Enhanced Magnetic Hyperthermia of Magnetoferritin through Synthesis at Elevated Temperature
Abstract
1. Introduction
2. Results and Discussion
2.1. Preparation and Characterization of MPfFn
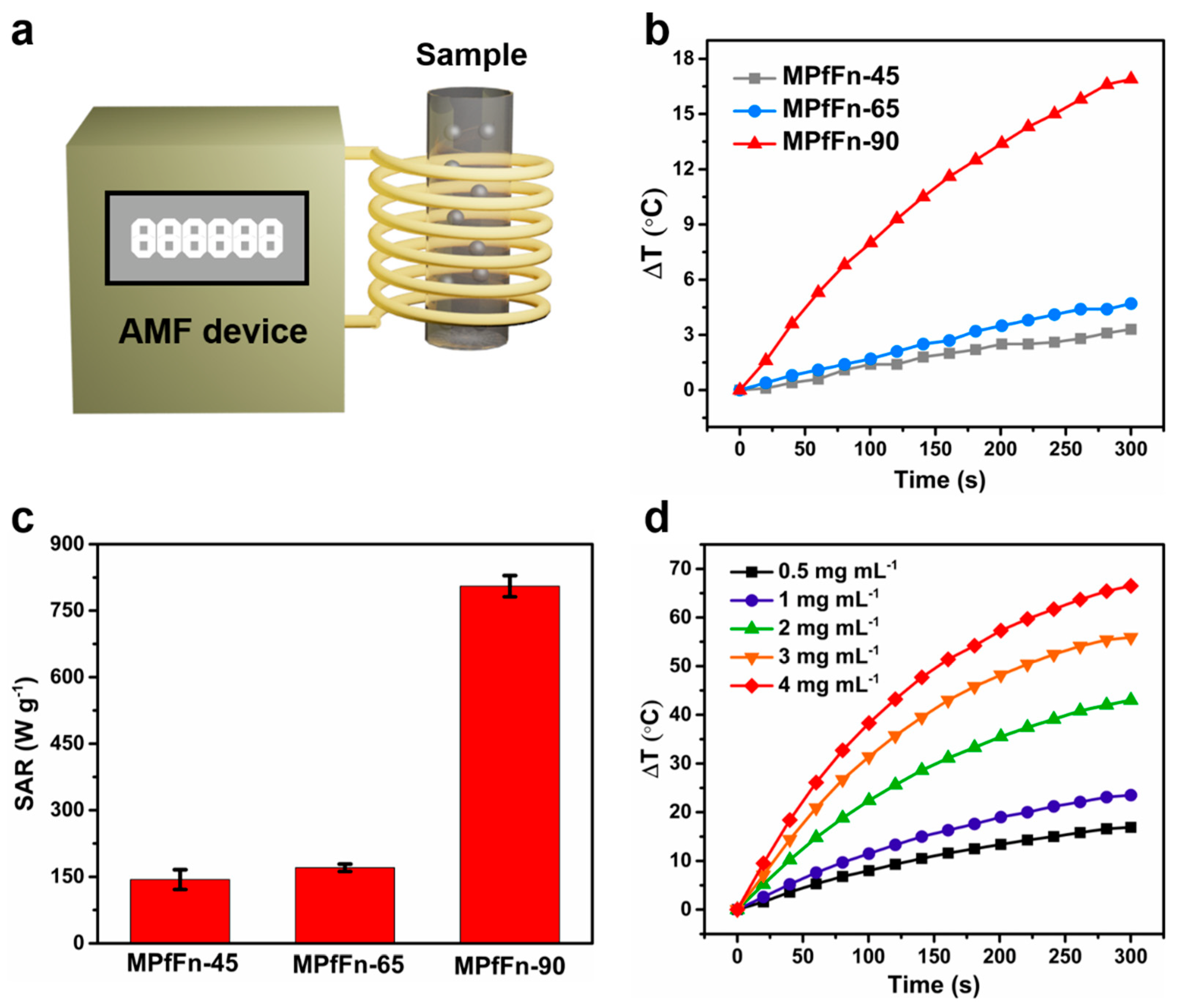
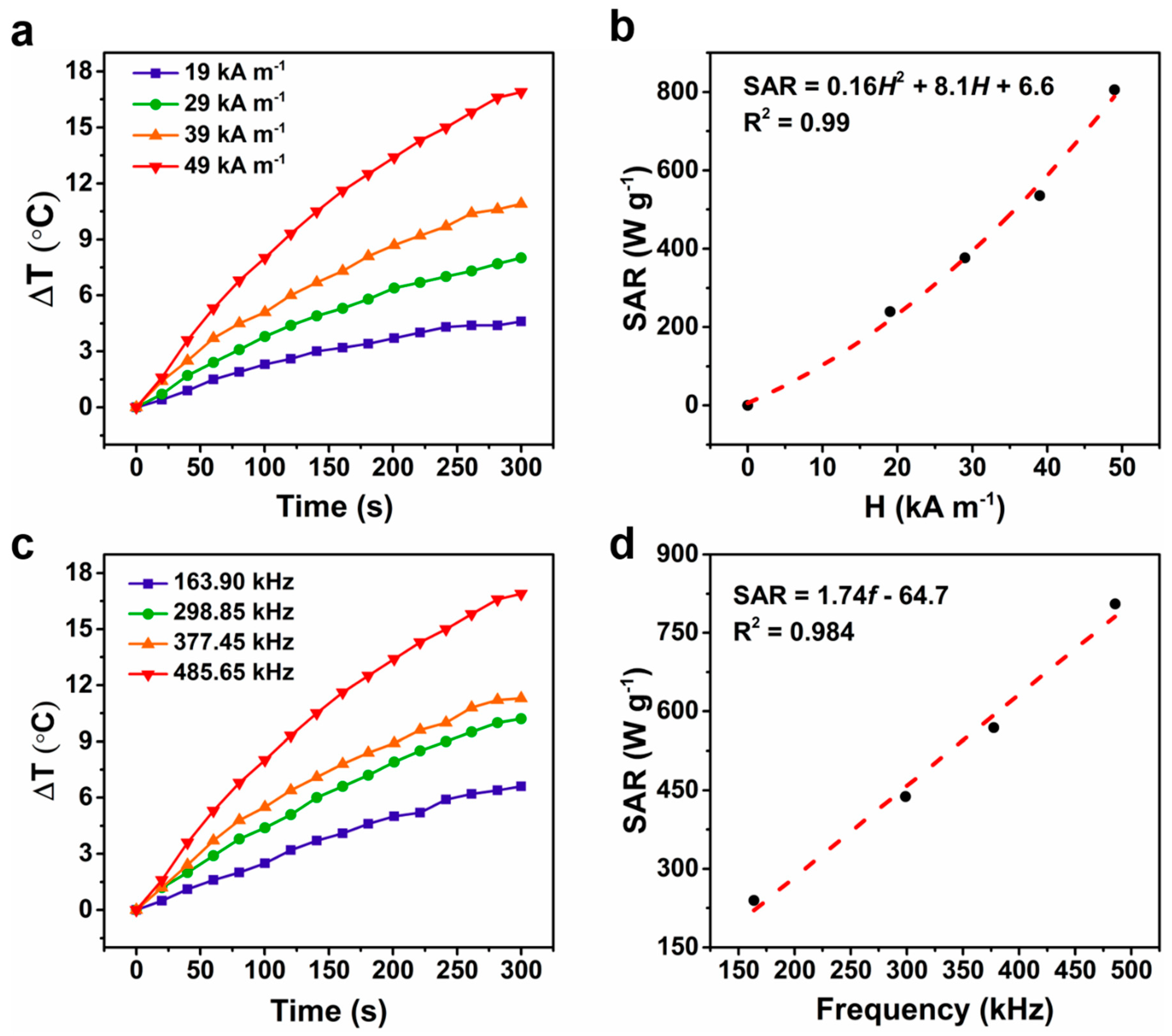
2.2. Magnetic Hyperthermia of Magnetoferritin
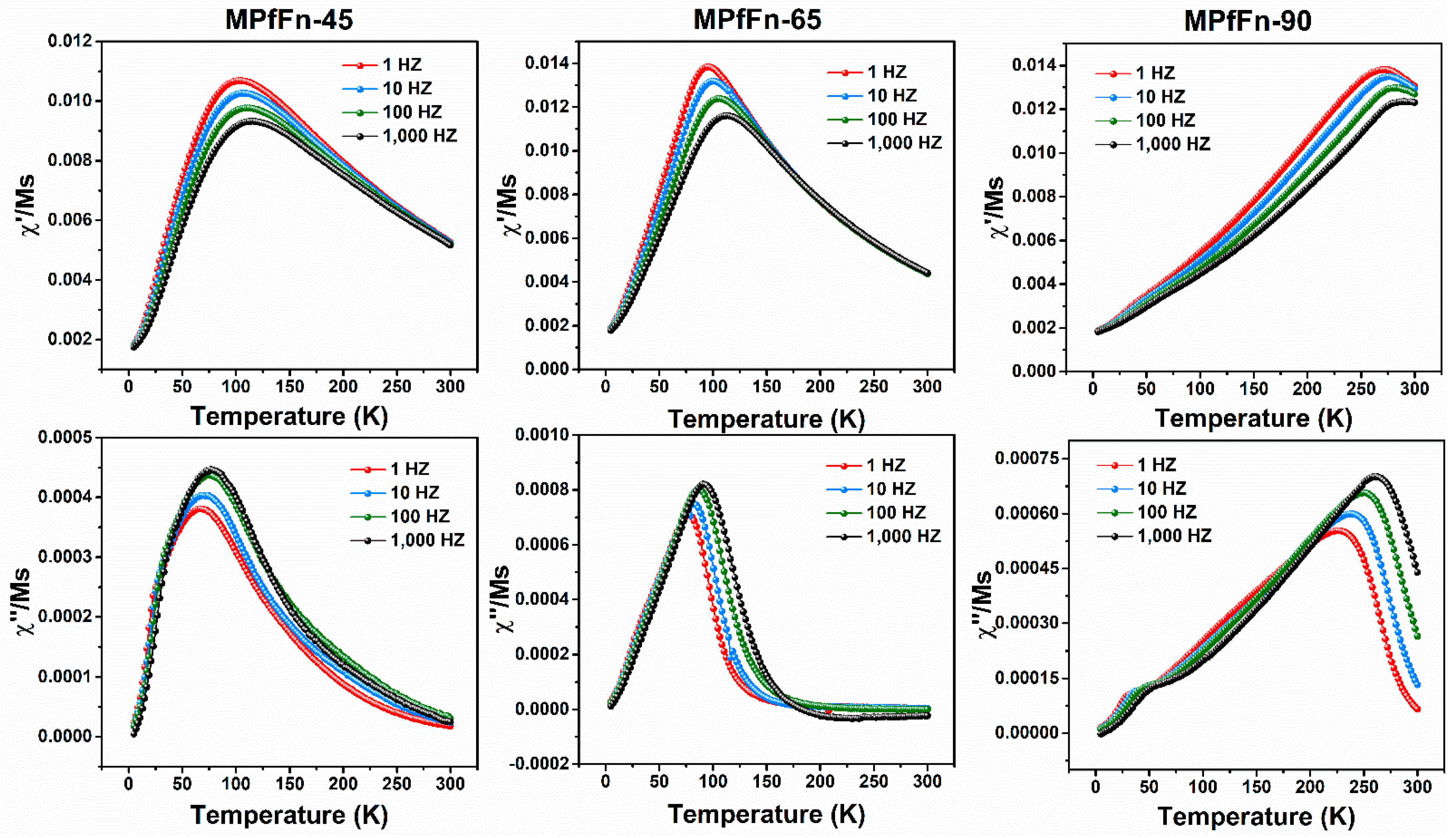
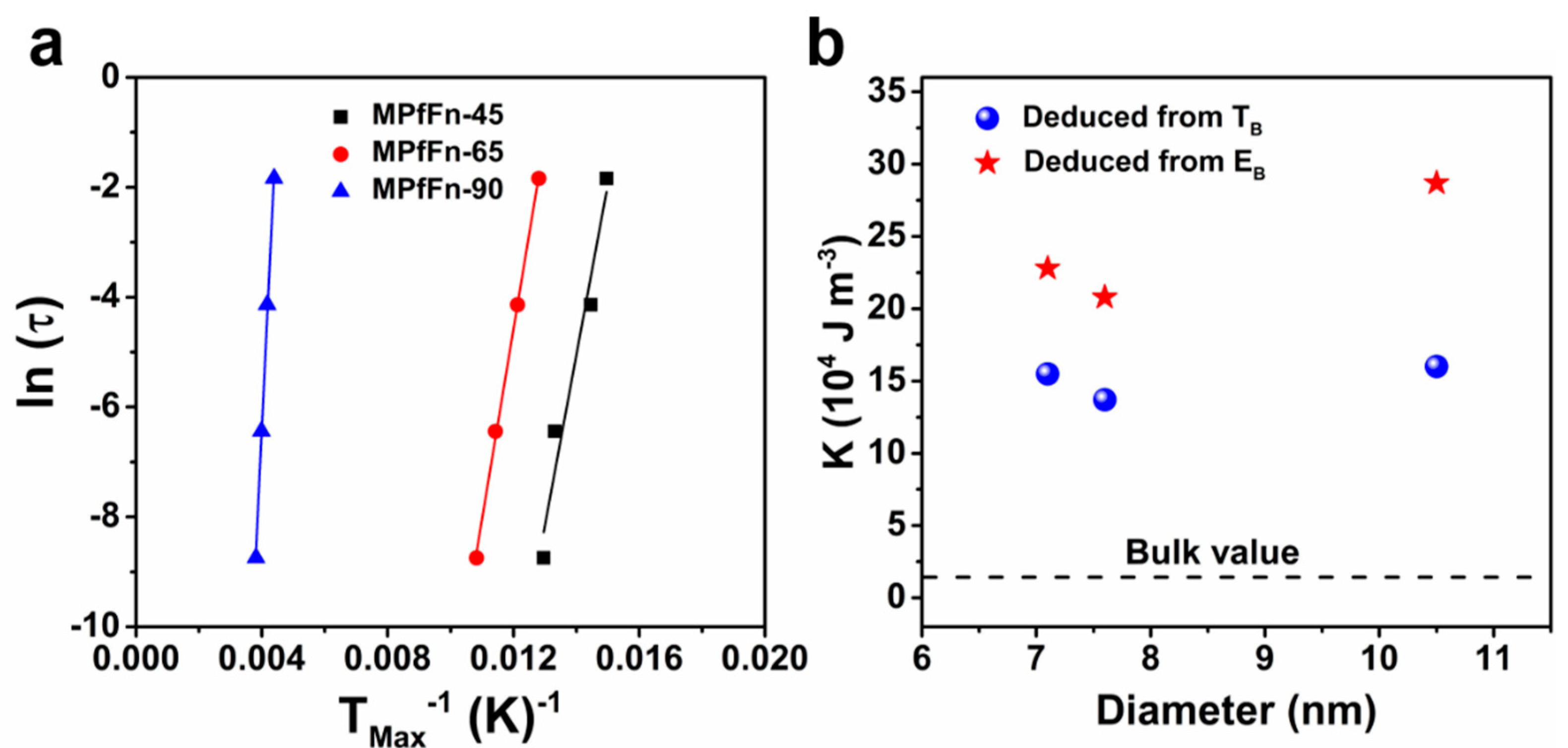
2.3. Magnetic Properties of Magnetoferritin Nanoparticles
3. Materials and Methods
3.1. Materials
3.2. Synthesis of MPfFn under Different Temperatures
3.3. Characterization of MPfFn Particles
3.4. Magnetic Measurements of MPfFn
3.5. Hyperthermic Efficiency Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, N.; Yoo, D.; Ling, D.; Cho, M.H.; Hyeon, T.; Cheon, J. Iron Oxide Based Nanoparticles for Multimodal Imaging and Magnetoresponsive Therapy. Chem. Rev. 2015, 115, 10637–10689. [Google Scholar] [CrossRef] [PubMed]
- Rosensweig, R. Heating magnetic fluid with alternating magnetic field. J. Magn. Magn. Mater. 2002, 252, 370–374. [Google Scholar] [CrossRef]
- Blanco-Andujar, C.; Walter, A.; Cotin, G.; Bordeianu, C.; Mertz, D.; Felder-Flesch, D.; Begin-Colin, S. Design of iron oxide-based nanoparticles for MRI and magnetic hyperthermia. Nanomedicine 2016, 11, 1889–1910. [Google Scholar] [CrossRef] [PubMed]
- Hergt, R.; Dutz, S.; Müller, R.; Zeisberger, M. Magnetic particle hyperthermia: Nanoparticle magnetism and materials development for cancer therapy. J. Phys. Condens. Matter 2006, 18, S2919–S2934. [Google Scholar] [CrossRef]
- Fortin, J.P.; Wilhelm, C.; Servais, J.; Ménager, C.; Bacri, J.C.; Gazeau, F. Size-Sorted Anionic Iron Oxide Nanomagnets as Colloidal Mediators for Magnetic Hyperthermia. J. Am. Chem. Soc. 2007, 129, 2628–2635. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.H.; Yang, X.; Ling, D.; Yan, X.; Dong, L.; Xu, Y.; Yang, Y.; Jiang, K.; Lu, Y.; Li, D.; et al. Ferrimagnetic mPEG-b-PHEP copolymer micelles loaded with iron oxide nanocubes and emodin for enhanced magnetic hyperthermia–chemotherapy. Natl. Sci. Rev. 2020, 7, 723–736. [Google Scholar]
- Tian, L.; Cao, C.; Pan, Y. The influence of reaction temperature on biomineralization of ferrihydrite cores in human H-ferritin. BioMetals 2011, 25, 193–202. [Google Scholar] [CrossRef]
- Theil, E.C. Ferritin: Structure, gene regulation, and cellular function in animals, plants, and microorganisms. Annu. Rev. Biochem. 1987, 56, 289–315. [Google Scholar] [CrossRef]
- Liu, X.; Jin, W.; Theil, E.C. Opening protein pores with chaotropes enhances Fe reduction and chelation of Fe from the ferritin biomineral. Proc. Natl. Acad. Sci. USA 2003, 100, 3653–3658. [Google Scholar] [CrossRef]
- Fan, K.; Cao, C.; Pan, Y.; Lu, D.; Yang, D.; Feng, J.; Song, L.; Liang, M.; Yan, X. Magnetoferritin nanoparticles for targeting and visualizing tumour tissues. Nat. Nanotechnol. 2012, 7, 459–464. [Google Scholar] [CrossRef]
- Cao, C.; Wang, X.; Cai, Y.; Sun, L.; Tian, L.; Wu, H.; He, X.; Lei, H.; Liu, W.; Chen, G.; et al. Targeted In Vivo Imaging of Microscopic Tumors with Ferritin-based Nanoprobes across Biological Barriers. Adv. Mater. 2014, 26, 2566–2571. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Wang, Y.; Xu, H.; Cao, C.; Zhu, R.; Tang, X.; Zhang, T.; Pan, Y. Positive magnetic resonance angiography using ultrafine ferritin-based iron oxide nanoparticles. Nanoscale 2019, 11, 2644–2654. [Google Scholar] [CrossRef] [PubMed]
- Fantechi, E.; Innocenti, C.; Zanardelli, M.; Fittipaldi, M.; Falvo, E.; Carbo, M.; Shullani, V.; Mannelli, L.D.C.; Ghelardini, C.; Ferretti, A.M.; et al. A Smart Platform for Hyperthermia Application in Cancer Treatment: Cobalt-Doped Ferrite Nanoparticles Mineralized in Human Ferritin Cages. ACS Nano 2014, 8, 4705–4719. [Google Scholar] [CrossRef] [PubMed]
- Balejcikova, L.; Molcan, M.; Kovac, J.; Kubovcikova, M.; Saksl, K.; Mitroova, Z.; Timko, M.; Kopcansky, P. Hyperthermic effect in magnetoferritin aqueous colloidal solution. J. Mol. Liq. 2019, 283, 39–44. [Google Scholar] [CrossRef]
- Xu, H.; Pan, Y. Experimental Evaluation on the Heating Efficiency of Magnetoferritin Nanoparticles in an Alternating Magnetic Field. Nanomaterials 2019, 9, 1457. [Google Scholar] [CrossRef]
- Tong, S.; Quinto, C.A.; Zhang, L.; Mohindra, P.; Bao, G. Size-Dependent Heating of Magnetic Iron Oxide Nanoparticles. ACS Nano 2017, 11, 6808–6816. [Google Scholar] [CrossRef]
- Uchida, M.; Flenniken, M.L.; Allen, M.; Willits, D.A.; Crowley, B.E.; Brumfield, S.; Willis, A.F.; Jackiw, L.; Jutila, M.; Young, M.J.; et al. Targeting of Cancer Cells with Ferrimagnetic Ferritin Cage Nanoparticles. J. Am. Chem. Soc. 2006, 128, 16626–16633. [Google Scholar] [CrossRef]
- Pan, Y.; Cao, C.; He, X.; Yang, C.; Tian, L.; Zhu, R.; Cai, Y. Enhanced magnetic resonance imaging and staining of cancer cells using ferrimagnetic H-ferritin nanoparticles with increasing core size. Int. J. Nanomed. 2015, 10, 2619–2634. [Google Scholar] [CrossRef]
- Cao, C.; Tian, L.; Liu, Q.; Liu, W.; Chen, G.; Pan, Y. Magnetic characterization of noninteracting, randomly oriented, nanometer-scale ferrimagnetic particles. J. Geophys. Res. Earth Surf. 2010, 115, B07103. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, T.; Xu, H.; Dong, X.; Cai, Y.; Pan, Y.; Cao, C. Thermostable iron oxide nanoparticle synthesis within recombinant ferritins from the hyperthermophile Pyrococcus yayanosii CH1. RSC Adv. 2019, 9, 39381–39393. [Google Scholar] [CrossRef]
- Tatur, J.; Hagedoorn, P.L.; Overeijnder, M.L.; Hagen, W.R. A highly thermostable ferritin from the hyperthermophilic archaeal anaerobe Pyrococcus furiosus. Extremophiles 2005, 10, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Stefanini, S.; Cavallo, S.; Wang, C.Q.; Tataseo, P.; Vecchini, P.; Giartosio, A.; Chiancone, E. Thermal Stability of Horse Spleen Apoferritin and Human Recombinant H Apoferritin. Arch. Biochem. Biophys. 1996, 325, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Parker, M.J.; Allen, M.A.; Ramsay, B.; Klem, M.T.; Young, M.; Douglas, T. Expanding the Temperature Range of Biomimetic Synthesis Using a Ferritin from the HyperthermophilePyrococcus furiosus. Chem. Mater. 2008, 20, 1541–1547. [Google Scholar] [CrossRef]
- Fantechi, E.; Innocenti, C.; Ferretti, A.M.; Falvo, E.; Ceci, P.; Pineider, F.; Sangregorio, C. Increasing the Magnetic Anisotropy of a Natural System: Co-Doped Magnetite Mineralized in Ferritin Shells. J. Nanosci. Nanotechnol. 2019, 19, 4964–4973. [Google Scholar] [CrossRef]
- Pal, S.L.; Jana, U.; Manna, P.K.; Mohanta, G.P.; Manavalan, R. Nanoparticle: An overview of preparation and characterization. J. Appl. Pharm. Sci. 2011, 1, 228–234. [Google Scholar]
- Roy, D.; Park, J.W. Spatially nanoscale-controlled functional surfaces toward efficient bioactive platforms. J. Mater. Chem. B 2015, 3, 5135–5149. [Google Scholar] [CrossRef][Green Version]
- Uchida, M.; Terashima, M.; Cunningham, C.H.; Suzuki, Y.; Willits, D.A.; Willis, A.F.; Yang, P.C.; Tsao, P.S.; McConnell, M.V.; Young, M.J.; et al. A human ferritin iron oxide nano-composite magnetic resonance contrast agent. Magn. Reson. Med. 2008, 60, 1073–1081. [Google Scholar] [CrossRef]
- Melnikova, L.; Petrenko, V.I.; Avdeev, M.V.; Ivankov, O.I.; Bulavin, L.A.; Garamus, V.M.; Almásy, L.; Mitroova, Z.; Kopcansky, P. SANS contrast variation study of magnetoferritin structure at various iron loading. J. Magn. Magn. Mater. 2015, 377, 77–80. [Google Scholar] [CrossRef]
- Fiala, G.; Stetter, K.O. Pyrococcus-Furiosus Sp-Nov Represents a Novel Genus of Marine Heterotrophic Archaebacteria Growing Optimally at 100-Degrees C. Arch. Microbiol. 1986, 145, 56–61. [Google Scholar] [CrossRef]
- Melníková, L.; Petrenko, V.; Avdeev, M.; Garamus, V.; Almásy, L.; Ivankov, O.; Bulavin, L.; Mitroova, Z.; Kopcansky, P. Effect of iron oxide loading on magnetoferritin structure in solution as revealed by SAXS and SANS. Colloids Surf. B Biointerfaces 2014, 123, 82–88. [Google Scholar] [CrossRef]
- Viollier, E.; Inglett, P.W.; Hunter, K.; Roychoudhury, A.N.; Van Cappellen, P. The ferrozine method revisited: Fe (II)/Fe (III) determination in natural waters. Appl. Geochem. 2000, 15, 785–790. [Google Scholar] [CrossRef]
- Valero, E.; Tambalo, S.; Marzola, P.; Ortega-Muñoz, M.; López-Jaramillo, F.J.; Santoyo-González, F.; López, J.D.D.; Delgado, J.J.; Calvino, J.J.; Cuesta, R.; et al. Magnetic Nanoparticles-Templated Assembly of Protein Subunits: A New Platform for Carbohydrate-Based MRI Nanoprobes. J. Am. Chem. Soc. 2011, 133, 4889–4895. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Xie, J.; Niu, G.; Zhang, F.; Gao, H.; Yang, M.; Quan, Q.; Aronova, M.A.; Zhang, G.; Lee, S.; et al. Chimeric Ferritin Nanocages for Multiple Function Loading and Multimodal Imaging. Nano Lett. 2011, 11, 814–819. [Google Scholar] [CrossRef] [PubMed]
- Balejčíková, L.; Kováč, J.; Garamus, V.M.; Avdeev, M.V.; Petrenko, V.I.; Almásy, L.; Kopčanský, P. Influence of synthesis temperature on structural and magnetic properties of magnetoferritin. Mendeleev Commun. 2019, 29, 279–281. [Google Scholar] [CrossRef]
- Hikono, T.; Uraoka, Y.; Fuyuki, T.; Yamashita, I. Novel Method for Making Nanodot Arrays Using a Cage-like Protein. Jpn. J. Appl. Phys. 2003, 42, L398–L399. [Google Scholar] [CrossRef]
- Cai, Y.; Wang, Y.; Zhang, T.; Pan, Y. Gadolinium-Labeled Ferritin Nanoparticles as T1 Contrast Agents for Magnetic Resonance Imaging of Tumors. ACS Appl. Nano Mater. 2020, 3, 8771–8783. [Google Scholar] [CrossRef]
- Cao, J.; Ng, E.S.; McNaughton, D.; Stanley, E.G.; Elefanty, A.G.; Tobin, M.J.; Heraud, P. The Characterisation of Pluripotent and Multipotent Stem Cells Using Fourier Transform Infrared Microspectroscopy. Int. J. Mol. Sci. 2013, 14, 17453–17476. [Google Scholar] [CrossRef]
- Durazzo, A.; Kiefer, J.; Lucarini, M.; Camilli, E.; Marconi, S.; Gabrielli, P.; Aguzzi, A.; Gambelli, L.; Lisciani, S.; Marletta, L. Qualitative Analysis of Traditional Italian Dishes: FTIR Approach. Sustainability 2018, 10, 4112. [Google Scholar] [CrossRef]
- Kallumadil, M.; Tada, M.; Nakagawa, T.; Abe, M.; Southern, P.; Pankhurst, Q.A. Suitability of commercial colloids for magnetic hyperthermia. J. Magn. Magn. Mater. 2009, 321, 1509–1513. [Google Scholar] [CrossRef]
- Guardia, P.; Di Corato, R.; Lartigue, L.; Wilhelm, C.; Espinosa, A.; Garcia-Hernandez, M.; Gazeau, F.; Manna, L.; Pellegrino, T. Water-Soluble Iron Oxide Nanocubes with High Values of Specific Absorption Rate for Cancer Cell Hyperthermia Treatment. ACS Nano 2012, 6, 3080–3091. [Google Scholar] [CrossRef]
- Kandasamy, G.; Sudame, A.; Luthra, T.; Saini, K.; Maity, D. Functionalized Hydrophilic Superparamagnetic Iron Oxide Nanoparticles for Magnetic Fluid Hyperthermia Application in Liver Cancer Treatment. ACS Omega 2018, 3, 3991–4005. [Google Scholar] [CrossRef] [PubMed]
- Gawali, S.L.; Barick, B.; Barick, K.; Hassan, P. Effect of sugar alcohol on colloidal stabilization of magnetic nanoparticles for hyperthermia and drug delivery applications. J. Alloy. Compd. 2017, 725, 800–806. [Google Scholar] [CrossRef]
- Ji, W.C.; Hu, P.; Wang, X.Y.; Chen, B.; Chang, T.; Yang, F.F.; Cao, Q.G.; Zhang, W.; Dang, R.; Wang, K.S. High heating ability of one-step carbothermal reduction method of Fe3O4 nanoparticles upon magnetic field. J. Alloy. Compd. 2021, 866, 158952. [Google Scholar] [CrossRef]
- Smolkova, I.S.; Kazantseva, N.E.; Babayan, V.; Smolka, P.; Parmar, H.; Vilcakova, J.; Schneeweiss, O.; Pizurova, N. Alternating magnetic field energy absorption in the dispersion of iron oxide nanoparticles in a viscous medium. J. Magn. Magn. Mater. 2015, 374, 508–515. [Google Scholar] [CrossRef]
- Gawali, S.L.; Shelar, S.B.; Gupta, J.; Barick, K.; Hassan, P. Immobilization of protein on Fe3O4 nanoparticles for magnetic hyperthermia application. Int. J. Biol. Macromol. 2021, 166, 851–860. [Google Scholar] [CrossRef]
- Vamvakidis, K.; Maniotis, N.; Dendrinou-Samara, C. Magneto-fluorescent nanocomposites: Experimental and theoretical linkage for the optimization of magnetic hyperthermia. Nanoscale 2021, 13, 6426–6438. [Google Scholar] [CrossRef]
- Zhang, T.; Pan, Y. Constraining the magnetic properties of ultrafine- and fine-grained biogenic magnetite. Earth Planets Space 2018, 70, 206. [Google Scholar] [CrossRef]
- Wohlfarth, E.P. Relations between Different Modes of Acquisition of the Remanent Magnetization of Ferromagnetic Particles. J. Appl. Phys. 1958, 29, 595–596. [Google Scholar] [CrossRef]
- Lee, J.H.; Jang, J.T.; Choi, J.S.; Moon, S.H.; Noh, S.H.; Kim, J.W.; Kim, J.G.; Kim, I.S.; Park, K.I.; Cheon, J. Exchange-coupled magnetic nanoparticles for efficient heat induction. Nat. Nanotechnol. 2011, 6, 418–422. [Google Scholar] [CrossRef]
- Jiang, K.; Zhang, Q.; Hinojosa, D.T.; Zhang, L.; Xiao, Z.; Yin, Y.; Tong, S.; Colvin, V.L.; Bao, G. Controlled oxidation and surface modification increase heating capacity of magnetic iron oxide nanoparticles. Appl. Phys. Rev. 2021, 8, 031407. [Google Scholar] [CrossRef]
- Demortiere, A.; Panissod, P.; Pichon, B.P.; Pourroy, G.; Guillon, D.; Donnio, B.; Begin-Colin, S. Size-dependent properties of magnetic iron oxide nanocrystals. Nanoscale 2011, 3, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Bedanta, S.; Kleemann, W. Supermagnetism. J. Phys. D Appl. Phys. 2009, 42, 013001. [Google Scholar] [CrossRef]



| Sample | TEM Size (nm) | H (kA m−1) | f (kHz) | SAR (W g−1) | ILP (nHm2 kg−1) | Reference |
|---|---|---|---|---|---|---|
| HFt5 | 6.8 | 12.4 | 183 | 2.81 | 0.1 | [13] |
| PfFt10 | 12.4 | 183 | 4.9 | 0.17 | [24] | |
| MHFn | 4.8 | 19.5 | 805.5 | 51.3 | 0.17 | [15] |
| AFF-3 | 10 | 13.9 | 175.2 | 48.6 | 1.4 | [41] |
| MMNPs | 10 | 40.56 | 300 | 127.7 | 0.26 | [42] |
| Fe3O4 | 6.5 | 4 | 165.3 | 10.3 | 3.8 | [43] |
| MNPs | 13 | 13.8 | 114 | 14.1 | 0.65 | [44] |
| Pro-Glu-MNPs | 4.5 | 42.3 | 300 | 69 | 0.2 | [45] |
| S4-Zn0.53 Fe2.47O4@PEG | 20 | 24 | 765 | 380 | 0.86 | [46] |
| MPfFn-45 | 7.1 | 49 | 485.7 | 143.6 | 0.12 | This work |
| MPfFn-65 | 7.6 | 49 | 485.7 | 170.4 | 0.15 | This work |
| MPfFn-90 | 10.3 | 49 | 485.7 | 805.3 | 0.70 | This work |
| MPfFn-90 | 10.3 | 39 | 485.7 | 535.4 | 0.72 | This work |
| MPfFn-90 | 10.3 | 29 | 485.7 | 376.8 | 0.92 | This work |
| MPfFn-90 | 10.3 | 19 | 485.7 | 239.2 | 1.36 | This work |
| Arrhenius Law | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Samples | Core Size (nm) | HD (nm) | Ms (300 K) (emu g−1) | Ms (5 K) (emu g−1) | Hc (5 K) (Oe) | TB (K) | R | EB/kB (K) | τ0 (s) |
| MPfFn-45 | 7.1 | 15.4 | 29.4 | 38.7 | 256.9 | 82.9 | 0.38 | 3102.5 | 8.6 × 10−22 |
| MPfFn-65 | 7.6 | 16.0 | 32.4 | 41.0 | 273.7 | 90.1 | 0.36 | 3461.0 | 9.5 × 10−21 |
| MPfFn-90 | 10.3 | 25.0 | 42.3 | 49.6 | 240.1 | 261.5 | 0.33 | 11,913.0 | 3.2 × 10−24 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, J.; Cao, C.; Fang, F.; Pan, Y. Enhanced Magnetic Hyperthermia of Magnetoferritin through Synthesis at Elevated Temperature. Int. J. Mol. Sci. 2022, 23, 4012. https://doi.org/10.3390/ijms23074012
Yu J, Cao C, Fang F, Pan Y. Enhanced Magnetic Hyperthermia of Magnetoferritin through Synthesis at Elevated Temperature. International Journal of Molecular Sciences. 2022; 23(7):4012. https://doi.org/10.3390/ijms23074012
Chicago/Turabian StyleYu, Jiacheng, Changqian Cao, Fengjiao Fang, and Yongxin Pan. 2022. "Enhanced Magnetic Hyperthermia of Magnetoferritin through Synthesis at Elevated Temperature" International Journal of Molecular Sciences 23, no. 7: 4012. https://doi.org/10.3390/ijms23074012
APA StyleYu, J., Cao, C., Fang, F., & Pan, Y. (2022). Enhanced Magnetic Hyperthermia of Magnetoferritin through Synthesis at Elevated Temperature. International Journal of Molecular Sciences, 23(7), 4012. https://doi.org/10.3390/ijms23074012






