Nutraceutical Concepts and Dextrin-Based Delivery Systems
Abstract
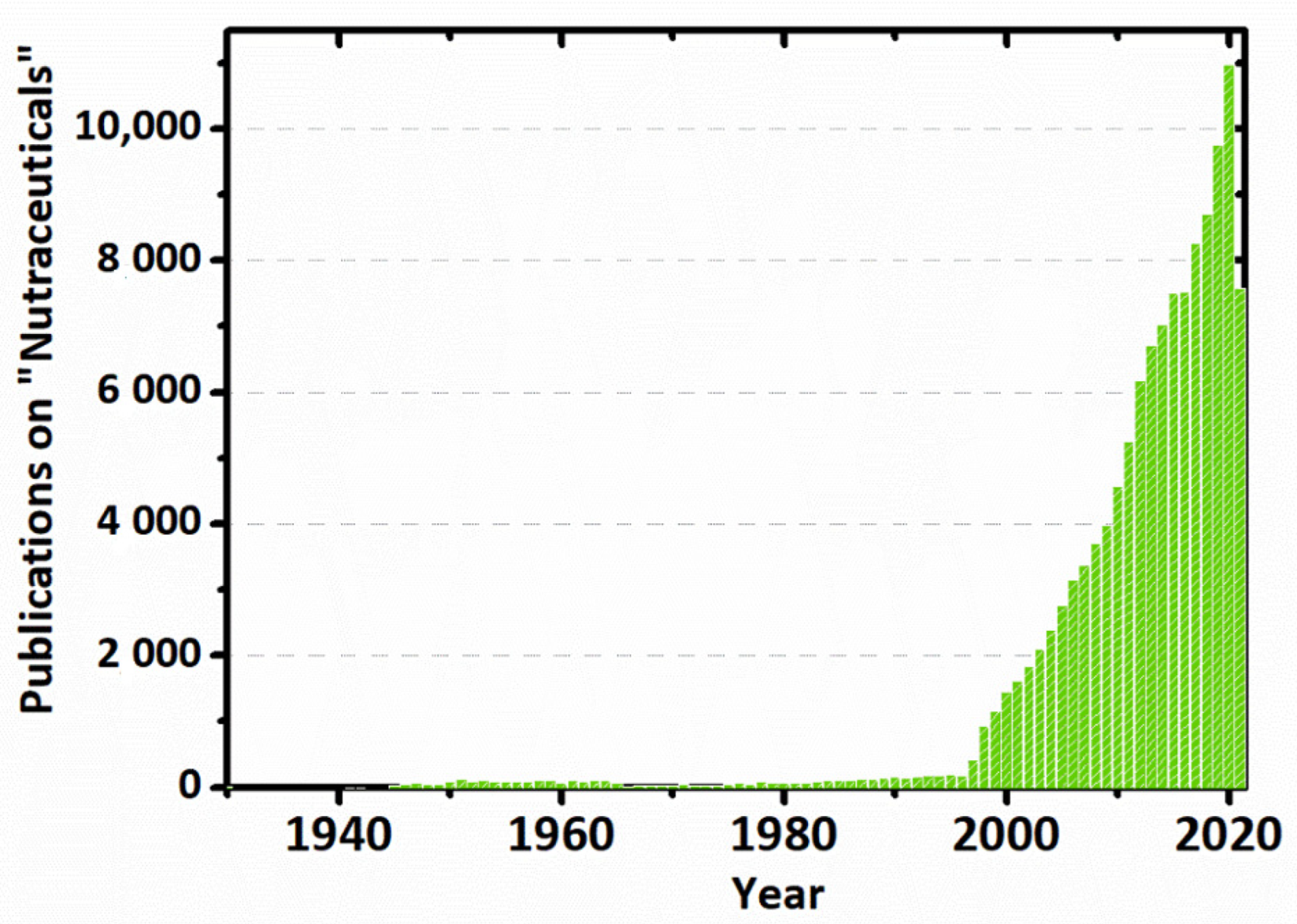
1. Introduction
2. Nutraceuticals
2.1. What Are Nutraceuticals?
2.2. Nutraceuticals vs. Other Definitions/Regulations
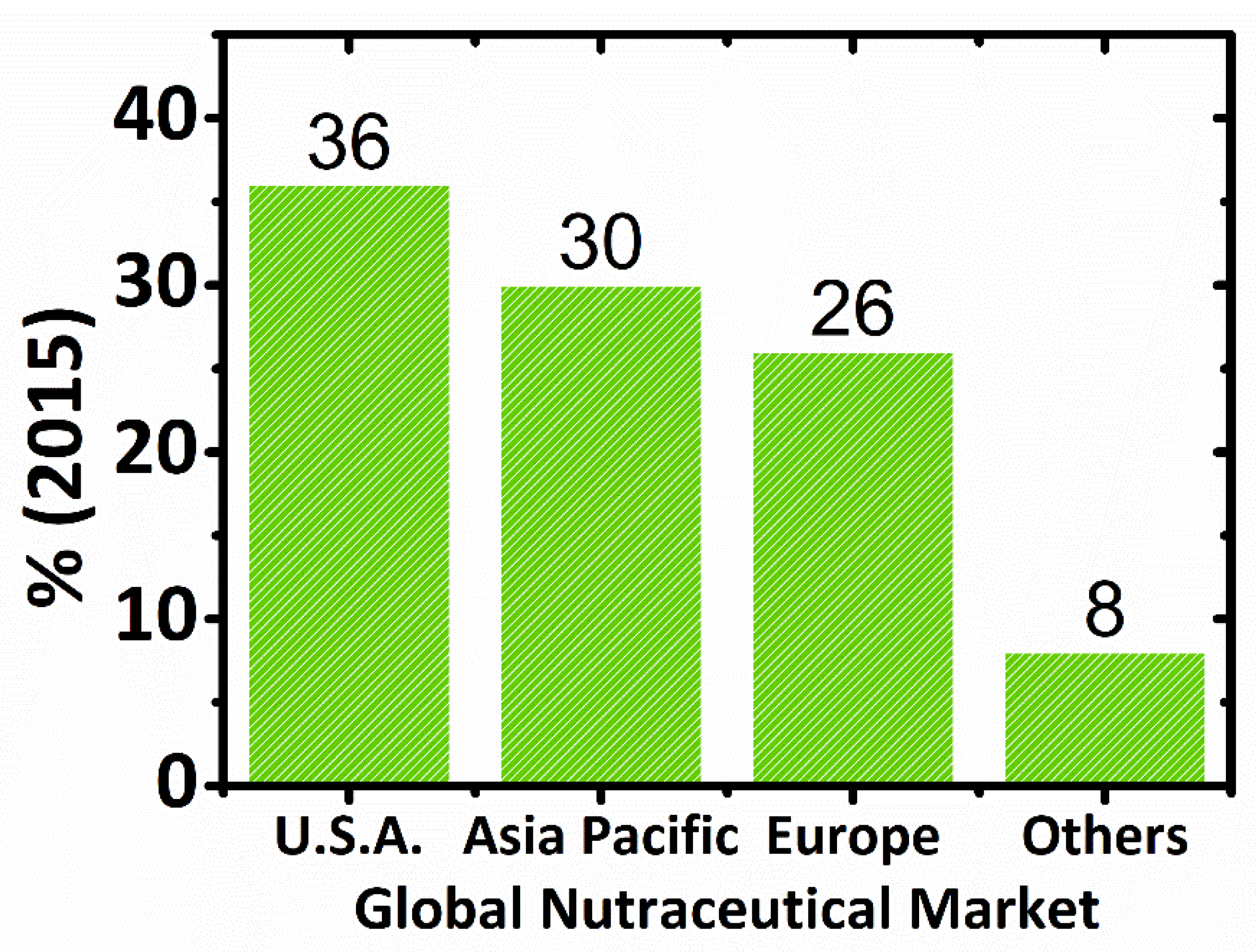
2.3. Global Market of Nutraceuticals
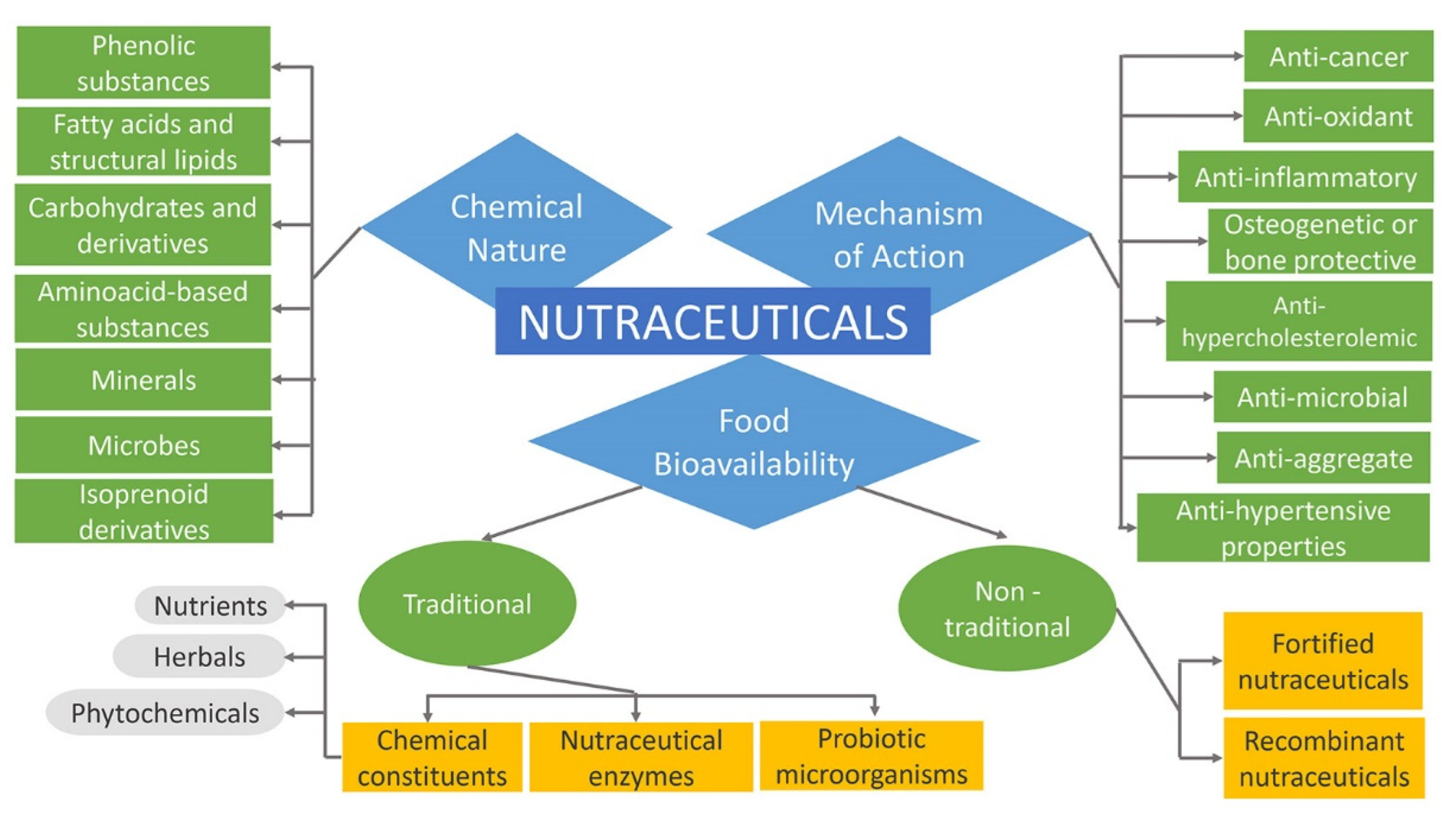
2.4. Classification of Nutraceuticals
2.4.1. Nutraceuticals Based on Food Bioavailability
2.4.2. Nutraceuticals Based on Chemical Nature
Isoprenoid Derivatives
Phenolic Substances
Fatty Acids and Structural Lipids
Carbohydrate Derivatives
Amino Acid Derivatives
Microbes and Minerals
2.4.3. Nutraceuticals Based on Mechanism of Action
Nutraceuticals and Health Benefits
Anti-Microbial Activity
Anti-Oxidant Activity
Anti-Hypertensive Activity
Anti-Inflammatory Activity
Anti-Hypercholesterolemic Activity
Anti-Aggregate Activity
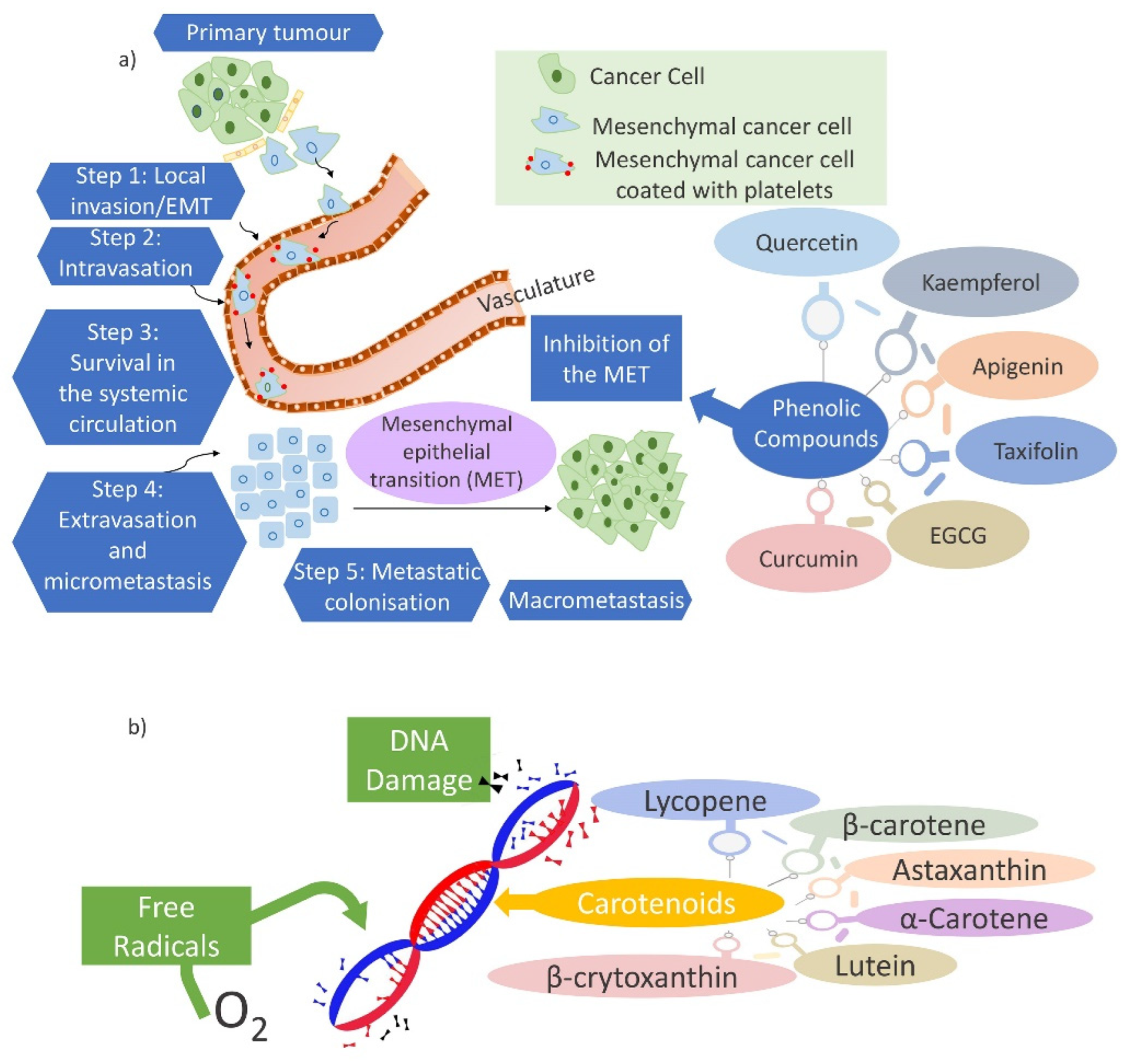
Anti-Carcinogenic Activity
Bone Protective Activity
3. Delivery Systems for Nutraceuticals
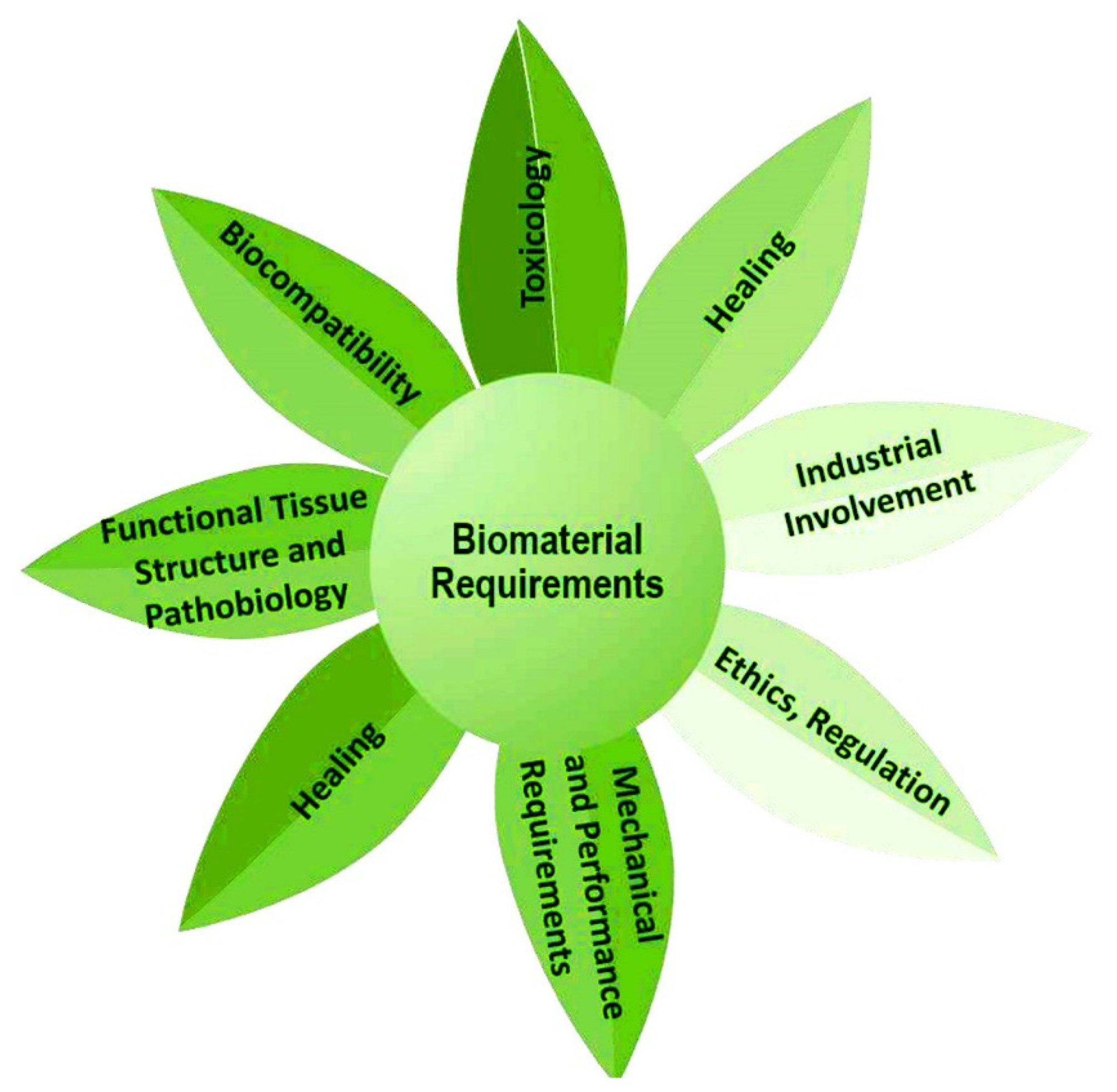
3.1. Advisable Features of Delivery Systems
3.1.1. Encapsulation and Controlled Release Capacity
3.1.2. Solubility
3.1.3. Bioavailability
3.2. Delivery Systems Design
3.2.1. Biopolymer-Based Delivery Systems
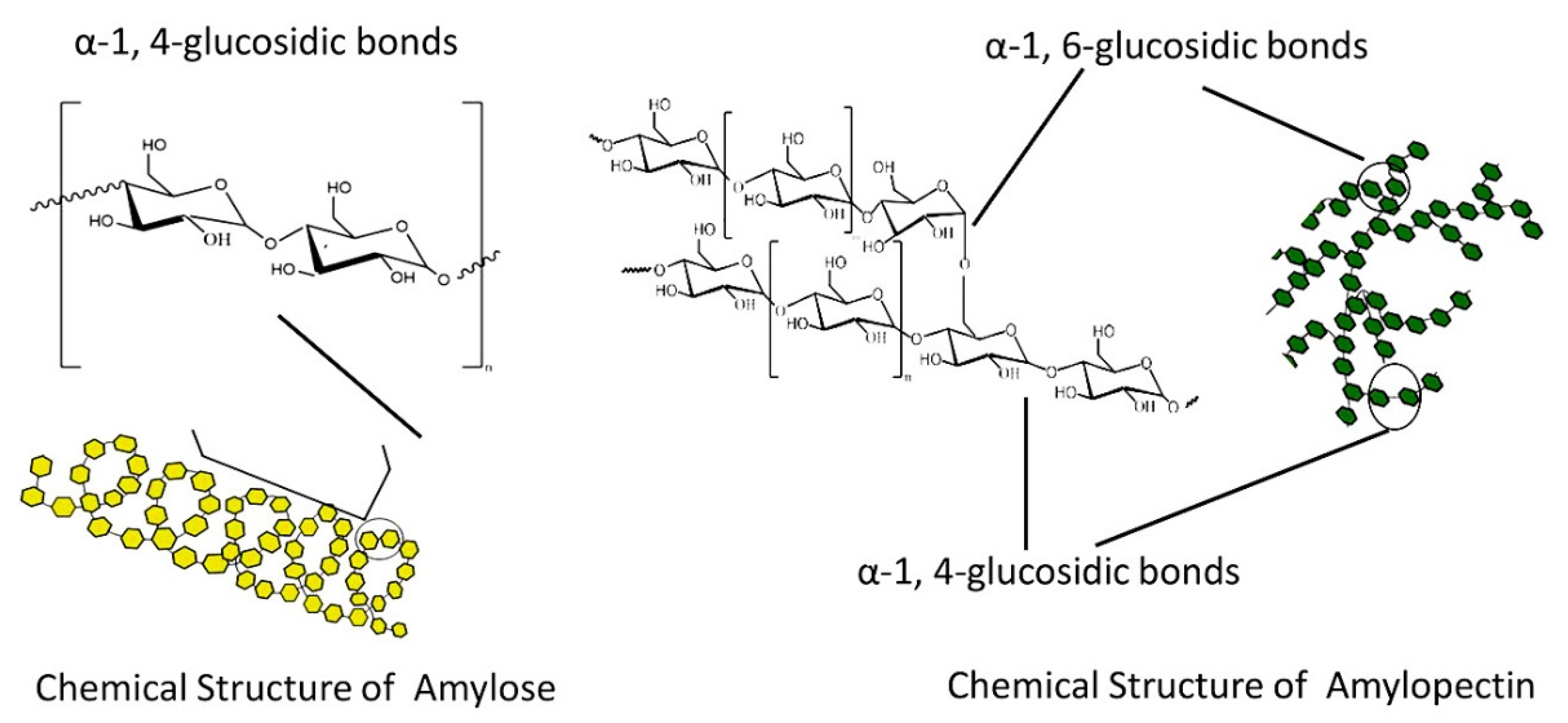
Starch
Dextrin
Nanoparticle-Cell Interactions In Vitro, and In Vivo
3.2.2. General Features of Cyclodextrins and Cyclodextrin-Based Polymers as Delivery Systems Matrices
Historical Developments of Cyclodextrin-Based Nanosponges as Delivery Systems Matrices
The First Generation of Cyclodextrin Nanosponges
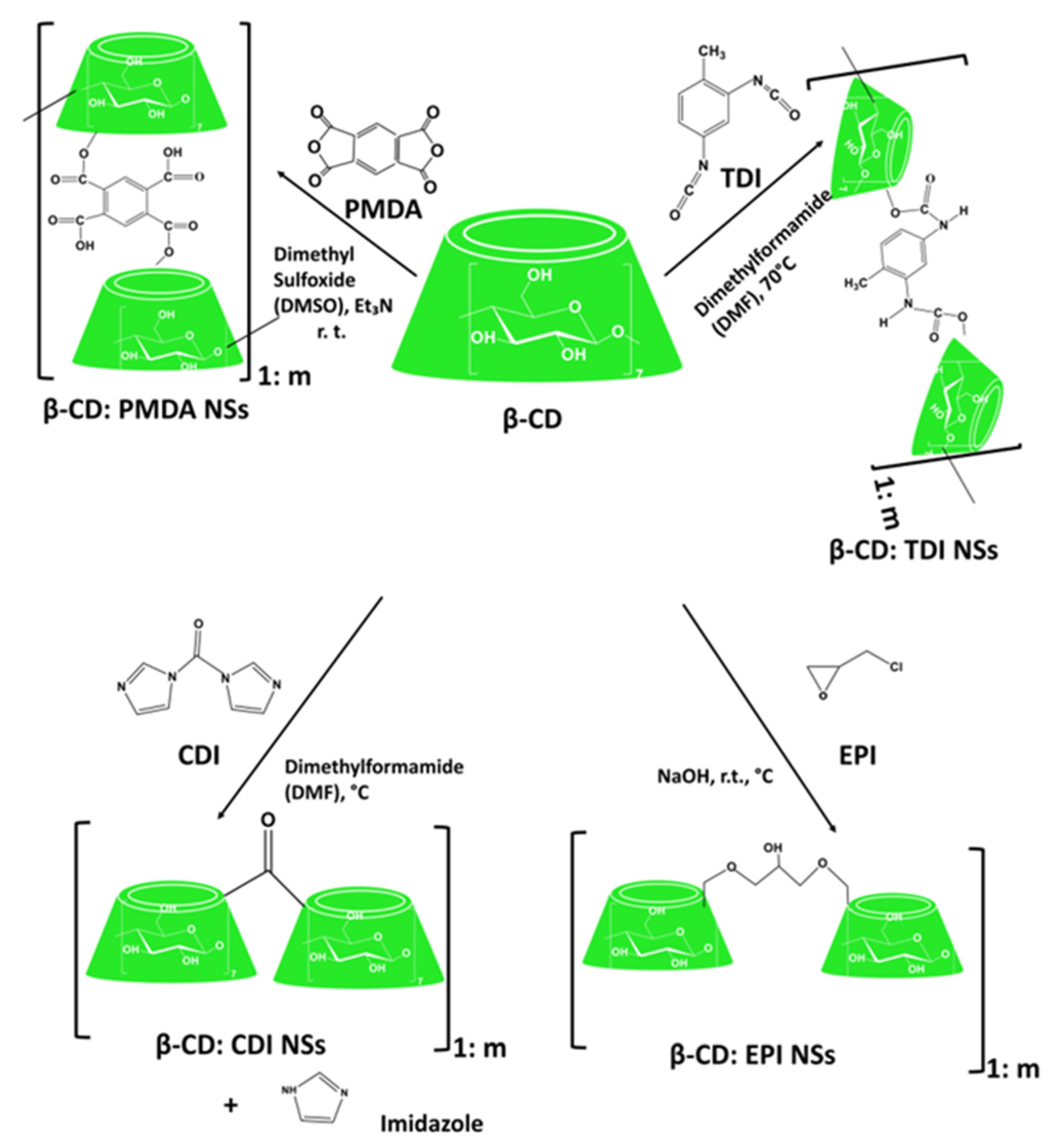
Cyclodextrin-Based Urethane Nanosponges
Cyclodextrin-Based Carbonate Nanosponges
Cyclodextrin-Based Ether Nanosponges
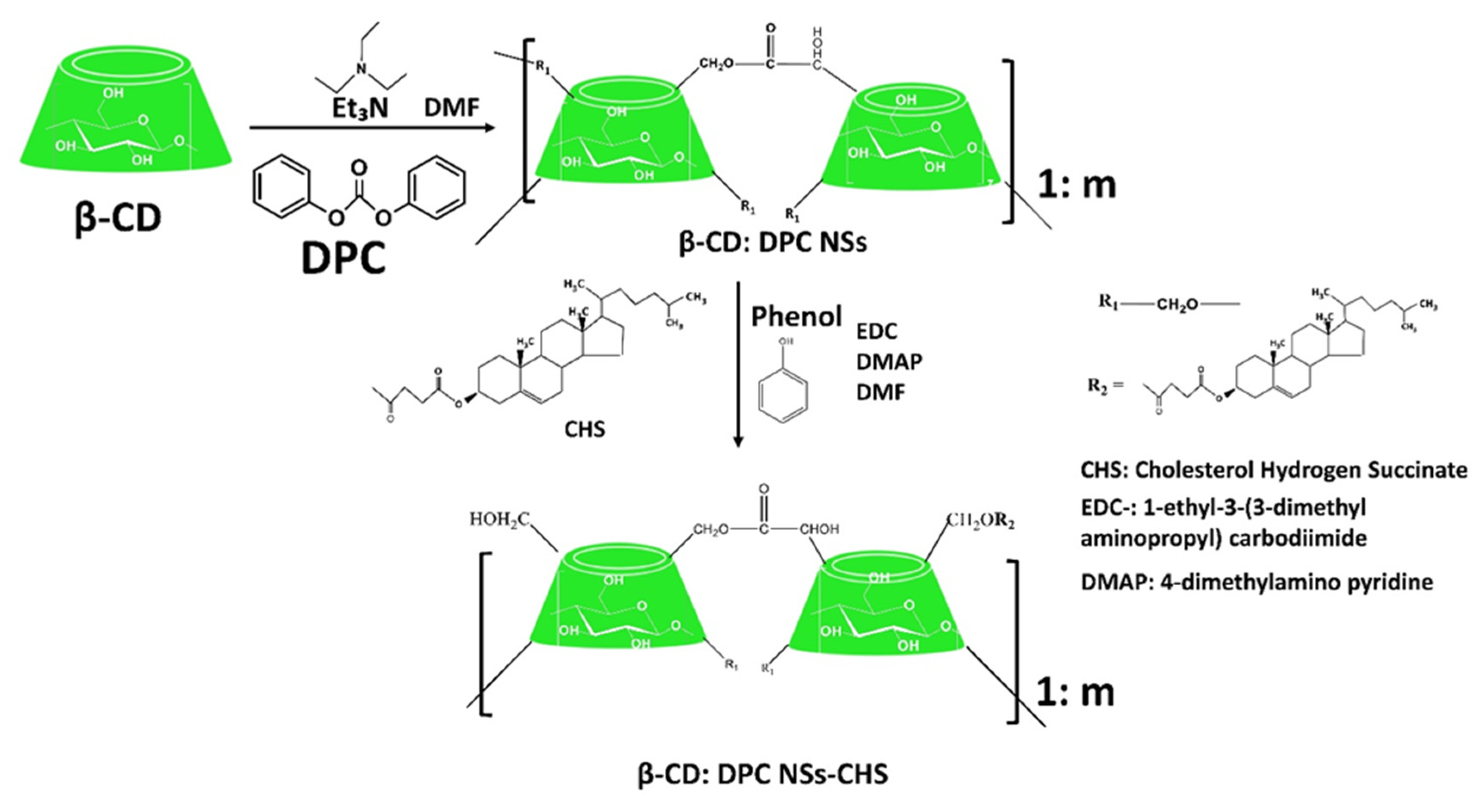
Cyclodextrin-Based Ester Nanosponges
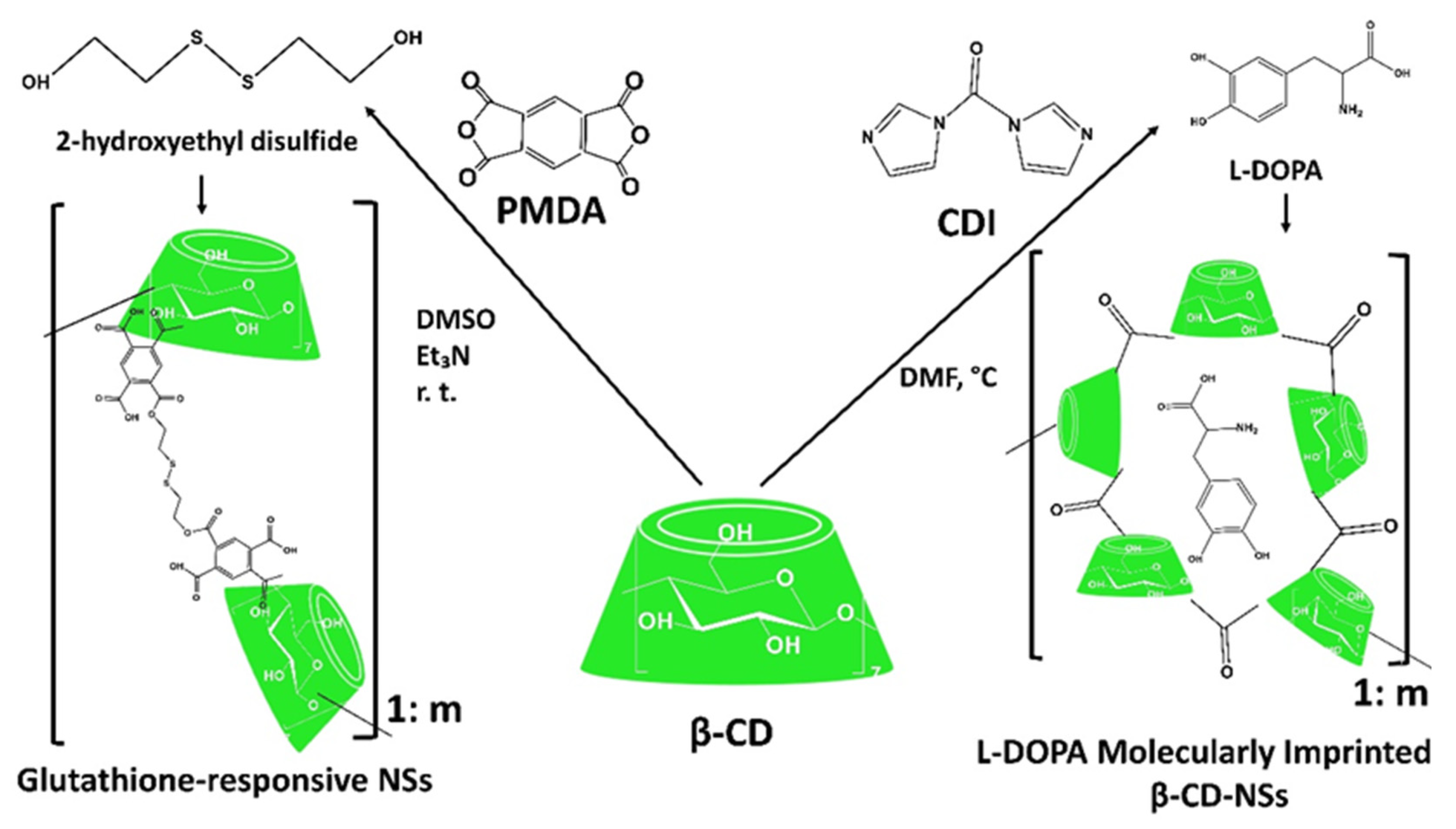
Other Generations of Cyclodextrin Nanosponges
3.2.3. General Features of Maltodextrins and Recent Trends in Their Applications as Delivery Systems Matrices
4. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Prabu, S.; Suriyaprakash, T.K.; Kumar, C.; Kumar, S. Nutraceuticals and their medicinal importance. Int. J. Health Allied Sci. 2012, 1, 7. [Google Scholar] [CrossRef]
- Pandey, M.; Verma, R.K.; Saraf, S.A. Nutraceuticals: New era of medicine and health. Asian J. Pharm. Clin. Res. 2010, 3, 4. [Google Scholar]
- Prabu, S.L.; Suriyaprakash, T.N.K.; Kumar, C.D.; Sureshkumar, S.; Ragavendran, T. Nutraceuticals: A review. Elixir Pharm. 2012, 46, 8372–8377. [Google Scholar]
- Kumari, M.; Jain, S.; Singh, J. Nutraceutical—Medicine of the future. J. Glob. Biosci. 2015, 4, 2790–2794. [Google Scholar]
- Zhao, J. Nutraceuticals, Nutritional Therapy, Phytonutrients, and Phytotherapy for Improvement of Human Health: A Perspective on Plant Biotechnology Application. Recent Pat. Biotechnol. 2007, 1, 75–97. [Google Scholar] [CrossRef]
- Das, L.; Bhaumik, E.; Raychaudhuri, U.; Chakraborty, R. Role of nutraceuticals in human health. J. Food Sci. Technol. 2012, 49, 173–183. [Google Scholar] [CrossRef]
- Agarwal, S.; Hordvik, S.; Morar, S. Nutritional claims for functional foods and supplements. Toxicology 2006, 221, 44–49. [Google Scholar] [CrossRef]
- Shahidi, F. Nutraceuticals and functional foods: Whole versus processed foods. Trends Food Sci. Technol. 2009, 20, 376–387. [Google Scholar] [CrossRef]
- Gupta, R.C. Nutraceuticals: Efficacy, Safety and Toxicity; Gupta, R.C., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2016; ISBN 9780128021477. [Google Scholar]
- Paolino, D.; Mancuso, A.; Cristiano, M.C.; Froiio, F.; Lammari, N.; Celia, C.; Fresta, M. Nanonutraceuticals: The New Frontier of Supplementary Food. Nanomaterials 2021, 11, 792. [Google Scholar] [CrossRef]
- Shekhar, V.; Jha, A.K.; Dangi, J.S. Nutraceuticals: A Re-emerging Health Aid. In Proceedings of the International Conference on Food, Biological and Medical Sciences (FBMS-2014), Bangkok, Thailand, 28–29 January 2014; pp. 105–107. [Google Scholar] [CrossRef]
- Ward, P.M.L.; Fasitsas, S.; Katz, S.E. Inhibition, Resistance Development, and Increased Antibiotic and Antimicrobial Resistance Caused by Nutraceuticals. J. Food Prot. 2002, 65, 528–533. [Google Scholar] [CrossRef]
- Vargason, A.M.; Anselmo, A.C.; Mitragotri, S. The evolution of commercial drug delivery technologies. Nat. Biomed. Eng. 2021, 5, 951–967. [Google Scholar] [CrossRef] [PubMed]
- Paolino, D.; Fresta, M.; Sinha, P.; Ferrari, M. Drug Delivery Systems. In Encyclopedia of Medical Devices and Instrumentation; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2006; pp. 437–495. ISBN 0471732877. [Google Scholar]
- Sung, Y.K.; Kim, S.W. Recent advances in polymeric drug delivery systems. Biomater. Res. 2020, 24, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Das, D.; Pal, S. Modified biopolymer-dextrin based crosslinked hydrogels: Application in controlled drug delivery. RSC Adv. 2015, 5, 25014–25050. [Google Scholar] [CrossRef]
- Santini, A.; Cammarata, S.M.; Capone, G.; Ianaro, A.; Tenore, G.C.; Pani, L.; Novellino, E. Nutraceuticals: Opening the debate for a regulatory framework. Br. J. Clin. Pharmacol. 2018, 84, 659–672. [Google Scholar] [CrossRef] [PubMed]
- Laurent, T.; Kacem, I.; Blanchemain, N.; Cazaux, F.; Neut, C.; Hildebrand, H.F.; Martel, B. Cyclodextrin and maltodextrin finishing of a polypropylene abdominal wall implant for the prolonged delivery of ciprofloxacin. Acta Biomater. 2011, 7, 3141–3149. [Google Scholar] [CrossRef]
- Molinos, M.; Carvalho, V.; Silva, D.M.; Gama, F.M. Development of a Hybrid Dextrin Hydrogel Encapsulating Dextrin Nanogel As Protein Delivery System. Biomacromolecules 2012, 13, 517–527. [Google Scholar] [CrossRef]
- Xie, H.; Ma, X.; Lin, W.; Dong, S.; Liu, Q.; Chen, Y.; Gao, Q. Linear Dextrin as Potential Insulin Delivery System: Effect of Degree of Polymerization on the Physicochemical Properties of Linear Dextrin–Insulin Inclusion Complexes. Polymers 2021, 13, 4187. [Google Scholar] [CrossRef]
- Hreczuk-Hirst, D.; Chicco, D.; German, L.; Duncan, R. Dextrins as potential carriers for drug targeting: Tailored rates of dextrin degradation by introduction of pendant groups. Int. J. Pharm. 2001, 230, 57–66. [Google Scholar] [CrossRef]
- Saavedra-Leos, Z.; Leyva-Porras, C.; Araujo-Díaz, S.B.; Toxqui-Terán, A.; Borrás-Enríquez, A.J. Technological Application of Maltodextrins According to the Degree of Polymerization. Molecules 2015, 20, 21067–21081. [Google Scholar] [CrossRef]
- Kurkov, S.V.; Loftsson, T. Cyclodextrins. Int. J. Pharm. 2013, 453, 167–180. [Google Scholar] [CrossRef]
- Trotta, F.; Dianzani, C.; Caldera, F.; Mognetti, B.; Cavalli, R. The application of nanosponges to cancer drug delivery. Expert Opin. Drug Deliv. 2014, 11, 931–941. [Google Scholar] [CrossRef] [PubMed]
- Takeiti, C.Y.; Kieckbusch, T.G.; Collares-Queiroz, F.P. Morphological and Physicochemical Characterization of Commercial Maltodextrins with Different Degrees of Dextrose-Equivalent. Int. J. Food Prop. 2010, 13, 411–425. [Google Scholar] [CrossRef]
- Sun, J.; Zhao, R.; Zeng, J.; Li, G.; Li, X. Characterization of Destrins with Different Dextrose Equivalents. Molecules 2010, 15, 5162–5173. [Google Scholar] [CrossRef] [PubMed]
- Marchal, L.M.; Beeftink, H.H.; Tramper, J. Towards a rational design of commercial maltodextrins. Trends Food Sci. Technol. 1999, 10, 345–355. [Google Scholar] [CrossRef]
- Sundari, S.; Raman, B.; Balasubramanian, D. Hydrophobic surfaces in oligosaccharides: Linear dextrins are amphiphilic chains. Biochim. Biophys. Acta 1991, 1065, 35–41. [Google Scholar] [CrossRef]
- Shukla, A.; Singh, A.P.; Maiti, P. Injectable hydrogels of newly designed brush biopolymers as sustained drug-delivery vehicle for melanoma treatment. Signal Transduct. Target. Ther. 2021, 6, 63. [Google Scholar] [CrossRef]
- Brewster, M.E.; Loftsson, T. The use of chemically modified cyclodextrins in the development of formulations for chemical delivery systems. Pharmazie 2002, 57, 94–101. [Google Scholar]
- Cumpstey, I. Chemical Modification of Polysaccharides. Chem. Funct. Prop. Food Sacch. 2013, 2013, 417672. [Google Scholar] [CrossRef]
- Trotta, F.; Zanetti, M.; Cavalli, R. Cyclodextrin-based nanosponges as drug carriers. Beilstein J. Org. Chem. 2012, 8, 2091–2099. [Google Scholar] [CrossRef]
- Krabicová, I.; Appleton, S.L.; Tannous, M.; Hoti, G.; Caldera, F.; Pedrazzo, A.R.; Cecone, C.; Cavalli, R.; Trotta, F. History of cyclodextrin nanosponges. Polymers 2020, 12, 1122. [Google Scholar] [CrossRef]
- Tejashri, G.; Amrita, B.; Darshana, J. Cyclodextrin based nanosponges for pharmaceutical use: A review. Acta Pharm. 2013, 63, 335–358. [Google Scholar] [CrossRef] [PubMed]
- Hoti, G.; Caldera, F.; Cecone, C.; Rubin Pedrazzo, A.; Anceschi, A.; Appleton, S.L.; Monfared, Y.K.; Trotta, F. Effect of the Cross-linking Density on the Swelling and Rheological Behavior of Ester-Bridged β-Cyclodextrin Nanosponges. Materials 2021, 14, 478. [Google Scholar] [CrossRef]
- Hoare, T.R.; Kohane, D.S. Hydrogels in drug delivery: Progress and challenges. Polymer 2008, 49, 1993–2007. [Google Scholar] [CrossRef]
- Matencio, A.; Hoti, G.; Monfared, Y.K.; Rezayat, A.; Pedrazzo, A.R.; Caldera, F.; Trotta, F. Cyclodextrin Monomers and Polymers for Drug Activity Enhancement. Polymers 2021, 13, 1684. [Google Scholar] [CrossRef] [PubMed]
- Gander, B.; Gurny, R.; Doelker, E.; Peppas, N.A. Effect of Polymeric Network Structure on Drug Release from Cross-Linked Poly(Vinyl Alcohol) Micromatrices. Pharm. Res. 1989, 6, 578–584. [Google Scholar] [CrossRef] [PubMed]
- De Stéfano, J.C.Q.; Abundis-Correa, V.; Herrera-Flores, S.D.; Alvarez, A.J. pH-Sensitive Starch-Based Hydrogels: Synthesis and Effect of Molecular Components on Drug Release Behavior. Polymers 2020, 12, 1974. [Google Scholar] [CrossRef] [PubMed]
- Anandam, S.; Selvamuthukumar, S. Fabrication of cyclodextrin nanosponges for quercetin delivery: Physicochemical characterization, photostability, and antioxidant effects. J. Mater Sci. 2014, 49, 8140–8153. [Google Scholar] [CrossRef]
- Darandale, S.S.; Vavia, P.R. Cyclodextrin-based nanosponges of curcumin: Formulation and physicochemical characterization. J. Incl. Phenom. Macrocycl. Chem. 2013, 75, 315–322. [Google Scholar] [CrossRef]
- Ansari, K.A.; Vavia, P.R.; Trotta, F.; Cavalli, R. Cyclodextrin-Based Nanosponges for Delivery of Resveratrol: In vitro Characterisation, Stability, Cytotoxicity and Permeation Study. AAPS PharmSciTech 2011, 12, 279–286. [Google Scholar] [CrossRef]
- Rezaei, A.; Khavari, S.; Sami, M. Incorporation of thyme essential oil into the β-cyclodextrin nanosponges: Preparation, characterization, and antibacterial activitiy. J. Mol. Liq. 2021, 1241, 1–8. [Google Scholar] [CrossRef]
- Mihailiasa, M.; Caldera, F.; Li, J.; Peila, R.; Ferri, A.; Trotta, F. Preparation of functionalized cotton fabrics by means of melatonin loaded β-cyclodextrin nanosponges. Carbohydr. Polym. 2016, 142, 24–30. [Google Scholar] [CrossRef] [PubMed]
- DeFelice, S.L. The nutraceutical revolution: Its impact on food industry R&D. Trends Food Sci. Technol. 1995, 6, 59–61. [Google Scholar] [CrossRef]
- Andlauer, W.; Fürst, P. Nutraceuticals: A piece of history, present status and outlook. Food Res. Int. 2002, 35, 171–176. [Google Scholar] [CrossRef]
- Shahidi, F. Nutraceuticals, functional foods and dietary supplements in health and disease. J. Food Drug Anal. 2012, 20, 226–230. [Google Scholar] [CrossRef]
- Singh, J.; Sinha, S. Classification, Regulatory Acts and Applications of Nutraceuticals for Health. Int. J. Pharm. Biol. Sci. 2012, 2, 177–187. [Google Scholar]
- Kalra, E.K. Nutraceutical—Definition and Introduction. AAPS J. 2003, 5, 1–2. [Google Scholar] [CrossRef]
- Chauhan, B.; Kumar, G.; Kalam, N.; Ansari, S.H. Current concepts and prospects of herbal nutraceutical: A review. J. Adv. Pharm. Technol. Res. 2013, 4, 4–8. [Google Scholar] [CrossRef]
- Cencic, A.; Chingwaru, W. The role of functional foods, nutraceuticals, and food supplements in intestinal health. Nutrients 2010, 2, 611–625. [Google Scholar] [CrossRef]
- Ganapathy, M.; Bhunia, S. Nutraceuticals: The New Generation Therapeutics. Adv. Tech. Biol. Med. 2015, 4, 2. [Google Scholar] [CrossRef]
- Aronson, J.K. Defining ‘nutraceuticals’: Neither nutritious nor pharmaceutical. Br. J. Clin. Pharmacol. 2016, 83, 12. [Google Scholar] [CrossRef]
- DeFelice, S.L. Nutrition Stymied: The Nutraceutical Solution. XXV National Congress of the Italian Chemical Society-SCI-The University of Calabria. Available online: https://fimdefelice.org/video (accessed on 27 April 2020).
- Daliu, P.; Santini, A.; Novellino, E. From pharmaceuticals to nutraceuticals: Bridging disease prevention and management. Expert Rev. Clin. Pharmacol. 2018, 12, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Martirosyan, D.M.; Singh, J. A new definition of functional food by FFC: What Makes a New Definition Unique? Funct. Foods Health Dis. 2015, 5, 209–223. [Google Scholar] [CrossRef]
- Public Law 101–535; 101st Congress Nutrition Labeling and Education Act of 1990. Library of Congress: Washington, DC, USA, 1990; p. 15. [CrossRef][Green Version]
- Young, A.L.; Bass, I.S. The Dietary Supplement Health and Education Act. Food Drug Law J. 1995, 50, 285–292. [Google Scholar] [PubMed]
- Marwick, C. Implementing the FDA modernization act. J. Am. Med. Assoc. 1998, 279, 815–816. [Google Scholar] [CrossRef]
- Glade, M.J. The Dietary Supplement Health and Education Act of 1994—Focus on labeling issues. Nutrition 1997, 13, 999–1001. [Google Scholar] [CrossRef]
- Quinones, R.L.; Winsor, R.D.; Patino, A.; Hoffmann, P. The Regulation of Dietary Supplements Within the United States: Flawed Attempts at Mending a Defective Consumer Safety Mechanism. J. Consum. Aff. 2013, 47, 328–357. [Google Scholar] [CrossRef]
- Bagchi, D. Nutraceutical and Functional Food Regulations in the United States and around the World, 2nd ed.; Bagchi, D., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Halsted, C.H. Dietary supplements and functional foods: 2 sides of a coin? Am. J. Clin. Nutr. 2003, 77, 1001S–1007S. [Google Scholar] [CrossRef]
- Gupta, S.; Chauhan, D.; Mehla, K.; Sood, P.; Nair, A. An overview of nutraceuticals: Current scenario. J. Basic Clin. Pharm. 2010, 1, 55–62. [Google Scholar]
- Hasler, C.M. Functional Foods: Benefits, Concerns and Challenges—A Position Paper from the American Council on Science and Health. J. Nutr. 2002, 132, 3772–3781. [Google Scholar] [CrossRef]
- Ross, S. Functional foods: The Food and Drug Administration perspective. Am. J. Clin. Nutr. 2000, 71, 1735S–1738S. [Google Scholar] [CrossRef]
- Golodner, L.F. The US Food and Drug Administration Modernization Act of 1997: Impact on consumers. Clin. Ther. 1998, 20, C20–C25. [Google Scholar] [CrossRef]
- Borchers, A.T.; Keen, C.L.; Gershwin, M.E. The Basis of Structure/Function Claims of Nutraceuticals. Clin. Rev. Allergy Immunol. 2016, 51, 370–382. [Google Scholar] [CrossRef] [PubMed]
- Muredzi, P. Food Is Medicine—An Introduction to Nutraceuticals. LAP Lambert Academic Publishing: Saarbrücken, Germany, 2013; ISBN 978-3659437106.
- Zeisel, S.H. Regulation of “ Nutraceuticals”. Sci. Compass 1999, 285, 1853–1855. [Google Scholar] [CrossRef]
- Ghosh, D.; Bagchi, D.; Konishi, T. Clinical Aspects of Functional Foods and Nutraceuticals; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2015; ISBN 9781466569164. [Google Scholar]
- Irene Boye, J. Nutraceutical and Functional Food Processing Technology; Wiley-Blackwell: Hoboken, NJ, USA, 2015; ISBN 9781118504949. [Google Scholar]
- Dillard, C.J.; Bruce German, J. Phytochemicals: Nutraceuticals and human health. J. Sci. Food Agric. 2000, 80, 1744–1756. [Google Scholar] [CrossRef]
- Valls, J.; Pasamontes, N.; Pantaleon, A.; Vinaixa, S.; Vaque, M.; Soler, A.; Millan, S.; Gomez, X. Prospects of Functional Foods/Nutraceuticals and Markets. Nat. Prod. 2013, 2491–2525. [Google Scholar] [CrossRef]
- Ahmad, M.F.; Amir Ashraf, S.; Ali Ahmad, F.; Akhtar Ansari, J.; Ahmad Siddiquee, M.R. Nutraceutical Market and its Regulation. Am. J. Food Technol. 2011, 6, 342–347. [Google Scholar] [CrossRef]
- Singh, U.K.; Deshmukh, S.N. Nutraceuticals. MIT Int. J. Pharm. Sci. 2016, 2, 43–52. [Google Scholar]
- Brower, V. Nutraceuticals: Poised for a healthy slice of the healthcare market? Nat. Biotechnol. 1998, 16, 728–731. [Google Scholar] [CrossRef]
- Helal, N.A.; Eassa, H.A.; Amer, A.M.; Eltokhy, M.A.; Edafiogho, I.; Nounou, M.I. Nutraceuticals’ Novel Formulations: The Good, the Bad, the Unknown and Patents Involved. Recent Pat. Drug Deliv. Formul. 2019, 13, 105–156. [Google Scholar] [CrossRef]
- Bagchi, D. Nutraceuticals and functional foods regulations in the United States and around the world. Toxicology 2006, 221, 1–3. [Google Scholar] [CrossRef]
- Dev, R.; Kumar, S.; Singh, J.; Chauhan, B. Potential role of nutraceuticals in present scenerio: A review. J. Appl. Pharm. Sci. 2011, 1, 26–28. [Google Scholar]
- Nicoletti, M. Nutraceuticals and botanicals: Overview and perspectives. Int. J. Food Sci. Nutr. 2012, 63, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Pagliaro, M. Italy’s Nutraceutical Industry: A Process and Bioeconomy Perspective into a Key Area of the Global Economy. Biofuels Bioprod. Biorefining 2019, 14, 1–7. [Google Scholar] [CrossRef]
- Lupsea, S. Nutraceuticals and Food Supplement Sector in Japan—Opportunities for European Produces? EU-Japan Centre for Industrial Cooperation: Tokyo, Japan, 2016; Available online: https://www.eu-japan.eu/publications/nutraceuticals-and-food-supplements-sector-japan (accessed on 18 January 2022).
- Chanda, S.; Tiwari, R.K.; Kumar, A.; Singh, K. Nutraceuticals inspiring the current therapy for lifestyle diseases. Adv. Pharmacol. Sci. 2019, 2019, 6908716. [Google Scholar] [CrossRef]
- Ali, A.; Ahmad, U.; Akhtar, J.; Badruddeen; Khan, M.M. Engineered nano scale formulation strategies to augment efficiency of nutraceuticals. J. Funct. Foods 2019, 62, 16. [Google Scholar] [CrossRef]
- Kumar, C.G.; Sripada, S.; Poornachandra, Y. Status and Future Prospects of Fructooligosaccharides as Nutraceuticals. In Role of Materials Science in Food Bioengineering; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 451–503. ISBN 9780128114483. [Google Scholar]
- Bhaskarachary, K.; Vemula, S.R.; Gavaravarapu, S.R.M.; Joshi, A.K.R. Traditional foods, functional foods and nutraceuticals. Proc. Indian Natl. Sci. Acad. 2016, 82, 1565–1577. [Google Scholar] [CrossRef]
- Keservani, R.K.; Sharma, A.K.; Kesharwani, R.K. Nutraceutical and Functional Foods in Disease Prevention; IGI Global Book Series; Advances in Human Services and Public Health: Hershey, PA, USA, 2019; ISBN 2475-6571. [Google Scholar]
- Holzapfel, W.H.; Haberer, P.; Geisen, R.; Björkroth, J.; Schillinger, U. Taxonomy and important features of probiotic microorganisms in food and nutrition. Am. J. Clin. Nutr. 2001, 73, 366S–373S. [Google Scholar] [CrossRef]
- Martínez Cruz, P.; Ibáñez, A.L.; Monroy Hermosillo, O.A.; Ramírez Saad, H.C. Use of Probiotics in Aquaculture. ISRN Microbiol. 2012, 2021, 916845. [Google Scholar] [CrossRef]
- DellaPenna, D. Nutritional Genomics: Manipulating Plant Micronutrients to Improve Human Health. Plant Biotechnol. Food Feed 1991, 285, 375–379. [Google Scholar] [CrossRef]
- Galanakis, C.M. Nutraceuticals and Natural Product Pharmaceuticals; Academic Press: Cambridge, MA, USA, 2019; ISBN 9780128164501. [Google Scholar]
- Srivastava, R.K. Need Of Nutraceuticals / Functional Food Products for Health Benefits to World-Wide People. J. Biotechnol. Biomed. Sci. 2018, 1, 13. [Google Scholar] [CrossRef]
- Wildman, R.E.C. Nutraceuticals and Functional Foods. In Handbook of Nutraceuticals and Functional Foods; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2020; ISBN 9781498703727. [Google Scholar]
- Stipanuk, M.H.; Caudill, M.A. Biochemical, Physiological, and Molecular Aspects of Human Nutrition, 3rd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2013; ISBN 9781437709599. [Google Scholar]
- Chemler, J.A.; Yan, Y.; Koffas, M.A.G. Biosynthesis of isoprenoids, polyunsaturated fatty acids and flavonoids in Saccharomyces cerevisiae. Microb. Cell Fact. 2006, 5, 9. [Google Scholar] [CrossRef] [PubMed]
- Sacchettini, J.C.; Poulter, C.D. Creating lsoprenoid Diversity. Science 1997, 277, 1788–1789. [Google Scholar] [CrossRef] [PubMed]
- Bhuyan, D.J.; Basu, A. Phenolic Compounds Potential Health Benefits and Toxicity. In Utilisation of Bioactive Compounds from Agricultural and Food Production Waste; Vuong, Q.V., Ed.; CRC Press, Taylor & Francis: Boca Raton, FL, USA, 2017; pp. 27–59. ISBN 97813151540. [Google Scholar]
- Tanase, C.; Cosarcă, S.; Muntean, D.-L. A Critical Review of Phenolic Compounds Extracted from the Bark of Woody Vascular Plants and their Potential Biological Activity. Molecules 2019, 24, 1182. [Google Scholar] [CrossRef] [PubMed]
- Tsao, R. Chemistry and Biochemistry of Dietary Polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef]
- Pérez-Jiménez, J.; Neveu, V.; Vos, F.; Scalbert, A. Identification of the 100 richest dietary sources of polyphenols: An application of the Phenol-Explorer database. Eur. J. Clin. Nutr. 2010, 64, S112–S120. [Google Scholar] [CrossRef]
- Ross, J.A.; Kasum, C.M. DIETARY FLAVONOIDS: Bioavailability, Metabolic Effects, and Safety. Annu. Rev. Nutr. 2002, 22, 19–34. [Google Scholar] [CrossRef]
- Giada, M.D.L.R. Food Phenolic Compounds: Main Classes, Sources and Their Antioxidant Power. In Oxidative Stress and Chronic Degenerative Diseases—A Role for Antioxidants; IntechOpen: London, UK, 2013; pp. 87–112. [Google Scholar] [CrossRef]
- Lachance, P.A.; Das, Y.T. Nutraceuticals. In Comprehensive Medicinal Chemistry II; Elsevier Ltd.: Amsterdam, The Netherlands, 2007; pp. 449–461. ISBN 0-08-044514-4. [Google Scholar]
- Burdge, G.C.; Calder, P.C. Introduction to Fatty Acids and Lipids. World Rev. Nutr. Diet. 2015, 112, 1–16. [Google Scholar] [CrossRef]
- Kresge, N.; Simoni, R.D.; Hill, R.L. JBC Historical Perspectives: Lipid Biochemistry. 2010. Available online: https://moam.info/lipid-biochemistry-the-journal-of-biological-chemistry_59e02dbf1723dd1a56a74f84.html (accessed on 18 January 2022).
- Fahy, E.; Cotter, D.; Sud, M.; Subramaniam, S. Lipid classification, structures and tools. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2011, 1811, 637–647. [Google Scholar] [CrossRef]
- Fahy, E.; Subramaniam, S.; Brown, H.A.; Glass, C.K.; Merrill, A.H.; Murphy, R.C.; Raetz, C.R.H.; Russell, D.W.; Seyama, Y.; Shaw, W.; et al. A comprehensive classification system for lipids. J. Lipid Res. 2005, 46, 839–861. [Google Scholar] [CrossRef]
- Nagy, K.; Tiuca, I.-D. Importance of Fatty Acids in Physiopathology of Human Body. IntechOpen 2017, 3–22. [Google Scholar] [CrossRef]
- Rustan, A.C.; Drevon, C.A. Fatty Acids: Structures and Properties. Encycl. Life Sci. 2005, 7. [Google Scholar] [CrossRef]
- Orsavova, J.; Misurcova, L.; Vavra Ambrozova, J.; Vicha, R.; Mlcek, J. Fatty Acids Composition of Vegetable Oils and its Contribution to Dietary Energy Intake and Dependence of Cardiovascular Mortality on Dietary Intake of Fatty Acids. Int. J. Mol. Sci. 2015, 16, 12871–12890. [Google Scholar] [CrossRef] [PubMed]
- Tvrzicka, E.; Kremmyda, L.-S.; Stankova, B.; Zak, A. Fatty Acids as Biocompounds: Their Role in Human Metabolism, Health and Disease—A Review. Part 1: Classification, Dietary Sources and Biological Functions. Biomed. Pap. 2011, 155, 117–130. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.; Bradford, C. Omega-3, Omega-6 and Omega-9 Fatty Acids: Implications for Cardiovascular and Other Diseases. J. Glycom. Lipidom. 2014, 4, 8. [Google Scholar] [CrossRef]
- Gerschenson, L.N.; Rojas, A.M.; Fissore, E.N. Carbohydrates. In Nutraceutical and Functional Food Components: Effects of Innovative Processing Techniques; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 39–101. ISBN 9780128052570. [Google Scholar]
- Lovegrove, A.; Edwards, C.H.; De Noni, I.; Patel, H.; El, S.N.; Grassby, T.; Zielke, C.; Ulmius, M.; Nilsson, L.; Butterworth, P.J.; et al. Role of polysaccharides in food, digestion, and health. Crit. Rev. Food Sci. Nutr. 2017, 57, 237–253. [Google Scholar] [CrossRef]
- Cummings, J.H.; Stephen, A.M. Carbohydrate terminology and classification. Eur. J. Clin. Nutr. 2007, 61, S5–S18. [Google Scholar] [CrossRef]
- Voragen, A.G.J.; Coenen, G.-J.; Verhoef, R.P.; Schols, H.A. Pectin, a versatile polysaccharide present in plant cell walls. Struct. Chem. 2009, 20, 263–275. [Google Scholar] [CrossRef]
- Wang, T.; Zabotina, O.; Hong, M. Pectin-Cellulose Interactions in the Arabidopsis Primary Cell Wall from Two-Dimensional Magic-Angle-Spinning Solid-State Nuclear Magnetic Resonance. Biochemistry 2012, 51, 9846–9856. [Google Scholar] [CrossRef]
- Aspinall, G.O. Chemistry of Cell Wall Polysaccharides. In The Biochemistry of Plants; Preiss, J., Ed.; Academic Press, Inc.: Cambridge, MA, USA, 1980; Volume 3, pp. 473–500. [Google Scholar]
- Zykwinska, A.; Thibault, J.F.; Ralet, M.C. Organization of pectic arabinan and galactan side chains in association with cellulose microfibrils in primary cell walls and related models envisaged. J. Exp. Bot. 2007, 58, 1795–1802. [Google Scholar] [CrossRef]
- Sinha, A.K.; Kumar, V.; Makkar, H.P.S.; De Boeck, G.; Becker, K. Non-starch polysaccharides and their role in fish nutrition—A review. Food Chem. 2011, 127, 1409–1426. [Google Scholar] [CrossRef]
- Waldron, K.W.; Parker, M.L.; Smith, A.C. Plant Cell Walls and Food Quality. Compr. Rev. Food Sci. Food Saf. 2003, 2, 128–146. [Google Scholar] [CrossRef] [PubMed]
- Scheller, H.V.; Ulvskov, P. Hemicelluloses. Annu. Rev. Plant Biol. 2010, 61, 263–289. [Google Scholar] [CrossRef] [PubMed]
- Wu, G. Dietary protein intake and human health. Food Funct. 2016, 7, 1251–1265. [Google Scholar] [CrossRef] [PubMed]
- Osborne, T.B.; Mendel, L.B. Amino-Acids in nutrition and growth. J. Biol. Chem. 1914, 17, 325–349. [Google Scholar] [CrossRef]
- Ray, P.D.; Fry, R.C. The Cell: The Fundamental Unit in Systems Biology. In Systems Biology in Toxicology and Environmental Health; Elsevier Inc.: Amsterdam, The Netherlands, 2015; pp. 11–42. ISBN 9780128015681. [Google Scholar]
- Carpenter, K.J. A Short History of Nutritional Science: Part 1 (1785–1885). J. Nutr. 2003, 133, 638–645. [Google Scholar] [CrossRef]
- Leuchtenberger, W.; Huthmacher, K.; Drauz, K. Biotechnological production of amino acids and derivatives: Current status and prospects. Appl. Microbiol. Biotechnol. 2005, 69, 1–8. [Google Scholar] [CrossRef]
- Wu, G. Amino acids: Metabolism, functions, and nutrition. Amino Acids 2009, 37, 1–17. [Google Scholar] [CrossRef]
- Wu, G. Functional Amino Acids in Growth, Reproduction and Health. Adv. Nutr. 2010, 1, 31–37. [Google Scholar] [CrossRef]
- Bhagavan, N.V.; Ha, C.-E. Amino Acids. In Essentials of Medical Biochemistry; Academic Press: Cambridge, MA, USA, 2015; pp. 21–29. [Google Scholar] [CrossRef]
- Wang, J.; Guleria, S.; Koffas, M.A.G.; Yan, Y. Microbial production of value-added nutraceuticals. Curr. Opin. Biotechnol. 2016, 37, 97–104. [Google Scholar] [CrossRef]
- Liong, M.-T. Beneficial Microorganisms in Food and Nutraceuticals; Springer: Berlin, Germany, 2015; Volume 27. [Google Scholar] [CrossRef]
- Joint FAO/WHO Expert Consultation. Probiotics in Food; Health and Nutritional Properties and Guidelines for Evaluation; FAO/WHO: Geneva, Switzerland, 2001. [Google Scholar] [CrossRef]
- Soccol, C.R.; de Souza Vandenberghe, L.P.; Spier, M.R.; Medeiros, A.B.P.; Yamaguishi, C.T.; De Dea Lindner, J.; Pandey, A.; Thomaz-Soccol, V. The Potential of Probiotics: A Review. Food Technol. Biotechnol. 2010, 48, 413–434. [Google Scholar]
- Sharma, S.; Agarwal, N.; Verma, P. Probiotics: The Emissaries of Health from Microbial World. J. Appl. Pharm. Sci. 2012, 2, 138–143. [Google Scholar]
- Zhang, Y.Y.; Panozzo, J.; Hall, M.S.; Ajlouni, S. Bioaccessibility of Some Essential Minerals in Three Selected Australian Pulse Varieties Using an In Vitro Gastrointestinal Digestion Model. J. Food Sci. 2018, 83, 2873–2881. [Google Scholar] [CrossRef] [PubMed]
- Gupta, U.C.; Gupta, S.C. Sources and Deficiency Diseases of Mineral Nutrients in Human Health and Nutrition: A Review. Pedosphere 2014, 24, 13–38. [Google Scholar] [CrossRef]
- Park, Y.W. Bioactive Components in Milk and Dairy Products; Park, Y.W., Ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2009; Volume 66, ISBN 9780813819822. [Google Scholar]
- Anderson, J.J.B.; Allen, J.C. Nutrition of Macrominerals and Trace Minerals. In Functional Foods: Designer Foods, Pharmafoods, Nutraceuticals; Goldberg, I., Ed.; Springer: New York, NY, USA, 1994; pp. 323–354. ISBN 9781461358619. [Google Scholar]
- Górska-Warsewicz, H.; Rejman, K.; Laskowski, W.; Czeczotko, M. Milk and Dairy Products and Their Nutritional Contribution to the Average Polish Diet. Nutrients 2019, 11, 1771. [Google Scholar] [CrossRef]
- Mahantesh, P.; Patil, C.S. Nutraceuticals and Functional Foods in Health Promotion and Disease Risk Management. In Proceedings of the International Conference on Biomedical Engineering and Technology, Kuala Lumpur, Malaysia, 4–5 June 2011; Volume 11, pp. 7–14. [Google Scholar]
- Galasso, C.; Gentile, A.; Orefice, I.; Ianora, A.; Bruno, A.; Noonan, D.M.; Sansone, C.; Albini, A.; Brunet, C. Microalgal Derivatives as Potential Nutraceutical and Food Supplements for Human Health: A Focus on Cancer Prevention and Interception. Nutrients 2019, 11, 1226. [Google Scholar] [CrossRef]
- Diem, G.; Brownson, R.C.; Grabauskas, V.; Shatchkute, A.; Stachenko, S. Prevention and control of noncommunicable diseases through evidence-based public health: Implementing the NCD 2020 action plan. Glob. Health Promot. 2014, 23, 5–13. [Google Scholar] [CrossRef]
- Probst-Hensch, N.; Tanner, M.; Kessler, C.; Burri, C.; Künzli, N. Prevention—A cost-effective way to fight the non-communicable disease epidemic. Eur. J. Med. Sci. 2011, 141, 1–8. [Google Scholar] [CrossRef]
- Anjali; Garg, V.; Dhiman, A.; Dutt, R.; Ranga, S. Health benefits of nutraceuticals. Pharma Innov. J. 2018, 7, 178–181. [Google Scholar]
- Sharma, R.; Singh, R.B. Bioactive Foods and Nutraceutical Supplementation Criteria in Cardiovascular Protection. Open Nutraceuticals J. 2010, 3, 141–153. [Google Scholar] [CrossRef][Green Version]
- Pellett, P.L. World essential amino acid supply with special attention to South-East Asia. Food Nutr. Bull. 1996, 17, 31. [Google Scholar] [CrossRef]
- Bravo, L. Polyphenols: Chemistry, Dietary Sources, Metabolism, and Nutritional Significance. Nutr. Rev. 2009, 56, 317–333. [Google Scholar] [CrossRef] [PubMed]
- Adeboye, P.T.; Bettiga, M.; Olsson, L. The chemical nature of phenolic compounds determines their toxicity and induces distinct physiological responses in Saccharomyces cerevisiae in lignocellulose hydrolysates. AMB Express 2014, 4, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Gutteridge, J.M.C. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem. J. 1984, 219, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, D.L. Oxygen and Living Processes, 1st ed.; Springer: New York, NY, USA, 1981; ISBN 9781461258926. [Google Scholar]
- Bartosz, G. Food Oxidants and Antioxidants Chemical, Biological and Functional Properties; CRC Press, Taylor & Francis: Boca Raton, FL, USA, 2014; Volume 236, ISBN 0021701319376. [Google Scholar]
- Gerschman, R.; Gilbert, D.L.; Nye, S.W.; Dwyer, P.; Fenn, W.O. Oxygen Poisoning and X-irradiation: A Mechanism in Common. Science 1954, 119, 623–626. [Google Scholar] [CrossRef] [PubMed]
- Palmer, H.J.; Paulson, K.E. Reactive Oxygen Species and Antioxidants in Signal Transduction and Gene Expression. Nutr. Rev. 1997, 55, 353–361. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 1–13. [Google Scholar] [CrossRef]
- Nunes, X.P.; Silva, F.S.; da Souza Almeida, J.R.G.; de Lima, J.T.; de Araújo Ribeiro, L.A.; Junior, L.J.Q.; Filho, J.M.B. Biological Oxidations and Antioxidant Activity of Natural Products. In Phytochemicals as Nutraceuticals—Global Approaches to Their Role in Nutrition and Health; IntechOpen: London, UK, 2011. [Google Scholar] [CrossRef]
- Tian, Y.; Zou, B.; Li, C.; Yang, J.; Xu, S.; Hagerman, A.E. High molecular weight persimmon tannin is a potent antioxidant both ex vivo and in vivo. Food Res. Int. 2012, 45, 26–30. [Google Scholar] [CrossRef]
- Stahl, W.; Sies, H. Antioxidant activity of carotenoids. Mol. Aspects Med. 2003, 24, 345–351. [Google Scholar] [CrossRef]
- Dreher, D.; Junod, A.F. Role of Oxygen Free Radicals in Cancer Development. Eur. J. Cancer 1996, 32A, 30–38. [Google Scholar] [CrossRef]
- Yamauchi, R. Vitamin E: Mechanism of its antioxidant activity. Food Sci. Technol. 1997, 3, 301–309. [Google Scholar] [CrossRef]
- Silva, E.M.; Souza, J.N.S.; Rogez, H.; Rees, J.F.; Larondelle, Y. Antioxidant activities and polyphenolic contents of fifteen selected plant species from the Amazonian region. Food Chem. 2007, 101, 1012–1018. [Google Scholar] [CrossRef]
- Gonzalez-Burgos, E.; Gomez-Serranillos, M.P. Terpene Compounds in Nature: A Review of Their Potential Antioxidant Activity. Curr. Med. Chem. 2012, 19, 5319–5341. [Google Scholar] [CrossRef] [PubMed]
- Saura-Calixto, F. Antioxidant Dietary Fiber Product: A New Concept and a Potential Food Ingredient. J. Agric. Food Chem. 1998, 46, 4303–4306. [Google Scholar] [CrossRef]
- Działo, M.; Mierziak, J.; Korzun, U.; Preisner, M.; Szopa, J.; Kulma, A. The Potential of Plant Phenolics in Prevention and Therapy of Skin Disorders. Int. J. Mol. Sci. 2016, 17, 160. [Google Scholar] [CrossRef] [PubMed]
- Houston, M.C. The role of cellular micronutrient analysis, nutraceuticals, vitamins, antioxidants and minerals in the prevention and treatment of hypertension and cardiovascular disease. Ther. Adv. Cardiovasc. Dis. 2010, 4, 165–183. [Google Scholar] [CrossRef]
- Vercruysse, L.; Smagghe, G.; Herregods, G.; Van Camp, J. ACE Inhibitory Activity in Enzymatic Hydrolysates of Insect Protein. J. Agric. Food Chem. 2005, 53, 5207–5211. [Google Scholar] [CrossRef]
- Chen, Z.-Y.; Peng, C.; Jiao, R.; Wong, Y.-M.; Yang, N.; Huang, Y. Anti-hypertensive Nutraceuticals and Functional Foods. J. Agric. Food Chem. 2009, 57, 4485–4499. [Google Scholar] [CrossRef]
- Rosen, C.J. Vitamin D Insufficiency. N. Engl. J. Med. 2011, 364, 248–254. [Google Scholar] [CrossRef]
- Boldo, A.; Campbell, P.; Luthra, P.; White, W.B. Should the Concentration of Vitamin D be Measured in All Patients with Hypertension? J. Clin. Hypertens. 2010, 12, 149–152. [Google Scholar] [CrossRef]
- Houston, M. The role of nutrition and nutraceutical supplements in the treatment of hypertension. World J. Cardiol. 2014, 6, 38–66. [Google Scholar] [CrossRef]
- Theodotou, M.; Fokianos, K.; Mouzouridou, A.; Konstantinou, C.; Aristotelous, A.; Prodromou, D.; Chrysikou, A. The effect of resveratrol on hypertension: A clinical trial. Exp. Ther. Med. 2017, 13, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Houston, M.C. Treatment of Hypertension with Nutrition and Nutraceutical Supplements: Part 2. Altern. Complement. Ther. 2019, 25, 23–36. [Google Scholar] [CrossRef]
- Mozos, I.; Stoian, D.; Caraba, A.; Malainer, C.; Horbanczuk, J.O.; Atanasov, A.G. Lycopene and Vascular Health. Front. Pharmacol. 2018, 9, 521. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Santos, P.; Carrón, R.; Montero, M.J.; Sevilla, M.Á. The antihypertensive and antihypertrophic effect of lycopene is not affected by and is independent of age. J. Funct. Foods 2021, 85, 104656. [Google Scholar] [CrossRef]
- Tetali, S.D. Terpenes and isoprenoids: A wealth of compounds for global use. Planta 2018, 249, 8. [Google Scholar] [CrossRef]
- Calder, P.C. Polyunsaturated Fatty Acids, Inflammation, and Immunity. Lipids 2001, 36, 1007–1024. [Google Scholar] [CrossRef]
- Ahmad, T.B.; Rudd, D.; Kotiw, M.; Liu, L.; Benkendorff, K. Correlation between Fatty Acid Profile and Anti-Inflammatory Activity in Common Australian Seafood by-Products. Mar. Drugs 2019, 17, 155. [Google Scholar] [CrossRef]
- Calder, P.C. n-3 Polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am. J. Clin. Nutr. 2006, 83, 1505S–1519S. [Google Scholar] [CrossRef]
- Mori, T.A.; Beilin, L.J. Omega-3 Fatty Acids and Inflammation. Curr. Atheroscler. Rep. 2004, 6, 461–467. [Google Scholar] [CrossRef]
- Endo, J.; Arita, M. Cardioprotective mechanism of omega-3 polyunsaturated fatty acids. J. Cardiol. 2015, 67, 22–27. [Google Scholar] [CrossRef]
- Kinsella, J.E.; Lokesh, B.; Stone, R.A. Dietary n-3 polyunsaturated fatty acids and amelioration of cardiovascular disease: Possible mechanisms. Am. J. Clin. Nutr. 1990, 52, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Sokoła-Wysoczańska, E.; Wysoczański, T.; Wagner, J.; Czyż, K.; Bodkowski, R.; Lochyński, S.; Patkowska-Sokoła, B. Polyunsaturated fatty acids and their potential therapeutic role in cardiovascular system disorders—A review. Nutrients 2018, 10, 1561. [Google Scholar] [CrossRef] [PubMed]
- Medeiros-De-Moraes, I.M.; Gonçalves-De-Albuquerque, C.F.; Kurz, A.R.M.; De Jesus Oliveira, F.M.; Pereira de Abreu, V.H.; Torres, R.C.; Carvalho, V.F.; Estato, V.; Bozza, P.T.; Sperandio, M.; et al. Omega-9 Oleic Acid, the Main Compound of Olive oil, Mitigates Inflammation during Experimental Sepsis. Oxid. Med. Cell. Longev. 2018, 2018, 13. [Google Scholar] [CrossRef] [PubMed]
- Feghali, C.A.; Wright, T.M. Cytokines in acute and chronic inflammation. Front. Biosci. 1997, 2, 12–26. [Google Scholar]
- Kawata, A.; Murakami, Y.; Suzuki, S.; Fujisawa, S. Anti-inflammatory Activity of β-Carotene, Lycopene and Tri-n-butylborane, a Scavenger of Reactive Oxygen Species. In Vivo 2018, 32, 255–264. [Google Scholar] [CrossRef]
- Magno, S.; Ceccarini, G.; Pelosini, C.; Jaccheri, R.; Vitti, J.; Fierabracci, P.; Salvetti, G.; Airoldi, G.; Minale, M.; Saponati, G.; et al. LDL-cholesterol lowering effect of a new dietary supplement: An open label, controlled, randomized, cross-over clinical trial in patients with mild-to-moderate hypercholesterolemia. Lipids Health Dis. 2018, 17, 8. [Google Scholar] [CrossRef]
- Ahangari, N.; Ghayour Mobarhan, M.; Sahebkar, A.; Pasdar, A. Molecular aspects of hypercholesterolemia treatment: Current perspectives and hopes. Ann. Med. 2018, 50, 303–311. [Google Scholar] [CrossRef]
- Venkadeswaran, K.; Muralidharan, A.R.; Annadurai, T.; Ruban, V.V.; Sundararajan, M.; Anandhi, R.; Thomas, P.A.; Geraldine, P. Antihypercholesterolemic and Antioxidative Potential of an Extract of the Plant, Piper betle, and its Active Constituent, Eugenol, in Triton WR-1339-Induced Hypercholesterolemia in Experimental Rats. Evid.-Based Complement. Altern. Med. 2014, 2014, 478973. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh Fokou, P.V.; Azzini, E.; Peluso, I.; et al. Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front. Physiol. 2020, 11, 21. [Google Scholar] [CrossRef]
- Mytilinaiou, M.; Kyrou, I.; Khan, M.; Grammatopoulos, D.K.; Randeva, H.S. Familial hypercholesterolemia: New horizons for diagnosis and effective management. Front. Pharmacol. 2018, 9, 29. [Google Scholar] [CrossRef]
- Goldberg, A.C.; Hopkins, P.N.; Toth, P.P.; Ballantyne, C.M.; Rader, D.J.; Robinson, J.G.; Daniels, S.R.; Gidding, S.S.; de Ferranti, S.D.; Ito, M.K.; et al. Familial Hypercholesterolemia: Screening, diagnosis and management of pediatric and adult patients. J. Clin. Lipidol. 2011, 5, S1–S8. [Google Scholar] [CrossRef] [PubMed]
- Scholle, J.M.; Baker, W.L.; Talati, R.; Coleman, C.I. The Effect of Adding Plant Sterols or Stanols to Statin Therapy in Hypercholesterolemic Patients: Systematic Review and Meta-Analysis. J. Am. Coll. Nutr. 2009, 28, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Santini, A.; Novellino, E. Nutraceuticals in hypercholesterolaemia: An overview. Br. J. Pharmacol. 2017, 174, 1450–1463. [Google Scholar] [CrossRef] [PubMed]
- Rana, A.; Westein, E.; Niego, B.; Hagemeyer, C.E. Shear-Dependent Platelet Aggregation: Mechanisms and Therapeutic Opportunities. Front. Cardiovasc. Med. 2019, 6, 141. [Google Scholar] [CrossRef] [PubMed]
- Olas, B. Dietary Supplements with Antiplatelet Activity: A Solution for Everyone? Adv. Nutr. 2018, 9, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Oh, S.J.; Liu, Y.; Lee, M.-Y. A Comparative Study of the Anti-Platelet Effects of cis- and trans-Resveratrol. Biomol. Ther. 2011, 19, 201–205. [Google Scholar] [CrossRef]
- Olas, B.; Wachowicz, B. Resveratrol, a phenolic antioxidant with effects on blood platelet functions. Platelets 2005, 16, 251–260. [Google Scholar] [CrossRef]
- Giordo, R.; Zinellu, A.; Hussein Eid, A.; Pintus, G. Therapeutic Potential of Resveratrol in Covid-19-Associated Hemostatic Disorders. Molecules 2021, 26, 856. [Google Scholar] [CrossRef]
- Dutra, L.A.; Guanaes, J.F.O.; Johmann, N.; Lopes Pires, M.E.; Chin, C.M.; Marcondes, S.; Dos Santos, J.L. Synthesis, antiplatelet and antithrombotic activities of resveratrol derivatives with NO-donor properties. Bioorg. Med. Chem. Lett. 2017, 27, 2450–2453. [Google Scholar] [CrossRef]
- Tungmunnithum, D.; Thongboonyou, A.; Pholboon, A.; Yangsabai, A. Flavonoids and Other Phenolic Compounds from Medicinal Plants for Pharmaceutical and Medical Aspects: An Overview. Medicines 2018, 5, 93. [Google Scholar] [CrossRef]
- Benetou, V.; Lagiou, A.; Lagiou, P. Chemoprevention of cancer: Current evidence and future prospects. F1000Research 2015, 4, 10. [Google Scholar] [CrossRef] [PubMed]
- Key, T.J.; Bradbury, K.E.; Perez-Cornago, A.; Sinha, R.; Tsilidis, K.K.; Tsugane, S. Diet, nutrition, and cancer risk: What do we know and what is the way forward? BMJ 2020, 368, m511. [Google Scholar] [CrossRef] [PubMed]
- Aruoma, O.I. Free radicals, oxidative stress, and antioxidants in human health and disease. J. Am. Oil Chem. Soc. 1998, 75, 199–212. [Google Scholar] [CrossRef] [PubMed]
- Brusselmans, K.; Vrolix, R.; Verhoeven, G.; Swinnen, J.V. Induction of Cancer Cell Apoptosis by Flavonoids Is Associated with Their Ability to Inhibit Fatty Acid Synthase Activity. J. Biol. Chem. 2005, 280, 5636–5645. [Google Scholar] [CrossRef]
- Danciu, C.; Vlaia, L.; Fetea, F.; Hancianu, M.; Coricovac, D.E.; Ciurlea, S.A.; Şoica, C.M.; Marincu, I.; Vlaia, V.; Dehelean, C.A.; et al. Evaluation of phenolic profile, antioxidant and anticancer potential of two main representants of Zingiberaceae family against B164A5 murine melanoma cells. Biol. Res. 2015, 48, 1. [Google Scholar] [CrossRef]
- Abusnina, A.; Keravis, T.; Yougbaré, I.; Bronner, C.; Lugnier, C. Anti-proliferative effect of curcumin on melanoma cells is mediated by PDE1A inhibition that regulates the epigenetic integrator UHRF1. Mol. Nutr. Food Res. 2011, 55, 1677–1689. [Google Scholar] [CrossRef]
- Basli, A.; Belkacem, N.; Amrani, I. Health Benefits of Phenolic Compounds Against Cancers. In Phenolic Compounds; Soto-Hernandez, M., Palma-Tenango, M., Garcia-Mateos, M.D.R., Eds.; IntechOpen: London, UK, 2017; pp. 193–210. [Google Scholar] [CrossRef]
- Amawi, H.; Ashby, C.R., Jr.; Samuel, T.; Peraman, R.; Tiwari, A.K. Polyphenolic Nutrients in Cancer Chemoprevention and Metastasis: Role of the Epithelial-to-Mesenchymal (EMT) Pathway. Nutrients 2017, 9, 911. [Google Scholar] [CrossRef]
- Johnson, E.J. The Role of Carotenoids in Human Health. Nutr. Clin. Care 2002, 5, 56–65. [Google Scholar] [CrossRef]
- Yao, D.; Dai, C.; Peng, S. Mechanism of the Mesenchymal-Epithelial Transition and Its Relationship with Metastatic Tumor Formation. Mol Cancer Res. 2011, 9, 1608–1620. [Google Scholar] [CrossRef]
- Datta, A.; Deng, S.; Gopal, V.; Yap, K.C.-H.; Halim, C.E.; Lye, M.L.; Ong, M.S.; Tan, T.Z.; Sethi, G.; Hooi, S.C.; et al. Cytoskeletal Dynamics in Epithelial-Mesenchymal Transition: Insights into Therapeutic Targets for Cancer Metastasis. Cancers 2021, 13, 1882. [Google Scholar] [CrossRef]
- Lin, Y.; Kazlova, V.; Ramakrishnan, S.; Murray, M.A.; Fast, D.; Chandra, A.; Gellenbeck, K.W. Bone health nutraceuticals alter microarray mRNA gene expression: A randomized, parallel, open-label clinical study. Phytomedicine 2016, 23, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, S.; Pal, S.; Mohamed, R.; Singh, P.; Chattopadhyay, S.; Pal China, S.; Porwal, K.; Sanyal, S.; Gayen, J.R.; Chattopadhyay, N. A nutraceutical composition containing diosmin and hesperidin has osteogenic and anti-resorptive effects and expands the anabolic window of teriparatide. Biomed. Pharmacother. 2019, 118, 109207. [Google Scholar] [CrossRef] [PubMed]
- Seely, K.D.; Kotelko, C.A.; Douglas, H.; Bealer, B.; Brooks, A.E. The Human Gut Microbiota: A Key Mediator of Osteoporosis and Osteogenesis. Int. J. Mol. Sci. 2021, 22, 9452. [Google Scholar] [CrossRef] [PubMed]
- Rajput, R.; Wairkar, S.; Gaud, R. Nutraceuticals for better management of osteoporosis: An overview. J. Funct. Foods 2018, 47, 480–490. [Google Scholar] [CrossRef]
- Ratner, B.D.; Hoffman, A.S.; Schoen, F.J.; Lemons, J. Introduction—Biomaterials Science: A Multidisciplinary Endeavor. Biomater. Sci. 2004, 20. [Google Scholar] [CrossRef]
- Kumar, S.; Nehra, M.; Kedia, D.; Dilbaghi, N.; Tankeshwar, K.; Kim, K.-H. Nanotechnology-based biomaterials for orthopaedic applications: Recent advances and future prospects. Mater. Sci. Eng. C 2020, 106, 1–25. [Google Scholar] [CrossRef]
- Pillai, S.C.; Borah, A.; Jacob, E.M.; Kumar, D.S. Nanotechnological approach to delivering nutraceuticals as promising drug candidates for the treatment of atherosclerosis. Drug Deliv. 2021, 28, 550–568. [Google Scholar] [CrossRef]
- Lidia, A.-V.; Carlos, Z.-M.; Alicia, R.-M.; Amalia, V.; Jose, V.-B. Nutraceuticals: Definition, applied nanoengineering in their production and applications. Int. J. Biosens. Bioelectron. 2019, 5, 56–61. [Google Scholar] [CrossRef]
- Favaro-Trindade, C.S.; de Matos Junior, F.E.; Okuro, P.K.; Dias-Ferreira, J.; Cano, A.; Severino, P.; Zielińska, A.; Souto, E.B. Encapsulation of Active Pharmaceutical Ingredients in Lipid Micro/Nanoparticles for Oral Administration by Spray-Cooling. Pharmaceutics 2021, 13, 1186. [Google Scholar] [CrossRef]
- Augustin, M.A.; Sanguansri, L. Challenges and Solutions to Incorporation of Nutraceuticals in Foods. Annu. Rev. Food Sci. Technol. 2015, 6, 463–477. [Google Scholar] [CrossRef]
- Reque, P.M.; Brandelli, A. Encapsulation of probiotics and nutraceuticals: Applications in functional food industry. Trends Food Sci. Technol. 2021, 114, 1–10. [Google Scholar] [CrossRef]
- Flores, F.P.; Kong, F. In Vitro Release Kinetics of Microencapsulated Materials and the Effect of the Food Matrix. Annu. Rev. Food Sci. Technol. 2017, 8, 237–259. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Yeo, Y. Controlled drug release from pharmaceutical nanocarriers. Chem. Eng. Sci. 2015, 125, 75–84. [Google Scholar] [CrossRef]
- Savjani, K.T.; Gajjar, A.K.; Savjani, J.K. Drug Solubility: Importance and Enhancement Techniques. ISRN Pharm. 2012, 2012, 195727. [Google Scholar] [CrossRef] [PubMed]
- Williams, H.D.; Trevaskis, N.L.; Charman, S.A.; Shanker, R.M.; Charman, W.N.; Pouton, C.W.; Porter, C.J.H. Strategies to address low drug solubility in discovery and development. Pharmacol. Rev. 2013, 65, 315–499. [Google Scholar] [CrossRef]
- Porter, C.J.H.; Trevaskis, N.L.; Charman, W.N. Lipids and lipid-based formulations: Optimizing the oral delivery of lipophilic drugs. Nat. Rev. Drug Discov. 2007, 6, 231–249. [Google Scholar] [CrossRef]
- Tambosi, G.; Coelho, P.F.; Soares, L.; Lenschow, I.C.S.; Zétola, M.; Stulzer, H.K.; Pezzini, B.R. Challenges to improve the biopharmaceutical properties of poorly water-soluble drugs and the application of the solid dispersion technology. Rev. Mater. 2018, 23. [Google Scholar] [CrossRef]
- Coltescu, A.-R.; Butnariu, M.; Sarac, I. The Importance of Solubility for New Drug Molecules. Biomed. Pharmacol. J. 2020, 13, 577–583. [Google Scholar] [CrossRef]
- Dima, C.; Assadpour, E.; Dima, S.; Jafari, S.M. Bioavailability and bioaccessibility of food bioactive compounds; overview and assessment by in vitro methods. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2862–2884. [Google Scholar] [CrossRef]
- Al-Obaidi, J.R.; Alobaidi, K.H.; Al-Taie, B.S.; Wee, D.H.S.; Hussain, H.; Jambari, N.N.; Ahmad-Kamil, E.I.; Ariffin, N.S. Uncovering Prospective Role and Applications of Existing and New Nutraceuticals from Bacterial, Fungal, Algal and Cyanobacterial, and Plant Sources. Sustainability 2021, 13, 3671. [Google Scholar] [CrossRef]
- Pressman, P.; Clemens, R.A.; Hayes, A.W. Bioavailability of micronutrients obtained from supplements and food: A survey and case study of the polyphenols. Toxicol. Res. Appl. 2017, 1, 1–7. [Google Scholar] [CrossRef]
- Sietsema, W.K. The absolute oral bioavailability of selected drugs. Int. J. Clin. Pharmacol. Ther. Toxicol. 1989, 27, 179–211. [Google Scholar] [PubMed]
- Dima, C.; Assadpour, E.; Dima, S.; Jafari, S.M. Bioavailability of nutraceuticals: Role of the food matrix, processing conditions, the gastrointestinal tract, and nanodelivery systems. Compr. Rev. Food Sci. Food Saf. 2020, 19, 954–994. [Google Scholar] [CrossRef] [PubMed]
- Ting, Y.; Jiang, Y.; Ho, C.-T.; Huang, Q. Common delivery systems for enhancing in vivo bioavailability and biological efficacy of nutraceuticals. J. Funct. Foods 2014, 7, 112–128. [Google Scholar] [CrossRef]
- Zare, M.; Dziemidowicz, K.; Williams, G.R.; Ramakrishna, S. Encapsulation of Pharmaceutical and Nutraceutical Active Ingredients Using Electrospinning Processes. Nanomaterials 2021, 11, 1968. [Google Scholar] [CrossRef]
- Subramanian, P. Lipid-Based Nanocarrier System for the Effective Delivery of Nutraceuticals. Molecules 2021, 26, 5510. [Google Scholar] [CrossRef]
- McClements, D.J.; Decker, E.A.; Park, Y.; Weiss, J. Structural Design Principles for Delivery of Bioactive Components in Nutraceuticals and Functional Foods. Crit. Rev. Food Sci. Nutr. 2009, 49, 577–606. [Google Scholar] [CrossRef]
- Gheorghita, R.; Anchidin-Norocel, L.; Filip, R.; Dimian, M.; Covasa, M. Applications of Biopolymers for Drugs and Probiotics Delivery. Polymers 2021, 13, 2729. [Google Scholar] [CrossRef]
- Hu, B.; Huang, Q.-R. Biopolymer Based Nano-Delivery Systems for Enhancing Bioavailability of Nutraceuticals. Chinese J. Polym. Sci. 2013, 31, 1190–1203. [Google Scholar] [CrossRef]
- Malafaya, P.B.; Silva, G.A.; Reis, R.L. Natural-origin polymers as carriers and scaffolds for biomolecules and cell delivery in tissue engineering applications. Adv. Drug Deliv. Rev. 2007, 59, 207–233. [Google Scholar] [CrossRef]
- Torres, F.G.; Troncoso, O.P.; Pisani, A.; Gatto, F.; Bardi, G. Natural Polysaccharide Nanomaterials: An Overview of Their Immunological Properties. Int. J. Mol. Sci. 2019, 20, 5092. [Google Scholar] [CrossRef] [PubMed]
- Jacob, J.; Haponiuk, J.T.; Thomas, S.; Gopi, S. Biopolymer based nanomaterials in drug delivery systems: A review. Mater. Today Chem. 2018, 9, 43–55. [Google Scholar] [CrossRef]
- Gopinath, V.; Saravanan, S.; Al-Maleki, A.R.; Ramesh, M.; Vadivelu, J. A review of natural polysaccharides for drug delivery applications: Special focus on cellulose, starch and glycogen. Biomed. Pharmacother. 2018, 107, 96–108. [Google Scholar] [CrossRef] [PubMed]
- Hosseinpourpia, R.; Echart, A.S.; Adamopoulos, S.; Gabilondo, N.; Eceiza, A. Modification of Pea Starch and Dextrin Polymers with Isocyanate Functional Groups. Polymers 2018, 10, 939. [Google Scholar] [CrossRef]
- Alcázar-Alay, S.C.; Meireles, M.A.A. Physicochemical properties, modifications and applications of starches from different botanical sources. Food Sci. Technol. 2015, 35, 215–236. [Google Scholar] [CrossRef]
- Beneke, C.E.; Viljoen, A.M.; Hamman, J.H. Polymeric Plant-derived Excipients in Drug Delivery. Molecules 2009, 14, 2602–2620. [Google Scholar] [CrossRef]
- Serrero, A.; Trombotto, S.; Cassagnau, P.; Bayon, Y.; Gravagna, P.; Montanari, S.; David, L. Polysaccharide Gels Based on Chitosan and Modified Starch: Structural Characterization and Linear Viscoelastic Behavior. Biomacromolecules 2010, 11, 1534–1543. [Google Scholar] [CrossRef]
- Van Der Maarel, M.J.E.C.; Van Der Veen, B.; Uitdehaag, J.C.M.; Leemhuis, H.; Dijkhuizen, L. Properties and applications of starch-converting enzymes of the α-amylase family. J. Biotechnol. 2002, 94, 137–155. [Google Scholar] [CrossRef]
- Carvalho, J.; Gonçalves, C.; Gil, A.M.; Gama, F.M. Production and characterization of a new dextrin based hydrogel. Eur. Polym. J. 2007, 43, 3050–3059. [Google Scholar] [CrossRef]
- Gonçalves, C.; Moreira, S.M.; Carvalho, V.; Silva, D.M.; Gama, M. Dextrin. Encycl. Biomed. Polym. Polym. Biomater. 2016, 2634–2649. [Google Scholar] [CrossRef]
- Silva, D.M.; Nunes, C.; Pereira, I.; Moreira, A.S.P.; Domingues, M.R.M.; Coimbra, M.A.; Gama, F.M. Structural analysis of dextrins and characterization of dextrin-based biomedical hydrogels. Carbohydr. Polym. 2014, 114, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Klein, S. Polysaccharides in Oral Drug Delivery—Recent Applications and Future Perspectives. In ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2009; pp. 13–30. ISBN 9780841269866. [Google Scholar]
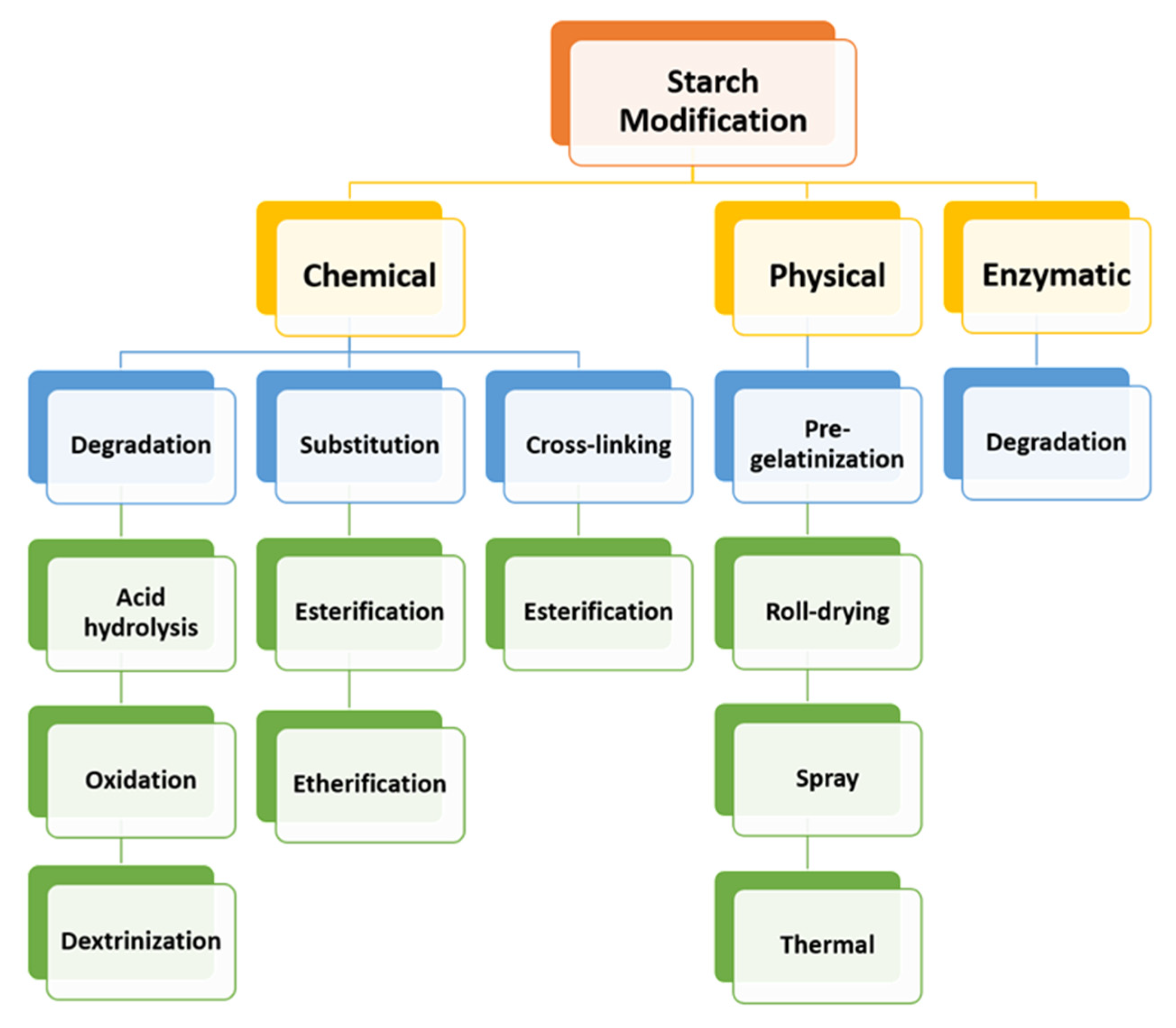
- Lewicka, K.; Siemion, P.; Kurcok, P. Chemical Modifications of Starch: Microwave Effect. Int. J. Polym. Sci. 2015, 2015, 867697. [Google Scholar] [CrossRef]
- Haq, F.; Yu, H.; Wang, L.; Teng, L.; Haroon, M.; Khan, R.U.; Mehmood, S.; Bilal-Ul-Amin; Ullah, R.S.; Khan, A.; et al. Advances in chemical modifications of starches and their applications. Carbohydr. Res. 2019, 476, 12–35. [Google Scholar] [CrossRef] [PubMed]
- Mailänder, V.; Landfester, K. Interaction of Nanoparticles with Cells. Biomacromolecules 2009, 10, 2379–2400. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Li, Q.; Mo, J.; Dai, H. Drug-Loaded Polymeric Nanoparticles for Cancer Stem Cell Targeting. Front. Pharmacol. 2017, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 1. [Google Scholar] [CrossRef]
- Lin, J.; Miao, L.; Zhong, G.; Lin, C.H.; Dargazangy, R.; Alexander-Katz, A. Understanding the synergistic effect of physicochemical properties of nanoparticles and their cellular entry pathways. Commun. Biol. 2020, 3, 205. [Google Scholar] [CrossRef]
- Brewster, M.E.; Loftsson, T. Cyclodextrins as pharmaceutical solubilizers. Adv. Drug Deliv. Rev. 2007, 59, 645–666. [Google Scholar] [CrossRef]
- Osmani, R.A.; Kulkarni, P.; Manjunatha, S.; Gowda, V.; Hani, U.; Vaghela, R.; Bhosale, R. Chapter 9 Cyclodextrin Nanosponges in Drug Delivery and Nanotherapeutics. In Environmental Nanotechnology; Dasgupta, N., Ranjan, S., Lichtfouse, E., Eds.; Springer: Berlin/Heidelberg, Germany, 2018; p. 405. ISBN 978-3-319-76090-2. [Google Scholar]
- Sherje, A.P.; Dravyakar, B.R. Cyclodextrin-based nanosponges: A critical review. Carbohydr. Polym. 2017, 173, 37–49. [Google Scholar] [CrossRef]
- Martin, J.; Díaz-Montaña, E.J.; Asuero, A.G. Cyclodextrins: Past and Present; IntechOpen: London, UK, 2008. [Google Scholar] [CrossRef]
- Liu, L.; Guo, Q.X. The driving forces in the inclusion complexation of cyclodextrins. J. Incl. Phenom. 2002, 42, 1–14. [Google Scholar] [CrossRef]
- Matencio, A.; Navarro-Orcajada, S.; García-Carmona, F.; López-Nicolás, J.M. Applications of cyclodextrins in food science. A review. Trends Food Sci. Technol. 2020, 104, 132–143. [Google Scholar] [CrossRef]
- Stella, V.J.; Rajewski, R.A. Cyclodextrins: Their Future in Drug Formulation and Delivery. Pharm. Res. 1997, 14N, 11. [Google Scholar] [CrossRef]
- Rousseau, J.; Menuel, S.; Rousseau, C.; Hapiot, F.; Monflier, E. Cyclodextrins as Porous Material for Catalysis; Elsevier Inc.: Amsterdam, The Netherlands, 2016; ISBN 9780128018101. [Google Scholar]
- Hedges, A.R. Industrial applications of cyclodextrins. Chem. Rev. 1998, 98, 2035–2044. [Google Scholar] [CrossRef] [PubMed]
- Szejtli, J. Past, present, and future of cyclodextrin research. Pure Appl. Chem. 2004, 76, 1825–1845. [Google Scholar] [CrossRef]
- Jambhekar, S.S.; Breen, P. Cyclodextrins in pharmaceutical formulations I: Structure and physicochemical properties, formation of complexes, and types of complex. Drug Discov. Today 2016, 21, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Bekers, O.; Uijtendaal, E.V.; Beijnen, J.H.; Bult, A.; Underberg, W.J.M. Cyclodextrins in the pharmaceutical Field. Drug Dev. Ind. Pharm. 1991, 17, 1503–1549. [Google Scholar] [CrossRef]
- Chilajwar, S.V.; Pednekar, P.P.; Jadhav, K.R.; Gupta, G.J.; Kadam, V.J. Cyclodextrin-based nanosponges: A propitious platform for enhancing drug delivery. Expert Opin. Drug Deliv. 2014, 11, 111–120. [Google Scholar] [CrossRef]
- Berto, S.; Bruzzoniti, M.C.; Cavalli, R.; Perrachon, D.; Prenesti, E.; Sarzanini, C.; Trotta, F.; Tumiatti, W. Synthesis of new ionic β-cyclodextrin polymers and characterization of their heavy metals retention. J. Incl. Phenom. Macrocycl. Chem. 2007, 57, 631–636. [Google Scholar] [CrossRef]
- Zhang, D.; Lv, P.; Zhou, C.; Zhao, Y.; Liao, X.; Yang, B. Cyclodextrin-based delivery systems for cancer treatment. Mater. Sci. Eng. C 2019, 96, 872–886. [Google Scholar] [CrossRef]
- Swaminathan, S.; Vavia, P.R.; Trotta, F.; Cavalli, R. Nanosponges encapsulating dexamethasone for ocular delivery: Formulation design, physicochemical characterization, safety and corneal permeability assessment. J. Biomed. Nanotechnol. 2013, 9, 998–1007. [Google Scholar] [CrossRef]
- Mognetti, B.; Barberis, A.; Marino, S.; Berta, G.; De Francia, S.; Trotta, F.; Cavalli, R. In vitro enhancement of anticancer activity of paclitaxel by a Cremophor free cyclodextrin-based nanosponge formulation. J. Incl. Phenom. Macrocycl. Chem. 2012, 74, 201–210. [Google Scholar] [CrossRef]
- Ferro, M.; Castiglione, F.; Punta, C.; Melone, L.; Panzeri, W.; Rossi, B.; Trotta, F.; Mele, A. Anomalous diffusion of ibuprofen in cyclodextrin nanosponge hydrogels: An HRMAS NMR study. Beilstein J. Org. Chem. 2014, 10, 2715–2723. [Google Scholar] [CrossRef] [PubMed]
- Trotta, F.; Cavalli, R. Characterization and applications of new hyper-cross-linked cyclodextrins. Compos. Interfaces 2009, 16, 39–48. [Google Scholar] [CrossRef]
- Santos, A.C.; Costa, D.; Ferreira, L.; Guerra, C.; Pereira-Silva, M.; Pereira, I.; Peixoto, D.; Ferreira, N.R.; Veiga, F. Cyclodextrin-based delivery systems for in vivo-tested anticancer therapies. Drug Deliv. Transl. Res. 2021, 11, 49–71. [Google Scholar] [CrossRef]
- Petitjean, M.; García-Zubiri, I.X.; Isasi, J.R. History of cyclodextrin-based polymers in food and pharmacy: A review. Environ. Chem. Lett. 2021, 19, 3465–3476. [Google Scholar] [CrossRef]
- Gambini, J.; Inglès, M.; Olaso, G.; Lopez-Grueso, R.; Bonet-Costa, V.; Gimeno-Mallench, L.; Mas-Bargues, C.; Abdelaziz, K.M.; Gomez-Cabrera, M.C.; Vina, J.; et al. Properties of Resveratrol: In Vitro and In Vivo Studies about Metabolism, Bioavailability, and Biological Effects in Animal Models and Humans. Oxid. Med. Cell. Longev. 2015, 2015, 837042. [Google Scholar] [CrossRef]
- Gandhi, S.R.; Quintans, J.D.S.S.; Gandhi, G.R.; Araujo, A.A.D.S.; Junior, L.J.Q. The use of cyclodextrin inclusion complexes to improve anticancer drug profiles: A systematic review. Expert Opin. Drug Deliv. 2020, 17, 1069–1080. [Google Scholar] [CrossRef]
- Hoti, G.; Appleton, S.L.; Rubin Pedrazzo, A.; Cecone, C.; Matencio, A.; Trotta, F.; Caldera, F. Strategies to Develop Cyclodextrin-Based Nanosponges for Smart Drug Delivery. In Smart Drug Delivery; IntechOpen: London, UK, 2021; pp. 1–22. [Google Scholar] [CrossRef]
- Caldera, F.; Tannous, M.; Cavalli, R.; Zanetti, M.; Trotta, F. Evolution of Cyclodextrin Nanosponges. Int. J. Pharm. 2017, 531, 470–479. [Google Scholar] [CrossRef]
- Thatiparti, T.R.; Von Recum, H.A. Cyclodextrin Complexation for Affinity-Based Antibiotic Delivery. Macromol. Biosci. 2010, 10, 82–90. [Google Scholar] [CrossRef]
- Merritt, S.R.; Velasquez, G.; Von Recum, H.A. Adjustable release of mitomycin C for inhibition of scar tissue formation after filtration surgery. Exp. Eye Res. 2013, 116, 9–16. [Google Scholar] [CrossRef]
- Jullian, C.; Moyano, L.; Yañez, C.; Olea-Azar, C. Complexation of quercetin with three kinds of cyclodextrins: An antioxidant study. Spectrochim. Acta-Part A 2007, 67, 230–234. [Google Scholar] [CrossRef] [PubMed]
- Dhakar, N.K.; Matencio, A.; Caldera, F.; Argenziano, M.; Cavalli, R.; Dianzani, C.; Zanetti, M.; López-Nicolás, J.M.; Trotta, F. Comparative Evaluation of Solubility, Cytotoxicity and Photostability Studies of Resveratrol and Oxyresveratrol Loaded Nanosponges. Pharmaceutics 2019, 11, 545. [Google Scholar] [CrossRef] [PubMed]
- Matencio, A.; Dhakar, N.K.; Bessone, F.; Musso, G.; Cavalli, R.; Dianzani, C.; García-Carmona, F.; López-Nicolás, J.M.; Trotta, F. Study of oxyresveratrol complexes with insoluble cyclodextrin based nanosponges: Developing a novel way to obtain their complexation constants and application in an anticancer study. Carbohydr. Polym. 2020, 231, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Mendes, C.; Meirelles, G.C.; Barp, C.G.; Assreuy, J.; Silva, M.A.S.; Ponchel, G. Cyclodextrin based nanosponge of norfloxacin: Intestinal permeation enhancement and improved antibacterial activity. Carbohydr. Polym. 2018, 195, 586–592. [Google Scholar] [CrossRef]
- Dhakar, N.K.; Caldera, F.; Bessone, F.; Cecone, C.; Pedrazzo, A.R.; Cavalli, R.; Dianzani, C.; Trotta, F. Evaluation of solubility enhancement, antioxidant activity, and cytotoxicity studies of kynurenic acid loaded cyclodextrin nanosponge. Carbohydr. Polym. 2019, 224, 1–9. [Google Scholar] [CrossRef]
- Torne, S.; Darandale, S.; Vavia, P.; Trotta, F.; Cavalli, R. Cyclodextrin-based nanosponges: Effective nanocarrier for Tamoxifen delivery. Pharm. Dev. Technol. 2013, 18, 619–625. [Google Scholar] [CrossRef]
- Zainuddin, R.; Zaheer, Z.; Sangshetti, J.N.; Momin, M. Enhancement of oral bioavailability of anti- HIV drug rilpivirine HCl through nanosponge formulation. Drug Dev. Ind. Pharm. 2017, 43, 2076–2084. [Google Scholar] [CrossRef]
- Lembo, D.; Swaminathan, S.; Donalisio, M.; Civra, A.; Pastero, L.; Aquilano, D.; Vavia, P.; Trotta, F.; Cavalli, R. Encapsulation of Acyclovir in new carboxylated cyclodextrin-based nanosponges improves the agent’s antiviral efficacy. Int. J. Pharm. 2013, 443, 262–272. [Google Scholar] [CrossRef]
- Swaminathan, S.; Pastero, L.; Serpe, L.; Trotta, F.; Vavia, P.; Aquilano, D.; Trotta, M.; Zara, G.; Cavalli, R. Cyclodextrin-based nanosponges encapsulating camptothecin: Physicochemical characterization, stability and cytotoxicity. Eur. J. Pharm. Biopharm. 2010, 74, 193–201. [Google Scholar] [CrossRef]
- Rao, M.; Bajaj, A.; Khole, I.; Munjapara, G.; Trotta, F. In vitro and in vivo evaluation of β-cyclodextrin-based nanosponges of telmisartan. J. Incl. Phenom. Macrocycl. Chem. 2013, 77, 135–145. [Google Scholar] [CrossRef]
- Kumar, S.; Pooja; Trotta, F.; Rao, R. Encapsulation of Babchi Oil in Cyclodextrin-Based Nanosponges: Physicochemical Characterization, Photodegradation, and In Vitro Cytotoxicity Studies. Pharmaceutics 2018, 10, 169. [Google Scholar] [CrossRef] [PubMed]
- Sundararajan, M.; Thomas, P.A.; Venkadeswaran, K.; Jeganathan, K.; Geraldine, P. Synthesis and characterization of chrysin-loaded β-cyclodextrin-based nanosponges to enhance in-vitro solubility, photostability, drug release, antioxidant effects and antitumorous efficacy. J. Nanosci. Nanotechnol. 2017, 17, 8742–8751. [Google Scholar] [CrossRef]
- Yaşayan, G.; Şatıroğlu Sert, B.; Tatar, E.; Küçükgüzel, İ. Fabrication and characterisation studies of cyclodextrin-based nanosponges for sulfamethoxazole delivery. J. Incl. Phenom. Macrocycl. Chem. 2020, 97, 175–186. [Google Scholar] [CrossRef]
- Rezaei, A.; Varshosaz, J.; Fesharaki, M.; Farhang, A.; Jafari, S.M. Improving the solubility and in vitro cytotoxicity (anticancer activity) of ferulic acid by loading it into cyclodextrin nanosponges. Int. J. Nanomed. 2019, 14, 4589–4599. [Google Scholar] [CrossRef]
- Salehi, O.; Masoud, S.; Rezaei, A. Limonene loaded cyclodextrin nanosponge: Preparation, characterization, antibacterial activity and controlled release. Food Biosci. 2021, 42, 1–9. [Google Scholar] [CrossRef]
- Kumar, A.; Rao, R. Enhancing efficacy and safety of azelaic acid via encapsulation in cyclodextrin nanosponges: Development, characterization and evaluation. Polym. Bull. 2021, 78, 5275–5302. [Google Scholar] [CrossRef]
- Sherje, A.P.; Surve, A.; Shende, P. CDI cross-linked β-cyclodextrin nanosponges of paliperidone: Synthesis and physicochemical characterization. J. Mater. Sci. Mater. Med. 2019, 30, 1–7. [Google Scholar] [CrossRef]
- Omar, S.M.; Ibrahim, F.; Ismail, A. Formulation and evaluation of cyclodextrin-based nanosponges of griseofulvin as pediatric oral liquid dosage form for enhancing bioavailability and masking bitter taste. Saudi Pharm. J. 2020, 28, 349–361. [Google Scholar] [CrossRef]
- Allahyari, S.; Esmailnezhad, N.; Valizadeh, H.; Ghorbani, M.; Jelvehgari, M.; Ghazi, F.; Zakeri-Milani, P. In-vitro characterization and cytotoxicity study of flutamide loaded cyclodextrin nanosponges. J. Drug Deliv. Sci. Technol. 2021, 61, 1–7. [Google Scholar] [CrossRef]
- Srivastava, S.; Mahor, A.; Singh, G.; Bansal, K.; Singh, P.P.; Gupta, R.; Dutt, R.; Alanazi, A.M.; Khan, A.A.; Kesharwani, P. Formulation Development, In Vitro and In Vivo Evaluation of Topical Hydrogel Formulation of Econazole Nitrate-Loaded β-Cyclodextrin Nanosponges. J. Pharm. Sci. 2021, 110, 3702–3714. [Google Scholar] [CrossRef]
- Guineo-Alvarado, J.; Quilaqueo, M.; Hermosilla, J.; González, S.; Medina, C.; Rolleri, A.; Lim, L.-T.; Rubilar, M. Degree of crosslinking in β-cyclodextrin-based nanosponges and their effect on piperine encapsulation. Food Chem. 2021, 340, 128132. [Google Scholar] [CrossRef] [PubMed]
- Machín, R.; Isasi, J.R.; Vélaz, I. β-Cyclodextrin hydrogels as potential drug delivery systems. Carbohydr. Polym. 2012, 87, 2024–2030. [Google Scholar] [CrossRef]
- Cassidy, J.; Berner, B.; Chan, K.; John, V.; Toon, S.; Holt, B.; Rowland, M. Human Transbuccal Absorption of Diclofenac Sodium from a Prototype Hydrogel Delivery Device. Pharm. Res. 1993, 10, 126–129. [Google Scholar] [CrossRef]
- Rodriguez-Tenreiro, C.; Alvarez-Lorenzo, C.; Rodriguez-Perez, A.; Concheiro, A.; Torres-Labandeira, J.J. New Cyclodextrin Hydrogels Cross-Linked with Diglycidylethers with a High Drug Loading and Controlled Release Ability. Pharm. Res. 2006, 23, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Gami, P.; Kundu, D.; Seera, S.D.K.; Banerjee, T. Chemically crosslinked xylan–β-Cyclodextrin hydrogel for the in vitro delivery of curcumin and 5-Fluorouracil. Int. J. Biol. Macromol. 2020, 158, 18–31. [Google Scholar] [CrossRef] [PubMed]
- Lucia Appleton, S.; Tannous, M.; Argenziano, M.; Muntoni, E.; Carolina Rosa, A.; Rossi, D.; Caldera, F.; Scomparin, A.; Trotta, F.; Cavalli, R. Nanosponges as protein delivery systems: Insulin, a case study. Int. J. Pharm. 2020, 590, 1–11. [Google Scholar] [CrossRef]
- Argenziano, M.; Haimhoffer, A.; Bastiancich, C.; Jicsinszky, L.; Caldera, F.; Trotta, F.; Scutera, S.; Alotto, D.; Fumagalli, M.; Musso, T.; et al. In Vitro Enhanced Skin Permeation and Retention of Imiquimod Loaded in β-Cyclodextrin Nanosponge Hydrogel. Pharmaceutics 2019, 11, 138. [Google Scholar] [CrossRef]
- Shende, P.K.; Trotta, F.; Gaud, R.S.; Deshmukh, K.; Cavalli, R.; Biasizzo, M. Influence of different techniques on formulation and comparative characterization of inclusion complexes of ASA with β-cyclodextrin and inclusion complexes of ASA with PMDA cross-linked β-cyclodextrin nanosponges. J. Incl. Phenom. Macrocycl. Chem. 2012, 74, 447–454. [Google Scholar] [CrossRef]
- Argenziano, M.; Gigliotti, C.L.; Clemente, N.; Boggio, E.; Ferrara, B.; Trotta, F.; Pizzimenti, S.; Barrera, G.; Boldorini, R.; Bessone, F.; et al. Improvement in the Anti-Tumor Efficacy of Doxorubicin Nanosponges in In Vitro and in Mice Bearing Breast Tumor Models. Cancers 2020, 12, 162. [Google Scholar] [CrossRef]
- Shende, P.K.; Gaud, R.S.; Bakal, R.; Patil, D. Effect of inclusion complexation of meloxicam with β-cyclodextrin- and β-cyclodextrin-based nanosponges on solubility, in vitro release and stability studies. Colloids Surfaces B Biointerfaces 2015, 136, 105–110. [Google Scholar] [CrossRef]
- Rao, M.R.P.; Chaudhari, J.; Trotta, F.; Caldera, F. Investigation of Cyclodextrin-Based Nanosponges for Solubility and Bioavailability Enhancement of Rilpivirine. AAPS PharmSciTech 2018, 19, 2358–2368. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Ren, X.; Guo, T.; Wu, L.; Shakkya, S.; He, Y.; Wang, C.; Maharjan, A.; Singh, V.; Zhang, J. Biofunctionalization of β-cyclodextrin nanosponges using cholesterol. Carbohydr. Polym. 2018, 190, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Asela, I.; Donoso-González, O.; Yutronic, N.; Sierpe, R. β-Cyclodextrin-Based Nanosponges Functionalized With Drugs and Gold Nanoparticles. Pharmaceutics 2021, 13, 513. [Google Scholar] [CrossRef] [PubMed]
- Daga, M.; de Graaf, I.A.M.; Argenziano, M.; Martinez Barranco, A.S.; Loeck, M.; Al-Adwi, Y.; Angele Cucci, M.; Caldera, F.; Trotta, F.; Barrera, G.; et al. Glutathione-responsive cyclodextrin-nanosponges as drug delivery systems for doxorubicin: Evaluation of toxicity and transport mechanisms in the liver. Toxicol. Vitr. 2020, 65, 1–10. [Google Scholar] [CrossRef]
- Coviello, V.; Sartini, S.; Quattrini, L.; Baraldi, C.; Gamberini, M.C.; La Motta, C. Cyclodextrin-based nanosponges for the targeted delivery of the anti-restenotic agent DB103: A novel opportunity for the local therapy of vessels wall subjected to percutaneous intervention. Eur. J. Pharm. Biopharm. 2017, 117, 276–285. [Google Scholar] [CrossRef]
- Deshmukh, K.; Tanwar, Y.S.; Sharma, S.; Shende, P.; Cavalli, R. Functionalized nanosponges for controlled antibacterial and antihypocalcemic actions. Biomed. Pharmacother. 2016, 84, 485–494. [Google Scholar] [CrossRef]
- Deshmukh, K.; Tanwar, Y.S.; Shende, P.; Cavalli, R. Biomimetic estimation of glucose using non-molecular and molecular imprinted polymer nanosponges. Int. J. Pharm. 2015, 494, 244–248. [Google Scholar] [CrossRef]
- Trotta, F.; Caldera, F.; Cavalli, R.; Soster, M.; Riedo, C.; Biasizzo, M.; Uccello Barretta, G.; Balzano, F.; Brunella, V. Molecularly imprinted cyclodextrin nanosponges for the controlled delivery of L-DOPA: Perspectives for the treatment of Parkinson’s disease. Expert Opin. Drug Deliv. 2016, 13, 1671–1680. [Google Scholar] [CrossRef]
- Cecone, C.; Hoti, G.; Krabicova, I.; Appleton, S.L.; Caldera, F.; Bracco, P.; Zanetti, M.; Trotta, F. Sustainable synthesis of cyclodextrin-based polymers exploiting natural deep eutectic solvents. Green Chem. 2020, 22, 5806–5814. [Google Scholar] [CrossRef]
- Pedrazzo, A.R.; Caldera, F.; Zanetti, M.; Appleton, S.L.; Dahkar, N.K.; Trotta, F. Mechanochemical green synthesis of hyper-crosslinked cyclodextrin polymers. Beilstein J. Org. Chem. 2020, 16, 1554–1563. [Google Scholar] [CrossRef]
- Tannous, M.; Trotta, F.; Cavalli, R. Nanosponges for combination drug therapy: State-of-the-art and future directions. Nanomedicine 2020, 15, 643–646. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-J.; Wang, L. Structures and Properties of Commercial Maltodextrins from Corn, Potato, and Rice Starches. Starch/Stärke 2000, 52, 296–304. [Google Scholar] [CrossRef]
- Guntero, V.A.; Peralta, M.; Noriega, P.; Kneeteman, M.N.; Ferretti, C.A. One-Pot Selective Functionalization of Polysaccharides with Urea. Chem. Proc. 2021, 3, 2–6. [Google Scholar]
- Siemons, I.; Politiek, R.G.A.; Boom, R.M.; Van der Sman, R.G.M.; Schutyser, M.A.I. Dextrose equivalence of maltodextrins determines particle morphology development during single sessile droplet drying. Food Res. Int. 2020, 131, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Barthold, S.; Hittinger, M.; Primavessy, D.; Zapp, A.; Groß, H.; Schneider, M. Preparation of maltodextrin nanoparticles and encapsulation of bovine serum albumin—Influence of formulation parameters. Eur. J. Pharm. Biopharm. 2019, 142, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Helal, H.M.; Samy, W.M.; El-Fakharany, E.M.; Kamoun, E.; Mortada, S.M.; Sallam, M.A. Maltodextrin-α-tocopherol conjugates of vitamin E: Influence of degree of derivatization on physicochemical properties and biological evaluation. J. Drug Deliv. Sci. Technol. 2020, 60, 1–12. [Google Scholar] [CrossRef]
- Hofman, D.L.; van Buul, V.J.; Brouns, F.J.P.H. Nutrition, Health, and Regulatory Aspects of Digestible Maltodextrins. Crit. Rev. Food Sci. Nutr. 2016, 56, 2091–2100. [Google Scholar] [CrossRef]
- Rezende, G.; Hashizume, L.N. Maltodextrin and dental caries: A literature review. Rev. Gaúch Odontol. 2018, 66, 257–262. [Google Scholar] [CrossRef]
- Sun, X.; Wu, X.; Chen, X.; Guo, R.; Kou, Y.; Li, X.; Sheng, Y.; Wu, Y. Casein-maltodextrin Maillard conjugates encapsulation enhances the antioxidative potential of proanthocyanidins: An in vitro and in vivo evaluation. Food Chem. 2021, 346, 1–8. [Google Scholar] [CrossRef]
- Okumuş, E.; Bakkalbaşı, E.; Javidipour, I.; Meral, R.; Ceylan, Z. A novel coating material: Ellagitannins-loaded maltodextrin and lecithin-based nanomaterials. Food Biosci. 2021, 42, 1–7. [Google Scholar] [CrossRef]
- Gurturk, Z.; Tezcaner, A.; Dalgic, A.D.; Korkmaz, S.; Keskin, D. Maltodextrin modified liposome for drug delivery through blood-brain barrier. Medchemcomm 2017, 8, 1337–1345. [Google Scholar] [CrossRef] [PubMed]
- Lai, F.; Franceschini, I.; Corrias, F.; Sala, M.C.; Cilurzo, F.; Sinico, C.; Pini, E. Maltodextrin fast dissolving films for quercetin nanocrystal delivery. A feasibility study. Carbohydr. Polym. 2015, 121, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Blazek-Welsh, A.I.; Rhodes, D.G. SEM Imaging Predicts Quality of Niosomes from Maltodextrin-Based Proniosomes. Pharm. Res. 2001, 18, 656–661. [Google Scholar] [CrossRef] [PubMed]
- Shruthi, P.A.; Pushpadass, H.A.; Franklin, M.E.E.; Battula, S.N.; Naik, N.L. Resveratrol-loaded proniosomes: Formulation, characterization and fortification. LWT-Food Sci. Technol. 2020, 134, 1–12. [Google Scholar] [CrossRef]
- Loftsson, T.; Duchêne, D. Cyclodextrins and their pharmaceutical applications. Int. J. Pharm. 2007, 329, 1–11. [Google Scholar] [CrossRef]
- Castro-Cabado, M.; Casado, A.L.; San Román, J. Bio-based thermosets: Effect of the structure of polycarboxylic acids on the thermal crosslinking of maltodextrins. Eur. Polym. J. 2016, 78, 91–105. [Google Scholar] [CrossRef]
- Cecone, C.; Costamagna, G.; Ginepro, M.; Trotta, F. One-step sustainable synthesis of cationic high-swelling polymers obtained from starch-derived maltodextrins. RSC Adv. 2021, 11, 7653–7662. [Google Scholar] [CrossRef]
- Meléndez-Ortiz, H.I.; Betancourt-Galindo, R.; Puente-Urbina, B.; Ledezma, A.; Rodríguez-Fernández, O. Synthesis and characterization of hydrogels based on maltodextrins with antimicrobial properties. Int. J. Polym. Mater. Polym. Biomater. 2021, 1–10. [Google Scholar] [CrossRef]
- Yan, S.; Ren, J.; Jian, Y.; Wang, W.; Yun, W.; Yin, J. Injectable Maltodextrin-Based Micelle/Hydrogel Composites for Simvastatin-Controlled Release. Biomacromolecules 2018, 19, 4554–4564. [Google Scholar] [CrossRef]
- Demasi, S.; Caser, M.; Caldera, F.; Dhakar, N.K.; Vidotto, F.; Trotta, F.; Scariot, V. Functionalized dextrin-based nanosponges as effective carriers for the herbicide ailanthone. Ind. Crops Prod. 2021, 164, 113346. [Google Scholar] [CrossRef]
- Pedrazzo, A.R.; Smarra, A.; Caldera, F.; Musso, G.; Dhakar, N.K.; Cecone, C.; Hamedi, A.; Corsi, I.; Trotta, F. Eco-Friendly β-cyclodextrin and Linecaps Polymers for the Removal of Heavy Metals. Polymers 2019, 11, 1658. [Google Scholar] [CrossRef] [PubMed]

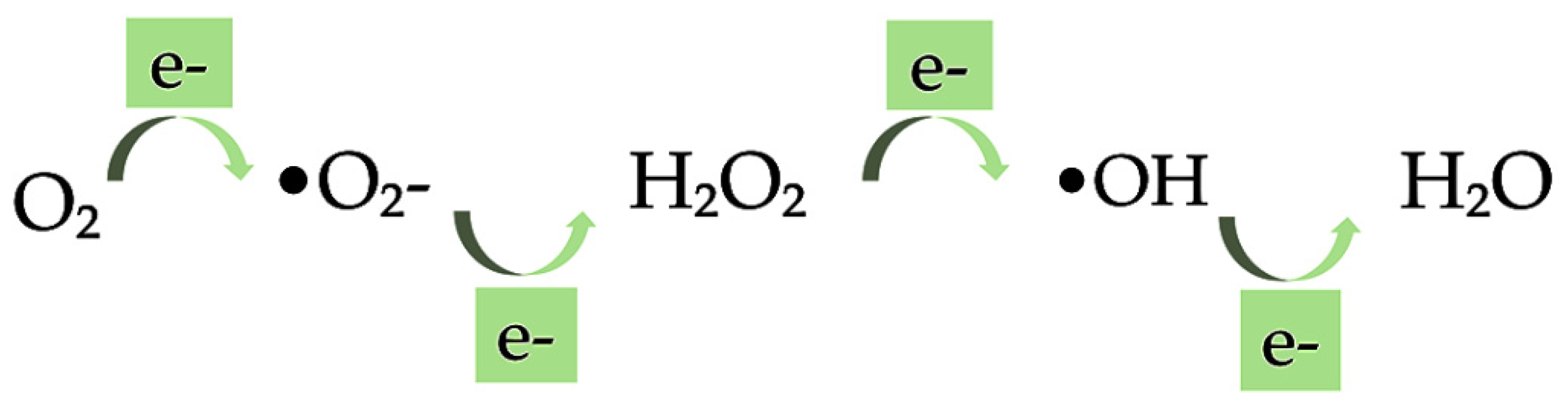


| 1O2 | + | β-carotene |  | O2 | + | β-carotene * |
| β-carotene * |  | β-carotene | + | energy (heat) | ||
| β-carotene * |  | all-trans-β-carotene | ||||
| Vitamin E | + | RO |  | Vitamin E | + | ROH |
| 2 Vitamin E | + | ascorbate |  | 2 Vitamin E | + | dehydroascorbate |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoti, G.; Matencio, A.; Rubin Pedrazzo, A.; Cecone, C.; Appleton, S.L.; Khazaei Monfared, Y.; Caldera, F.; Trotta, F. Nutraceutical Concepts and Dextrin-Based Delivery Systems. Int. J. Mol. Sci. 2022, 23, 4102. https://doi.org/10.3390/ijms23084102
Hoti G, Matencio A, Rubin Pedrazzo A, Cecone C, Appleton SL, Khazaei Monfared Y, Caldera F, Trotta F. Nutraceutical Concepts and Dextrin-Based Delivery Systems. International Journal of Molecular Sciences. 2022; 23(8):4102. https://doi.org/10.3390/ijms23084102
Chicago/Turabian StyleHoti, Gjylije, Adrián Matencio, Alberto Rubin Pedrazzo, Claudio Cecone, Silvia Lucia Appleton, Yousef Khazaei Monfared, Fabrizio Caldera, and Francesco Trotta. 2022. "Nutraceutical Concepts and Dextrin-Based Delivery Systems" International Journal of Molecular Sciences 23, no. 8: 4102. https://doi.org/10.3390/ijms23084102
APA StyleHoti, G., Matencio, A., Rubin Pedrazzo, A., Cecone, C., Appleton, S. L., Khazaei Monfared, Y., Caldera, F., & Trotta, F. (2022). Nutraceutical Concepts and Dextrin-Based Delivery Systems. International Journal of Molecular Sciences, 23(8), 4102. https://doi.org/10.3390/ijms23084102











