Type XX Collagen Is Elevated in Circulation of Patients with Solid Tumors
Abstract
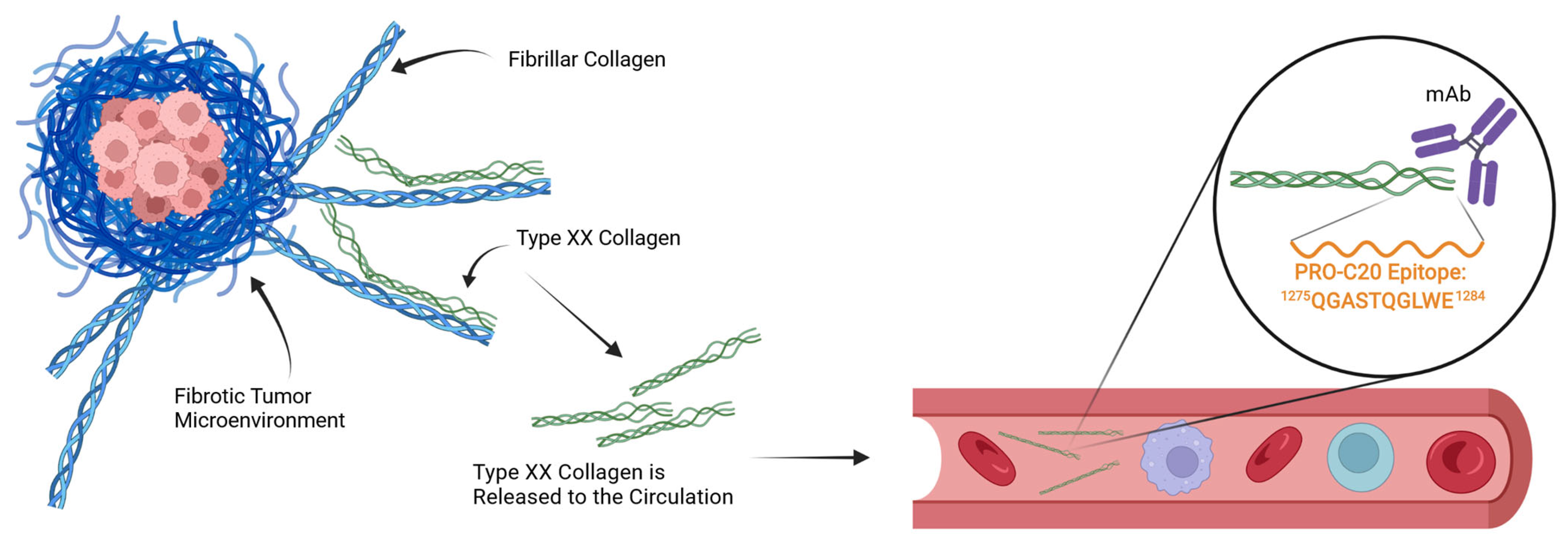
:1. Introduction
2. Results
2.1. PRO-C20 ELISA Development and Validation
2.2. PRO-C20 in Serum of Patients with Solid Cancers
2.3. PRO-C20 in Serum of Patients with PDAC
3. Discussion
4. Materials and Methods
4.1. Generation of Monoclonal Antibodies Targeting the PRO-C20 Epitope
4.2. PRO-C20 ELISA Protocol
4.3. Technical Validation of the PRO-C20 ELISA
4.4. PRO-C1 ELISA Protocol
4.5. Patient Samples
4.6. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Marrugo-Ramírez, J.; Mir, M.; Samitier, J. Blood-Based Cancer Biomarkers in Liquid Biopsy: A Promising Non-Invasive Alternative to Tissue Biopsy. Int. J. Mol. Sci. 2018, 19, 2877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alix-Panabières, C.; Pantel, K. Clinical Applications of Circulating Tumor Cells and Circulating Tumor DNA as Liquid Biopsy. Cancer Discov. 2016, 6, 479–491. [Google Scholar] [CrossRef] [Green Version]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jensen, C.; Nissen, N.I.; Von Arenstorff, C.S.; Karsdal, M.A.; Willumsen, N. Serological Assessment of Collagen Fragments and Tumor Fibrosis May Guide Immune Checkpoint Inhibitor Therapy. J. Exp. Clin. Cancer Res. 2021, 40, 326. [Google Scholar] [CrossRef]
- Walker, C.; Mojares, E.; del Río Hernández, A. Role of Extracellular Matrix in Development and Cancer Progression. Int. J. Mol. Sci. 2018, 19, 3028. [Google Scholar] [CrossRef] [Green Version]
- Xu, S.; Xu, H.; Wang, W.; Li, S.; Li, H.; Li, T.; Zhang, W.; Yu, X.; Liu, L. The Role of Collagen in Cancer: From Bench to Bedside. J. Transl. Med. 2019, 17, 309. [Google Scholar] [CrossRef] [Green Version]
- Armstrong, T.; Packham, G.; Murphy, L.B.; Bateman, A.C.; Conti, J.A.; Fine, D.R.; Johnson, C.D.; Benyon, R.C.; Iredale, J.P. Type I Collagen Promotes the Malignant Phenotype of Pancreatic Ductal Adenocarcinoma. Clin. Cancer Res. 2004, 10, 7427–7437. [Google Scholar] [CrossRef] [Green Version]
- Shintani, Y.; Hollingsworth, M.A.; Wheelock, M.J.; Johnson, K.R. Collagen I Promotes Metastasis in Pancreatic Cancer by Activating C-Jun NH 2 -Terminal Kinase 1 and Up-Regulating N-Cadherin Expression. Cancer Res. 2006, 66, 11745–11753. [Google Scholar] [CrossRef] [Green Version]
- Shields, M.A.; Dangi-Garimella, S.; Krantz, S.B.; Bentrem, D.J.; Munshi, H.G. Pancreatic Cancer Cells Respond to Type I Collagen by Inducing Snail Expression to Promote Membrane Type 1 Matrix Metalloproteinase-Dependent Collagen Invasion. J. Biol. Chem. 2011, 286, 10495–10504. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharjee, S.; Hamberger, F.; Ravichandra, A.; Miller, M.; Nair, A.; Affo, S.; Filliol, A.; Chin, L.; Savage, T.M.; Yin, D.; et al. Tumor Restriction by Type I Collagen Opposes Tumor-Promoting Effects of Cancer-Associated Fibroblasts. J. Clin. Investig. 2021, 131, e146987. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Kim, J.; Yang, S.; Wang, H.; Wu, C.-J.; Sugimoto, H.; LeBleu, V.S.; Kalluri, R. Type I Collagen Deletion in ASMA+ Myofibroblasts Augments Immune Suppression and Accelerates Progression of Pancreatic Cancer. Cancer Cell 2021, 39, 548–565.e6. [Google Scholar] [CrossRef] [PubMed]
- Peng, D.H.; Rodriguez, B.L.; Diao, L.; Chen, L.; Wang, J.; Byers, L.A.; Wei, Y.; Chapman, H.A.; Yamauchi, M.; Behrens, C.; et al. Collagen Promotes Anti-PD-1/PD-L1 Resistance in Cancer through LAIR1-Dependent CD8+ T Cell Exhaustion. Nat. Commun. 2020, 11, 4520. [Google Scholar] [CrossRef]
- Willumsen, N.; Jensen, C.; Green, G.; Nissen, N.I.; Neely, J.; Nelson, D.M.; Pedersen, R.S.; Frederiksen, P.; Chen, I.M.; Boisen, M.K.; et al. Fibrotic Activity Quantified in Serum by Measurements of Type III Collagen Pro-Peptides Can Be Used for Prognosis across Different Solid Tumor Types. Cell. Mol. Life Sci. 2022, 79, 204. [Google Scholar] [CrossRef] [PubMed]
- Huilgol, D.; Venkataramani, P.; Nandi, S.; Bhattacharjee, S. Transcription Factors That Govern Development and Disease: An Achilles Heel in Cancer. Genes 2019, 10, 794. [Google Scholar] [CrossRef] [Green Version]
- Manzo, G. Similarities between Embryo Development and Cancer Process Suggest New Strategies for Research and Therapy of Tumors: A New Point of View. Front. Cell Dev. Biol. 2019, 7, 20. [Google Scholar] [CrossRef]
- Bonnans, C.; Chou, J.; Werb, Z. Remodelling the Extracellular Matrix in Development and Disease. Nat. Rev. Mol. Cell Biol. 2014, 15, 786–801. [Google Scholar] [CrossRef]
- Izzi, V.; Heljasvaara, R.; Heikkinen, A.; Karppinen, S.-M.; Koivunen, J.; Pihlajaniemi, T. Exploring the Roles of MACIT and Multiplexin Collagens in Stem Cells and Cancer. Semin. Cancer Biol. 2020, 62, 134–148. [Google Scholar] [CrossRef]
- Thorlacius-Ussing, J.; Manon-Jensen, T.; Sun, S.; Leeming, D.J.; Sand, J.M.; Karsdal, M.; Willumsen, N. Serum Type XIX Collagen Is Significantly Elevated in Non-Small Cell Lung Cancer: A Preliminary Study on Biomarker Potential. Cancers 2020, 12, 1510. [Google Scholar] [CrossRef]
- Koch, M.; Foley, J.E.; Hahn, R.; Zhou, P.; Burgeson, R.E.; Gerecke, D.R.; Gordon, M.K. Alpha 1(Xx) Collagen, a New Member of the Collagen Subfamily, Fibril-Associated Collagens with Interrupted Triple Helices. J. Biol. Chem. 2001, 276, 23120–23126. [Google Scholar] [CrossRef] [Green Version]
- Uhlén, M.; Fagerberg, L.; Hallström, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, Å.; Kampf, C.; Sjöstedt, E.; Asplund, A.; et al. Tissue-Based Map of the Human Proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, E.; Takahashi, S.; Fukaya, R.; Ohta, S.; Yoshida, K.; Toda, M. Identification of KLRC2 as a Candidate Marker for Brain Tumor-Initiating Cells. Neurol. Res. 2019, 41, 1043–1049. [Google Scholar] [CrossRef] [PubMed]
- Vitanza, N.A.; Biery, M.C.; Myers, C.; Ferguson, E.; Zheng, Y.; Girard, E.J.; Przystal, J.M.; Park, G.; Noll, A.; Pakiam, F.; et al. Optimal Therapeutic Targeting by HDAC Inhibition in Biopsy-Derived Treatment-Naïve Diffuse Midline Glioma Models. Neuro. Oncol. 2021, 23, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-C.; Tu, S.-H.; Lien, H.-H.; Jeng, J.-Y.; Huang, C.-S.; Huang, C.-J.; Lai, L.-C.; Chuang, E.Y. Concurrent Gene Signatures for Han Chinese Breast Cancers. PLoS ONE 2013, 8, e76421. [Google Scholar] [CrossRef] [Green Version]
- Gorlov, I.P.; Byun, J.; Gorlova, O.Y.; Aparicio, A.M.; Efstathiou, E.; Logothetis, C.J. Candidate Pathways and Genes for Prostate Cancer: A Meta-Analysis of Gene Expression Data. BMC Med. Genom. 2009, 2, 48. [Google Scholar] [CrossRef] [Green Version]
- Nissen, N.I.; Karsdal, M.; Willumsen, N. Collagens and Cancer Associated Fibroblasts in the Reactive Stroma and Its Relation to Cancer Biology. J. Exp. Clin. Cancer Res. 2019, 38, 115. [Google Scholar] [CrossRef] [Green Version]
- Leeming, D.J.; Koizumi, M.; Qvist, P.; Barkholt, V.; Zhang, C.; Henriksen, K.; Byrjalsen, I.; Karsdal, M.A. Serum N-Terminal Propeptide of Collagen Type I Is Associated with the Number of Bone Metastases in Breast and Prostate Cancer and Correlates to Other Bone Related Markers. Biomark. Cancer 2011, 3, BIC-S6484. [Google Scholar] [CrossRef] [Green Version]
- Klein, A.P. Pancreatic Cancer Epidemiology: Understanding the Role of Lifestyle and Inherited Risk Factors. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 493–502. [Google Scholar] [CrossRef]
- Eyre, D.R.; Pietka, T.; Weis, M.A.; Wu, J.-J. Covalent Cross-Linking of the NC1 Domain of Collagen Type IX to Collagen Type II in Cartilage. J. Biol. Chem. 2004, 279, 2568–2574. [Google Scholar] [CrossRef] [Green Version]
- Chiquet, M.; Birk, D.E.; Bönnemann, C.G.; Koch, M. Collagen XII: Protecting Bone and Muscle Integrity by Organizing Collagen Fibrils. Int. J. Biochem. Cell Biol. 2014, 53, 51–54. [Google Scholar] [CrossRef] [Green Version]
- Wenstrup, R.J.; Florer, J.B.; Brunskill, E.W.; Bell, S.M.; Chervoneva, I.; Birk, D.E. Type V Collagen Controls the Initiation of Collagen Fibril Assembly. J. Biol. Chem. 2004, 279, 53331–53337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elia, I.; Rossi, M.; Stegen, S.; Broekaert, D.; Doglioni, G.; van Gorsel, M.; Boon, R.; Escalona-Noguero, C.; Torrekens, S.; Verfaillie, C.; et al. Breast Cancer Cells Rely on Environmental Pyruvate to Shape themetastatic Niche. Nature 2019, 568, 117. [Google Scholar] [CrossRef] [PubMed]
- Karagiannis, G.S.; Petraki, C.; Prassas, I.; Saraon, P.; Musrap, N.; Dimitromanolakis, A.; Diamandis, E.P. Proteomic Signatures of the Desmoplastic Invasion Front Reveal Collagen Type XII as a Marker of Myofibroblastic Differentiation During Colorectal Cancer Metastasis. Oncotarget 2012, 3, 267–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amenta, P.S.; Hadad, S.; Lee, M.T.; Barnard, N.; Li, D.; Myers, J.C. Loss of Types XV and XIX Collagen Precedes Basement Membrane Invasion in Ductal Carcinoma of the Female Breast. J. Pathol. 2003, 199, 298–308. [Google Scholar] [CrossRef] [PubMed]
- Schönborn, K.; Willenborg, S.; Schulz, J.-N.; Imhof, T.; Eming, S.A.; Quondamatteo, F.; Brinckmann, J.; Niehoff, A.; Paulsson, M.; Koch, M.; et al. Role of Collagen XII in Skin Homeostasis and Repair. Matrix Biol. 2020, 94, 57–76. [Google Scholar] [CrossRef]
- Sun, M.; Zafrullah, N.; Devaux, F.; Hemmavanh, C.; Adams, S.; Ziebarth, N.M.; Koch, M.; Birk, D.E.; Espana, E.M. Collagen XII Is a Regulator of Corneal Stroma Structure and Function. Investig. Opthalmol. Vis. Sci. 2020, 61, 61. [Google Scholar] [CrossRef]
- Kuczek, D.E.; Larsen, A.M.H.; Thorseth, M.-L.; Carretta, M.; Kalvisa, A.; Siersbæk, M.S.; Simões, A.M.C.; Roslind, A.; Engelholm, L.H.; Noessner, E.; et al. Collagen Density Regulates the Activity of Tumor-Infiltrating T Cells. J. Immunother. Cancer 2019, 7, 68. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.-H.; Chen, Y.-L.; Lai, T.-Y.; Ko, Y.-C.; Chou, Y.-F.; Chen, P.-R.; Hsiao, J.-R.; Chang, J.-Y.; Shiah, S.-G.; Lee, J.-W.; et al. Identification of Prognostic Biomarkers Originating From the Tumor Stroma of Betel Quid-Associated Oral Cancer Tissues. Front. Oncol. 2021, 11, 769665. [Google Scholar] [CrossRef]
- Sun, M.; Koudouna, E.; Cogswell, D.; Avila, M.Y.; Koch, M.; Espana, E.M. Collagen XII Regulates Corneal Stromal Structure by Modulating TGF-β Activity. Am. J. Pathol. 2021, 192, 308–319. [Google Scholar] [CrossRef]
- Ishihara, J.; Ishihara, A.; Sasaki, K.; Lee, S.S.Y.; Williford, J.M.; Yasui, M.; Abe, H.; Potin, L.; Hosseinchi, P.; Fukunaga, K.; et al. Targeted Antibody and Cytokine Cancer Immunotherapies through Collagen Affinity. Sci. Transl. Med. 2019, 11, 3259. [Google Scholar] [CrossRef]
- Holländer, N.; Sauerbrei, W.; Schumacher, M. Confidence Intervals for the Effect of a Prognostic Factor after Selection of an ‘Optimal’ Cutpoint. Stat. Med. 2004, 23, 1701–1713. [Google Scholar] [CrossRef] [PubMed]
- Leeming, D.J.; Larsen, D.V.; Zhang, C.; Hi, Y.; Veidal, S.S.; Nielsen, R.H.; Henriksen, K.; Zheng, Q.; Barkholt, V.; Riis, B.J.; et al. Enzyme-Linked Immunosorbent Serum Assays (ELISAs) for Rat and Human N-Terminal pro-Peptide of Collagen Type I (PINP)—Assessment of Corresponding Epitopes. Clin. Biochem. 2010, 43, 1249–1256. [Google Scholar] [CrossRef] [PubMed]
- Nissen, N.I.; Kehlet, S.; Johansen, A.Z.; Chen, I.M.; Karsdal, M.; Johansen, J.S.; Diab, H.M.H.; Jørgensen, L.N.; Sun, S.; Manon-Jensen, T.; et al. Noninvasive Prognostic Biomarker Potential of Quantifying the Propeptides of Type XI Collagen Alpha-1 Chain (PRO-C11) in Patients with Pancreatic Ductal Adenocarcinoma. Int. J. Cancer 2021, 149, 228–238. [Google Scholar] [CrossRef] [PubMed]





| Test | Result |
|---|---|
| IC50 | 1.82 nM |
| Measurement range | 0.84–27.3 nM |
| Detection range | 0.20–14.9 nM |
| Dilution recovery of human serum (undiluted to 1:2) | 86.8% |
| Spiking recovery of peptide in serum | 101.3% |
| Spiking recovery of serum in serum | 95.5% |
| Hemoglobin interference recovery, low/high conc. | 104.9/103.7% |
| Lipids interference recovery, low/high conc. | 103.2/109.2% |
| Biotin interference recovery, low/high conc. | 98.1/85.4% |
| Inter-assay variation | 5.4% |
| Intra-assay variation | 5.9% |
| Analyte stability (48 h 4 °C/48 h 20 °C) | 89.5/87.1% |
| Freeze–thaw stability up to four cycles | 89.8% |
| Characteristic | Cancer, n = 219 | Healthy, n = 33 |
|---|---|---|
| Diagnosis, n (%) | ||
| Bladder Cancer | 20 (9.1) | - |
| Breast Cancer | 20 (9.1) | - |
| Colorectal Cancer | 20 (9.1) | - |
| Head and Neck Cancer | 20 (9.1) | - |
| Kidney Cancer | 20 (9.1) | - |
| Lung Cancer | 20 (9.1) | - |
| Melanoma | 20 (9.1) | - |
| Ovarian Cancer | 19 (8.7) | - |
| Pancreatic Cancer | 20 (9.1) | - |
| Prostate Cancer | 20 (9.1) | - |
| Stomach Cancer | 20 (9.1) | - |
| Healthy | - | 33 (100) |
| Cancer Stages, n (%) | ||
| I | 7 (3.2) | - |
| II | 46 (21) | - |
| III | 93 (42) | - |
| IV | 73 (33) | - |
| Age, Mean (SD) | 59 (11) | 58 (6) |
| Sex, n (%) | ||
| Male | 119 (54) | 21 (64) |
| Female | 100 (46) | 12 (36) |
| Diagnosis | AUC | Cutoff | Youden | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|---|---|---|
| Lung Cancer | 0.92 | 2.03 | 0.82 | 1.00 | 0.82 | 0.77 | 1.00 |
| Colorectal Cancer | 0.90 | 1.73 | 0.76 | 1.00 | 0.76 | 0.71 | 1.00 |
| Kidney Cancer | 0.88 | 1.71 | 0.71 | 0.95 | 0.76 | 0.70 | 0.96 |
| Breast Cancer | 0.87 | 1.77 | 0.76 | 1.00 | 0.76 | 0.71 | 1.00 |
| Bladder Cancer | 0.86 | 2.35 | 0.65 | 0.80 | 0.85 | 0.76 | 0.88 |
| Stomach Cancer | 0.83 | 1.28 | 0.64 | 1.00 | 0.64 | 0.62 | 1.00 |
| Pancreatic Cancer | 0.82 | 0.77 | 0.61 | 1.00 | 0.61 | 0.61 | 1.00 |
| Ovarian Cancer | 0.80 | 1.41 | 0.61 | 0.95 | 0.67 | 0.62 | 0.96 |
| Prostate Cancer | 0.79 | 1.19 | 0.59 | 0.95 | 0.64 | 0.61 | 0.95 |
| Head and Neck Cancer | 0.76 | 1.15 | 0.54 | 0.90 | 0.64 | 0.60 | 0.91 |
| Melanoma | 0.76 | 1.53 | 0.55 | 0.85 | 0.70 | 0.63 | 0.88 |
| Characteristic | PDAC, n = 36 | Pancreatitis, n = 11 | Healthy, n = 20 |
|---|---|---|---|
| Age, Mean (SD) | 66 (8) | 61 (9) | 57 (6) |
| Sex, n (%) | |||
| Female | 17 (47) | 1 (9.1) | 10 (50) |
| Male | 19 (53) | 10 (91) | 10 (50) |
| BMI, Mean (SD) | 23.7 (3.7) | - | - |
| Diabetes, n (%) | 7 (19) | 3 (27) | - |
| Tobacco, n (%) | |||
| Ever | 23 (64) | 8 (73) | - |
| Never | 13 (36) | 3 (27) | - |
| Stage, n (%) | |||
| 1b | 3 (8.3) | - | - |
| 2a | 3 (8.3) | - | - |
| 2b | 11 (31) | - | - |
| 4 | 19 (53) | - | - |
| Metastases, n (%) | |||
| Liver Metastasis | 15 (79) | - | - |
| Other Metastasis | 4 (21) | - | - |
| Performance Status, n (%) | |||
| 0 | 15 (45) | - | - |
| 1 | 14 (42) | - | - |
| 2 | 4 (12) | - | - |
| Unknown | 3 | - | - |
| Positive Classifier | Negative Classifier | AUC | Cutoff | Youden | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|---|---|---|---|
| PDAC | Healthy | 0.92 | 1.71 | 0.77 | 0.97 | 0.80 | 0.90 | 0.94 |
| Pancreatitis | Healthy | 0.91 | 1.69 | 0.80 | 1.00 | 0.80 | 0.73 | 1.00 |
| PDAC | Pancreatitis | 0.63 | 2.33 | 0.35 | 0.44 | 0.91 | 0.94 | 0.33 |
| Characteristic | HR (95% CI) 1 | p-Value |
|---|---|---|
| PRO-C20: Above 2.57 nM | 4.25 (1.52 to 11.9) | 0.006 |
| Metastasis: Yes | 5.00 (1.93 to 13.0) | <0.001 |
| Age: Continuous | 1.08 (1.01 to 1.15) | 0.025 |
| Sex: Male | 2.71 (1.13 to 6.48) | 0.025 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thorlacius-Ussing, J.; Jensen, C.; Madsen, E.A.; Nissen, N.I.; Manon-Jensen, T.; Chen, I.M.; Johansen, J.S.; Diab, H.M.H.; Jørgensen, L.N.; Karsdal, M.A.; et al. Type XX Collagen Is Elevated in Circulation of Patients with Solid Tumors. Int. J. Mol. Sci. 2022, 23, 4144. https://doi.org/10.3390/ijms23084144
Thorlacius-Ussing J, Jensen C, Madsen EA, Nissen NI, Manon-Jensen T, Chen IM, Johansen JS, Diab HMH, Jørgensen LN, Karsdal MA, et al. Type XX Collagen Is Elevated in Circulation of Patients with Solid Tumors. International Journal of Molecular Sciences. 2022; 23(8):4144. https://doi.org/10.3390/ijms23084144
Chicago/Turabian StyleThorlacius-Ussing, Jeppe, Christina Jensen, Emilie A. Madsen, Neel I. Nissen, Tina Manon-Jensen, Inna M. Chen, Julia S. Johansen, Hadi M. H. Diab, Lars N. Jørgensen, Morten A. Karsdal, and et al. 2022. "Type XX Collagen Is Elevated in Circulation of Patients with Solid Tumors" International Journal of Molecular Sciences 23, no. 8: 4144. https://doi.org/10.3390/ijms23084144
APA StyleThorlacius-Ussing, J., Jensen, C., Madsen, E. A., Nissen, N. I., Manon-Jensen, T., Chen, I. M., Johansen, J. S., Diab, H. M. H., Jørgensen, L. N., Karsdal, M. A., & Willumsen, N. (2022). Type XX Collagen Is Elevated in Circulation of Patients with Solid Tumors. International Journal of Molecular Sciences, 23(8), 4144. https://doi.org/10.3390/ijms23084144






