The Anti-Cancer Potential of Heat-Killed Lactobacillus brevis KU15176 upon AGS Cell Lines through Intrinsic Apoptosis Pathway
Abstract
:1. Introduction
2. Results
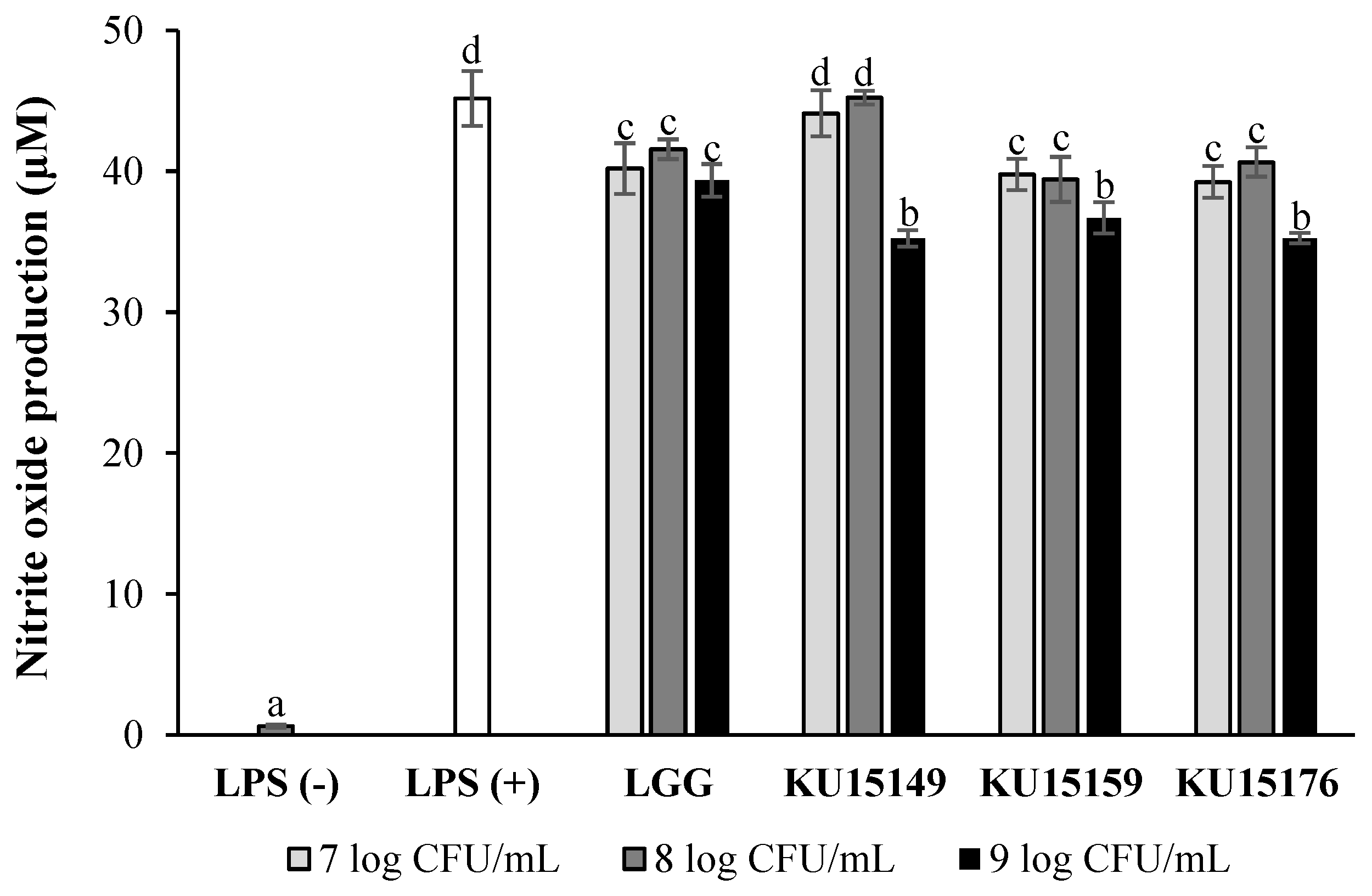
2.1. Nitric Oxide (NO) Production in RAW 264.7 Cells
2.2. Cell Proliferation Assay
2.3. Microscopic Analysis
2.4. RNA Expression Using Semi-Quantitative Real-Time PCR
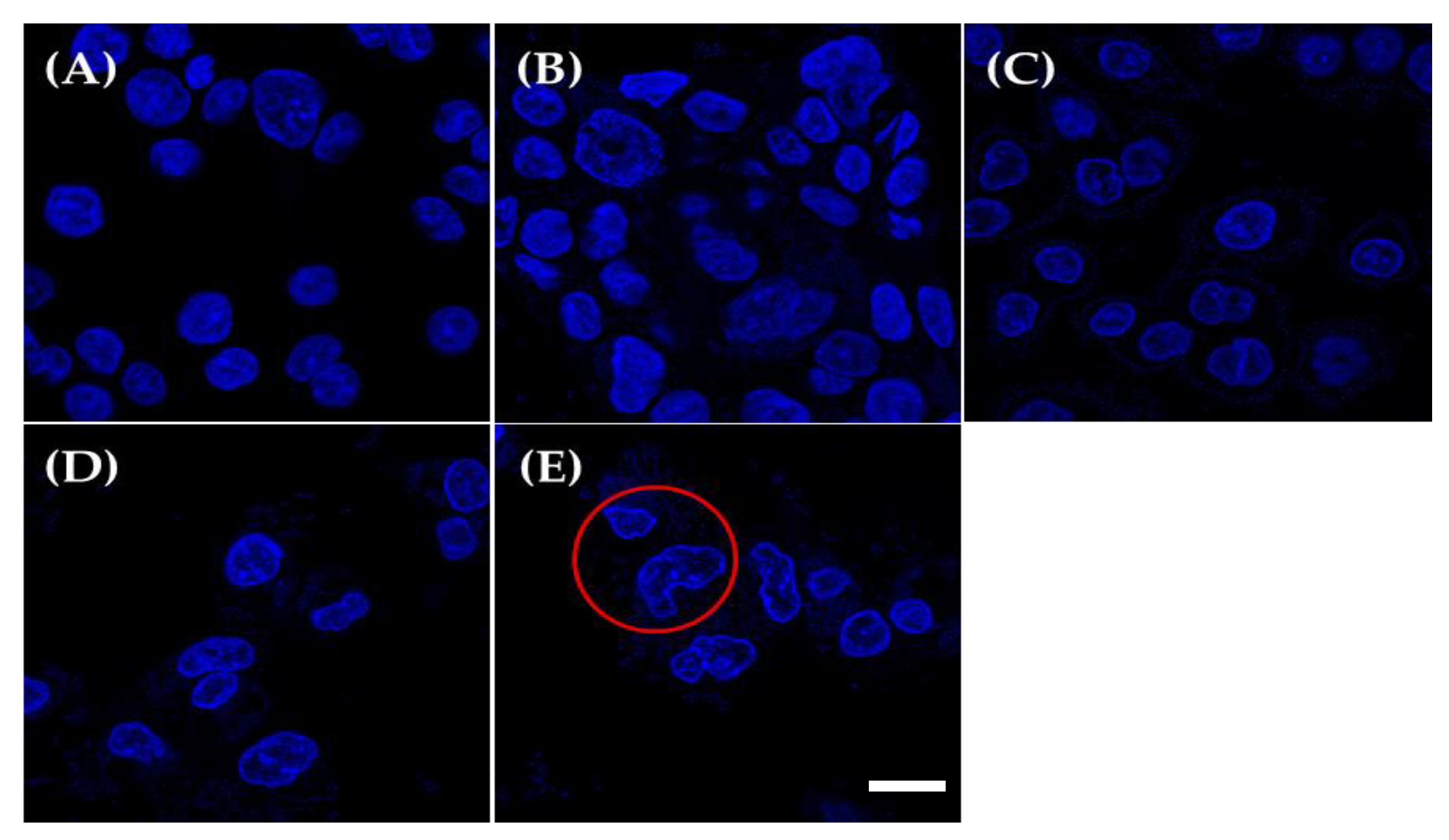
2.5. DAPI Staining
2.6. Flow Cytometry Analyses
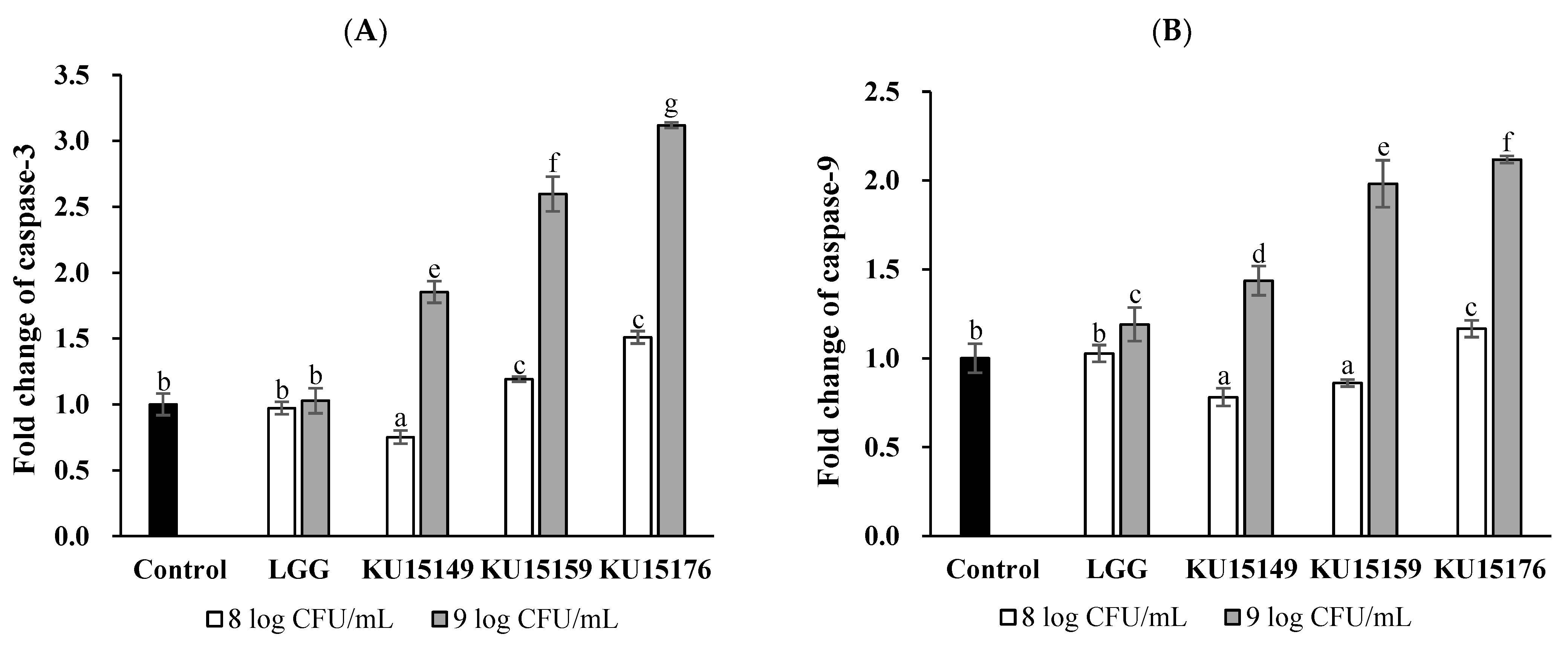
2.7. Caspase Colorimetric Assay
3. Discussion
4. Materials and Methods
4.1. Culture Media and Reagents
4.2. Bacteria, Incubation Conditions, and Sample Preparation
4.3. Normal and Cancer Cell Lines and Culture Condition
4.4. Nitric Oxide Production in RAW 264.7 Cells
4.5. Cell Proliferation Assay
4.6. Microscopic Analysis
4.7. RNA Extraction and Semi-Quantitative Real-Time PCR
4.8. DAPI Staining
4.9. Apoptosis Assay
4.10. Caspase-3 and Caspase-9 Colorimetric Assay
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| LAB | lactic acid bacteria |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| NO | Nitric oxide |
| Bax | Bcl-2-associated X protein |
| Bcl-2 | B-cell lymphoma |
| DAPI | 4,6-diamidino-2-phenylindole |
References
- Son, S.H.; Yang, S.J.; Jeon, H.L.; Yu, H.S.; Lee, N.K.; Park, Y.S.; Paik, H.D. Antioxidant and immunostimulatory effect of potential probiotic Lactobacillus paraplantarum SC61 isolated from Korean traditional fermented food, jangajji. Microb. Pathog. 2018, 125, 486–492. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.H.; Tsai, W.H.; Wu, H.Y.; Chen, C.Y.; Yeh, W.L.; Chen, Y.H.; Hsu, H.Y.; Chen, W.W.; Chen, Y.W.; Chang, W.W.; et al. Probiotic Lactobacillus spp. act against Helicobacter pylori-induced inflammation. J. Clin. Med. 2019, 8, 90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, J.I.; Kim, Y.G.; Kang, C.H.; Imm, J.Y. Effects of Lactobacillus curvatus MG5246 on inflammatory markers in Porphyromonas gingivalis lipopolysaccharide-sensitized human gingival fibroblasts and periodontitis rat model. Food Sci. Biotechnol. 2022, 31, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Shakya, S.; Danshiitsoodol, N.; Sugimoto, S.; Noda, M.; Sugiyama, M. Anti-oxidant and anti-inflammatory substance generated newly in Paeoniae Radix Alba extract fermented with plant-derived Lactobacillus brevis 174A. Antioxidants 2021, 10, 1071. [Google Scholar] [CrossRef]
- Kariyawasam, K.; Yang, S.J.; Lee, N.K.; Paik, H.D. Probiotic properties of Lactobacillus brevis KU200019 and synergistic activity with fructooligosaccharides in antagonistic activity against foodborne pathogens. Food Sci. Anim. Resour. 2020, 40, 297–310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foysal, J.; Fotedar, R.; Siddik, M.A.B.; Tay, A. Lactobacillus acidophilus and L. plantarum improve health status, modulate gut microbiota and innate immune response of marron (Cherax cainii). Sci. Rep. 2020, 10, 1–13. [Google Scholar]
- Kim, K.T.; Yang, S.J.; Paik, H.D. Probiotic properties of novel probiotic Levilactobacillus brevis KU15147 isolated from radish kimchi and its antioxidant and immune-enhancing activities. Food Sci. Biotechnol. 2021, 30, 257–265. [Google Scholar] [CrossRef]
- Lee, J.E.; Lee, N.K.; Paik, H.D. Antimicrobial and anti-biofilm effects of probiotic Lactobacillus plantarum KU200656 isolated from kimchi. Food Sci. Biotechnol. 2021, 30, 97–106. [Google Scholar] [CrossRef]
- Yan, F.; Li, N.; Shi, J.; Li, H.; Yue, Y.; Jiao, W.; Wang, N.; Song, Y.; Huo, G.; Li, B. Lactobacillus acidophilus alleviates type 2 diabetes by regulating hepatic glucose, lipid metabolism and gut microbiota in mice. Food Funct. 2019, 10, 5804–5815. [Google Scholar] [CrossRef]
- Laslo, V.; Pinzaru, S.C.; Zaguła, G.; Kluz, M.; Vicas, S.I.; Cavalu, S. Synergic effect of selenium nanoparticles and lactic acid bacteria in reduction cadmium toxicity. J. Mol. Struct. 2022, 1247, 131325. [Google Scholar] [CrossRef]
- Nami, Y.; Abdullah, N.; Haghshenas, B.; Radiah, D.; Rosli, R.; Khosroushahi, A.Y. Probiotic potential and biotherapeutic effects of newly isolated vaginal Lactobacillus acidophilus 36YL strain on cancer cells. Anaerobe 2014, 28, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Riaz Rajoka, M.S.; Zhao, H.; Lu, Y.; Lian, Z.; Li, N.; Hussain, N.; Shao, D.; Jin, M.; Li, Q.; Shi, J. Anticancer potential against cervix cancer (HeLa) cell line of probiotic Lactobacillus casei and Lactobacillus paracasei strains isolated from human breast milk. Food Funct. 2018, 9, 2705–2715. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.E.; Lee, J.; Kim, J.H.; Cho, N.; Lee, S.H.; Park, S.B.; Koh, B.; Kang, D.; Kim, S.; Yoo, H.M. Characterization of the anti-cancer activity of the probiotic bacterium Lactobacillus fermentum using 2D vs. 3D culture in colorectal cancer cells. Biomolecules 2019, 9, 557. [Google Scholar] [CrossRef] [Green Version]
- Battistelli, M.; Falcieri, E. Apoptotic bodies: Particular extracellular vesicles involved in intercellular communication. Biology 2020, 9, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfeffer, C.M.; Singh, A.T.K. Apoptosis: A target for anticancer therapy. Int. J. Mol. Sci. 2018, 19, 448. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Kwak, A.; Lee, M.; Seo, J.; Cho, S.; Yoon, G.; Chae, J.; Joo, S.H.; Shim, J. Picropodophyllotoxin induces g1 cell cycle arrest and apoptosis in human colorectal cancer cells via ROS generation and activation of p38 MAPK signaling pathway. J. Microbiol. Biotechnol. 2021, 31, 1615–1623. [Google Scholar] [CrossRef]
- Snydman, D.R. The safety of probiotics. Clin. Infect. Dis. 2008, 46, S104–S111. [Google Scholar] [CrossRef] [Green Version]
- Han, K.J.; Lee, J.E.; Lee, N.K.; Paik, H.D. Antioxidant and anti-inflammatory effect of probiotic Lactobacillus plantarum KU15149 derived from Korean homemade diced-radish Kimchi. J. Microbiol. Biotechnol. 2020, 30, 591–598. [Google Scholar] [CrossRef]
- Murata, S.; Takahama, Y.; Kasahara, M.; Tanaka, K. The immunoproteasome and thymoproteasome: Functions, evolution, and human disease. Nat. Immunol. 2018, 19, 923–931. [Google Scholar] [CrossRef]
- Maleki-Kakelar, H.; Dehghani, J.; Barzegari, A.; Barar, J.; Shirmohamadi, M.; Sadeghi, J.; Omidi, Y. Lactobacillus plantarum induces apoptosis in gastric cancer cells via modulation of signaling pathways in Helicobacter pylori. Bioimpacts 2020, 10, 65–72. [Google Scholar] [CrossRef] [Green Version]
- Legesse Bedada, T.; Feto, T.K.; Awoke, K.S.; Garedew, A.D.; Yifat, F.T.; Birri, D.J. Probiotics for cancer alternative prevention and treatment. Biomed. Pharmacother. 2020, 129, 110409. [Google Scholar] [CrossRef] [PubMed]
- Peter, M.E. Programmed cell death: Apoptosis meets necrosis. Nature 2011, 471, 310–312. [Google Scholar] [CrossRef] [PubMed]
- Sochalska, M.; Tuzlak, S.; Egle, A.; Villunger, A. Lessons from gain- and loss-of-function models of pro-survival Bcl2 family proteins: Implications for targeted therapy. FEBS J. 2015, 282, 834–849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, R.; Letai, A.; Sarosiek, K. Regulation of apoptosis in health and disease: The balancing act of BCL-2 family proteins. Nat. Rev. Mol. Cell Biol. 2019, 20, 175–193. [Google Scholar] [CrossRef] [PubMed]
- Mehrbod, P.; Ande, S.R.; Alizadeh, J.; Rahimizadeh, S.; Shariati, A.; Malek, H.; Hashemi, M.; Glover, K.K.M.; Sher, A.A.; Coombs, K.M.; et al. The roles of apoptosis, autophagy and unfolded protein response in arbovirus, influenza virus, and HIV infections. Virulence 2019, 10, 376–413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, I.; Choi, R.; Lee, J.H.; Seo, M.; Lee, H.J.; Kim, M.; Kim, S.H.; Kim, I.; Hwang, J.S. Anticancer activity of periplanetasin-5, an antimicrobial peptide from the cockroach Periplaneta americana. J. Microbiol. Biotechnol. 2021, 31, 1343–1349. [Google Scholar] [CrossRef]
- Maleki, E.H.; Bahrami, A.R.; Sadeghian, H.; Matin, M.M. Discovering the structure–activity relationships of different O-prenylated coumarin derivatives as effective anticancer agents in human cervical cancer cells. Toxicol. Vitr. 2020, 63, 104745. [Google Scholar] [CrossRef]
- Chung, Y.; Park, J.Y.; Lee, J.E.; Kim, K.T.; Paik, H.D. Antioxidant activity and inhibitory effect on nitric oxide production of hydroponic ginseng fermented with Lactococcus lactis KC24. Antioxidants 2021, 10, 1614. [Google Scholar] [CrossRef]
- Han, K.J.; Lee, N.K.; Park, H.; Paik, H.D. Anticancer and anti-inflammatory activity of probiotic Lactococcus lactis NK34. J. Microbiol. Biotechnol. 2015, 25, 1697–1701. [Google Scholar] [CrossRef] [Green Version]
- Jeon, E.B.; Son, S.H.; Jeewanthi, R.K.C.; Lee, N.K.; Paik, H.D. Characterization of Lactobacillus plantarum Lb41, an isolate from kimchi and its application as a probiotic in cottage cheese. Food Sci. Biotechnol. 2016, 25, 1129–1133. [Google Scholar] [CrossRef]
- Wang, S.; Han, X.; Zhang, L.; Zhang, Y.; Li, H.; Jiao, Y. Whole peptidoglycan extracts from the Lactobacillus paracasei subsp. paracasei M5 strain exert anticancer activity in vitro. Biomed. Res. Int. 2018, 2018, 2871710. [Google Scholar] [PubMed] [Green Version]
- Salmanzadeh, R.; Eskandani, M.; Mokhtarzadeh, A.; Vandghanooni, S.; Ilghami, R.; Maleki, H.; Saeeidi, N.; Omidi, Y. Propyl gallate (PG) and tert-butylhydroquinone (TBHQ) may alter the potential anti-cancer behavior of probiotics. Food Biosci. 2018, 24, 37–45. [Google Scholar] [CrossRef]
- Xu, C.; Wu, A.; Zhu, H.; Fang, H.; Xu, L.; Ye, J.; Shen, J. Melatonin is involved in the apoptosis and necrosis of pancreatic cancer cell line SW-1990 via modulating of Bcl-2/Bax balance. Biomed. Pharmacother. 2013, 67, 133–139. [Google Scholar] [CrossRef]
- Nami, Y.; Abdullah, N.; Haghshenas, B.; Radiah, D.; Rosli, R.; Khosroushahi, A.Y. Assessment of probiotic potential and anticancer activity of newly isolated vaginal bacterium Lactobacillus plantarum 5BL. Microbiol. Immunol. 2014, 58, 492–502. [Google Scholar] [CrossRef] [PubMed]
- Sakthivel, R.; Malar, D.S.; Devi, K.P. Phytol shows anti-angiogenic activity and induces apoptosis in A549 cells by depolarizing the mitochondrial membrane potential. Biomed. Pharmacother. 2018, 105, 742–752. [Google Scholar] [CrossRef]
- Gao, Q.X.; Qi, L.L.; Wu, T.X.; Wang, J.B. Ability of Clostridium butyricum to inhibit Escherichia coli-induced apoptosis in chicken embryo intestinal cells. Vet. Microbiol. 2012, 160, 395–402. [Google Scholar] [CrossRef]
- Yeh, Y.H.; Liang, C.Y.; Chen, M.L.; Tsai, F.M.; Lin, Y.Y.; Lee, M.C.; Wu, J.S.; Kuo, C.Y. Apoptotic effects of hsian-tsao (Mesona procumbens Hemsley) on hepatic stellate cells mediated by reactive oxygen species and ERK, JNK, and caspase-3 pathways. Food Sci. Nutr. 2019, 7, 1891–1898. [Google Scholar] [CrossRef] [Green Version]



| LAB | Viability (%) of MRC-5 a | |||
|---|---|---|---|---|
| LGG | L. plantarum KU15149 | L. brevis KU15159 | L. brevis KU15176 | |
| 8 log CFU/mL | 102.60 ± 2.18 | 113.04 ± 6.29 | 110.74 ± 2.06 | 124.54 ± 4.88 |
| 9 log CFU/mL | 113.19 ± 2.24 | 112.99 ± 5.76 | 102.85 ± 3.04 | 127.37 ± 1.59 |
| LAB | Cytotoxicity (%) a | |||||||
|---|---|---|---|---|---|---|---|---|
| LGG | L. plantarum KU15149 | L. brevis KU15159 | L. brevis KU15176 | |||||
| 8 log CFU/mL | 9 log CFU/mL | 8 log CFU/mL | 9 log CFU/mL | 8 log CFU/mL | 9 log CFU/mL | 8 log CFU/mL | 9 log CFU/mL | |
| AGS | 9.21 ± 1.47 | 31.94 ± 1.22 | 8.54 ± 0.61 | 35.52 ± 0.66 | 21.21 ± 0.62 | 37.60 ± 0.83 | 15.39 ± 0.80 | 41.34 ± 1.36 |
| HT-29 | 1.12 ± 0.60 | 1.34 ± 1.23 | 0.90 ± 0.18 | 7.36 ± 1.20 | 1.31 ± 0.19 | 3.19 ± 0.45 | 1.67 ± 1.27 | 3.32 ± 0.95 |
| DLD-1 | 49.37 ± 1.12 | 51.56 ± 3.02 | 37.28 ± 1.86 | 57.61 ± 3.61 | 45.85 ± 0.81 | 54.43 ± 0.93 | 42.22 ± 2.89 | 51.79 ± 3.10 |
| LoVo | 43.06 ± 1.94 | 48.14 ± 3.81 | 41.49 ± 4.84 | 61.34 ± 2.58 | 75.00 ± 2.22 | 76.00 ± 1.94 | 73.08 ± 3.35 | 69.56 ± 1.78 |
| Caco-2 | 9.41 ± 0.16 | 17.49 ± 0.80 | 17.06 ± 3.45 | 25.30 ± 1.18 | 13.25 ± 0.46 | 26.22 ± 3.12 | 11.47 ± 2.22 | 27.33 ± 1.35 |
| Primer | Sequence (5′ to 3′) | |
|---|---|---|
| β-Actin | (Forward) | TTCTGACGGCAACTTCAACT |
| (Reverse) | GTCCAGCCCATGATGGTTCT | |
| Bax | (Forward) | TCACCCTGAAGTACCCCATC |
| (Reverse) | GTCCAGCCCATGATGGTTCT | |
| Bcl-2 | (Forward) | CAGCTGCACCTGACGCCCTT |
| (Reverse) | GCCTCCGTTATCCTGGATCC | |
| Caspase-3 | (Forward) | TTTGTTTGTGTGCTTCTGAGCC |
| (Reverse) | ATTCTGTTGCCACCTTTCGG | |
| Caspase-9 | (Forward) | TGCTGCGTGGTGGTCATTCTC |
| (Reverse) | CCGACACAGGGCATCCATCTG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, C.-H.; Lee, N.-K.; Paik, H.-D. The Anti-Cancer Potential of Heat-Killed Lactobacillus brevis KU15176 upon AGS Cell Lines through Intrinsic Apoptosis Pathway. Int. J. Mol. Sci. 2022, 23, 4073. https://doi.org/10.3390/ijms23084073
Hwang C-H, Lee N-K, Paik H-D. The Anti-Cancer Potential of Heat-Killed Lactobacillus brevis KU15176 upon AGS Cell Lines through Intrinsic Apoptosis Pathway. International Journal of Molecular Sciences. 2022; 23(8):4073. https://doi.org/10.3390/ijms23084073
Chicago/Turabian StyleHwang, Chang-Hoon, Na-Kyoung Lee, and Hyun-Dong Paik. 2022. "The Anti-Cancer Potential of Heat-Killed Lactobacillus brevis KU15176 upon AGS Cell Lines through Intrinsic Apoptosis Pathway" International Journal of Molecular Sciences 23, no. 8: 4073. https://doi.org/10.3390/ijms23084073






