Anti-Obesity and Anti-Hyperglycemic Effects of Meretrix lusoria Protamex Hydrolysate in ob/ob Mice
Abstract
1. Introduction
2. Results
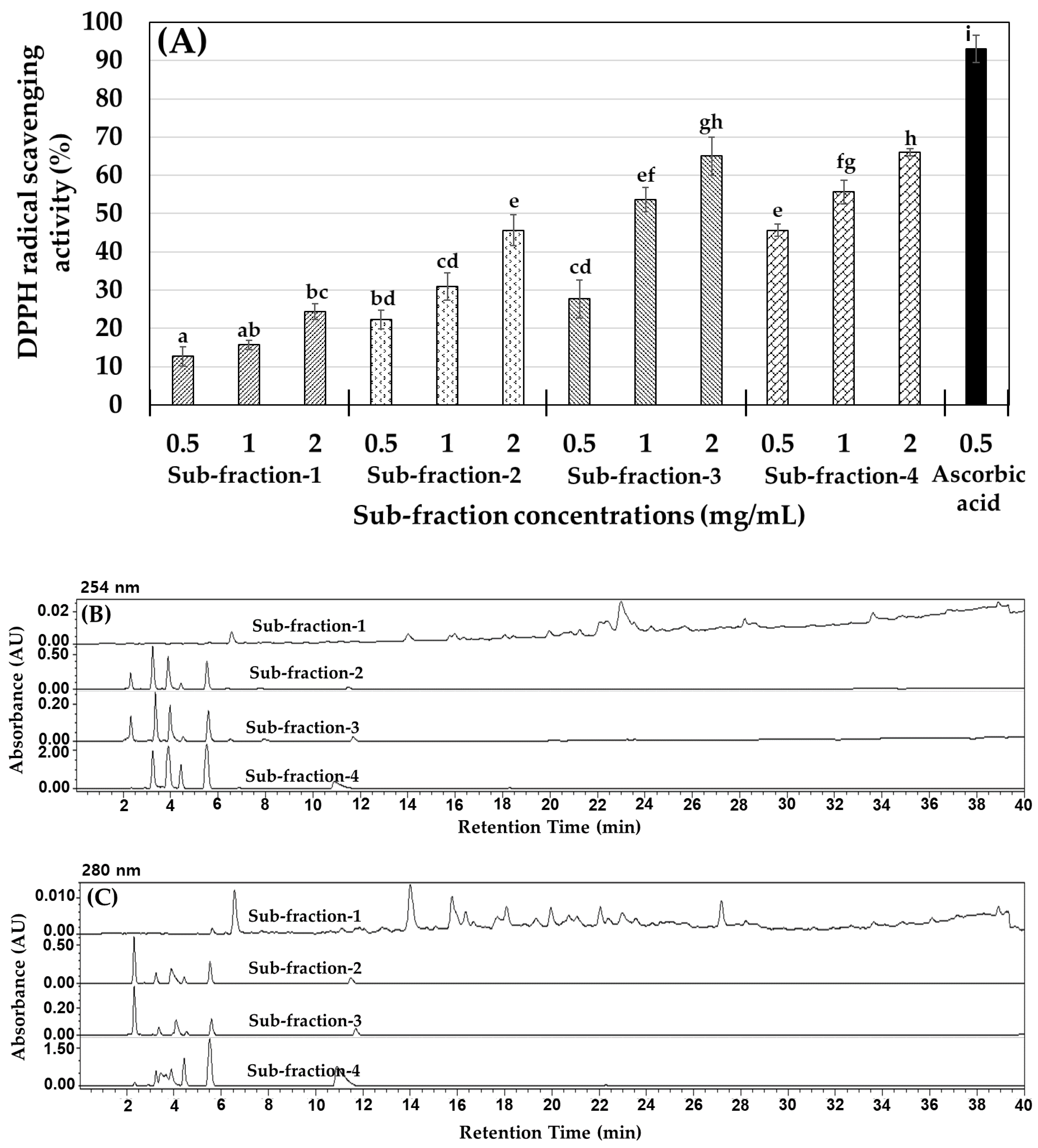
2.1. M. lusoria Enzymatic Hydrolysate Yield (%) and the Antioxidant Activity of Their Fractions
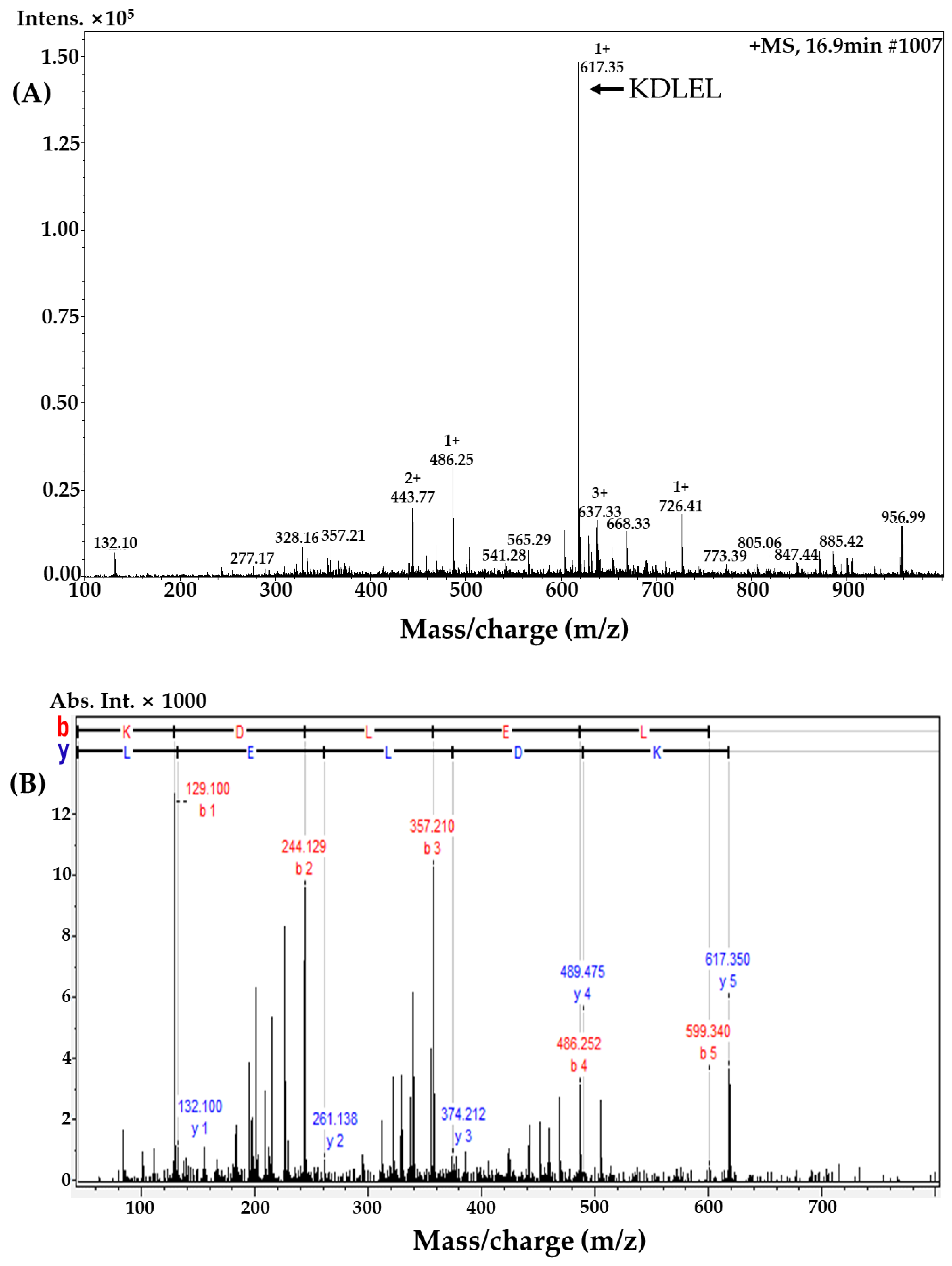
2.2. MLPH (≤1 kDa) Fraction Purification and Peptide Sequence Identification
2.3. Metabolic Characteristics of ob/ob Mice Fed a Standard Diet and Mice Administered MLPH
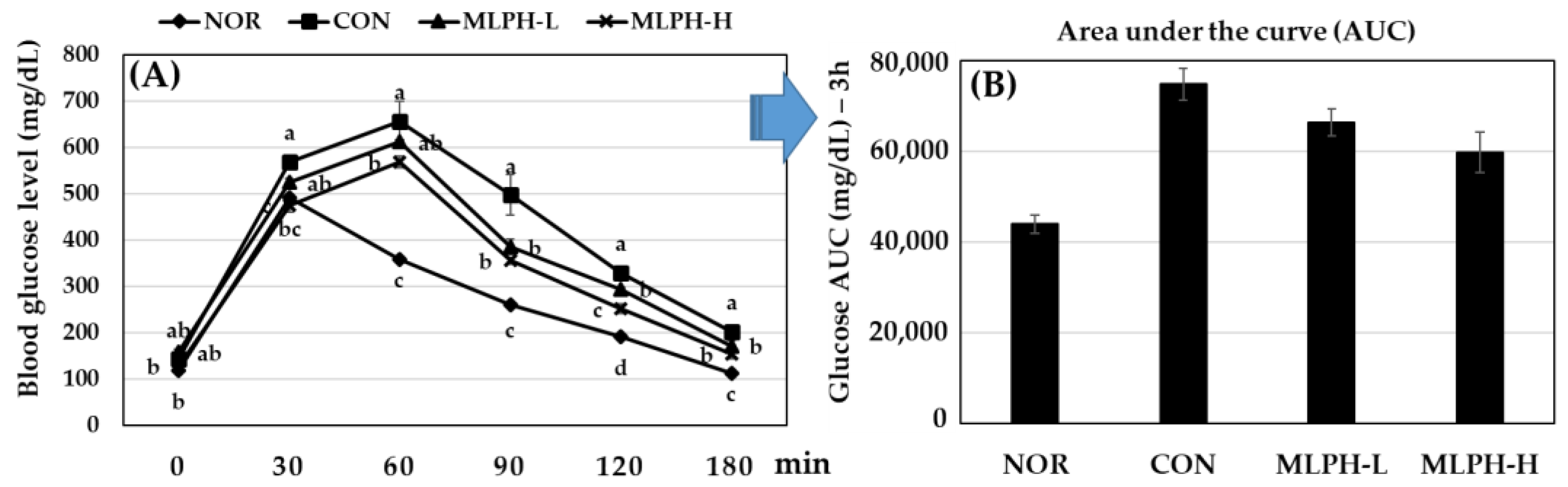
2.4. Effect of MLPH Fraction on Impaired Glucose Tolerance in ob/ob Mice
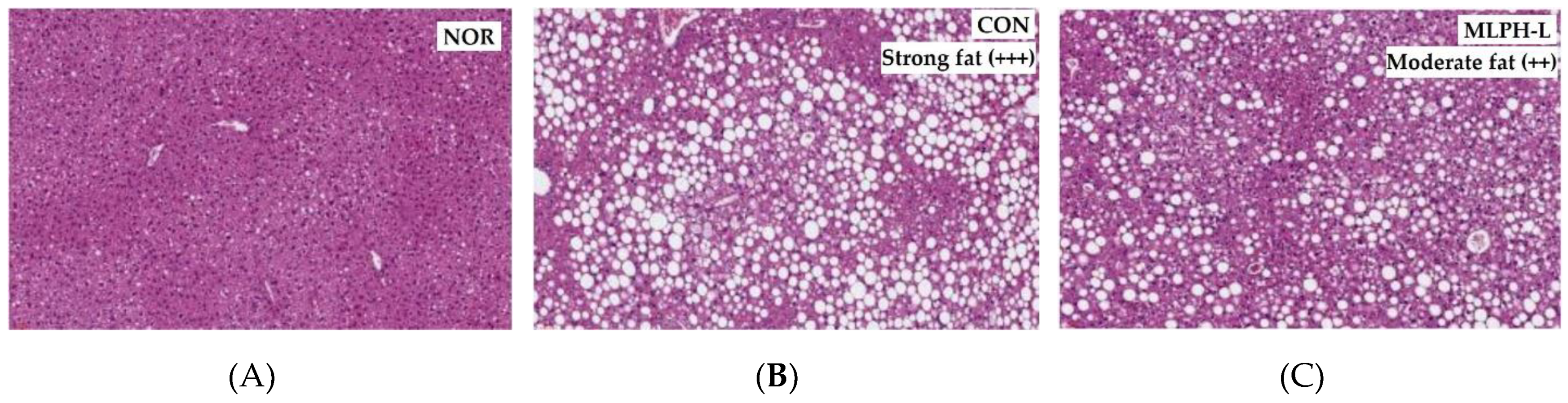
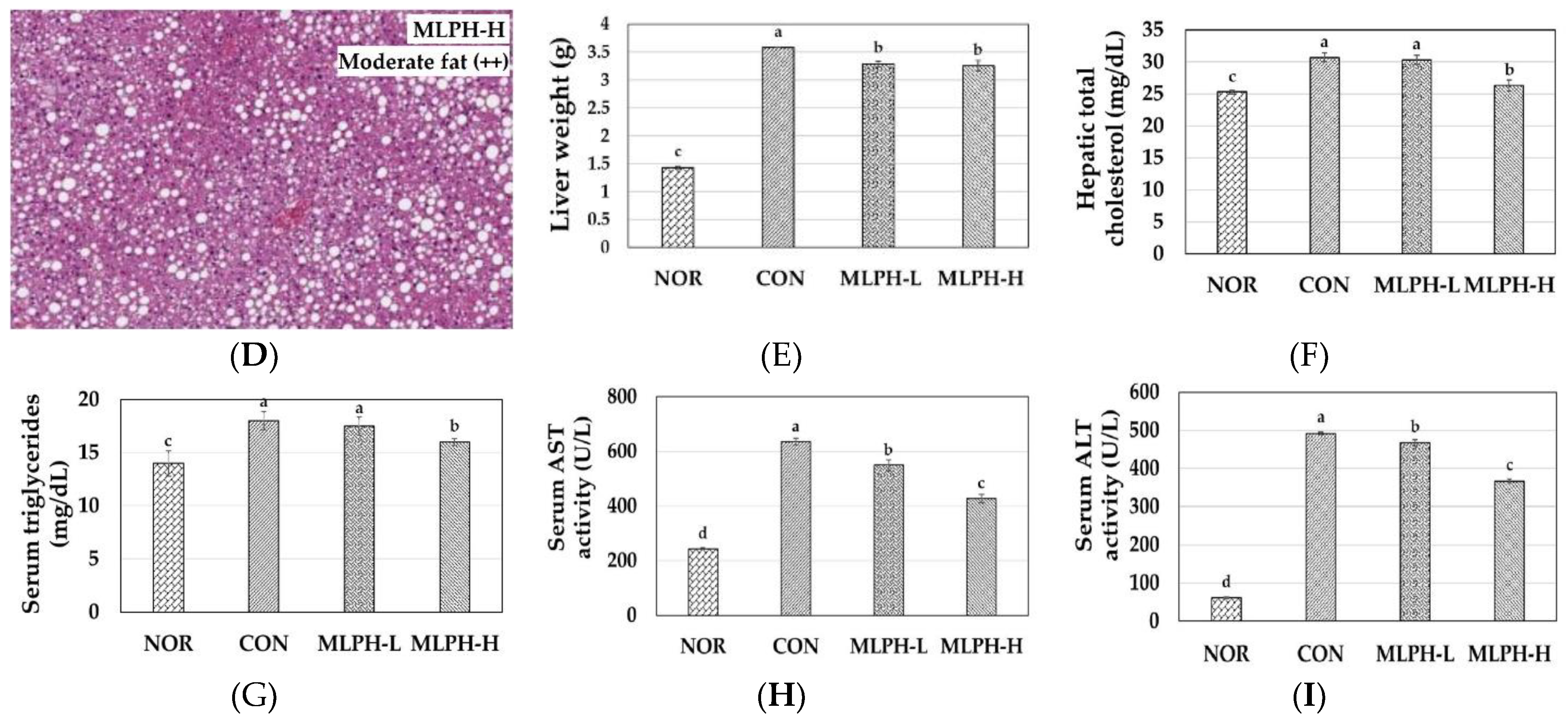
2.5. Hepatic Histological Changes and Marker Enzymes, including Antihyperlipidemic Effect, in MLPH treated ob/ob Mice
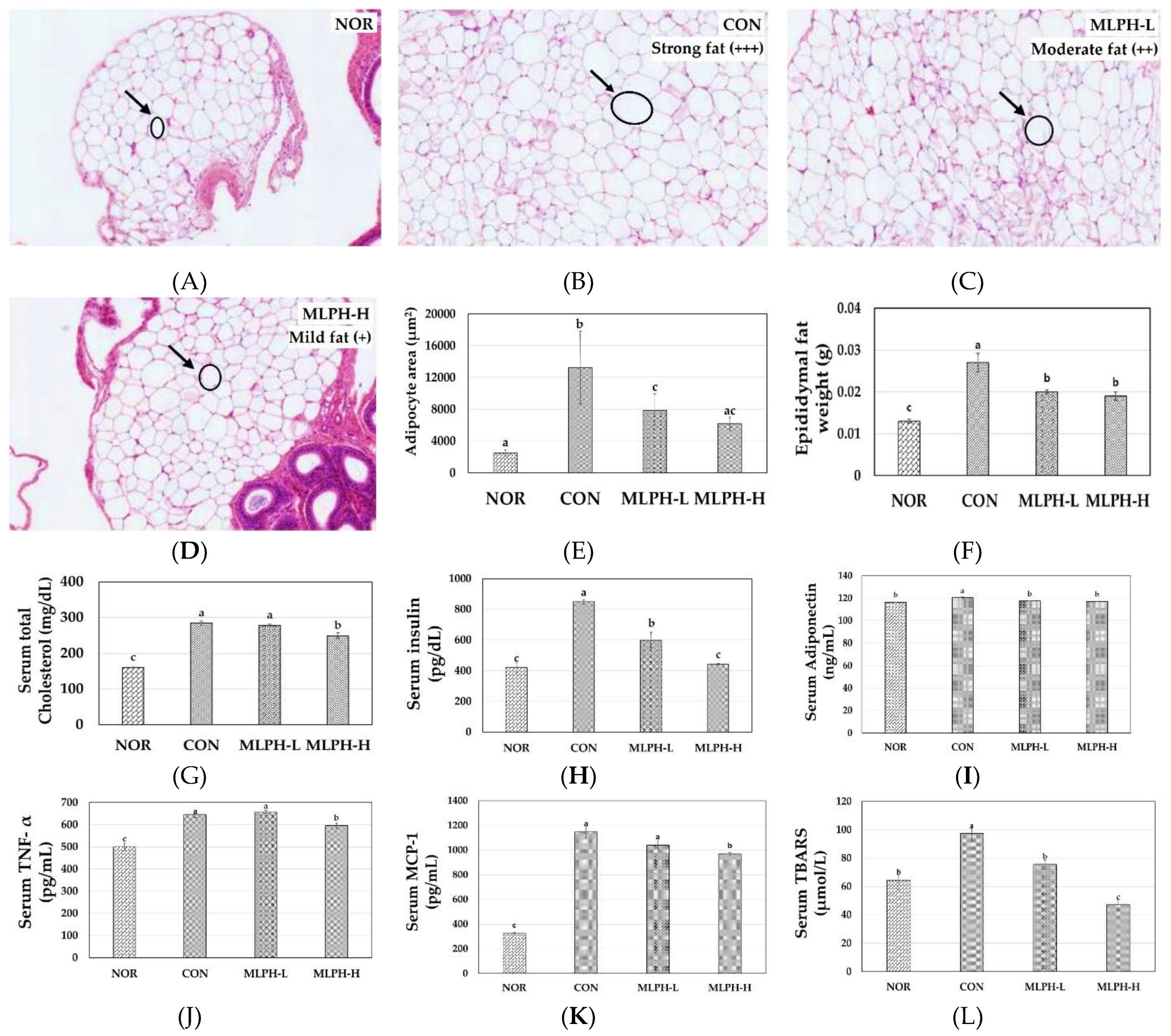
2.6. Epididymal Fat Changes and Metabolic Variables in MLPH Treated ob/ob Mice
2.7. Hepatic Antioxidant Enzyme Activity in Normal, Control, and MLPH Treated Mice
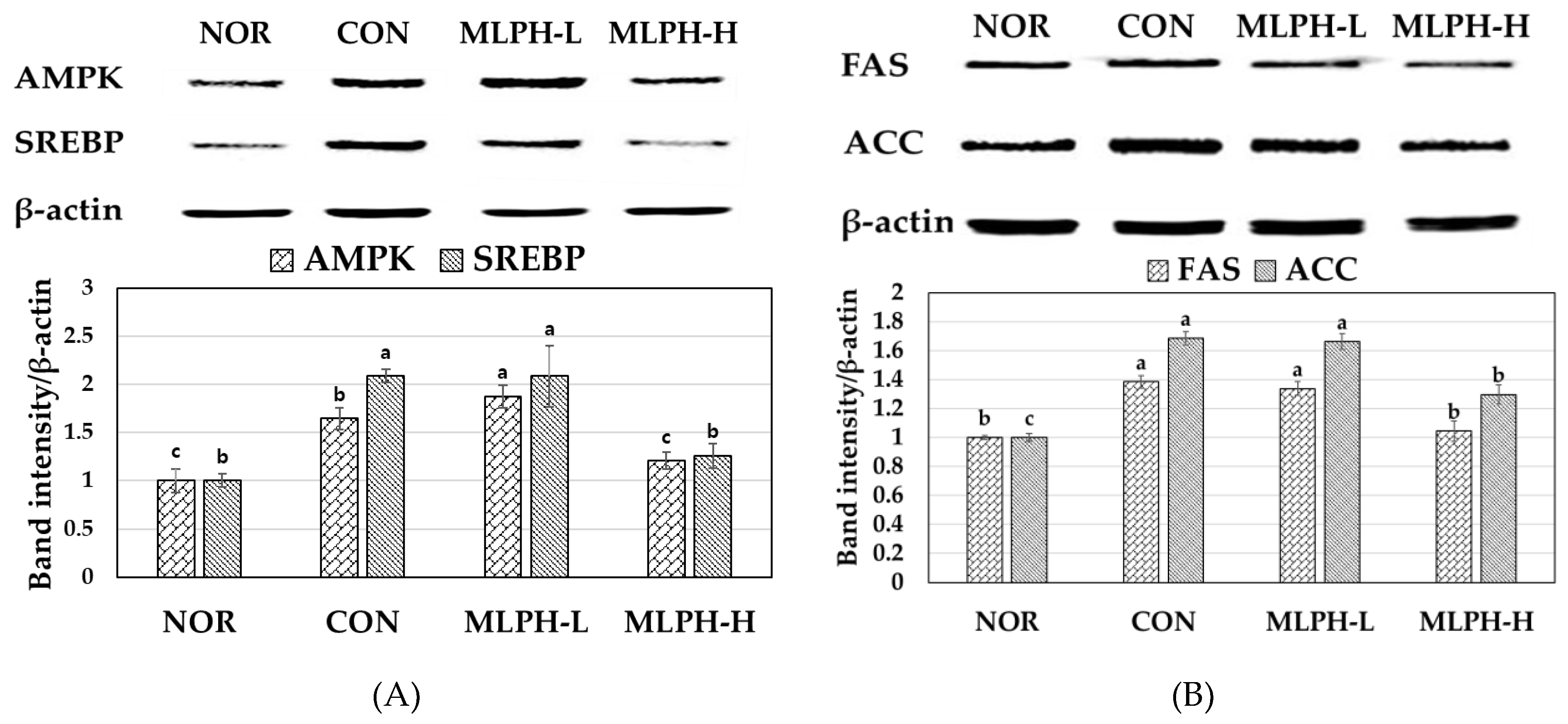
2.8. Effects of MLPH Fraction on AMPK, SREBP, FAS, and ACC Protein Expression in Control Obese Mice
2.9. Effects of MLPH on Hepatic mRNA Expression Levels Related to Lipogenesis and Gluconeogenesis in Obese Mice
3. Discussion
4. Materials and Methods
4.1. Preparation of Protein Hydrolysate Fractions from M. lusoria
4.2. Antioxidant Activity of M. lusoria Hydrolysate Fractions
4.3. Purification and Identification of MLPH Peptide Fraction Using LC-MS/MS Analysis
4.4. Animals
4.5. Experimental Design
4.6. Glucose Tolerance Test
4.7. Serum Biochemical Analysis
4.8. Total Cholesterol and Triglyceride Levels in Liver Tissues
4.9. Antioxidant Enzyme Activity in Liver Tissues
4.10. Liver Tissue Extracts and Western Blotting
4.11. Isolation and Quantification of RNA Using Real-Time RT-PCR
4.12. Hematoxylin and Eosin (H&E) Staining for Histological Analysis
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahmed, B.; Sultana, R.; Greene, M.W. Adipose tissue and insulin resistance in obese. Biomed. Pharmacother. 2021, 137, 111315. [Google Scholar] [CrossRef] [PubMed]
- Akheruzzaman, M.; Hegde, V.; Shin, A.C.; Dhurandhar, N.V. Reducing endogenous insulin is linked with protection against hepatic steatosis in mice. Nutr. Diabetes 2020, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.-W.; Lee, Y.-h.; Park, C.-Y.; Rhee, E.-J.; Lee, W.-Y.; Kim, N.-H.; Choi, K.M.; Park, K.-G.; Choi, Y.-K.; Cha, B.-S. Non-alcoholic fatty liver disease in patients with type 2 diabetes mellitus: A position statement of the Fatty Liver Research Group of the Korean Diabetes Association. Diabetes Metab. J. 2020, 44, 382–401. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Li, H.; Xia, N. The interplay between adipose tissue and vasculature: Role of oxidative stress in obesity. Front. Cardiovasc. Med. 2021, 8, 131. [Google Scholar] [CrossRef]
- Dommel, S.; Blüher, M. Does CC motif chemokine ligand 2 (CCL2) link obesity to a pro-inflammatory state? Int. J. Mol. Sci. 2021, 22, 1500. [Google Scholar] [CrossRef]
- Toan, N.L.; Van Hoan, N.; Cuong, D.V.; Dung, N.V.; Hang, N.T.; Chung, D.T.; Son, H.A.; Phong, P.X.; Lenon, G.B.; Van De, D. Adipose tissue-derived cytokines and their correlations with clinical characteristics in Vietnamese patients with type 2 diabetes mellitus. Diabetol. Metab. Syndr. 2018, 10, 1–14. [Google Scholar] [CrossRef]
- An, H.-J.; Kim, J.-Y.; Gwon, M.-G.; Gu, H.; Kim, H.-J.; Leem, J.; Youn, S.W.; Park, K.-K. Beneficial effects of Srebp decoy oligodeoxynucleotide in an animal model of hyperlipidemia. Int. J. Mol. Sci. 2020, 21, 552. [Google Scholar] [CrossRef]
- Zhu, L.; Yang, X.; Li, J.; Jia, X.; Bai, X.; Zhao, Y.; Cheng, W.; Shu, M.; Zhu, Y.; Jin, S. Leptin gene-targeted editing in ob/ob mouse adipose tissue based on the CRISPR/Cas9 system. J. Genet. Genom. 2021, 48, 134–146. [Google Scholar] [CrossRef]
- Batchuluun, B.; Pinkosky, S.L.; Steinberg, G.R. Lipogenesis inhibitors: Therapeutic opportunities and challenges. Nat. Rev. Drug Discov. 2022, 21, 283–305. [Google Scholar] [CrossRef]
- Lee, M.-H.; Hwang, J.-M.; Kang, E.-J.; Kim, H.-M.; Yoon, S.-W.; Chung, H.-C.; Lee, J.-H. Anti-Obesity Effect of Licorice Acetone Extract in a Mouse Model of Obesity Induced by a High-Fat Diet. Food Suppl. Biomater. Health 2021, 1, e8. [Google Scholar] [CrossRef]
- van der Vaart, J.I.; Boon, M.R.; Houtkooper, R.H. The Role of AMPK Signaling in Brown Adipose Tissue Activation. Cells 2021, 10, 1122. [Google Scholar] [CrossRef] [PubMed]
- Foretz, M.; Even, P.C.; Viollet, B. AMPK activation reduces hepatic lipid content by increasing fat oxidation in vivo. Int. J. Mol. Sci. 2018, 19, 2826. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.; Kim, C.Y. Natural products and obesity: A focus on the regulation of mitotic clonal expansion during adipogenesis. Molecules 2019, 24, 1157. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.G.; Park, C.-Y. Anti-obesity drugs: A review about their effects and safety. Diabetes Metab. J. 2012, 36, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Elam, E.; Feng, J.; Lv, Y.-M.; Ni, Z.-J.; Sun, P.; Thakur, K.; Zhang, J.-G.; Ma, Y.-L.; Wei, Z.-J. Recent advances on bioactive food derived anti-diabetic hydrolysates and peptides from natural resources. J. Funct. Foods 2021, 86, 104674. [Google Scholar] [CrossRef]
- Kang, N.; Kim, E.-A.; Kim, J.; Lee, S.-H.; Heo, S.-J. Identifying potential antioxidant properties from the viscera of sea snails (Turbo cornutus). Mar. Drugs 2021, 19, 567. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Nair, S.; Gagnon, J. Herring Milt and Herring Milt Protein Hydrolysate Are Equally Effective in Improving Insulin Sensitivity and Pancreatic Beta-Cell Function in Diet-Induced Obese-and Insulin-Resistant Mice. Mar. Drugs 2020, 18, 635. [Google Scholar] [CrossRef]
- Kim, M.-R.; Kim, J.-W.; Park, J.B.; Hong, Y.-K.; Ku, S.K.; Choi, J.-S. Anti-obesity effects of yellow catfish protein hydrolysate on mice fed a 45% kcal high-fat diet. Int. J. Mol. Med. 2017, 40, 784–800. [Google Scholar] [CrossRef]
- Greenwood, H.C.; Bloom, S.R.; Murphy, K.G. Peptides and their potential role in the treatment of diabetes and obesity. Rev. Diabet. Stud. RDS 2011, 8, 355. [Google Scholar] [CrossRef]
- Zhang, S.; Luo, L.; Sun, X.; Ma, A. Bioactive Peptides: A Promising Alternative to Chemical Preservatives for Food Preservation. J. Agric. Food Chem. 2021, 69, 12369–12384. [Google Scholar] [CrossRef]
- Hosseini, H.; Jafari, S.M. Introducing nano/microencapsulated bioactive ingredients for extending the shelf-life of food products. Adv. Colloid Interface Sci. 2020, 282, 102210. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.-J.; Choi, D.; Lee, J.; Jo, Y.-J. Encapsulation of a bioactive peptide in a formulation of W1/O/W2-type double emulsions: Formation and stability. Food Struct. 2020, 25, 100145. [Google Scholar] [CrossRef]
- Lu, T.H.; Yang, Y.F.; Chen, C.Y.; Wang, W.M.; Liao, C.M. Quantifying the impact of temperature variation on birnavirus transmission dynamics in hard clams Meretrix lusoria. J. Fish Dis. 2020, 43, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Smaal, A.C.; Ferreira, J.G.; Grant, J.; Petersen, J.K.; Strand, Ø. Goods and Services of Marine Bivalves; Springer Nature: Gewerbestrasse: Cham, Switzerland, 2019. [Google Scholar]
- Lee, Y.-C.; Lin, C.-S.; Zeng, W.-H.; Hwang, C.-C.; Chiu, K.; Ou, T.-Y.; Chang, T.-H.; Tsai, Y.-H. Effect of a Novel Microwave-Assisted Induction Heating (MAIH) Technology on the Quality of Prepackaged Asian Hard Clam (Meretrix lusoria). Foods 2021, 10, 2299. [Google Scholar] [CrossRef]
- Slizyte, R.; Rommi, K.; Mozuraityte, R.; Eck, P.; Five, K.; Rustad, T. Bioactivities of fish protein hydrolysates from defatted salmon backbones. Biotechnol. Rep. 2016, 11, 99–109. [Google Scholar] [CrossRef]
- Cheong, S.H.; Kim, E.K.; Hwang, J.W.; Kim, Y.S.; Lee, J.S.; Moon, S.H.; Jeon, B.T.; Pyo-Jam Park, P.J. Purification of a novel peptide derived from a shellfish, Crassostrea gigas, and evaluation of its anticancer property. J. Agric. Food Chem. 2013, 61, 11442–11446. [Google Scholar] [CrossRef]
- Je, J.Y.; Park, J.Y.; Jung, W.K.; Park, P.J.; Kim, S.K. Isolation of angiotensin I converting enzyme (ACE) inhibitor from fermented oyster sauce, Crassostrea gigas. Food Chem. 2005, 90, 809–814. [Google Scholar] [CrossRef]
- Lee, J.K.; Byun, H.-G. Characterization of antioxidative peptide purified from black eelpout (Lycodes diapterus) hydrolysate. Fish. Aquat. Sci. 2019, 22, 1–7. [Google Scholar] [CrossRef]
- Hunsakul, K.; Laokuldilok, T.; Prinyawiwatkul, W.; Utama-ang, N. Effects of thermal processing on antioxidant activities, amino acid composition and protein molecular weight distributions of jasmine rice bran protein hydrolysate. Int. J. Food Sci. Technol. 2021, 56, 3289–3298. [Google Scholar] [CrossRef]
- He, R.; Girgih, A.T.; Malomo, S.A.; Ju, X.; Aluko, R.E. Antioxidant activities of enzymatic rapeseed protein hydrolysates and the membrane ultrafiltration fractions. J. Funct. Foods 2013, 5, 219–227. [Google Scholar] [CrossRef]
- Choi, J.-S.; Kim, J.-W.; Park, J.B.; Pyo, S.E.; Hong, Y.-K.; Ku, S.K.; Kim, M.-R. Blood glycemia-modulating effects of melanian snail protein hydrolysates in mice with type II diabetes. Int. J. Mol. Med. 2017, 39, 1437–1451. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Piché, M.-E.; Tchernof, A.; Després, J.-P. Obesity phenotypes, diabetes, and cardiovascular diseases. Circ. Res. 2020, 126, 1477–1500. [Google Scholar] [CrossRef] [PubMed]
- Han, J.M.; Kim, H.I.; Lee, Y.-J.; Lee, J.W.; Kim, K.M.; Bae, J.C. Differing associations between fatty liver and dyslipidemia according to the degree of hepatic steatosis in Korea. J. Lipid Atheroscler. 2019, 8, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Garcés-Rimón, M.; González, C.; Uranga, J.; López-Miranda, V.; López-Fandiño, R.; Miguel, M. Pepsin egg white hydrolysate ameliorates obesity-related oxidative stress, inflammation and steatosis in Zucker fatty rats. PLoS ONE 2016, 11, e0151193. [Google Scholar] [CrossRef] [PubMed]
- Francisco, V.; Pino, J.; Gonzalez-Gay, M.A.; Mera, A.; Lago, F.; Gómez, R.; Mobasheri, A.; Gualillo, O. Adipokines and inflammation: Is it a question of weight? Br. J. Pharmacol. 2018, 175, 1569–1579. [Google Scholar] [CrossRef]
- Choi, H.M.; Doss, H.M.; Kim, K.S. Multifaceted physiological roles of adiponectin in inflammation and diseases. Int. J. Mol. Sci. 2020, 21, 1219. [Google Scholar] [CrossRef]
- Kojta, I.; Chacińska, M.; Błachnio-Zabielska, A. Obesity, bioactive lipids, and adipose tissue inflammation in insulin resistance. Nutrients 2020, 12, 1305. [Google Scholar] [CrossRef]
- Zhao, S.; Jiang, J.; Jing, Y.; Liu, W.; Yang, X.; Hou, X.; Gao, L.; Wei, L. The concentration of tumor necrosis factor-α determines its protective or damaging effect on liver injury by regulating Yap activity. Cell Death Dis. 2020, 11, 1–13. [Google Scholar] [CrossRef]
- Nagao, K.; Inoue, N.; Suzuki, K.; Shimizu, T.; Yanagita, T. The Cholesterol Metabolite Cholest-5-en-3-One Alleviates Hyperglycemia and Hyperinsulinemia in Obese (db/db) Mice. Metabolites 2022, 12, 26. [Google Scholar] [CrossRef]
- Lee, J.-H.; Kim, D.-Y.; Pantha, R.; Lee, E.-H.; Bae, J.-H.; Han, E.; Song, D.-K.; Kwon, T.K.; Im, S.-S. Identification of Pre-Diabetic Biomarkers in the Progression of Diabetes Mellitus. Biomedicines 2022, 10, 72. [Google Scholar] [CrossRef]
- Queck, A.; Bode, H.; Uschner, F.E.; Brol, M.J.; Graf, C.; Schulz, M.; Jansen, C.; Praktiknjo, M.; Schierwagen, R.; Klein, S. Systemic MCP-1 levels derive mainly from injured liver and are associated with complications in cirrhosis. Front. Immunol. 2020, 11, 354. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Fan, J.; Su, Q.; Yang, Z. Cytokines and abnormal glucose and lipid metabolism. Front. Endocrinol. 2019, 10, 703. [Google Scholar] [CrossRef] [PubMed]
- Montes-Nieto, R.; Insenser, M.; Murri, M.; Fernández-Durán, E.; Ojeda-Ojeda, M.; Martínez-García, M.Á.; Luque-Ramírez, M.; Escobar-Morreale, H.F. Plasma thiobarbituric acid reactive substances (TBARS) in young adults: Obesity increases fasting levels only in men whereas glucose ingestion, and not protein or lipid intake, increases postprandial concentrations regardless of sex and obesity. Mol. Nutr. Food Res. 2017, 61, 1700425. [Google Scholar] [CrossRef]
- Tsikas, D. Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: Analytical and biological challenges. Anal. Biochem. 2017, 524, 13–30. [Google Scholar] [CrossRef] [PubMed]
- Ghani, M.A.; Barril, C.; Bedgood Jr, D.R.; Prenzler, P.D. Measurement of antioxidant activity with the thiobarbituric acid reactive substances assay. Food Chem. 2017, 230, 195–207. [Google Scholar] [CrossRef] [PubMed]
- de Medeiros, A.F.; de Queiroz, J.L.C.; Maciel, B.L.L.; de Araújo Morais, A.H. Hydrolyzed Proteins and Vegetable Peptides: Anti-Inflammatory Mechanisms in Obesity and Potential Therapeutic Targets. Nutrients 2022, 14, 690. [Google Scholar] [CrossRef]
- Wondmkun, Y.T. Obesity, insulin resistance, and type 2 diabetes: Associations and therapeutic implications. Diabetes Metab. Syndr. Obes. Targets Ther. 2020, 13, 3611. [Google Scholar] [CrossRef]
- Smith, U.; Kahn, B.B. Adipose tissue regulates insulin sensitivity: Role of adipogenesis, de novo lipogenesis and novel lipids. J. Internal Med. 2016, 280, 465–475. [Google Scholar] [CrossRef]
- Thomas, D.D.; Corkey, B.E.; Istfan, N.W.; Apovian, C.M. Hyperinsulinemia: An early indicator of metabolic dysfunction. J. Endocr. Soc. 2019, 3, 1727–1747. [Google Scholar] [CrossRef]
- Panag, K.M.D.S.; Kaur, N.; Goyal, G. Correlation of insulin resistance by various methods with fasting insulin in obese. Int. J. Appl. Basic Med. Res. 2014, 4 (Suppl. S1), S41. [Google Scholar]
- Wang, T.; He, C. Pro-inflammatory cytokines: The link between obesity and osteoarthritis. Cytokine Growth Factor Rev. 2018, 44, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Marseglia, L.; Manti, S.; D’Angelo, G.; Nicotera, A.; Parisi, E.; Di Rosa, G.; Gitto, E.; Arrigo, T. Oxidative stress in obesity: A critical component in human diseases. Int. J. Mol. Sci. 2015, 16, 378–400. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, S.; Fujita, T.; Shimabukuro, M.; Iwaki, M.; Yamada, Y.; Nakajima, Y.; Nakayama, O.; Makishima, M.; Matsuda, M.; Shimomura, I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Investig. 2017, 114, 1752–1761. [Google Scholar] [CrossRef] [PubMed]
- Su, L.-J.; Zhang, J.-H.; Gomez, H.; Murugan, R.; Hong, X.; Xu, D.; Jiang, F.; Peng, Z.-Y. Reactive oxygen species-induced lipid peroxidation in apoptosis, autophagy, and ferroptosis. Oxid. Med. Cell. Longev. 2019, 2019, 5080843. [Google Scholar] [CrossRef]
- Jung, U.J.; Park, Y.B.; Kim, S.R.; Choi, M.-S. Supplementation of persimmon leaf ameliorates hyperglycemia, dyslipidemia and hepatic fat accumulation in type 2 diabetic mice. PLoS ONE 2012, 7, e49030. [Google Scholar]
- Santos-Sánchez, G.; Cruz-Chamorro, I.; Álvarez-Ríos, A.I.; Fernández-Santos, J.M.; Vázquez-Román, M.V.; Rodríguez-Ortiz, B.; Álvarez-Sánchez, N.; Álvarez-López, A.I.; Millán-Linares, M.d.C.; Millán, F. Lupinus angustifolius protein hydrolysates reduce abdominal adiposity and ameliorate metabolic associated fatty liver disease (MAFLD) in Western diet fed-ApoE−/− mice. Antioxidants 2021, 10, 1222. [Google Scholar] [CrossRef]
- Ströher, D.J.; de Oliveira, M.F.; Martinez-Oliveira, P.; Pilar, B.C.; Cattelan, M.D.P.; Rodrigues, E.; Bertolin, K.; Gonçalves, P.B.D.; Piccoli, J.d.C.E.; Manfredini, V. Virgin coconut oil associated with high-fat diet induces metabolic dysfunctions, adipose inflammation, and hepatic lipid accumulation. J. Med. Food 2020, 23, 689–698. [Google Scholar] [CrossRef]
- Juszczak, F.; Caron, N.; Mathew, A.V.; Declèves, A.-E. Critical role for AMPK in metabolic disease-induced chronic kidney disease. Int. J. Mol. Sci. 2020, 21, 7994. [Google Scholar] [CrossRef]
- Janzen, N.R.; Whitfield, J.; Murray-Segal, L.; Kemp, B.E.; Hawley, J.A.; Hoffman, N.J. Mice with Whole-Body Disruption of AMPK-Glycogen Binding Have Increased Adiposity, Reduced Fat Oxidation and Altered Tissue Glycogen Dynamics. Int. J. Mol. Sci. 2021, 22, 9616. [Google Scholar] [CrossRef]
- Lee, M.; Katerelos, M.; Gleich, K.; Galic, S.; Kemp, B.E.; Mount, P.F.; Power, D.A. Phosphorylation of acetyl-CoA carboxylase by AMPK reduces renal fibrosis and is essential for the anti-fibrotic effect of metformin. J. Am. Soc. Nephrol. 2018, 29, 2326–2336. [Google Scholar] [CrossRef]
- Lee, M.H.; Kim, H.M.; Chung, H.C.; Lee, J.H. Licorice extract suppresses adipogenesis through regulation of mitotic clonal expansion and adenosine monophosphate-activated protein kinase in 3T3-L1 cells. J. Food Biochem. 2020, 44, e13528. [Google Scholar] [CrossRef] [PubMed]
- Crewe, C.; Zhu, Y.; Paschoal, V.A.; Joffin, N.; Ghaben, A.L.; Gordillo, R.; Oh, D.Y.; Liang, G.; Horton, J.D.; Scherer, P.E. SREBP-regulated adipocyte lipogenesis is dependent on substrate availability and redox modulation of mTORC1. JCI Insight 2019, 4, e129397. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-C.; Chen, R.-J.; Peng, S.-Y.; Yu, W.C.; Chang, V.H.-S. Therapeutic Targeting of Nonalcoholic Fatty Liver Disease by Downregulating SREBP-1C Expression via AMPK-KLF10 Axis. Front. Mol. Biosci. 2021, 8, 751938. [Google Scholar] [CrossRef] [PubMed]
- Nejad, S.M.; Özgüneş, H.; Başaran, N. Pharmacological and toxicological properties of eugenol. Turk. J. Pharm. Sci. 2017, 14, 201. [Google Scholar] [CrossRef]
- Moslehi, A.; Hamidi-Zad, Z. Role of SREBPs in liver diseases: A mini-review. J. Clin. Transl. Hepatol. 2018, 6, 332. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Wang, D.; Zhang, L.; Kang, X.; Li, Y.; Zhou, X.; Yuan, G. Catalpol induces autophagy and attenuates liver steatosis in ob/ob and high-fat diet-induced obese mice. Aging 2019, 11, 9461. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-H.; Kim, M.-K.; Yeo, S.-H.; Kim, S. Short-term Cudrania tricuspidata fruit vinegar administration attenuates obesity in high-fat diet-fed mice by improving fat accumulation and metabolic parameters. Sci. Rep. 2020, 10, 1–19. [Google Scholar] [CrossRef]
- Lee, K.; Jin, H.; Chei, S.; Lee, J.-Y.; Oh, H.-J.; Lee, B.-Y. Dietary silk peptide prevents high-fat diet-induced obesity and promotes adipose browning by activating AMP-activated protein kinase in mice. Nutrients 2020, 12, 201. [Google Scholar] [CrossRef]
- Zhu, Y.-X.; Hu, H.-Q.; Zuo, M.-L.; Mao, L.; Song, G.-L.; Li, T.-M.; Dong, L.-C.; Yang, Z.-B.; Ali Sheikh, M.S. Effect of oxymatrine on liver gluconeogenesis is associated with the regulation of PEPCK and G6Pase expression and AKT phosphorylation. Biomed. Rep. 2021, 15, 1–10. [Google Scholar] [CrossRef]
- Wollaston-Hayden, E.E.; Harris, R.; Liu, B.; Bridger, R.; Xu, Y.; Wells, L. Global O-GlcNAc levels modulate transcription of the adipocyte secretome during chronic insulin resistance. Front. Endocrinol. 2015, 5, 223. [Google Scholar] [CrossRef][Green Version]
- Parker, G.J.; Lund, K.C.; Taylor, R.P.; McClain, D.A. Insulin resistance of glycogen synthase mediated byo-linked N-acetylglucosamine. J. Biol. Chem. 2003, 278, 10022–10027. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.; Li, N.; Li, J.; Li, W.; Liang, L.; Li, Q.; Wang, R.; Shi, H.; Storey, K.B.; Ding, L. Adenosine monophosphate-activated protein kinase signaling regulates lipid metabolism in response to salinity stress in the red-eared slider turtle Trachemys scripta elegans. Front. Physiol. 2019, 10, 962. [Google Scholar] [CrossRef] [PubMed]
- Ha, J.-H.; Jang, J.; Chung, S.-I.; Yoon, Y. AMPK and SREBP-1c mediate the anti-adipogenic effect of β-hydroxyisovalerylshikonin. Int. J. Mol. Med. 2016, 37, 816–824. [Google Scholar] [CrossRef] [PubMed]
- Mello, T.; Materozzi, M.; Galli, A. PPARs and mitochondrial metabolism: From NAFLD to HCC. PPAR Res. 2016, 2016, 7403230. [Google Scholar] [CrossRef]
- Kang, H.-J.; Min, B.-K.; Choi, W.-I.; Jeon, J.-H.; Kim, D.W.; Park, S.; Lee, Y.-K.; Kim, H.-j.; Byeon, J.-E.; Go, Y. Pyruvate dehydrogenase kinase 1 and 2 deficiency reduces high-fat diet-induced hypertrophic obesity and inhibits the differentiation of preadipocytes into mature adipocytes. Exp. Mol. Med. 2021, 53, 1390–1401. [Google Scholar] [CrossRef]
- Orellana-Gavaldà, J.M.; Herrero, L.; Malandrino, M.I.; Paneda, A.; Sol Rodríguez-Peña, M.; Petry, H.; Asins, G.; Van Deventer, S.; Hegardt, F.G.; Serra, D. Molecular therapy for obesity and diabetes based on a long-term increase in hepatic fatty-acid oxidation. Hepatology 2011, 53, 821–832. [Google Scholar] [CrossRef]
- Song, X.; Liu, J.; Kuang, F.; Chen, X.; Zeh III, H.J.; Kang, R.; Kroemer, G.; Xie, Y.; Tang, D. PDK4 dictates metabolic resistance to ferroptosis by suppressing pyruvate oxidation and fatty acid synthesis. Cell Rep. 2021, 34, 108767. [Google Scholar] [CrossRef]
- Park, S.Y.; Je, J.-Y.; Ahn, C.-B. Protein hydrolysates and ultrafiltration fractions obtained from krill (Euphausia superba): Nutritional, functional, antioxidant, and ACE-inhibitory characterization. J. Aquat. Food Prod. Technol. 2016, 25, 1266–1277. [Google Scholar] [CrossRef]
- Ko, W.; Lee, H.; Kim, N.; Jo, H.G.; Woo, E.-R.; Lee, K.; Han, Y.S.; Park, S.R.; Ahn, G.; Cheong, S.H. The Anti-Oxidative and Anti-Neuroinflammatory Effects of Sargassum horneri by Heme Oxygenase-1 Induction in BV2 and HT22 Cells. Antioxidants 2021, 10, 859. [Google Scholar] [CrossRef]
- Zhao, H.; Fan, W.; Dong, J.; Lu, J.; Chen, J.; Shan, L.; Lin, Y.; Kong, W. Evaluation of antioxidant activities and total phenolic contents of typical malting barley varieties. Food Chem. 2008, 107, 296–304. [Google Scholar] [CrossRef]
- Joel, C.H.; Sutopo, C.C.; Prajitno, A.; Su, J.-H.; Hsu, J.-L. Screening of angiotensin-I converting enzyme inhibitory peptides derived from Caulerpa lentillifera. Molecules 2018, 23, 3005. [Google Scholar] [CrossRef] [PubMed]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Reitman, S.; Frankel, S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am. J. Clin. Pathol. 1957, 28, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Allain, C.C.; Poon, L.S.; Chan, C.S.; Richmond, W.; Fu, P.C. Enzymatic determination of total serum cholesterol. Clin. Chem. 1974, 20, 470–475. [Google Scholar] [CrossRef]
- Park, S.Y.; Fernando, I.P.S.; Han, E.J.; Kim, M.J.; Jung, K.; Kang, D.-S.; Ahn, C.-B.; Ahn, G. In vivo hepatoprotective effects of a peptide fraction from krill protein hydrolysates against alcohol-induced oxidative damage. Mar. Drugs 2019, 17, 690. [Google Scholar] [CrossRef]
- Arunkumar, E.; Anuradha, C.V. Genistein promotes insulin action through adenosine monophosphate–activated protein kinase activation and p70 ribosomal protein S6 kinase 1 inhibition in the skeletal muscle of mice fed a high energy diet. Nutr. Res. 2012, 32, 617–625. [Google Scholar] [CrossRef]
- Minakawa, M.; Miura, Y.; Yagasaki, K. Piceatannol, a resveratrol derivative, promotes glucose uptake through glucose transporter 4 translocation to plasma membrane in L6 myocytes and suppresses blood glucose levels in type 2 diabetic model db/db mice. Biochem. Biophys. Res. Commun. 2012, 422, 469–475. [Google Scholar] [CrossRef]
- Kim, K.-N.; Kang, M.-C.; Kang, N.; Kim, S.-Y.; Hyun, C.-G.; Roh, S.W.; Ko, E.-Y.; Cho, K.; Jung, W.-K.; Ahn, G. 2, 4, 6-Trihydroxybenzaldehyde, a potential anti-obesity treatment, suppressed adipocyte differentiation in 3T3-L1 cells and fat accumulation induced by high-fat diet in C57BL/6 mice. Environ. Toxicol. Pharmacol. 2015, 39, 962–968. [Google Scholar] [CrossRef]

| Sample Name | Yield (%) | Enzymatic Hydrolysate Fractions | |||||||
|---|---|---|---|---|---|---|---|---|---|
| DPPH Radical IC50 Value (mg/mL) | ABTS Radical IC50 Value (mg/mL) | ||||||||
| ≤1 kDa | 1–3 kDa | 3–5 kDa | ≥5 kDa | ≤1 kDa | 1–3 kDa | 3–5 kDa | ≥5 kDa | ||
| Neutrase | 65.21 | 1.38 | 2.97 | 2.43 | 2.21 | 2.67 | 3.30 | 3.21 | 3.10 |
| Protamex | 64.08 | 1.38 | 2.93 | 2.10 | 1.63 | 1.58 | 1.51 | 1.71 | 1.50 |
| Alcalase | 59.73 | 1.34 | 2.07 | 2.48 | 2.20 | 1.27 | 1.56 | 1.53 | 1.56 |
| Flavourzyme | 59.89 | 1.28 | 2.77 | 1.80 | 1.82 | 1.50 | 1.48 | 1.80 | 1.82 |
| Measurement | NOR | CON | MLPH-L | MLPH-H |
|---|---|---|---|---|
| Initial body weight (g) | 22.33 ± 0.33 b | 38.33 ± 0.88 a | 38.67 ± 1.33 a | 37.67 ± 1.67 a |
| Water intake (mL/day) | 3.21 ± 0.03 b | 6.63 ± 0.09 a | 6.47 ± 0.61 a | 6.24 ± 0.19 a |
| Food intake (g/day) | 3.16 ± 0.027 b | 3.91 ± 0.077 a | 4.12 ± 0.140 a | 3.99 ± 0.035 a |
| Food efficiency ratio (FER) (g) | 1.48 ± 0.10 b | 2.47 ± 0.12 a | 2.60 ± 0.34 a | 2.83 ± 0.31 a |
| Body weight gain (g/42 days) | 4.67 ± 0.33 b | 9.67 ± 0.33 a | 10.67 ± 1.20 a | 11.33 ± 1.33 a |
| Heart (g) | 0.128 ± 0.001 b | 0.155 ± 0.009 a | 0.142 ± 0.007 ab | 0.136 ± 0.004 ab |
| Kidney (g) | 0.322 ± 0.002 b | 0.365 ± 0.011 a | 0.343 ± 0.008 ab | 0.323 ± 0.013 b |
| Liver (g) | 1.433 ± 0.021 c | 3.582 ± 0.007 a | 3.285 ± 0.052 b | 3.248 ± 0.093 b |
| Gastrocnemius muscle (g) | 0.170 ± 0.004 NS | 0.163 ± 0.010 | 0.174 ± 0.011 | 0.184 ± 0.009 |
| Fat mesenteric (g) | 0.552 ± 0.006 c | 1.733 ± 0.015 a | 1.307 ± 0.055 b | 1.219 ± 0.090 b |
| Fat retroperitoneal (g) | 0.881 ± 0.010 b | 3.109 ± 0.031 a | 2.916 ± 0.152 a | 2.997 ± 0.063 a |
| Fat epididymal (g) | 0.013 ± 0.0004 c | 0.027 ± 0.0023 a | 0.020 ± 0.0005 b | 0.019 ± 0.0010 b |
| Fat kidney (g) | 0.083 ± 0.004 c | 0.683 ± 0.050 a | 0.417 ± 0.056 b | 0.395 ± 0.010 b |
| Fat total (g) | 1.529 ± 0.008 c | 5.553 ± 0.079 a | 4.659 ± 0.106 b | 4.630 ± 0.108 b |
| Liver Analysis | NOR | CON | MLPH-L | MLPH-H |
|---|---|---|---|---|
| GSH (nmol/mg) | 456.16 ± 9.06 a | 202.26 ± 3.19 d | 259.45 ± 14.58 c | 335.46 ± 0.86 b |
| GST activity (U/mL) | 3.49 ± 0.38 a | 1.96 ± 0.21 b | 1.58 ± 0.36 b | 1.72 ± 0.19 b |
| GPx activity (U/mL) | 1.63 ± 0.35 c | 0.71 ± 0.10 d | 2.48 ± 0.23 b | 3.27 ± 0.38 a |
| GR activity (mU/mL) | 22.57 ± 0.95 a | 16.28 ± 3.14 b | 17.36 ± 1.28 b | 19.61 ± 0.64 ab |
| SOD activity (U/mL) | 3.08 ± 0.01 a | 2.69 ± 0.01 d | 2.86 ± 0.01 c | 3.02 ± 0.02 b |
| CAT colorimetric activity (U/mL) | 1.668 ± 0.007 b | 1.662 ± 0.001 b | 1.670 ± 0.004 b | 1.685 ± 0.001 a |
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| PEPCK | CTGGCACCTCAGTGAAGACA | TCGATGCCTTCCCAGTAAAC |
| G6Pase | ATGACTTTGGGATCCAGTCG | TGGAACCAGATGGGAAAGAG |
| GS | GACACTGAGCAGGGCTTTTC | GGGCCTGGGATACTTAAAGC |
| LGP | CCAGAGTGCTCTACCCCAAT | CCACAAAGTACTCCTGTTTCAGC |
| FAS | CCCTTGATGAAGAGGGATCA | ACTCCACAGGTGGGAACAAG |
| ACC | GACGTTCGCCATAACCAAGT | CTGCAGGTTCTCAATGCAAA |
| SCD | AGCTGGTGATGTTCCAGAGG | GTGGGCAGGATGAAGCAC |
| MCAD | AGCTGATTGGCAATGTCTCCAGCAAA | GATCGCAATGGGTGCTTTTGATAGAA |
| PDK | ATCTAACATCGCCAGAATTAAACC | GGAACGTACACAATGTGGATTG |
| CPT | GAGCCACGAAGCCCTCAAACACAT | GCTGTACAACATGGGCTTCCGACCTG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.J.; Chilakala, R.; Jo, H.G.; Lee, S.-J.; Lee, D.-S.; Cheong, S.H. Anti-Obesity and Anti-Hyperglycemic Effects of Meretrix lusoria Protamex Hydrolysate in ob/ob Mice. Int. J. Mol. Sci. 2022, 23, 4015. https://doi.org/10.3390/ijms23074015
Kim MJ, Chilakala R, Jo HG, Lee S-J, Lee D-S, Cheong SH. Anti-Obesity and Anti-Hyperglycemic Effects of Meretrix lusoria Protamex Hydrolysate in ob/ob Mice. International Journal of Molecular Sciences. 2022; 23(7):4015. https://doi.org/10.3390/ijms23074015
Chicago/Turabian StyleKim, Min Ju, Ramakrishna Chilakala, Hee Geun Jo, Seung-Jae Lee, Dong-Sung Lee, and Sun Hee Cheong. 2022. "Anti-Obesity and Anti-Hyperglycemic Effects of Meretrix lusoria Protamex Hydrolysate in ob/ob Mice" International Journal of Molecular Sciences 23, no. 7: 4015. https://doi.org/10.3390/ijms23074015
APA StyleKim, M. J., Chilakala, R., Jo, H. G., Lee, S.-J., Lee, D.-S., & Cheong, S. H. (2022). Anti-Obesity and Anti-Hyperglycemic Effects of Meretrix lusoria Protamex Hydrolysate in ob/ob Mice. International Journal of Molecular Sciences, 23(7), 4015. https://doi.org/10.3390/ijms23074015






