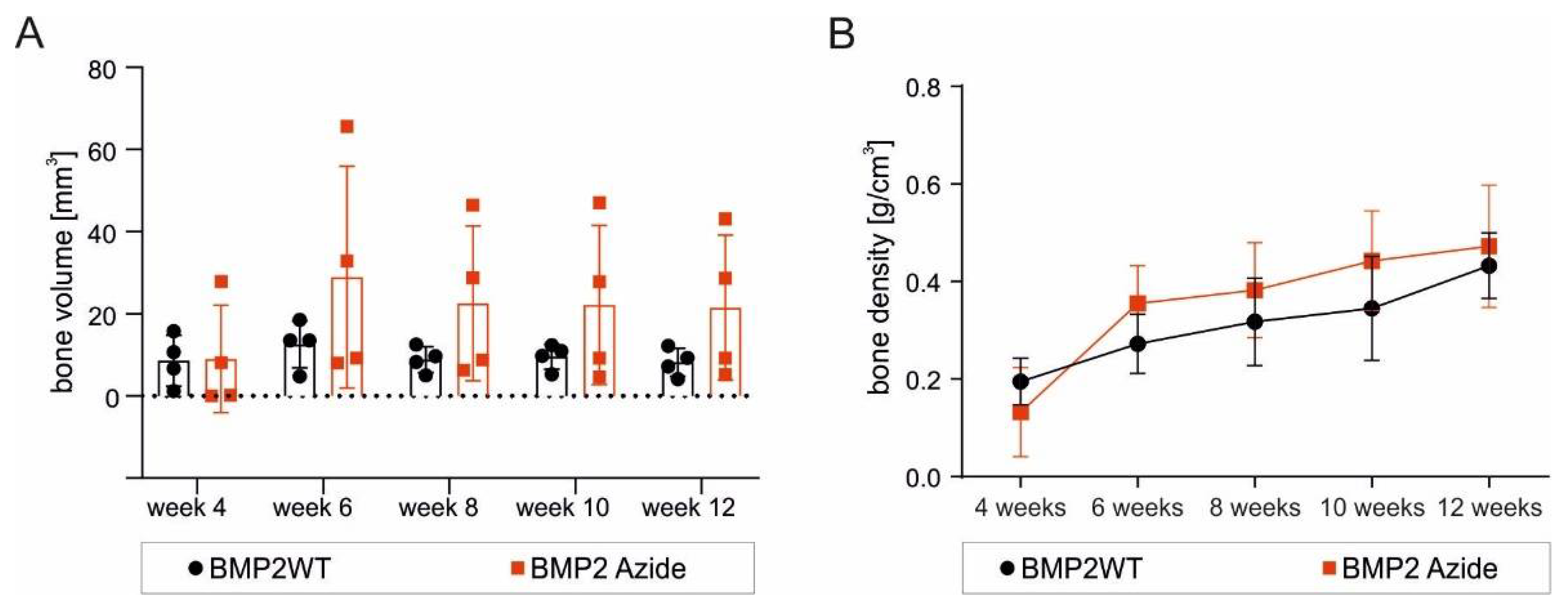
2.4. RCP Microspheres Functionalized with the Covalently Coupled BMP2 Azide Induce Ectopic Bone Formation with Similar Bone Volume and Density as Ad/Absorbed BMP2 WT
2.5. The Morphology of the De-Novo-Induced Bone Tissue Highly Varies Depending on the Specific Immobilization Technique
3. Discussion
3.1. BMP2 Azide: A New BMP2 Variant Optimized for Site-Directed Coupling to Scaffolds
3.2. Induction of ALP Expression in C2C12 Cells by RCP with Covalently Coupled BMP2 Azide Is Restricted to Cells in Direct Contact
3.3. The Delivery Methods Significantly Affect the Morphology of the Ectopically Formed Bone
4. Materials and Methods
4.1. Cloning, Expression and Purification of BMP2 Azide
4.2. Analyses of BMP2 Azide
4.2.1. SDS Gel Electrophoresis and Coomassie Brilliant Blue Staining
4.2.2. Alkaline Phosphatase Assay
4.2.3. Surface Plasmon Resonance (SPR) Spectroscopy
4.3. Coupling of BMP2 Azide by Strain-Promoted Azide–Alkyne Cycloaddition (SPAAC) to a Dibenzocyclooctyne (DBCO)-Functionalized Fluorophore
4.4. Coupling of BMP2 Azide to DBCO-Functionalized Microspheres
4.5. Quantification of Immobilized BMP2 Azide
4.6. Injection of a Paste Containing BMP2-Functionalized Microspheres in a Subcutaneous Rat Model
4.7. Micro-Computed Tomography (Micro-CT) Analysis
4.8. Evaluation of Bone Density and Bone Volume
4.9. Histological Analysis of Explanted Implants
4.10. Statistics
4.11. Study Approval
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nauth, A.; Schemitsch, E.; Norris, B.; Nollin, Z.; Watson, J.T. Critical-Size Bone Defects: Is There a Consensus for Diagnosis and Treatment? J. Orthop. Trauma 2018, 32 (Suppl. S1), S7–S11. [Google Scholar] [CrossRef] [PubMed]
- Schemitsch, E.H. Size Matters: Defining Critical in Bone Defect Size! J. Orthop. Trauma 2017, 31 (Suppl. S5), S20–S22. [Google Scholar] [CrossRef]
- Dimitriou, R.; Jones, E.; McGonagle, D.; Giannoudis, P.V. Bone regeneration: Current concepts and future directions. BMC Med. 2011, 9, 66. [Google Scholar] [CrossRef] [Green Version]
- Bishop, G.B.; Einhorn, T.A. Current and future clinical applications of bone morphogenetic proteins in orthopaedic trauma surgery. Int. Orthop. 2007, 31, 721–727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poynton, A.R.; Lane, J.M. Safety Profile for the Clinical Use of Bone Morphogenetic Proteins in the Spine. Spine 2002, 27, S40–S48. [Google Scholar] [CrossRef] [PubMed]
- Mumcuoglu, D.; Siverino, C.; Tabisz, B.; Kluijtmans, B.; Nickel, J. How to use BMP-2 for clinical applications? A review on pros and cons of existing delivery strategies. J. Transl. Sci. 2017, 3, 1–11. [Google Scholar] [CrossRef]
- Burkus, J.K.; Transfeldt, E.E.; Kitchel, S.H.; Watkins, R.G.; Balderston, R.A. Clinical and Radiographic Outcomes of Anterior Lumbar Interbody Fusion Using Recombinant Human Bone Morphogenetic Protein-2. Spine 2002, 27, 2396–2408. [Google Scholar] [CrossRef] [PubMed]
- Govender, S.; Csimma, C.; Genant, H.K.; Valentin-Opran, A.; Amit, Y.; Arbel, R.; Aro, H.; Atar, D.; Bishay, M.; Börner, M.G.; et al. Recombinant human bone morphogenetic protein-2 for treatment of open tibial fractures: A prospective, controlled, randomized study of four hundred and fifty patients. J. Bone Jt. Surg. Am. 2002, 84, 2123–2134. [Google Scholar] [CrossRef] [PubMed]
- Boyne, P.J.; Marx, R.E.; Nevins, M.; Triplett, G.; Lazaro, E.; Lilly, L.C.; Alder, M.; Nummikoski, P. A feasibility study evaluating rhBMP-2/absorbable collagen sponge for maxillary sinus floor augmentation. Int. J. Periodontics Restor. Dent. 1997, 17, 11–25. [Google Scholar]
- King, W.J.; Krebsbach, P.H. Growth factor delivery: How surface interactions modulate release in vitro and in vivo. Adv. Drug Deliv. Rev. 2012, 64, 1239–1256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seeherman, H.; Wozney, J.M. Delivery of bone morphogenetic proteins for orthopedic tissue regeneration. Cytokine Growth Factor Rev. 2005, 16, 329–345. [Google Scholar] [CrossRef] [PubMed]
- Gothard, D.; Smith, E.; Kanczler, J.; Rashidi, H.; Qutachi, O.; Henstock, J.; Rotherham, M.; El Haj, A.; Shakesheff, K.; Oreffo, R. Tissue engineered bone using select growth factors: A comprehensive review of animal studies and clinical translation studies in man. Eur. Cells Mater. 2014, 28, 166–208. [Google Scholar] [CrossRef]
- Agarwal, R.; García, A.J. Biomaterial strategies for engineering implants for enhanced osseointegration and bone repair. Adv. Drug Deliv. Rev. 2015, 94, 53–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bessa, P.C.; Casal, M.; Reis, R.L. Bone morphogenetic proteins in tissue engineering: The road from laboratory to clinic, part II (BMP delivery). J. Tissue Eng. Regen. Med. 2008, 2, 81–96. [Google Scholar] [CrossRef] [PubMed]
- Seeherman, H.; Wozney, J.; Li, R. Bone Morphogenetic Protein Delivery Systems. Spine 2002, 27, S16–S23. [Google Scholar] [CrossRef] [PubMed]
- Thanyaphoo, S.; Kaewsrichan, J. A new biocompatible delivery scaffold containing heparin and bone morphogenetic protein 2. Acta Pharm. 2016, 66, 373–385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gadalla, D.; Goldstein, A.S. Improving the Osteogenicity of PCL Fiber Substrates by Surface-Immobilization of Bone Morphogenic Protein-2. Ann. Biomed. Eng. 2019, 48, 1006–1015. [Google Scholar] [CrossRef] [PubMed]
- Terpe, K. Overview of tag protein fusions: From molecular and biochemical fundamentals to commercial systems. Appl. Microbiol. Biotechnol. 2003, 60, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, J.; Wang, X.; Chen, B.; Xiao, Z.; Shi, C.; Wei, Z.; Hou, X.; Wang, Q.; Dai, J. The osteogenic effect of bone morphogenetic protein-2 on the collagen scaffold conjugated with antibodies. J. Control. Release 2010, 141, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Pohl, T.L.M.; Schwab, E.H.; Cavalcanti-Adam, E.A. Covalent Binding of BMP-2 on Surfaces Using a Self-assembled Monolayer Approach. J. Vis. Exp. 2013, 78, e50842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, J.; Duppatla, V.; Harth, S.; Schmitz, W.; Sebald, W. Site-Specific PEGylation of Bone Morphogenetic Protein-2 Cysteine Analogues. Bioconjug. Chem. 2010, 21, 1762–1772. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Park, Y.J.; Lee, J.Y.; Ku, Y.; Lee, S.J.; Min, B.M.; Chung, C.P. Immobilization of bone morphogenetic protein-2 on a nanofibrous chitosan membrane for enhanced guided bone regeneration. Biotechnol. Appl. Biochem. 2006, 43, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Budiraharjo, R.; Neoh, K.G.; Kang, E.-T. Enhancing bioactivity of chitosan film for osteogenesis and wound healing by covalent immobilization of BMP-2 or FGF-2. J. Biomater. Sci. Polym. Ed. 2012, 24, 645–662. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Yu, Y.; Zhang, W.; Pan, Y.; Wang, J.; Xiao, Y.; Liu, C. Tuning the bioactivity of bone morphogenetic protein-2 with surface immobilization strategies. Acta Biomater. 2018, 80, 108–120. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Tan, B.; Bao, Z.; Wang, S.; Tang, R.; Wang, Z.; Chen, G.; Chen, S.; Lu, W.W.; Yang, D.; et al. Enhanced bone regeneration via spatiotemporal and controlled delivery of a genetically engineered BMP-2 in a composite Hydrogel. Biomaterials 2021, 277, 121117. [Google Scholar] [CrossRef] [PubMed]
- Wiemann, M.; Jennissen, H.P.; Rumpf, H.; Winkler, L.; Chatzinikolaidou, M.; Schmitz, I.; Bingmann, D. A reporter-cell assay for the detection of BMP-2 immobilized on porous and nonporous materials. J. Biomed. Mater. Res. 2002, 62, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Migliorini, E.; Valat, A.; Picart, C.; Cavalcanti-Adam, E.A. Tuning cellular responses to BMP-2 with material surfaces. Cytokine Growth Factor Rev. 2015, 27, 43–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Bialy, I.; Jiskoot, W.; Reza Nejadnik, M. Formulation, Delivery and Stability of Bone Morphogenetic Proteins for Effective Bone Regeneration. Pharm. Res. 2017, 34, 1152–1170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, J.; Schultz, P.G. A chemical toolkit for proteins—An expanded genetic code. Nat. Rev. Mol. Cell Biol. 2006, 7, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Gunnoo, S.B.; Madder, A. Chemical Protein Modification through Cysteine. ChemBioChem 2016, 17, 529–553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weiner, M.P.; Costa, G.L.; Schoettlin, W.; Cline, J.; Mathur, E.; Bauer, J.C. Site-directed mutagenesis of double-stranded DNA by the polymerase chain reaction. Gene 1994, 151, 119–123. [Google Scholar] [CrossRef]
- Foley, T.L.; Burkart, M.D. Site-specific protein modification: Advances and applications. Curr. Opin. Chem. Biol. 2007, 11, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Jennissen, H.P.; Laub, M. Development of an universal affinity fusion tag (Poly-DOPA) for immobilizing recombinant proteins on biomaterials. Mater. Und Werkst. 2007, 38, 1035–1039. [Google Scholar] [CrossRef]
- Tabisz, B.; Schmitz, W.; Schmitz, M.; Luehmann, T.; Heusler, E.; Rybak, J.-C.; Meinel, L.; Fiebig, J.E.; Mueller, T.D.; Nickel, J. Site-Directed Immobilization of BMP-2: Two Approaches for the Production of Innovative Osteoinductive Scaffolds. Biomacromolecules 2017, 18, 695–708. [Google Scholar] [CrossRef] [PubMed]
- McKay, C.; Finn, M. Click Chemistry in Complex Mixtures: Bioorthogonal Bioconjugation. Chem. Biol. 2014, 21, 1075–1101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tabisz, B. Site Directed Immobilization of BMP2: Two Approaches for the Production of Osteoinductive Scaffolds. Ph.D. Thesis, University Hospital Würzburg, Würzburg, Germany, 2016. [Google Scholar]
- Kim, E.; Koo, H. Biomedical applications of copper-free click chemistry: In vitro, in vivo, and ex vivo. Chem. Sci. 2019, 10, 7835–7851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weber, D.; Kotzsch, A.; Nickel, J.; Harth, S.; Seher, A.; Mueller, U.; Sebald, W.; Mueller, T.D. A silent H-bond can be mutationally activated for high-affinity interaction of BMP-2 and activin type IIB receptor. BMC Struct. Biol. 2007, 7, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Depprich, R.; Handschel, J.; Sebald, W.; Kubler, N.R.; Wurzler, K.K. Comparison of the osteogenic activity of bone morphogenetic protein (BMP) mutants. Mund Kiefer Gesichtschir. 2005, 9, 363–368. [Google Scholar] [CrossRef]
- Park, S.H.; Kwon, J.S.; Lee, B.S.; Park, J.H.; Lee, B.K.; Yun, J.-H.; Lee, B.Y.; Kim, J.H.; Min, B.H.; Yoo, T.H.; et al. BMP2-modified injectable hydrogel for osteogenic differentiation of human periodontal ligament stem cells. Sci. Rep. 2017, 7, 6603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, J.; Zhao, C.; Lin, W.; Hu, R.; Wang, Q.; Chen, H.; Li, L.; Chen, S.; Zheng, J. Binding characteristics between polyethylene glycol (PEG) and proteins in aqueous solution. J. Mater. Chem. B 2014, 2, 2983–2992. [Google Scholar] [CrossRef]
- Wang, Y.; Annunziata, O. Comparison between Protein−Polyethylene Glycol (PEG) Interactions and the Effect of PEG on Protein−Protein Interactions Using the Liquid−Liquid Phase Transition. J. Phys. Chem. B 2007, 111, 1222–1230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mumcuoglu, D.; de Miguel, L.; Jekhmane, S.; Siverino, C.; Nickel, J.; Mueller, T.D.; van Leeuwen, J.P.; van Osch, G.J.; Kluijtmans, S.G. Collagen I derived recombinant protein microspheres as novel delivery vehicles for bone morphogenetic protein-2. Mater. Sci. Eng. C 2018, 84, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Hartung, A.; Bitton-Worms, K.; Rechtman, M.M.; Wenzel, V.; Boergermann, J.H.; Hassel, S.; Henis, Y.I.; Knaus, P. Different Routes of Bone Morphogenic Protein (BMP) Receptor Endocytosis Influence BMP Signaling. Mol. Cell. Biol. 2006, 26, 7791–7805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paarmann, P.; Dörpholz, G.; Fiebig, J.; Amsalem, A.R.; Ehrlich, M.; Henis, Y.I.; Müller, T.; Knaus, P. Dynamin-dependent endocytosis of Bone Morphogenetic Protein2 (BMP2) and its receptors is dispensable for the initiation of Smad signaling. Int. J. Biochem. Cell Biol. 2016, 76, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.K.; Raggatt, L.-J.; Alexander, K.; Kuliwaba, J.; Fazzalari, N.L.; Schroder, K.; Maylin, E.R.; Ripoll, V.M.; Hume, D.; Pettit, A. Osteal Tissue Macrophages Are Intercalated throughout Human and Mouse Bone Lining Tissues and Regulate Osteoblast Function In Vitro and In Vivo. J. Immunol. 2008, 181, 1232–1244. [Google Scholar] [CrossRef] [Green Version]
- Miron, R.J.; Bosshardt, D.D. OsteoMacs: Key players around bone biomaterials. Biomaterials 2016, 82, 1–19. [Google Scholar] [CrossRef]
- Siverino, C.; Tabisz, B.; Lühmann, T.; Meinel, L.; Müller, T.; Walles, H.; Nickel, J. Site-Directed Immobilization of Bone Morphogenetic Protein 2 to Solid Surfaces by Click Chemistry. J. Vis. Exp. 2018, 29, e56616. [Google Scholar] [CrossRef]
- Katagiri, T.; Yamaguchi, A.; Komaki, M.; Abe, E.; Takahashi, N.; Ikeda, T.; Rosen, V.; Wozney, J.M.; Fujisawa-Sehara, A.; Suda, T. Bone morphogenetic protein-2 converts the differentiation pathway of C2C12 myoblasts into the osteoblast lineage. J. Cell Biol. 1994, 127, 1755–1766. [Google Scholar] [CrossRef] [Green Version]
- Confalonieri, D.; La Marca, M.; Van Dongen, E.M.W.M.; Walles, H.; Ehlicke, F. An Injectable Recombinant Collagen I Peptide–Based Macroporous Microcarrier Allows Superior Expansion of C2C12 and Human Bone Marrow-Derived Mesenchymal Stromal Cells and Supports Deposition of Mineralized Matrix. Tissue Eng. Part A 2017, 23, 946–957. [Google Scholar] [CrossRef]
- Pawelec, K.M.; Confalonieri, D.; Ehlicke, F.; Van Boxtel, H.A.; Walles, H.; Kluijtmans, S.G. Osteogenesis and mineralization of mesenchymal stem cells in collagen type I-based recombinant peptide scaffolds. J. Biomed. Mater. Res. Part A 2017, 105, 1856–1866. [Google Scholar] [CrossRef] [PubMed]
- Suarez Munoz, M.; Confalonieri, D.; Walles, H.; van Dongen, E.; Dandekar, G. Recombinant Collagen I Peptide Microcarriers for Cell Expansion and Their Potential Use As Cell Delivery System in a Bioreactor Model. J. Vis. Exp. 2018, 7, e57363. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siverino, C.; Fahmy-Garcia, S.; Mumcuoglu, D.; Oberwinkler, H.; Muehlemann, M.; Mueller, T.; Farrell, E.; van Osch, G.J.V.M.; Nickel, J. Site-Directed Immobilization of an Engineered Bone Morphogenetic Protein 2 (BMP2) Variant to Collagen-Based Microspheres Induces Bone Formation In Vivo. Int. J. Mol. Sci. 2022, 23, 3928. https://doi.org/10.3390/ijms23073928
Siverino C, Fahmy-Garcia S, Mumcuoglu D, Oberwinkler H, Muehlemann M, Mueller T, Farrell E, van Osch GJVM, Nickel J. Site-Directed Immobilization of an Engineered Bone Morphogenetic Protein 2 (BMP2) Variant to Collagen-Based Microspheres Induces Bone Formation In Vivo. International Journal of Molecular Sciences. 2022; 23(7):3928. https://doi.org/10.3390/ijms23073928
Chicago/Turabian StyleSiverino, Claudia, Shorouk Fahmy-Garcia, Didem Mumcuoglu, Heike Oberwinkler, Markus Muehlemann, Thomas Mueller, Eric Farrell, Gerjo J. V. M. van Osch, and Joachim Nickel. 2022. "Site-Directed Immobilization of an Engineered Bone Morphogenetic Protein 2 (BMP2) Variant to Collagen-Based Microspheres Induces Bone Formation In Vivo" International Journal of Molecular Sciences 23, no. 7: 3928. https://doi.org/10.3390/ijms23073928
APA StyleSiverino, C., Fahmy-Garcia, S., Mumcuoglu, D., Oberwinkler, H., Muehlemann, M., Mueller, T., Farrell, E., van Osch, G. J. V. M., & Nickel, J. (2022). Site-Directed Immobilization of an Engineered Bone Morphogenetic Protein 2 (BMP2) Variant to Collagen-Based Microspheres Induces Bone Formation In Vivo. International Journal of Molecular Sciences, 23(7), 3928. https://doi.org/10.3390/ijms23073928





