Isolation and Functional Characterization of a LEAFY Gene in Mango (Mangifera indica L.)
Abstract
:1. Introduction
2. Results
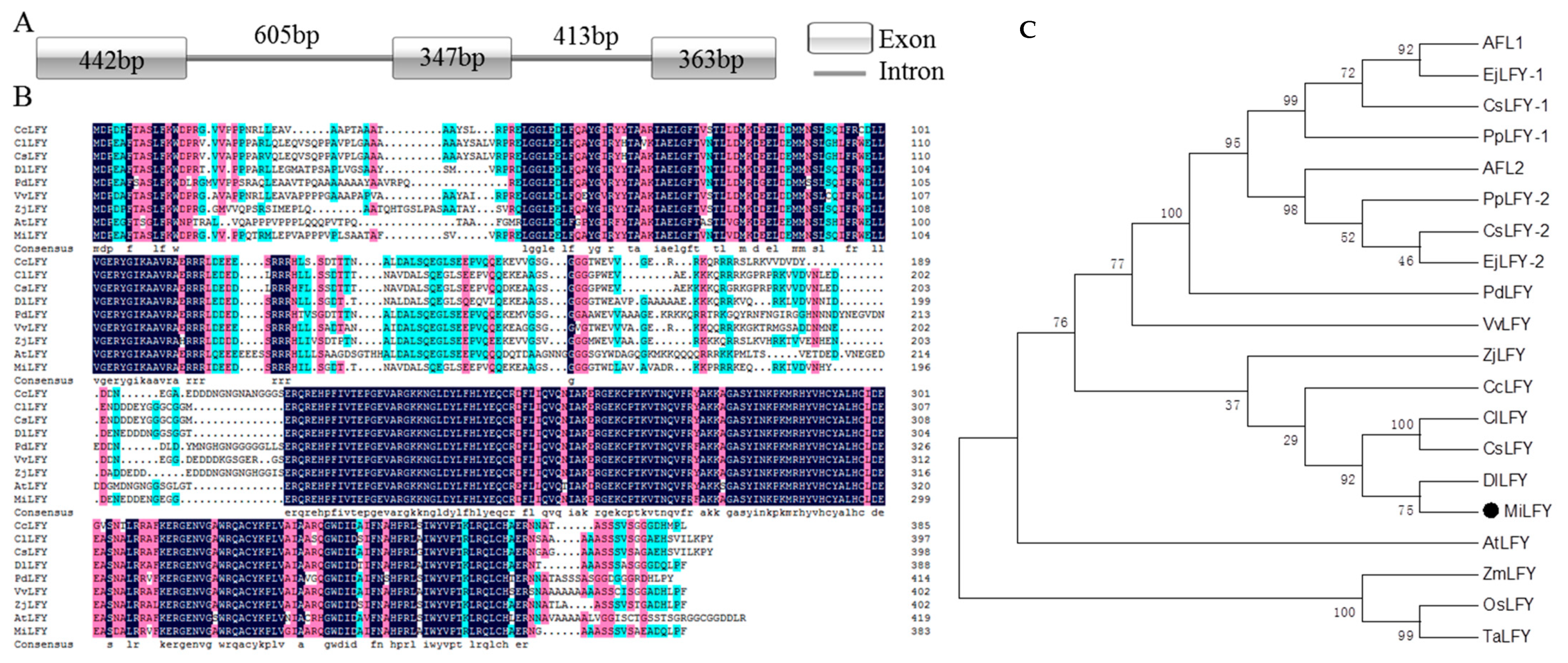
2.1. Sequence and Phylogenetic Analysis of LFY
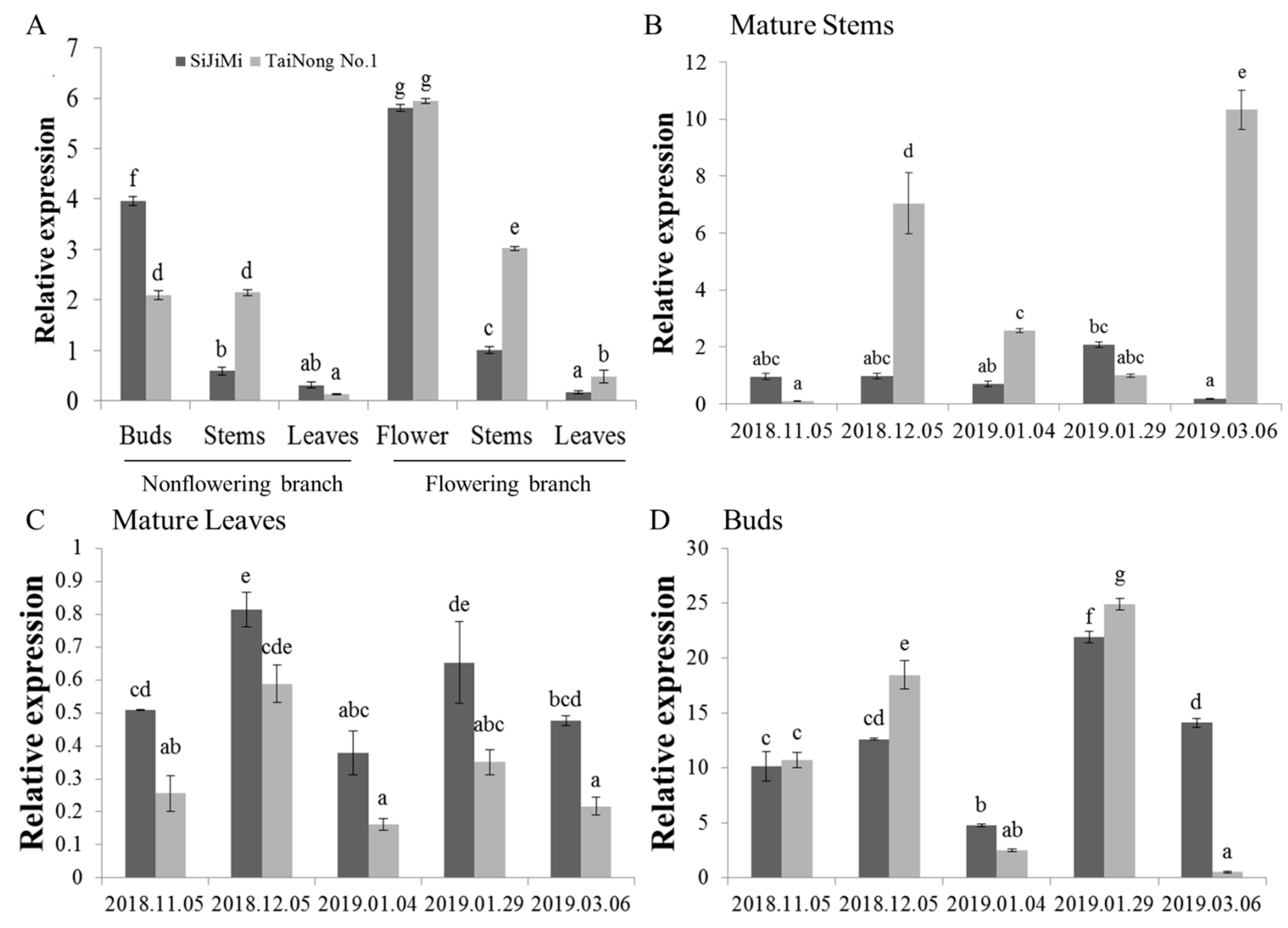
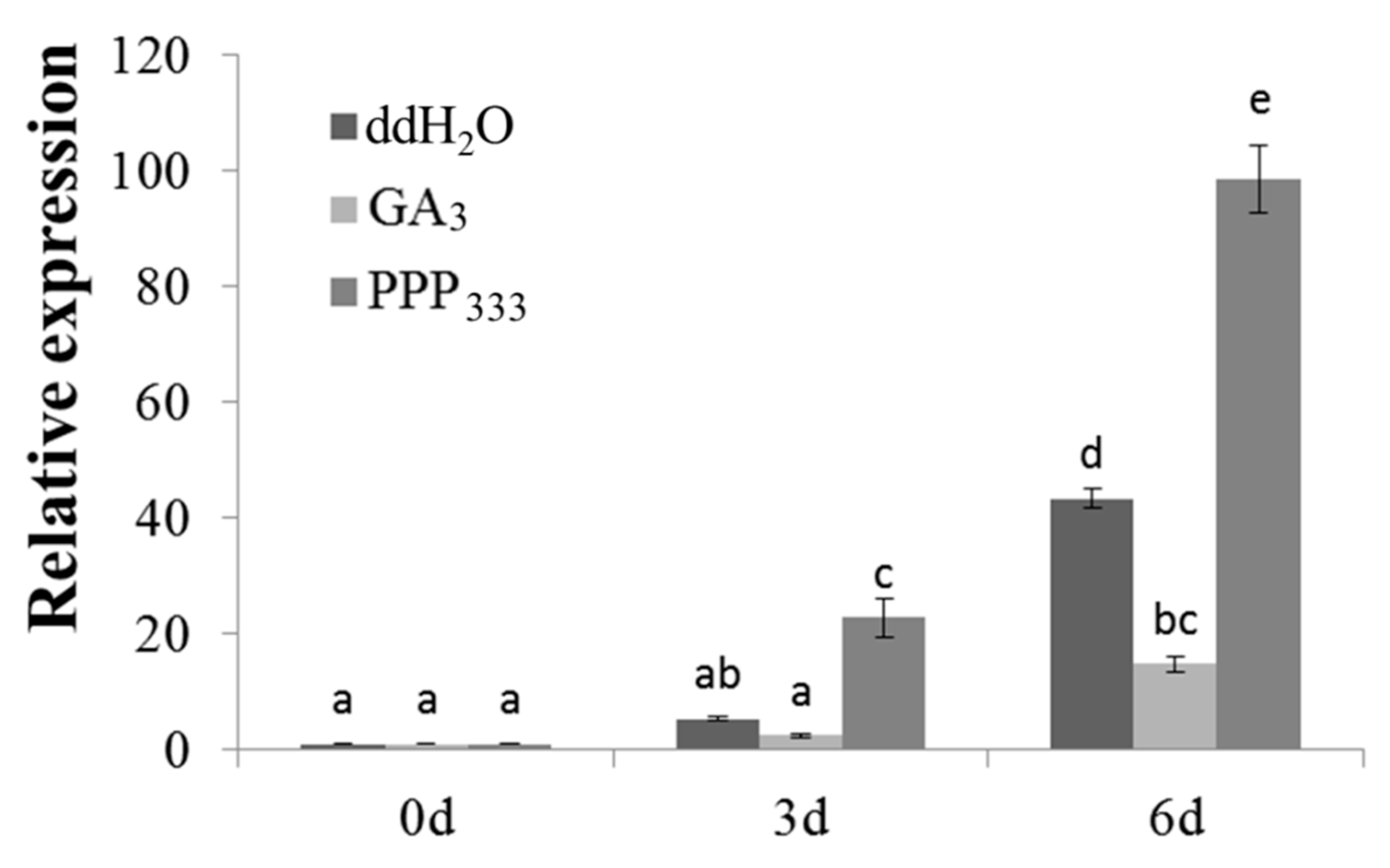
2.2. Analysis of MiLFY Gene Expression in Mango
2.3. Subcellular Localization of MiLFY
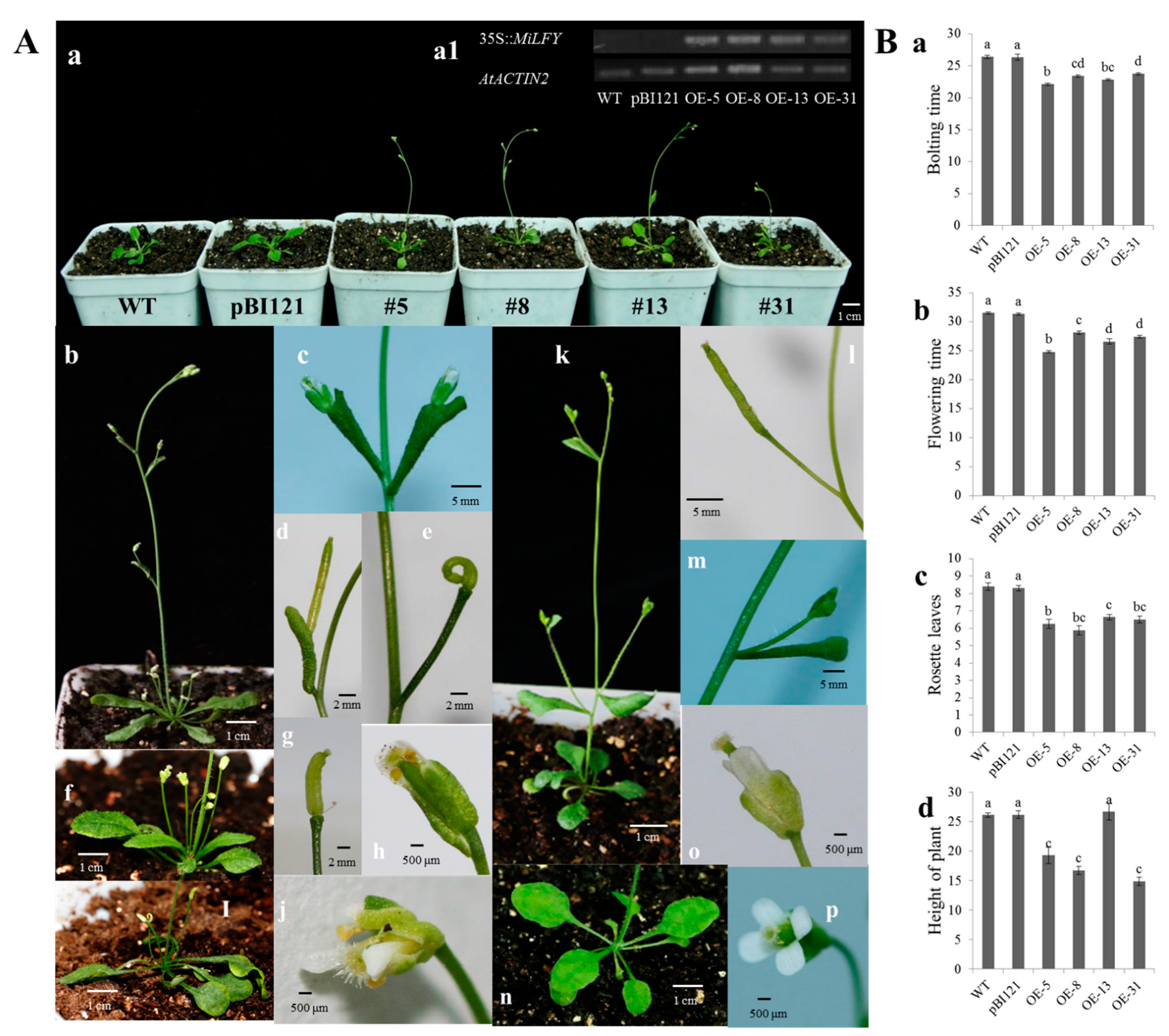
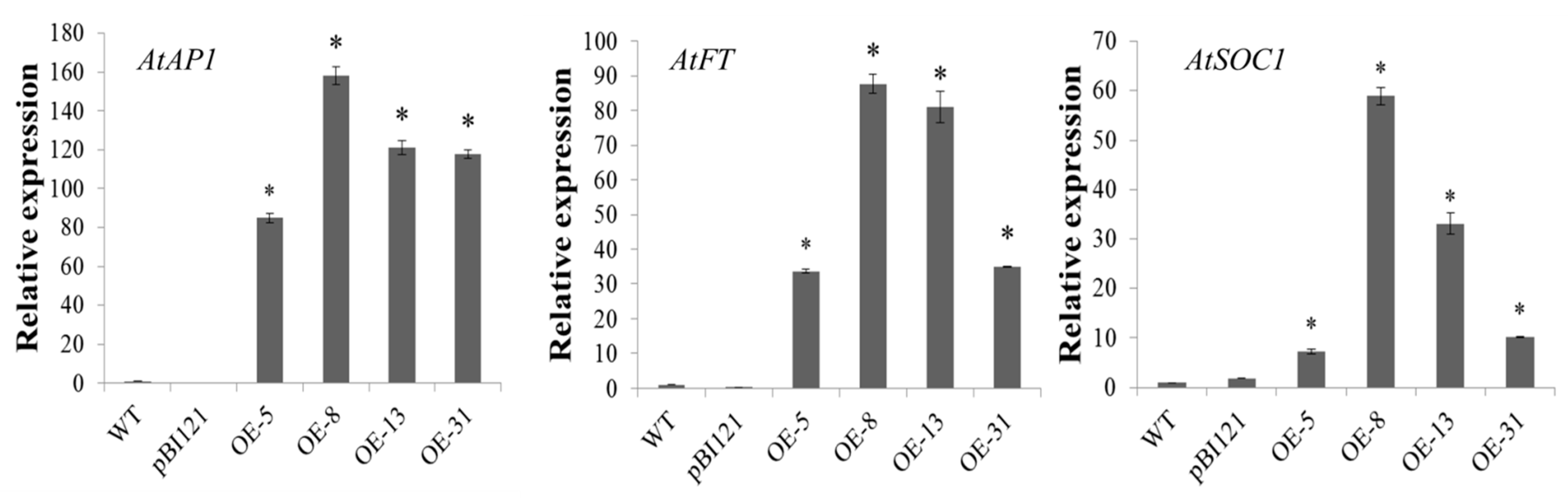
2.4. Phenotypic Analysis of Transgenic Plants with Overexpression
2.5. Activity of GUS Driven by the MiLFY Promoter in Transgenic Arabidopsis Lines
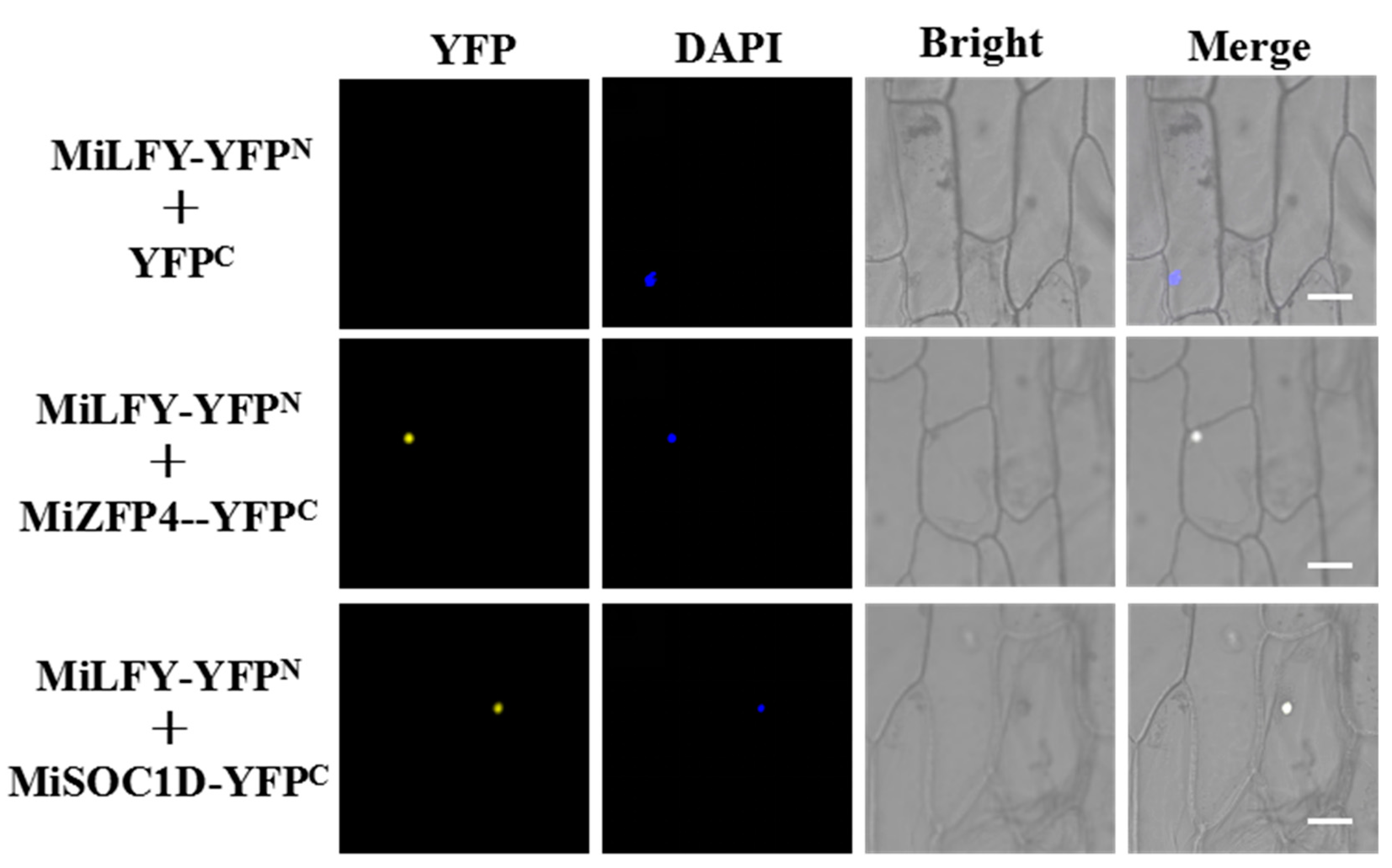
2.6. Yeast Two-Hybrid (Y2H) Screening and Confirmation by Bimolecular Fluorescence Complementation (BiFC) Assays
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Isolation of the MiLFY Gene
4.3. Sequence Alignment and Bioinformatic Analysis
4.4. Analysis of MiLFY Gene Expression
4.5. Subcellular Localization Analysis
4.6. Vector Construction and Transformation of Arabidopsis
4.7. MiLFY Promoter Activity Assay
4.8. Y2H Screening and Confirmation by BiFC Assay
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Luo, C.; Yu, H.X.; Fan, Y.; Zhang, X.J.; He, X.H. Research advance on the flowering mechanism of mango. Acta Hortic. 2019, 1, 17–22. [Google Scholar] [CrossRef]
- Wang, S.L.; An, H.R.; Tong, C.G.; Jang, S. Flowering and fowering genes: From model plants to orchids. Hortic. Environ. Biotechnol. 2021, 62, 135–148. [Google Scholar] [CrossRef]
- Moon, J.; Lee, H.; Kim, M.; Lee, I. Analysis of flowering pathway integrators in Arabidopsis. Plant Cell Physiol. 2005, 47, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Weigel, D.; Alvarez, J.P.; Smyth, D.R.; Yanofsky, M.F.; Meyerowitz, E.M. LEAFY controls floral meristem identity in Arabidopsis. Cell 1992, 79, 843–859. [Google Scholar] [CrossRef] [Green Version]
- Dornelas, M.C.; Rodriguez, A.P. A Floricaula/Leafy gene homolog is preferentially expressed in developing female cones of the tropical pine Pinus caribaea var. caribaea. Genet. Mol. Biol. 2005, 28, 299–307. [Google Scholar] [CrossRef] [Green Version]
- Pillitteri, L.; Lovatt, C.; Walling, L. Isolation and characterization of LEAFY and apetala1 homologues from citrus sinensis l. osbeck ‘washington’. J. Am. Soc. Hortic. Sci. 2004, 129, 847–857. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, S.; Li, Y.; Yang, Y.; Zhou, Y.; Zhao, K.; Zhang, Q. Isolation, functional characterization and evolutionary study of LFY1 gene in Prunus mume. Plant Cell Tissue Organ Cult. 2019, 136, 523–537. [Google Scholar] [CrossRef]
- Weigel, D.; Nilsson, O. A developmental switch sufficient for flower initiation in diverse plants. Nature 1995, 377, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.J.; Huang, J.Q.; Huang, Y.J.; Chen, F.; Zheng, B.S. Cloning and characterization of a homologue of the FLORICAULA/LEAFY gene in Hickory (Carya cathayensis Sarg). Plant Mol. Biol. Rep. 2012, 30, 794–805. [Google Scholar] [CrossRef]
- Peña, L.; Martín-Trillo, M.; Juárez, J.; Pina, J.A.; Navarro, L.; Martínez-Zapater, J.M. Constitutive expression of Arabidopsis LEAFY or APETALA1 genes in citrus reduces their generation time. Nat. Biotechnol. 2001, 19, 263–267. [Google Scholar] [CrossRef]
- Ahearn, K.P.; Johnson, H.A.; Weigel, D.; Wagner, D.R. NFL1, a Nicotiana tabacum LEAFY-like gene, controls meristem initiation and floral structure. Plant Cell Physiol. 2001, 42, 1130–1139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, H.X.; Luo, C.; Fan, Y.; Zhang, X.J.; Huang, F.; Li, M.; He, X.H. Isolation and characterization of two APETALA1-Like genes from mango (Mangifera indica L.). Sci. Hortic. Amst. Neth. 2020, 259, 08814. [Google Scholar] [CrossRef]
- Fan, Z.Y.; He, X.H.; Fan, Y.; Yu, H.X.; Wang, Y.H.; Xie, X.J.; Liu, Y.; Mo, X.; Wang, J.Y.; Luo, C. Isolation and functional characterization of three MiFTs genes from mango. Plant Physiol. Bioch. 2020, 155, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Luo, C.; Zhang, X.J.; Lu, X.X.; Yu, H.X.; Xie, X.J.; Fan, Z.Y.; Mo, X.; He, X.H. Overexpression of the mango MiCO gene delayed fowering time in transgenic Arabidopsis. Plant Cell Tissue Organ Cult. 2020, 143, 219–228. [Google Scholar] [CrossRef]
- Wang, Y.H.; He, X.H.; Yu, H.X.; Mo, X.; Fan, Y.; Fan, Z.Y.; Xie, X.J.; Liu, Y.; Luo, C. Overexpression of four MiTFL1 genes from mango delays the flowering time in transgenic Arabidopsis. BMC Plant Biol. 2021, 7, 407. [Google Scholar]
- Luo, C.; He, X.H.; Chen, H.; Hu, Y.; Ou, S.J. Molecular cloning and expression analysis of four actin genes (MiACT) from mango. Biol. Plantarum. 2013, 57, 238–244. [Google Scholar] [CrossRef]
- Luo, C.; He, X.H.; Tan, C.; Hu, Y. Cloning and sequence analysis of LEAFY promoter from Mango. Acta Hortic. 2013, 992, 309–317. [Google Scholar] [CrossRef]
- Guo, L.; Yu, Y.; Xia, X.; Yin, W. Identification and functional characterisation of the promoter of the calcium sensor gene CBL1 from the xerophyte Ammopiptanthus mongolicus. BMC Plant Biol. 2010, 10, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blázquez, M.A.; Soowal, L.N.; Lee, I.; Weigel, D. LEAFY expression and flower initiation in Arabidopsis. Development 1997, 124, 3835–3844. [Google Scholar] [CrossRef]
- Shangguan, L.; Wang, C.; Kayesh, E.; Zhang, Y.P.; Korir, N.K.; Han, J.; Fang, J. Review and structural analysis of the evolution of grapevine (Vitis vinifera L.) genes involved in flower and fruit development. J. Hortic. Sci. Biotechnol. 2012, 87, 243–249. [Google Scholar] [CrossRef]
- Meir, M.; Ransbotyn, V.; Raveh, E.; Barak, S.; Telzur, N.; Zaccai, M. Dormancy release and flowering time in Ziziphus jujuba Mill., a “direct flowering” fruit tree, has a facultative requirement for chilling. J. Plant Physiol. 2017, 192, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Jue, D.W.; Sang, X.L.; Liu, L.Q.; Shu, B.; Wang, Y.C.; Liu, C.M.; Wang, Y.; Xie, J.H.; Shi, S.Y. Comprehensive analysis of the longan transcriptome reveals distinct regulatory programs during the floral transition. BMC Genom. 2019, 20, 127. [Google Scholar] [CrossRef]
- Esumi, T.; Tao, R.; Yonemori, K. Isolation of LEAFY and TERMINAL FLOWER 1 homologues from six fruit tree species in the subfamily Maloideae of the Rosaceae. Sex. Plant Reprod. 2005, 17, 277–287. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhu, Y.; Zhang, L.; Su, W.; Peng, J.; Yang, X.; Song, H.; Gao, Y.; Lin, S. EjTFL1 genes promote growth but inhibit flower bud differentiation in loquat. Front. Plant Sci. 2020, 11, 576. [Google Scholar] [CrossRef]
- Yeoh, S.H.; Satake, A.; Numata, S.; Ichie, T.; Lee, S.L.; Basherudin, N.; Muhammad, N.; Kondo, T.; Otani, T.; Hashim, M.; et al. Unravelling proximate cues of mass flowering in the tropical forests of South-East Asia from gene expression analyses. Mol. Ecol. 2017, 27, 5074–5085. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Li, Z.; Zhang, J.; Yi, S.; Liu, L.; Bao, M.; Liu, G. Isolation and expression analysis of a LEAFY/FLORICAULA homolog and its promoter from London plane (Platanus acerifolia Willd.). Plant Cell Rep. 2012, 31, 1851–1865. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Z.; Li, M.; Liu, R.; Khan, M.R.; Hu, C.G. Possible involvement of locus-specific methylation on expression regulation of LEAFY homologous gene (CiLFY) during precocious trifoliate orange phase change process. PLoS ONE 2014, 9, e88558. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Tao, Y.B.; Fu, Q.; Song, Y.; Niu, L.; Xu, Z.F. An ortholog of LEAFY in Jatropha curcas regulates flowering time and floral organ development. Sci. Rep. 2016, 6, 37306. [Google Scholar] [CrossRef] [PubMed]
- An, L.; Lei, H.; Shen, X.; Li, T. Identification and characterization of PpLFL, a homolog of FLORICAULA/LEAFY in Peach (Prunus persica). Plant Mol. Biol. Rep. 2012, 30, 1488–1495. [Google Scholar] [CrossRef]
- An, X.M.; Wang, D.M.; Wang, Z.L.; Li, B.; Bo, W.H.; Cao, G.L.; Zhang, Z.Y. Isolation of a LEAFY homolog from Populus tomentosa: Expression of PtLFY in P. tomentosa floral buds and PtLFY-IR-mediated gene silencing in tobacco (Nicotiana tabacum). Plant Cell Rep. 2011, 30, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Ding, F.; Zhang, S.W.; Chen, H.B.; Peng, H.X.; Lu, J.; He, X.H.; Pan, J.C. Functional analysis of a homologue of the FLORICAULA/LEAFY gene in litchi (Litchi chinensis Sonn.) revealing its significance in early flowering process. Genes Genom. 2018, 40, 1259–1277. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ballard, H.E.; Sternberger, A.L.; Wyatt, S.E.; Stockinger, E.J.; Nadella, V. The potential role of two LEAFY orthologs in the chasmogamous cleistogamous mixed breeding system of Viola pubescens (Violaceae). J. Torrey Bot. Soc. 2017, 144, 206–217. [Google Scholar] [CrossRef]
- Hu, J.; Jin, Q.; Ma, Y. AfLFY, a LEAFY homolog in Argyranthemum frutescens, controls flowering time and leaf development. Sci. Rep. 2020, 10, 1616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Z.; Li, Z.; Huang, J.; Zheng, B.; Zhang, L.; Wang, Z. Genome-wide comparative analysis of LEAFY promoter sequence in angiosperms. Physiol. Mol. Biol. Plants 2017, 23, 23–33. [Google Scholar] [CrossRef] [Green Version]
- Jin, R.; Klasfeld, S.; Zhu, Y.; Garcia, M.F.; Xiao, J.; Han, S.K.; Konkol, A.; Wagner, D. LEAFY is a pioneer transcription factor and licenses cell reprogramming to floral fate. Nat. Commun. 2021, 12, 626. [Google Scholar] [CrossRef]
- Lyu, T.; Cao, J. Cys2/His2 Zinc-Finger Proteins in transcriptional regulation of flower development. Int. J. Mol. Sci. 2018, 19, 2589. [Google Scholar] [CrossRef] [Green Version]
- Wei, J.; Liu, D.; Liu, G.; Tang, J.; Chen, Y. Molecular cloning, characterization, and expression of MiSOC1: A homolog of the flowering gene SUPPRESSOR OF OVEREXPRESSION OF CONSTANS1 from mango (Mangifera indica L). Front. Plant Sci. 2016, 7, 1758. [Google Scholar] [CrossRef] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR, and the 2−ΔΔCT method. Methods 2002, 25, 402–408. [Google Scholar] [CrossRef]
- Mo, X.; Luo, C.; Yu, H.; Chen, J.; Liu, Y.; Xie, X.; Fan, Z.; He, X. Isolation and Functional Characterization of Two SHORT VEGETATIVE PHASE Homologous Genes from Mango. Int. J. Mol. Sci. 2021, 10, 9802. [Google Scholar] [CrossRef]
- Clough, S.J.; Bent, A.F. Floral dip: A simplifed method for Agrobacterium—Mediated transformation of Arabidopsis thaliana. Plant J. 1998, 16, 735–743. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Yu, H.; He, X.; Lu, T.; Huang, X.; Luo, C. Isolation and Functional Characterization of a LEAFY Gene in Mango (Mangifera indica L.). Int. J. Mol. Sci. 2022, 23, 3974. https://doi.org/10.3390/ijms23073974
Wang Y, Yu H, He X, Lu T, Huang X, Luo C. Isolation and Functional Characterization of a LEAFY Gene in Mango (Mangifera indica L.). International Journal of Molecular Sciences. 2022; 23(7):3974. https://doi.org/10.3390/ijms23073974
Chicago/Turabian StyleWang, Yihan, Haixia Yu, Xinhua He, Tingting Lu, Xing Huang, and Cong Luo. 2022. "Isolation and Functional Characterization of a LEAFY Gene in Mango (Mangifera indica L.)" International Journal of Molecular Sciences 23, no. 7: 3974. https://doi.org/10.3390/ijms23073974
APA StyleWang, Y., Yu, H., He, X., Lu, T., Huang, X., & Luo, C. (2022). Isolation and Functional Characterization of a LEAFY Gene in Mango (Mangifera indica L.). International Journal of Molecular Sciences, 23(7), 3974. https://doi.org/10.3390/ijms23073974





