Vitamin D3 Metabolic Enzymes in the Porcine Uterus: Expression, Localization and Autoregulation by 1,25(OH)2D3 In Vitro
Abstract
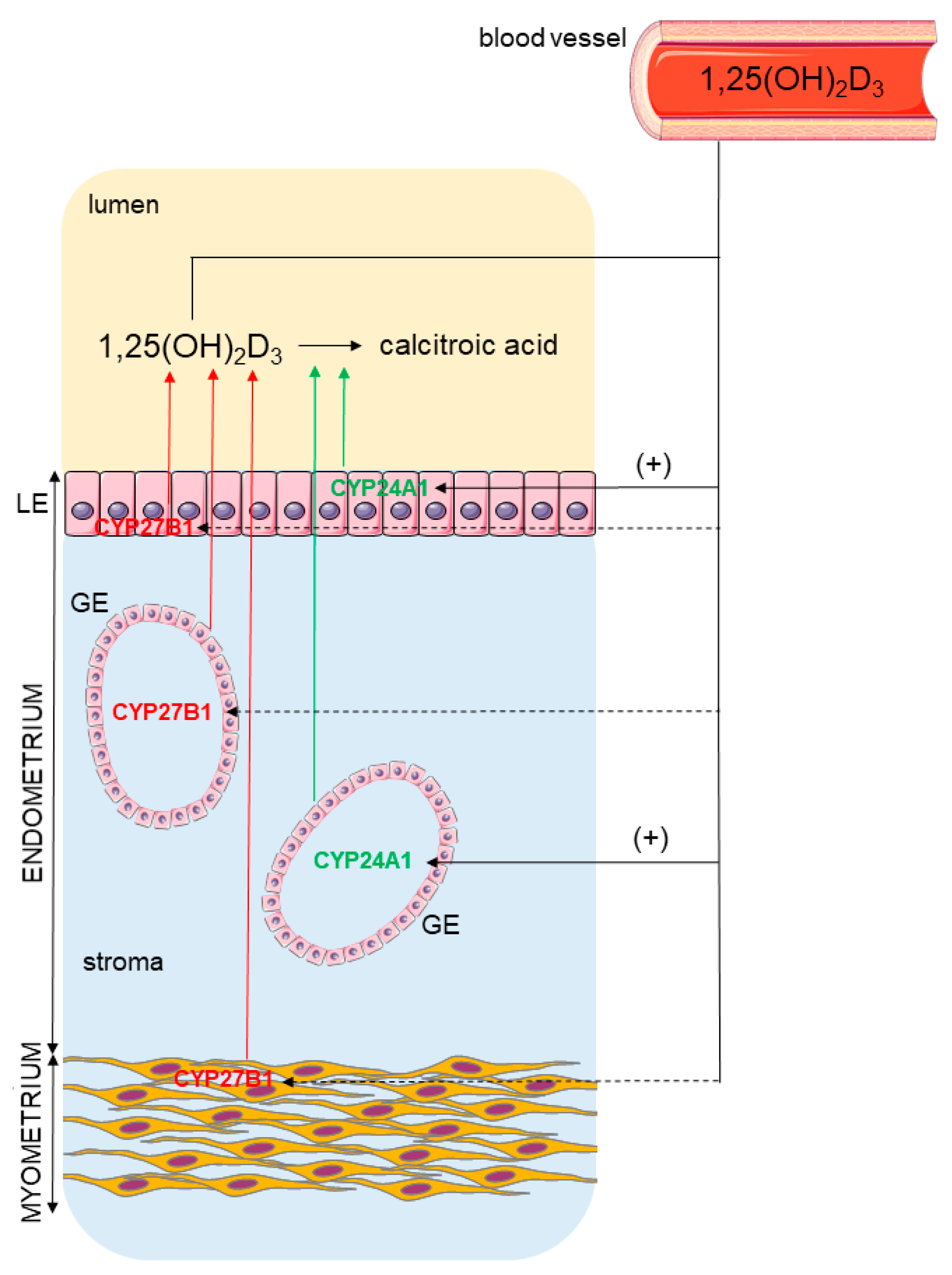
:1. Introduction
2. Results
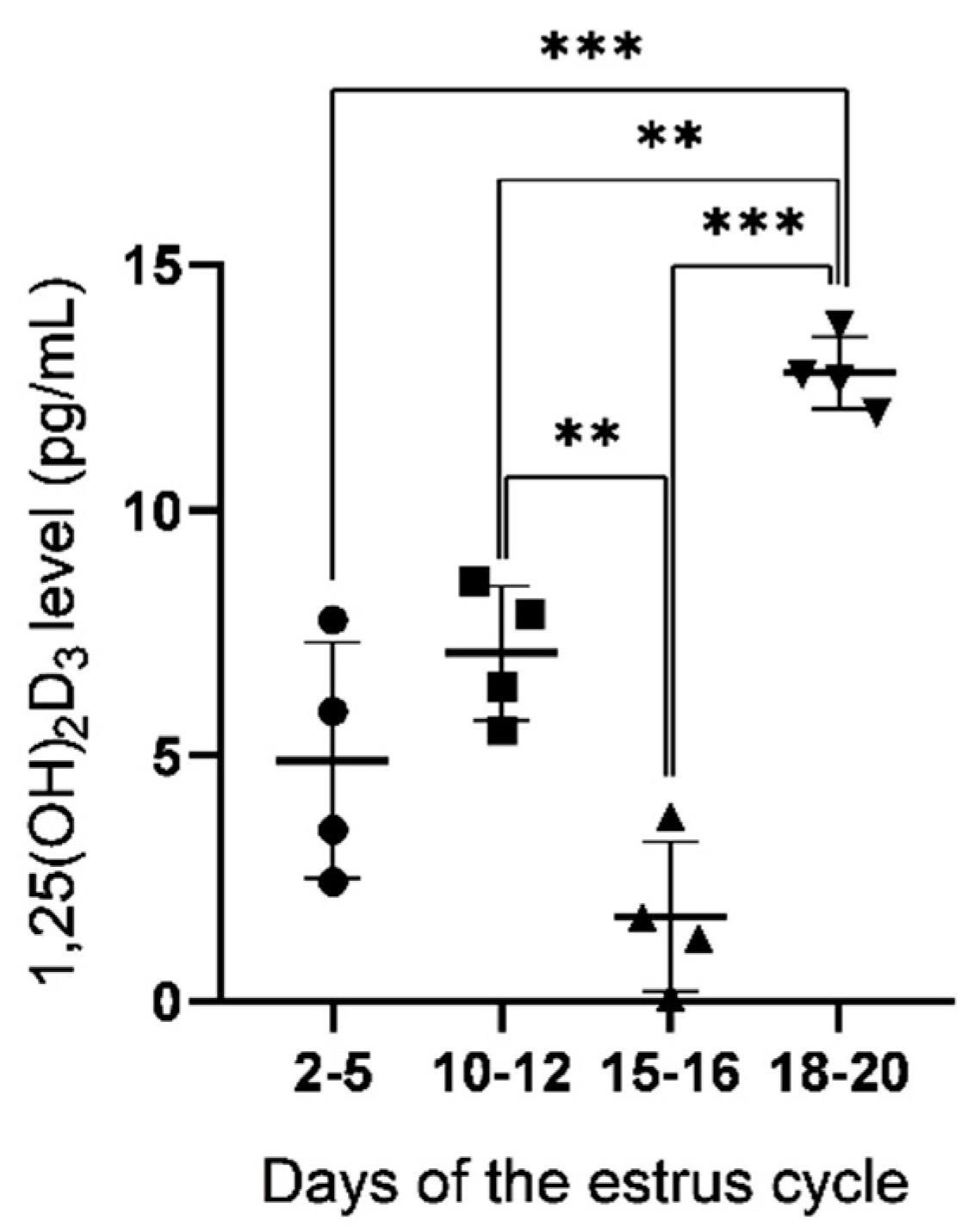
2.1. Concentration of 1,25(OH)2D3 in Uterine Flushings
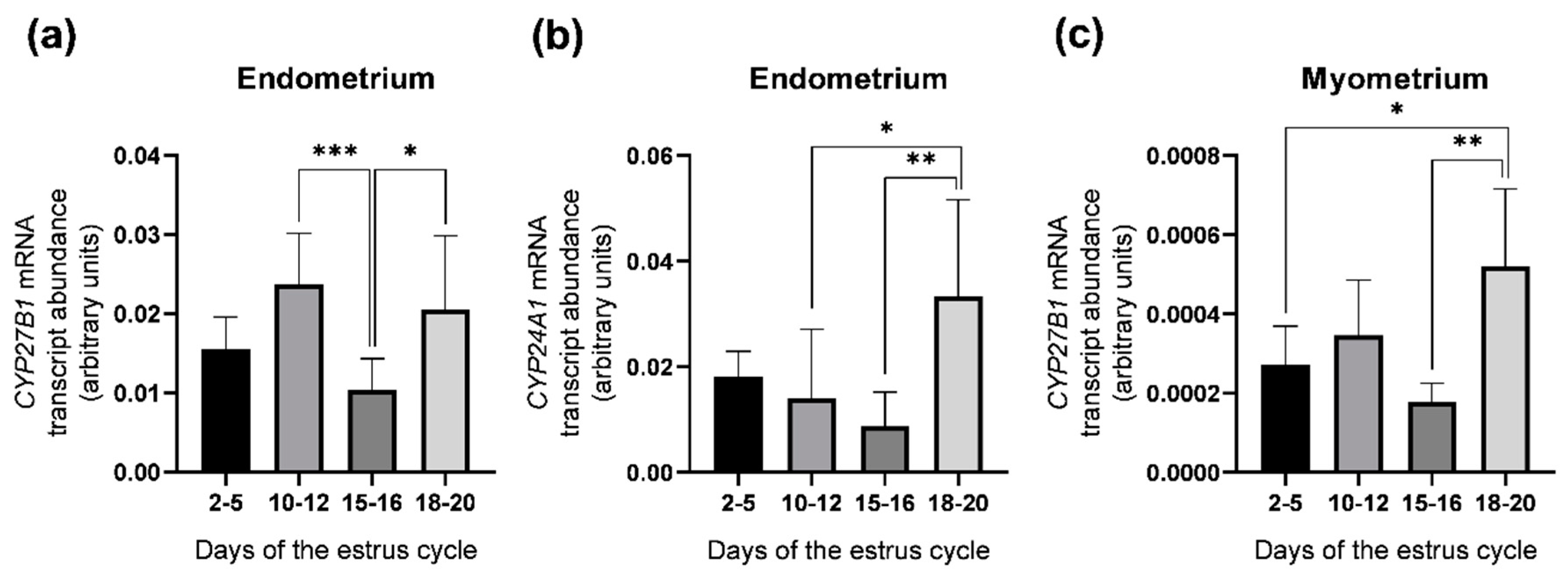
2.2. Abundance of CYP27B1 and CYP24A1 mRNA Transcripts in the Porcine Uterus
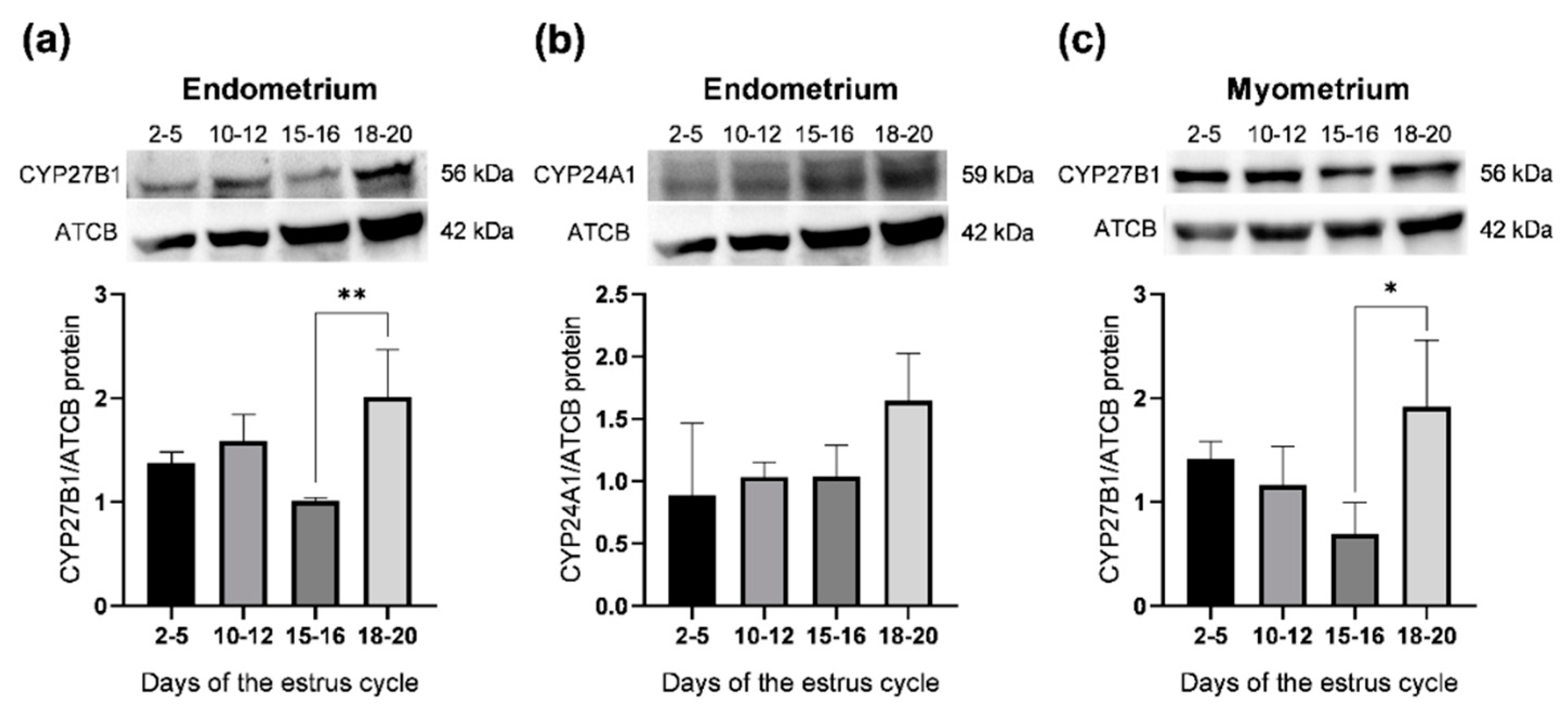
2.3. Abundance of CYP27B1 and CYP24A1 Proteins in the Porcine Uterus
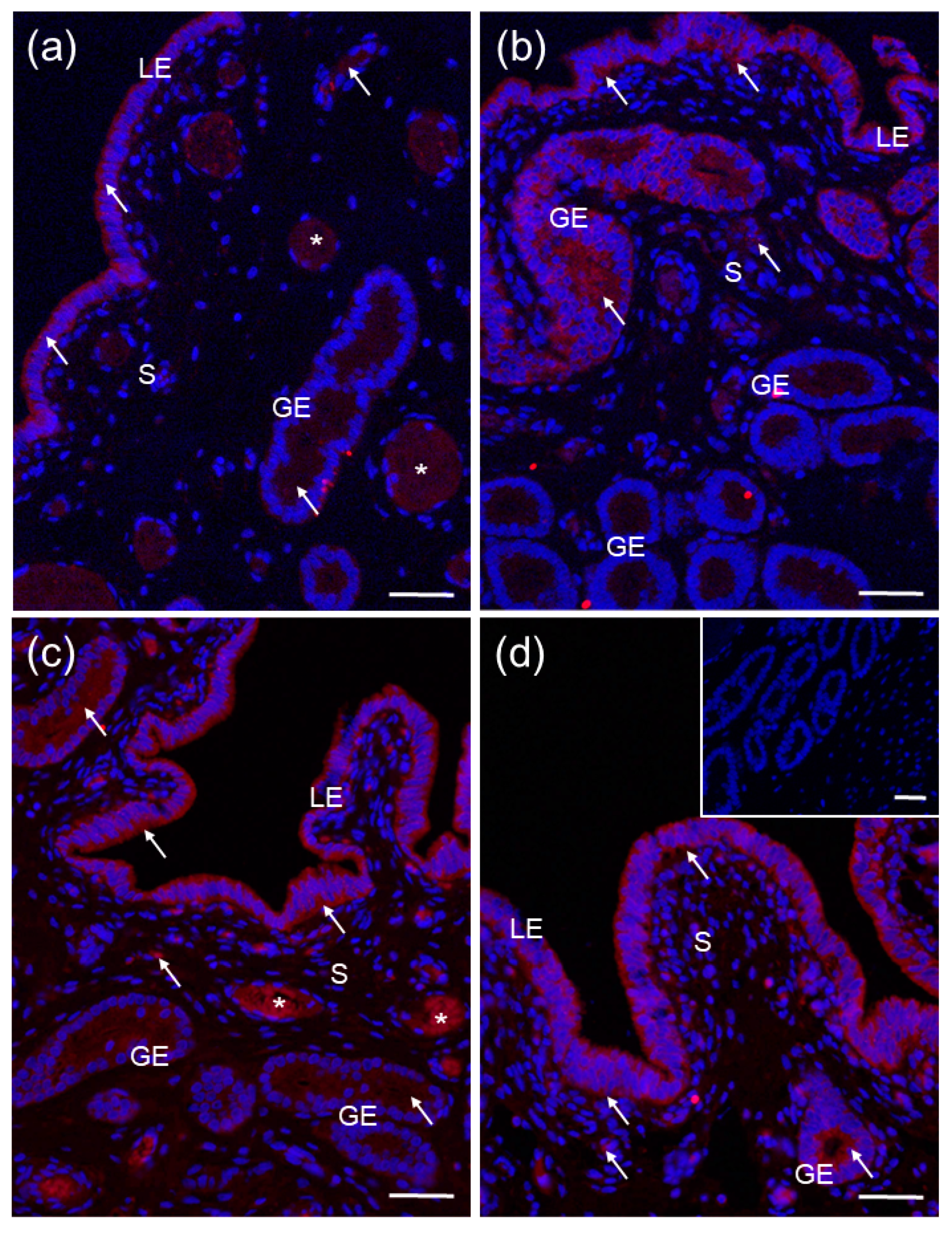
2.4. Localization of CYP27B1 and CYP24A1 in the Porcine Uterus
2.5. Effect of 1,25(OH)2D3 on CYP27B1 and CYP24A1 mRNA Transcript Abundance in Endometrial and Myometrial Slices
3. Discussion
4. Materials and Methods
4.1. Animals and Sample Collection
4.2. Incubation of Endometrial and Myometrial Slices In Vitro
4.3. Quantitative Real-Time PCR Analysis
4.4. Western Blot Analysis
4.5. Immunofluorescence
4.6. Analysis of 1,25(OH)2D3 Concentration in Uterine Flushings
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cermisoni, G.C.; Alteri, A.; Corti, L.; Rabellotti, E.; Papaleo, E.; Viganò, P.; Sanchez, A.M. Vitamin D and endometrium: A systematic review of a neglected area of research. Int. J. Mol. Sci. 2018, 19, 2320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grzesiak, M. Vitamin D3 action within the ovary—An updated review. Physiol. Res. 2020, 69, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Lopez, A.G.; Kerlan, V.; Desailloud, R. Non-classical effects of vitamin D: Non-bone effects of vitamin D. Ann. Endocrinol. 2021, 82, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Lorenzen, M.; Boisen, I.M.; Mortensen, L.J.; Lanske, B.; Juul, A.; Blomberg Jensen, M. Reproductive endocrinology of vitamin D. Mol. Cell. Endocrinol. 2017, 453, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Yoshizawa, T.; Handa, Y.; Uematsu, Y.; Takeda, S.; Sekine, K.; Yoshihara, Y.; Kawakami, T.; Arioka, K.; Sato, H.; Uchiyama, Y.; et al. Mice lacking the vitamin D receptor exhibit impaired bone formation, uterine hypoplasia and growth retardation after weaning. Nat. Genet. 1997, 16, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Panda, D.K.; Miao, D.; Tremblay, M.L.; Sirois, J.; Farookhi, R.; Hendy, G.N.; Goltzman, D. Targeted ablation of the 25-hydroxyvitamin D 1alpha -hydroxylase enzyme: Evidence for skeletal, reproductive, and immune dysfunction. Proc. Natl. Acad. Sci. USA 2001, 98, 7498–7503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ingles, S.A.; Wu, L.; Liu, B.T.; Chen, Y.; Wang, C.Y.; Templeman, C.; Brueggmann, D. Differential gene expression by 1,25(OH)2D3 in an endometriosis stromal cell line. J. Steroid Biochem. Mol. Biol. 2017, 173, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Othman, E.R.; Ahmed, E.; Sayed, A.A.; Hussein, M.; Abdelaal, I.I.; Fetih, A.N.; Abou-Taleb, H.A.; Yousef, A.A. Human uterine leiomyoma contains low levels of 1, 25-dihdroxyvitamin D3, and shows dysregulated expression of vitamin D metabolizing enzymes. Eur. J. Obstet. Gynecol. Reprod. Biol. 2018, 229, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Grzesiak, M.; Burzawa, G.; Kurowska, P.; Blaszczyk, K.; Szlaga, A.; Blasiak, A.; Sechman, A.; Rak, A. Altered vitamin D3 metabolism in the ovary and periovarian adipose tissue of rats with letrozole-induced PCOS. Histochem. Cell Biol. 2021, 155, 101–116. [Google Scholar] [CrossRef] [PubMed]
- Grzesiak, M.; Socha, M.; Hrabia, A. Altered vitamin D metabolic system in follicular cysts of sows. Reprod. Domest. Anim. 2021, 56, 193–196. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Vitamin D: A millenium perspective. J. Cell. Biochem. 2003, 88, 296–307. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D. Vitamin D metabolism, mechanism of action, and clinical applications. Chem. Biol. 2014, 21, 319–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christakos, S.; Dhawan, P.; Verstuyf, A.; Verlinden, L.; Carmeliet, G. Vitamin D: Metabolism, molecular mechanism of action, and pleiotropic effects. Physiol. Rev. 2016, 96, 365–408. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Kim, T.K.; Shehabi, H.Z.; Semak, I.; Tang, E.K.; Nguyen, M.N.; Benson, H.A.; Korik, E.; Janjetovic, Z.; Chen, J.; et al. In vivo evidence for a novel pathway of vitamin D₃ metabolism initiated by P450scc and modified by CYP27B1. FASEB J. 2012, 26, 3901–3915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slominski, A.T.; Li, W.; Kim, T.K.; Semak, I.; Wang, J.; Zjawiony, J.K.; Tuckey, R.C. Novel activities of CYP11A1 and their potential physiological significance. J. Steroid Biochem. Mol. Biol. 2015, 151, 25–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slominski, A.T.; Kim, T.K.; Li, W.; Postlethwaite, A.; Tieu, E.W.; Tang, E.; Tuckey, R.C. Detection of novel CYP11A1-derived secosteroids in the human epidermis and serum and pig adrenal gland. Sci. Rep. 2015, 5, 14875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bikle, D.D.; Patzek, S.; Wang, Y. Physiologic and pathophysiologic roles of extra renal CYP27b1: Case report and review. Bone Rep. 2018, 8, 255–267. [Google Scholar] [CrossRef]
- Olmos-Ortiz, A.; Avila, E.; Durand-Carbajal, M.; Díaz, L. Regulation of calcitriol biosynthesis and activity: Focus on gestational vitamin D deficiency and adverse pregnancy outcomes. Nutrients 2015, 7, 443–480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horst, R.L.; Reinhardt, T.A.; Reddy, G.S. Vitamin D metabolism. Vitam. D 2005, 1, 15–36. [Google Scholar]
- Zierold, C.; Darwish, H.M.; DeLuca, H.F. Two vitamin D response elements function in the rat 1,25-dihydroxyvitamin D 24-hydroxylase promoter. J. Biol. Chem. 1995, 270, 1675–1678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grzesiak, M.; Waszkiewicz, E.; Wojtas, M.; Kowalik, K.; Franczak, A. Expression of vitamin D receptor in the porcine uterus and effect of 1,25(OH)2D3 on progesterone and estradiol-17β secretion by uterine tissues in vitro. Theriogenology 2019, 125, 102–108. [Google Scholar] [CrossRef]
- Viganò, P.; Lattuada, D.; Mangioni, S.; Ermellino, L.; Vignali, M.; Caporizzo, E.; Panina-Bordignon, P.; Besozzi, M.; Di Blasio, A.M. Cycling and early pregnant endometrium as a site of regulated expression of the vitamin D system. J. Mol. Endocrinol. 2006, 36, 415–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, J.; Liu, S.; Wang, P.; Ren, H.; Li, Y. Characterization of VDR and CYP27B1 expression in the endometrium during the menstrual cycle before embryo transfer: Implications for endometrial receptivity. Reprod. Biol. Endocrinol. 2020, 18, 24. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.; Choi, Y.; Yoo, I.; Han, J.; Hong, J.S.; Kim, Y.Y.; Ka, H. Vitamin D-metabolic enzymes and related molecules: Expression at the maternal-conceptus interface and the role of vitamin D in endometrial gene expression in pigs. PLoS ONE 2017, 12, e0187221. [Google Scholar] [CrossRef] [Green Version]
- Yao, X.; Zhang, G.; Guo, Y.; Ei-Samahy, M.; Wang, S.; Wan, Y.; Han, L.; Liu, Z.; Wang, F.; Zhang, Y. Vitamin D receptor expression and potential role of vitamin D on cell proliferation and steroidogenesis in goat ovarian granulosa cells. Theriogenology 2017, 102, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Delissalde, F.; Hernández, M.A.; Barrón, A.; Bermejo, L.; Arias, J.; Halhali, A.; Castro, I. La vitamina D induce la proliferación de células en cultivo del endometrio de rata [Vitamin D induces proliferation in rat endometrium cultured cells]. Rev. Investig. Clin. 1998, 50, 113–118. [Google Scholar]
- Jeon, S.M.; Shin, E.A. Exploring vitamin D metabolism and function in cancer. Exp. Mol. Med. 2018, 50, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pike, J.W.; Meyer, M.B. Regulation of mouse Cyp24a1 expression via promoter-proximal and downstream-distal enhancers highlights new concepts of 1,25-dihydroxyvitamin D(3) action. Arch. Biochem. Biophys. 2012, 523, 2–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, Z.; Munson, S.J.; Huang, N.; Portale, A.A.; Miller, W.L.; Bikle, D.D. The mechanism of 1,25-dihydroxyvitamin D3 autoregulation in keratinocytes. J. Biol. Chem. 2002, 277, 36987–36990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avila, E.; Díaz, L.; Barrera, D.; Halhali, A.; Méndez, I.; González, L.; Zuegel, U.; Steinmeyer, A.; Larrea, F. Regulation of vitamin D hydroxylases gene expression by 1,25-dihydroxyvitamin D3 and cyclic AMP in cultured human syncytiotrophoblasts. J. Steroid Biochem. Mol. Biol. 2007, 103, 90–96. [Google Scholar] [CrossRef]
- Meyer, M.B.; Goetsch, P.D.; Pike, J.W. A downstream intergenic cluster of regulatory enhancers contributes to the induction of CYP24A1 expression by 1alpha,25-dihydroxyvitamin D3. J. Biol. Chem. 2010, 285, 15599–15610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Overbergh, L.; Stoffels, K.; Valckx, D.; Giulietti, A.; Bouillon, R.; Mathieu, C. Regulation of 25-hydroxyvitamin D-1alpha-hydroxylase by IFNgamma in human monocytic THP1 cells. J. Steroid Biochem. Mol. Biol. 2004, 89–90, 453–455. [Google Scholar] [CrossRef]
- Akins, E.L.; Morrisette, M.C. Gross ovarian changes during estrus cycle of swine. Am. J. Vet. Res. 1968, 29, 1953–1957. [Google Scholar] [PubMed]
- Franczak, A.; Zmijewska, A.; Kurowicka, B.; Wojciechowicz, B.; Kotwica, G. Interleukin 1β-induced synthesis and secretion of prostaglandin E2 in the porcine uterus during various periods of pregnancy and the estrous cycle. J. Physiol. Pharmacol. 2010, 61, 733–742. [Google Scholar] [PubMed]
- Grzesiak, M.; Knapczyk-Stwora, K.; Slomczynska, M. Vitamin D3 in ovarian antral follicles of mature gilts: Expression of its receptors and metabolic enzymes, concentration in follicular fluid and effect on steroid secretion in vitro. Theriogenology 2021, 160, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Durlej, M.; Knapczyk-Stwora, K.; Duda, M.; Kopera-Sobota, I.; Hejmej, A.; Bilinska, B.; Slomczynska, M. Prenatal and neonatal exposure to the antiandrogen flutamide alters connexin 43 gene expression in adult porcine ovary. Domest. Anim. Endocrinol. 2011, 40, 19–29. [Google Scholar] [CrossRef] [PubMed]


| Antibody | Serum | Host Species | Vendor | WB Dilution | IF Dilution |
|---|---|---|---|---|---|
| Anti–CYP27B1 | 5% NGS | Rabbit | Invitrogen, Carsband, CA, USA cat no. PA5-79128 | 1:3000 | 1:300 |
| Anti–CYP24A1 | 5% NGS | Rabbit | Invitrogen, Carsband, CA, USA cat. no. PA5-79127 | 1:1000 | 1:300 |
| Anti–β–actin | - | Mouse | Sigma-Aldrich, St. Louis, MO, USA cat. no. A2228 | 1:4000 | - |
| Anti–rabbit IgG, Cy3 | - | Goat | Thermo Fisher Scientific, DE, USA cat. no. A10520 | - | 1:100 |
| Anti–rabbit IgG | - | Goat | Invitrogen, Carsband, CA, USA cat. no. 31460 | 1:3000 | - |
| Anti–mouse IgG | - | Horse | Bio-Rad Laboratories Inc., GmbH, Munchen, Germany cat. no. 170-6516 | 1:3000 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grzesiak, M.; Kaminska, K.; Bodzioch, A.; Drzewiecka, E.M.; Franczak, A.; Knapczyk-Stwora, K. Vitamin D3 Metabolic Enzymes in the Porcine Uterus: Expression, Localization and Autoregulation by 1,25(OH)2D3 In Vitro. Int. J. Mol. Sci. 2022, 23, 3972. https://doi.org/10.3390/ijms23073972
Grzesiak M, Kaminska K, Bodzioch A, Drzewiecka EM, Franczak A, Knapczyk-Stwora K. Vitamin D3 Metabolic Enzymes in the Porcine Uterus: Expression, Localization and Autoregulation by 1,25(OH)2D3 In Vitro. International Journal of Molecular Sciences. 2022; 23(7):3972. https://doi.org/10.3390/ijms23073972
Chicago/Turabian StyleGrzesiak, Malgorzata, Kinga Kaminska, Aleksandra Bodzioch, Ewa M. Drzewiecka, Anita Franczak, and Katarzyna Knapczyk-Stwora. 2022. "Vitamin D3 Metabolic Enzymes in the Porcine Uterus: Expression, Localization and Autoregulation by 1,25(OH)2D3 In Vitro" International Journal of Molecular Sciences 23, no. 7: 3972. https://doi.org/10.3390/ijms23073972
APA StyleGrzesiak, M., Kaminska, K., Bodzioch, A., Drzewiecka, E. M., Franczak, A., & Knapczyk-Stwora, K. (2022). Vitamin D3 Metabolic Enzymes in the Porcine Uterus: Expression, Localization and Autoregulation by 1,25(OH)2D3 In Vitro. International Journal of Molecular Sciences, 23(7), 3972. https://doi.org/10.3390/ijms23073972






