Structural Comparison of Sulfonamide-Based Derivatives That Can Improve Anti-Coagulation Properties of Metformin
Abstract
1. Introduction
2. Results
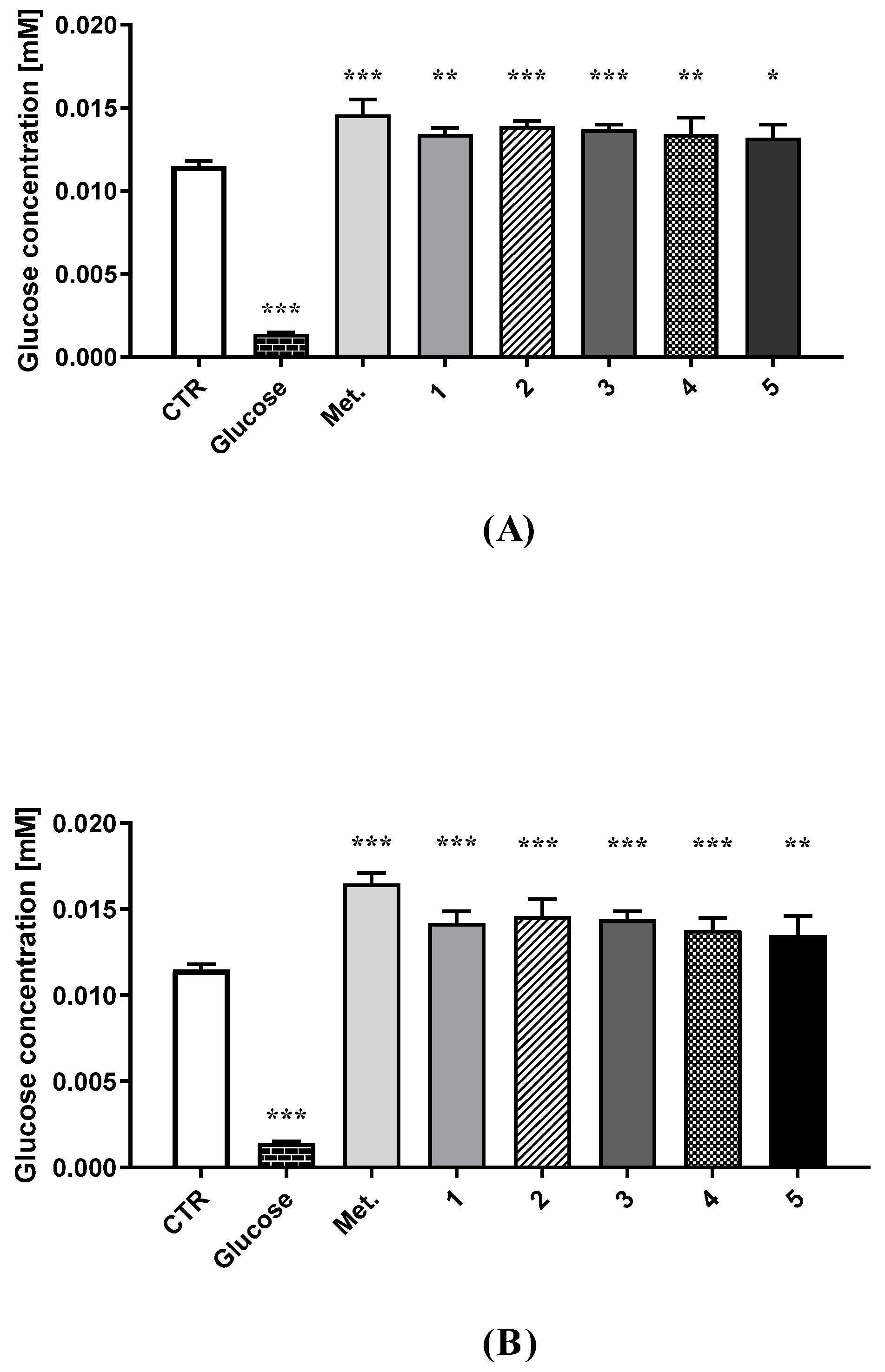
2.1. Intracellular Glucose Uptake
2.2. Viability of HUVEC and AoSMC Cells
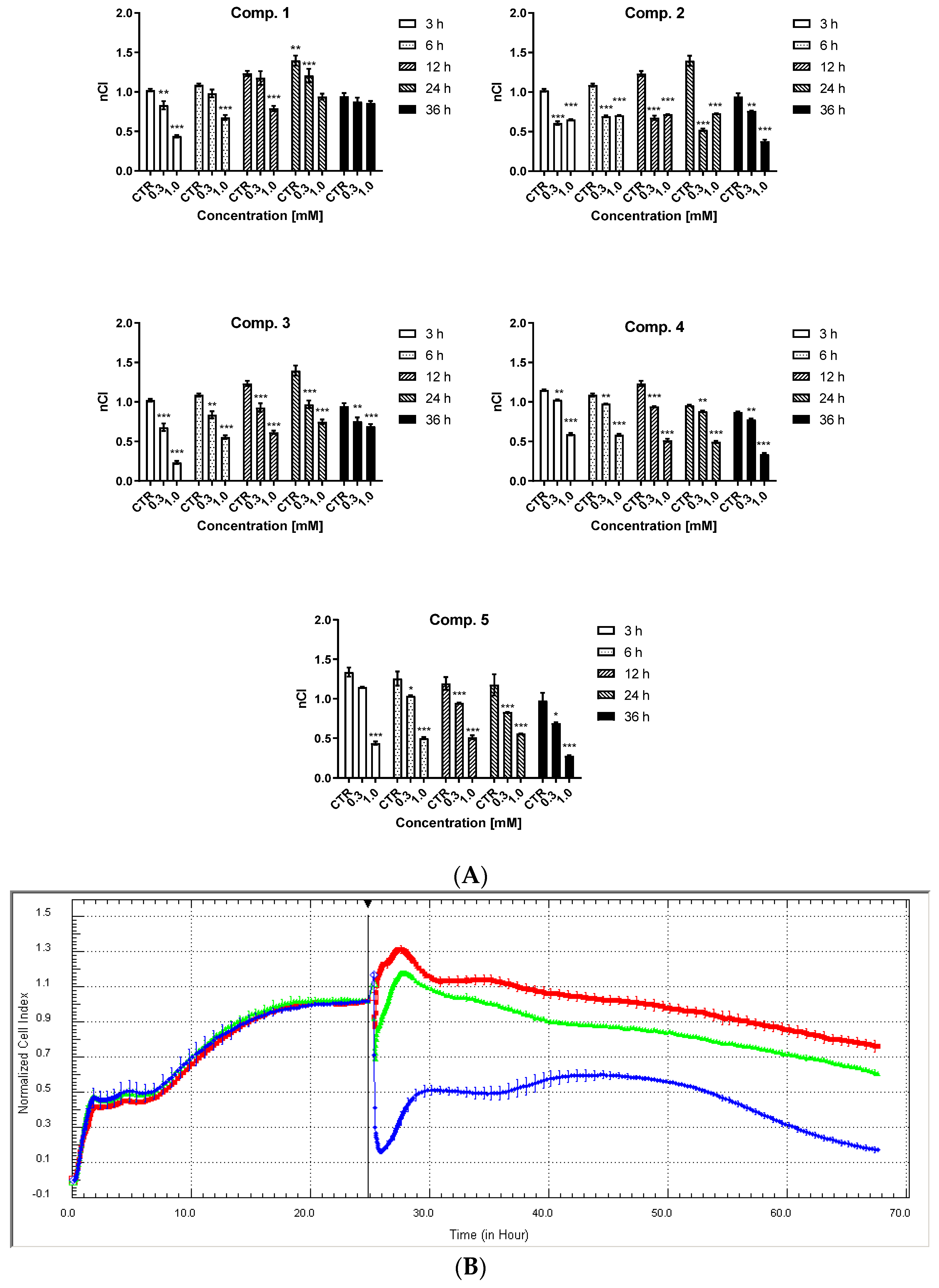
2.3. HUVEC Cell Integrity
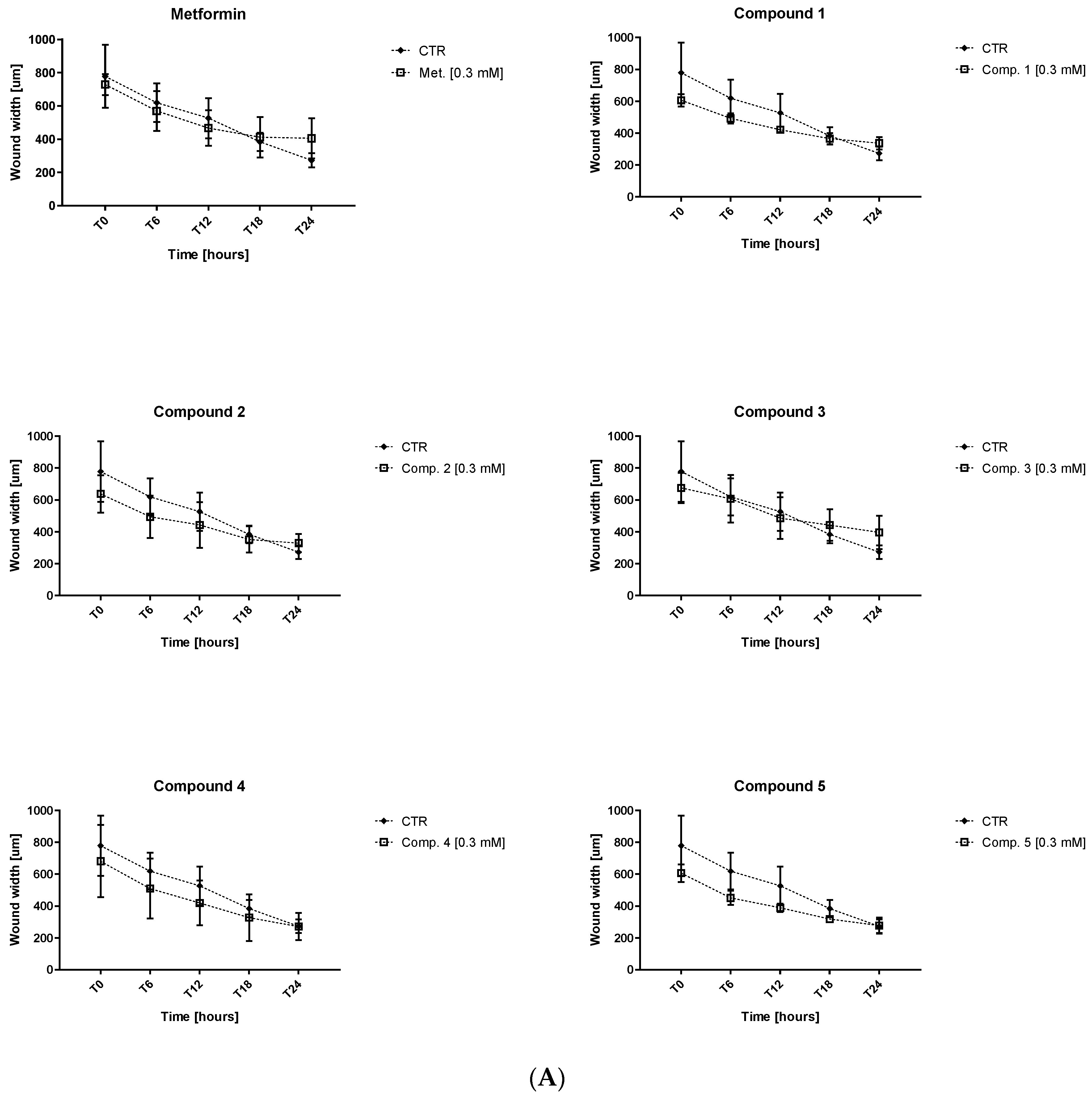
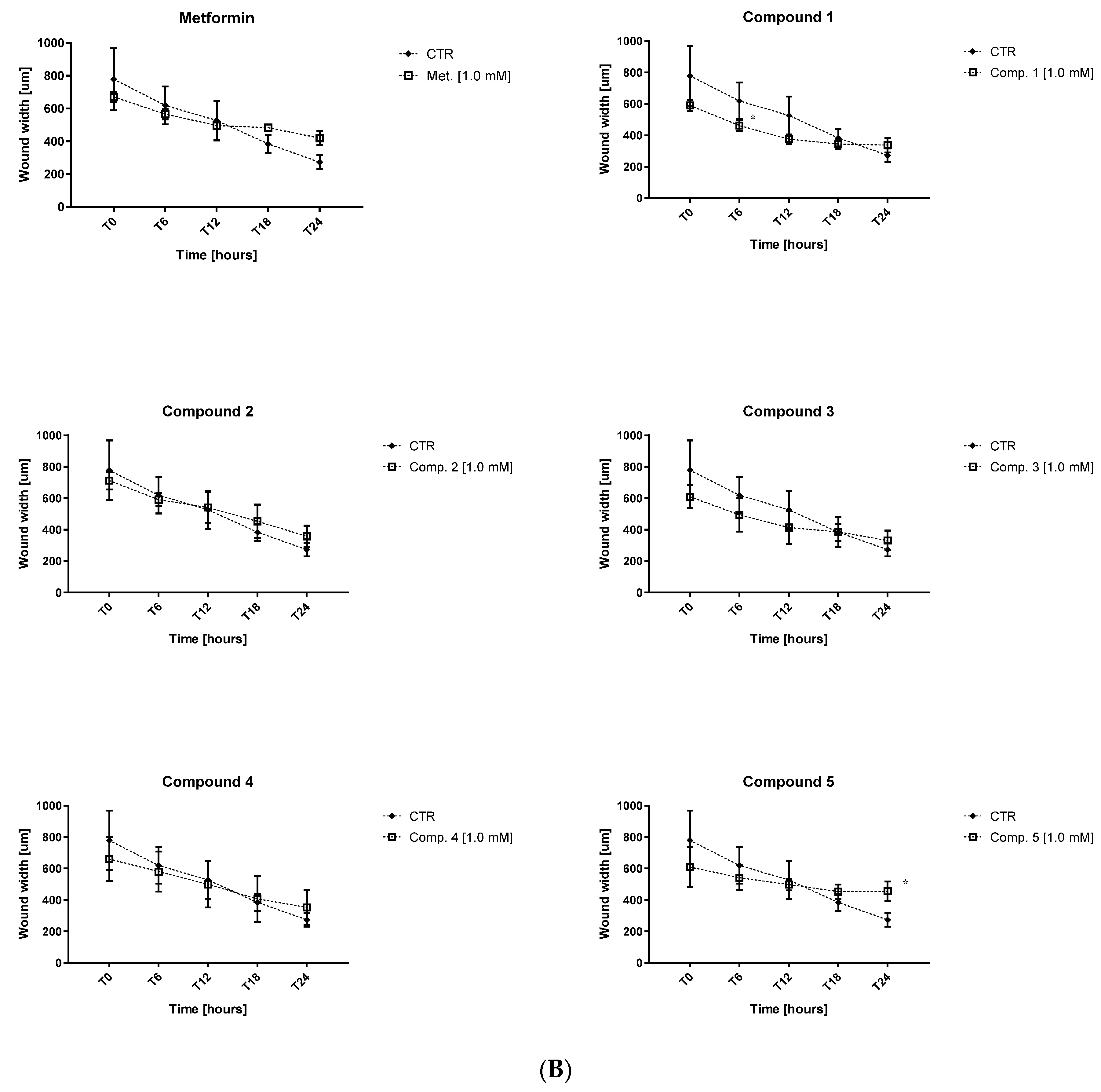
2.4. Endothelial Cell Migration
2.5. Tissue Plasminogen Activator (t-PA) Release from Human Umbilical Endothelial Cells
2.6. Basic Coagulation Studies
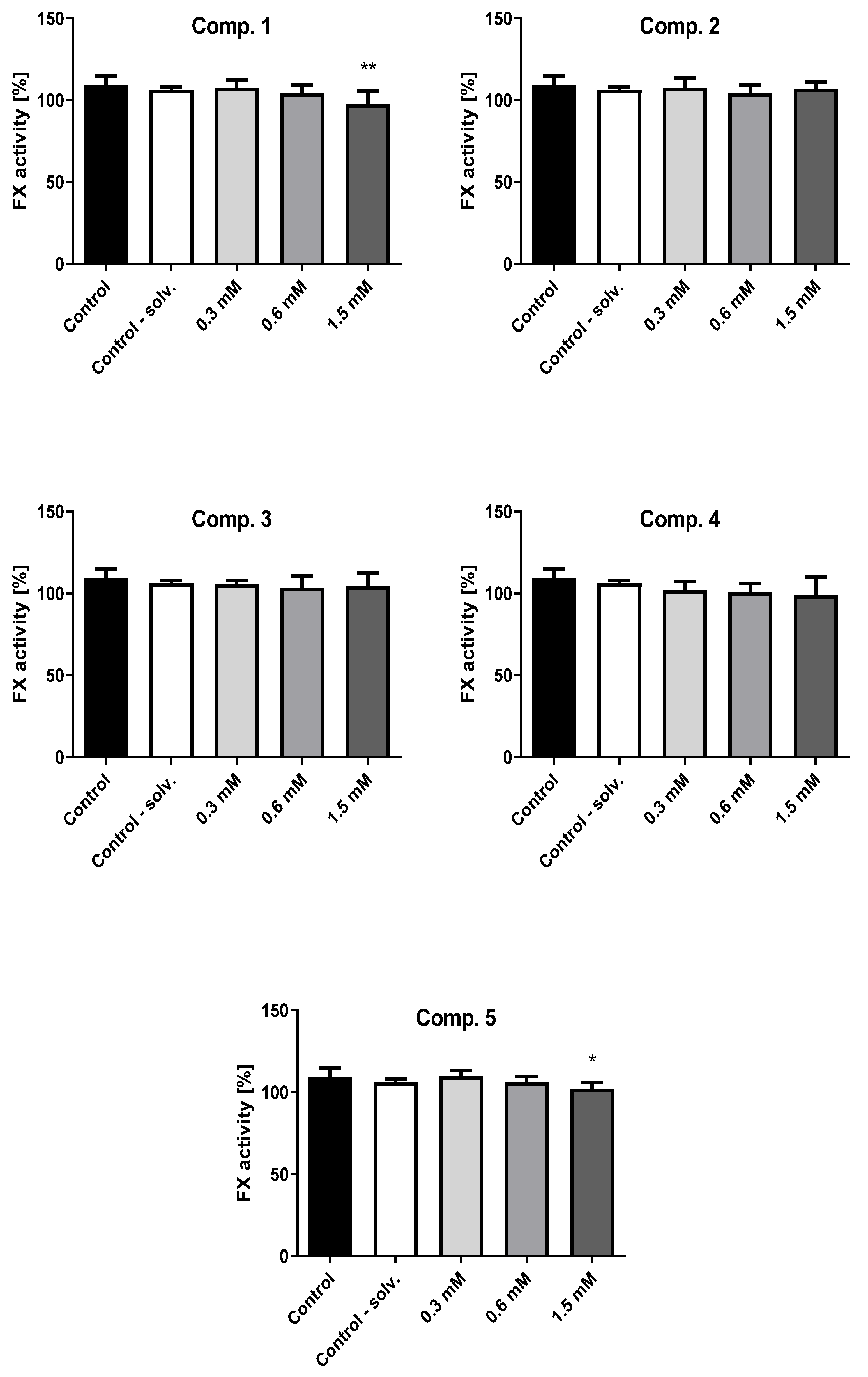
2.7. Factor X Activity
2.8. Antithrombin III (AT) Activity
2.9. Clot Formation and Lysis Test (CL-Test)
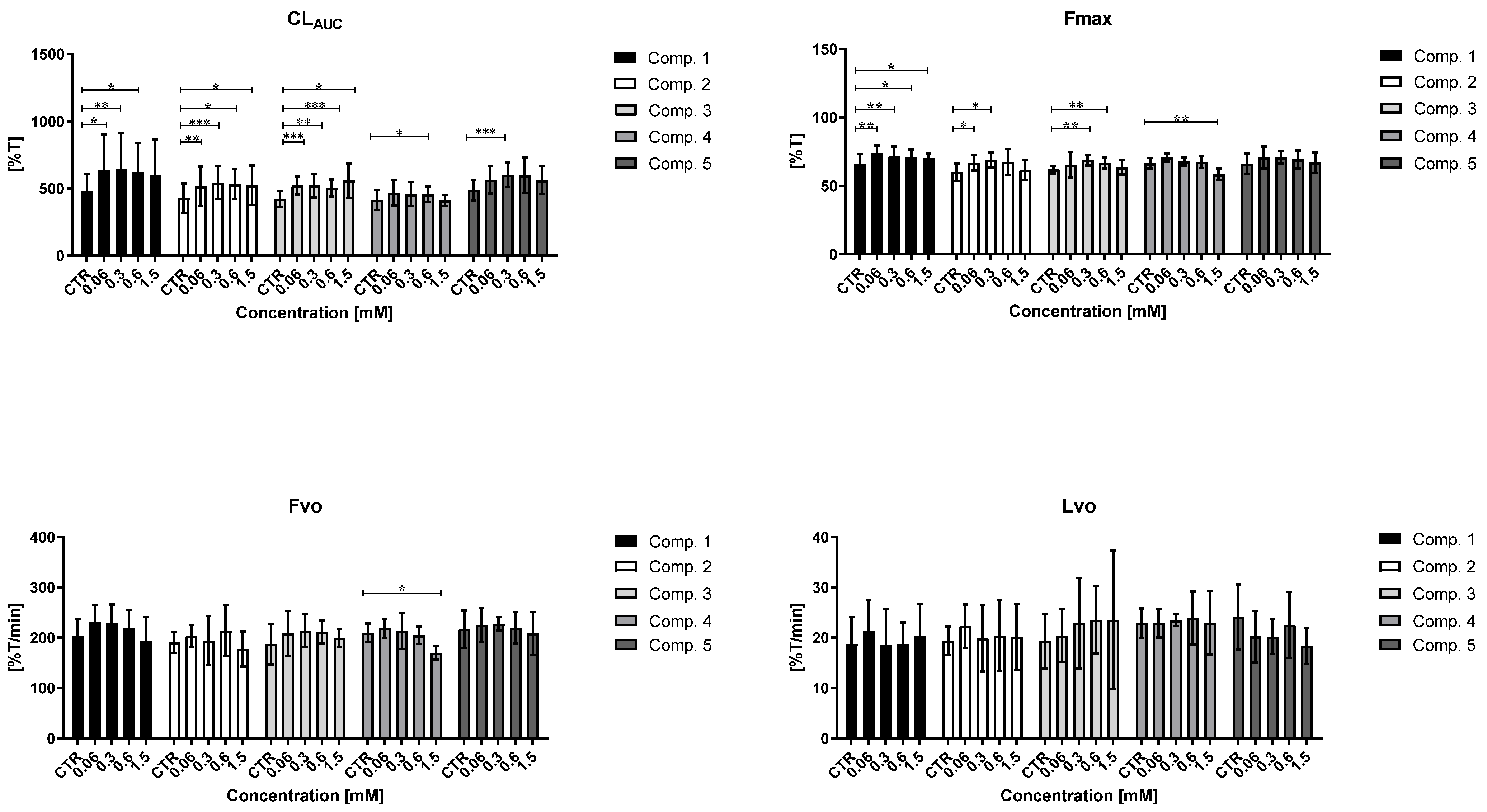
2.9.1. Overall Potential of Clot Formation and Fibrinolysis (CLAUC)
2.9.2. Kinetic Parameters of Clot Formation Phase
2.9.3. Kinetic Parameters of Clot Stabilization Phase
2.9.4. The Kinetic Parameters of the Fibrinolysis Phase
2.10. Coagulation Assay
3. Discussion
4. Materials and Methods
4.1. Materials
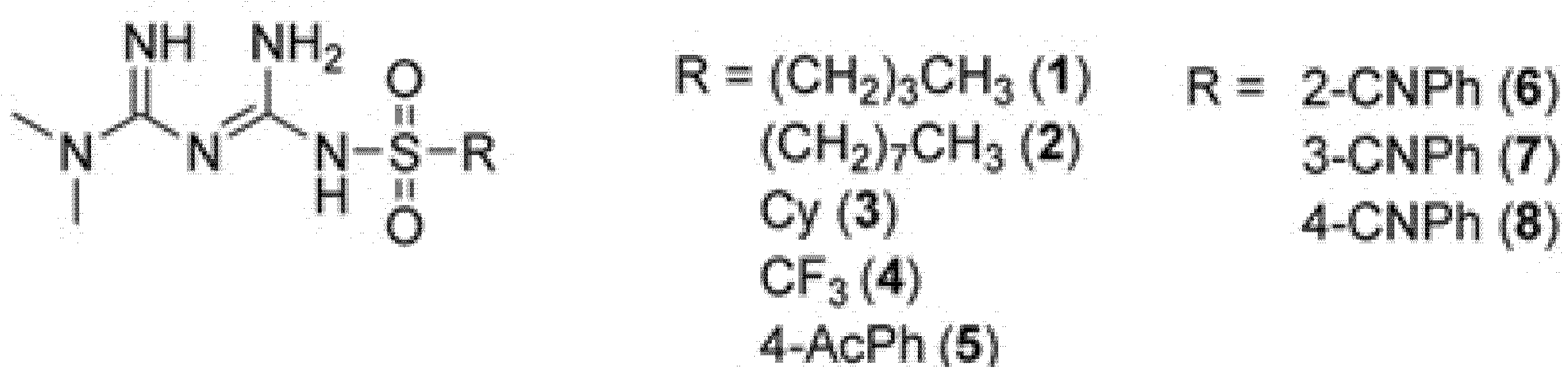
4.2. Studied Compounds
4.3. Plasma Preparation for Basic Coagulology Tests
4.4. Glucose Utilization Assay
4.5. Cell Viability Assay
4.6. HUVEC Integrity Studies
4.7. HUVEC Migration in Real Time
4.8. Tissue Plasminogen Activators Release from Endothelial Cells
4.9. Basic Coagulation Tests: PT, INR, APTT, TT
4.10. Factor X Activity
4.11. Activity of Antithrombin III (AT)
4.12. Clot Formation and Fibrinolysis Test (CL-Test)
4.13. Coagulation Assay
4.14. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Park, K. A review of computational drug repurposing. Transl. Clin. Pharmacol. 2019, 27, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Strittmatter, S.M. Overcoming Drug Development Bottlenecks with Repurposing: Old drugs learn new tricks. Nat. Med. 2014, 20, 590–591. [Google Scholar] [CrossRef] [PubMed]
- Papapetropoulos, A.; Szabo, C. Inventing new therapies without reinventing the wheel: The power of drug repurposing. Br. J. Pharmacol. 2018, 175, 165–167. [Google Scholar] [CrossRef]
- Juárez-López, D.; Schcolnik-Cabrera, A. Drug Repurposing: Considerations to Surpass While Re-directing Old Compounds for New Treatments. Arch. Med. Res. 2021, 52, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Fetro, C.; Scherman, D. Drug repurposing in rare diseases: Myths and reality. Therapies 2020, 75, 157–160. [Google Scholar] [CrossRef] [PubMed]
- Lakizadeh, A.; Hassan Mir-Ashrafi, S.M. Drug repurposing improvement using a novel data integration framework based on the drug side effect. Inform. Med. Unlocked 2021, 23, 100523. [Google Scholar] [CrossRef]
- Apaydın, S.; Török, M. Sulfonamide derivatives as multi-target agents for complex diseases. Bioorg. Med. Chem. Lett. 2019, 29, 2042–2050. [Google Scholar] [CrossRef]
- Mondal, S.; Malakar, S. Synthesis of sulfonamide and their synthetic and therapeutic applications: Recent advances. Tetrahedron 2020, 76, 131662. [Google Scholar] [CrossRef]
- Brnardic, E.J.; Ye, G.; Brooks, C.; Donatelli, C.; Barton, L.; McAtee, J.; Sanchez, R.M.; Shu, A.; Erhard, K.; Terrell, L.; et al. Discovery of Pyrrolidine Sulfonamides as Selective and Orally Bioavailable Antagonists of Transient Receptor Potential Vanilloid-4 (TRPV4). J. Med. Chem. 2018, 61, 9738–9755. [Google Scholar] [CrossRef]
- Pero, J.E.; Matthews, J.M.; Behm, D.J.; Brnardic, E.J.; Brooks, C.; Budzik, B.W.; Costell, M.H.; Donatelli, C.A.; Eisennagel, S.H.; Erhard, K.; et al. Design and Optimization of Sulfone Pyrrolidine Sulfonamide Antagonists of Transient Receptor Potential Vanilloid-4 with in Vivo Activity in a Pulmonary Edema Model. J. Med. Chem. 2018, 61, 11209–11220. [Google Scholar] [CrossRef]
- Filosa, J.A.; Yao, X.; Rath, G. TRPV4 and the Regulation of Vascular Tone. J. Cardiovasc. Pharmacol. 2013, 61, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Karakas, S.E.; Banaszewska, B.; Spaczynski, R.Z.; Pawelczyk, L.; Duleba, A. Free fatty acid binding protein-4 and retinol binding protein-4 in polycystic ovary syndrome: Response to simvastatin and metformin therapies. Gynecol. Endocrinol. 2013, 29, 483–487. [Google Scholar] [CrossRef]
- Majerczyk, M.; Olszanecka-Glinianowicz, M.; Puzianowska-Kuźnicka, M.; Chudek, J. Białko wiążące retinol typu 4 (RBP4) jako czynnik i marker uszkodzenia naczyń związany z insulino opornością. Postepy Hig. Med. Dosw. 2016, 70, 1267–1275. [Google Scholar] [CrossRef]
- Gao, D.-D.; Dou, H.-X.; Su, H.-X.; Zhang, M.-M.; Wang, T.; Liu, Q.-F.; Cai, H.-Y.; Ding, H.-P.; Yang, Z.; Zhu, W.-L.; et al. From hit to lead: Structure-based discovery of naphthalene-1-sulfonamide derivatives as potent and selective inhibitors of fatty acid binding protein 4. Eur. J. Med. Chem. 2018, 154, 44–59. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Sauter, E.R.; Li, B. FABP4: A New Player in Obesity-Associated Breast Cancer. Trends Mol. Med. 2020, 26, 437–440. [Google Scholar] [CrossRef] [PubMed]
- Becker, M.R.; Ewing, W.R.; Davis, R.S.; Pauls, H.W.; Ly, C.; Li, A.; Mason, H.J.; Choi-Sledeski, Y.M.; Spada, A.P.; Chu, V.; et al. Synthesis, SAR and in vivo activity of novel thienopyridine sulfonamide pyrrolidinones as factor Xa inhibitors. Bioorg. Med. Chem. Lett. 1999, 9, 2753–2758. [Google Scholar] [CrossRef]
- Takebayashi, K.; Suetsugu, M.; Matsumoto, S.; Aso, Y.; Inukai, T. Effects of Rosuvastatin and Colestimide on Metabolic Parameters and Urinary Monocyte Chemoattractant Protein-1 in Type 2 Diabetic Patients with Hyperlipidemia. South. Med. J. 2009, 102, 361–368. [Google Scholar] [CrossRef]
- Packer, M.; Mcmurray, J.; Massie, B.M.; Caspi, A.; Charlon, V.; Cohen-Solal, A.; Kiowski, W.; Kostuk, W.; Krum, H.; Levine, B.; et al. Clinical effects of endothelin receptor antagonism with bosentan in patients with severe chronic heart failure: Results of a pilot study. J. Card. Fail. 2005, 11, 12–20. [Google Scholar] [CrossRef]
- Packer, M.; Mcmurray, J.; Krum, H.; Kiowski, W.; Massie, B.M.; Caspi, A.; Pratt, C.M.; Petrie, M.C.; DeMets, D.; Kobrin, I.; et al. Long-Term Effect of Endothelin Receptor Antagonism with Bosentan on the Morbidity and Mortality of Patients with Severe Chronic Heart Failure: Primary Results of the ENABLE Trials. JACC Heart Fail. 2017, 5, 317–326. [Google Scholar] [CrossRef]
- Tellew, J.E.; Baska, R.A.F.; Beyer, S.M.; Carlson, K.E.; Cornelius, L.A.; Fadnis, L.; Gu, Z.; Kunst, B.L.; Kowala, M.C.; Monshizadegan, H.; et al. Discovery of 4′-[(Imidazol-1-yl)methyl]biphenyl-2-sulfonamides as dual endothelin/Angiotensin II receptor antagonists. Bioorg. Med. Chem. Lett. 2003, 13, 1093–1096. [Google Scholar] [CrossRef]
- Bai, R.; Wei, Z.; Liu, J.; Xie, W.; Yao, H.; Wu, X.; Jiang, J.; Wang, Q.; Xu, J. Synthesis and biological evaluation of 4′-[(benzimidazole-1-yl)methyl]biphenyl-2-sulfonamide derivatives as dual angiotensin II/endothelin A receptor antagonists. Bioorg. Med. Chem. 2012, 20, 4661–4667. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, Q.; Yang, X.; Xu, S.; Zhang, H.; Bai, R.; Yao, H.; Jiang, J.; Shen, M.; Wu, X.; et al. Design, synthesis, and biological evaluation of 1,2,4-triazole bearing 5-substituted biphenyl-2-sulfonamide derivatives as potential antihypertensive candidates. Bioorg. Med. Chem. 2013, 21, 7742–7751. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Xu, X.; Du, M.; Zhao, T.; Wang, J. A preclinical overview of metformin for the treatment of type 2 diabetes. Biomed. Pharmacother. 2018, 106, 1227–1235. [Google Scholar] [CrossRef] [PubMed]
- Rena, G.; Hardie, D.G.; Pearson, E.R. The mechanisms of action of metformin. Diabetologia 2017, 60, 1577–1585. [Google Scholar] [CrossRef] [PubMed]
- Sardu, C.; Paolisso, P.; Sacra, C.; Mauro, C.; Minicucci, F.; Portoghese, M.; Rizzo, M.R.; Barbieri, M.; Sasso, F.C.; D’Onofrio, N.; et al. Effects of Metformin Therapy on Coronary Endothelial Dysfunction in Patients with Prediabetes with Stable Angina and Nonobstructive Coronary Artery Stenosis: The CODYCE Multicenter Prospective Study. Diabetes Care 2019, 42, 1946–1955. [Google Scholar] [CrossRef] [PubMed]
- De Jager, J.; Kooy, A.; Schalkwijk, C.; Van Der Kolk, J.; Lehert, P.; Bets, D.; Wulffelé, M.G.; Donker, A.J.; Stehouwer, C.D.A. Long-term effects of metformin on endothelial function in type 2 diabetes: A randomized controlled trial. J. Intern. Med. 2014, 275, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Caballero, A.E.; Delgado, A.; Aguilar-Salinas, C.A.; Herrera, A.N.; Castillo, J.L.; Cabrera, T.; Gomez-Perez, F.J.; Rull, J.A. The Differential Effects of Metformin on Markers of Endothelial Activation and Inflammation in Subjects with Impaired Glucose Tolerance: A Placebo-Controlled, Randomized Clinical Trial. J. Clin. Endocrinol. Metab. 2004, 89, 3943–3948. [Google Scholar] [CrossRef]
- Markowicz-Piasecka, M.; Huttunen, K.M.; Broncel, M.; Sikora, J. Sulfenamide and Sulfonamide Derivatives of Metformin—A New Option to Improve Endothelial Function and Plasma Haemostasis. Sci. Rep. 2019, 9, 6573. [Google Scholar] [CrossRef]
- Witkowski, M.; Friebel, J.; Tabaraie, T.; Grabitz, S.; Dörner, A.; Taghipour, L.; Jakobs, K.; Stratmann, B.; Tschoepe, D.; Landmesser, U.; et al. Metformin Is Associated with Reduced Tissue Factor Procoagulant Activity in Patients with Poorly Controlled Diabetes. Cardiovasc. Drugs Ther. 2020, 35, 809–813. [Google Scholar] [CrossRef]
- Xin, G.; Wei, Z.; Ji, C.; Zheng, H.; Gu, J.; Ma, L.; Huang, W.; Morris-Natschke, S.L.; Yeh, J.-L.; Zhang, R.; et al. Metformin Uniquely Prevents Thrombosis by Inhibiting Platelet Activation and mtDNA Release. Sci. Rep. 2016, 6, 36222. [Google Scholar] [CrossRef]
- Markowicz-Piasecka, M.; Sikora, J.; Zajda, A.; Huttunen, K.M. Novel halogenated sulfonamide biguanides with anti-coagulation properties. Bioorg. Chem. 2020, 94, 103444. [Google Scholar] [CrossRef] [PubMed]
- Xin, G.; Ming, Y.; Ji, C.; Wei, Z.; Li, S.; Morris-Natschke, S.L.; Zhang, X.; Yu, K.; Li, Y.; Zhang, B.; et al. Novel potent antiplatelet thrombotic agent derived from biguanide for ischemic stroke. Eur. J. Med. Chem. 2020, 200, 112462. [Google Scholar] [CrossRef] [PubMed]
- Coagulation Factor X Activity Assay, Plasma. Available online: https://www.mayocliniclabs.com/test-catalog/Clinical+and+Interpretive/9066 (accessed on 5 October 2021).
- Găman, A.M.; Găman, G. Deficiency of Antithrombin III (AT III)—Case Report and Review of the Literature. Curr. Health Sci. J. 2014, 40, 141–143. [Google Scholar] [PubMed]
- Sniecinski, R.M.; Welsby, I.J.; Levi, M.M.; Levy, J.H. Antithrombin: Anti-inflammatory properties and clinical applications. Thromb. Haemost. 2016, 115, 712–728. [Google Scholar] [CrossRef]
- Kostka, B.; Para, J.; Sikora, J. A multiparameter test of clot formation and fibrinolysis for in-vitro drug screening. Blood Coagul. Fibrinolysis 2007, 18, 611–618. [Google Scholar] [CrossRef]
- Markowicz-Piasecka, M.; Sikora, J.; Mateusiak, Ł.; Mikiciuk-Olasik, E.; Huttunen, K.M. New prodrugs of metformin do not influence the overall haemostasis potential and integrity of the erythrocyte membrane. Eur. J. Pharmacol. 2017, 811, 208–221. [Google Scholar] [CrossRef]
- Pryor, R.; Cabreiro, F. Repurposing metformin: An old drug with new tricks in its binding pockets. Biochem. J. 2015, 471, 307–322. [Google Scholar] [CrossRef]
- Schneider, D.J. Factors Contributing to Increased Platelet Reactivity in People with Diabetes. Diabetes Care 2009, 32, 525–527. [Google Scholar] [CrossRef]
- Li, X.; Weber, N.; Cohn, D.; Hollmann, M.; DeVries, J.; Hermanides, J.; Preckel, B. Effects of Hyperglycemia and Diabetes Mellitus on Coagulation and Hemostasis. J. Clin. Med. 2021, 10, 2419. [Google Scholar] [CrossRef]
- Pretorius, E.; Lipinski, B.; Bester, J.; Vermeulen, N.; Soma, P. Albumin Stabilizes Fibrin Fiber Ultrastructure in Low Serum Albumin Type 2 Diabetes. Ultrastruct. Pathol. 2013, 37, 254–257. [Google Scholar] [CrossRef]
- Carr, M.E. Diabetes mellitus: A hypercoagulable state. J. Diabetes Complicat. 2001, 15, 44–54. [Google Scholar] [CrossRef]
- Erem, C.; Hacıhasanoğlu, A.; Çelik, Ş.; Ovalı, E.; Ersöz, H.Ö.; Ukinç, K.; Deger, O.; Telatar, M. Coagulation and Fibrinolysis Parameters in Type 2 Diabetic Patients with and without Diabetic Vascular Complications. Med. Princ. Pract. 2004, 14, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Schubert, M.; Hansen, S.; Leefmann, J.; Guan, K. Repurposing Antidiabetic Drugs for Cardiovascular Disease. Front. Physiol. 2020, 11, 568632. [Google Scholar] [CrossRef] [PubMed]
- Nesti, L.; Natali, A. Metformin effects on the heart and the cardiovascular system: A review of experimental and clinical data. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 657–669. [Google Scholar] [CrossRef]
- Markowicz-Piasecka, M.; Huttunen, K.M.; Mateusiak, L.; Mikiciuk-Olasik, E.; Sikora, J. Is Metformin a Perfect Drug? Updates in Pharmacokinetics and Pharmacodynamics. Curr. Pharm. Des. 2017, 23, 2532–2550. [Google Scholar] [CrossRef]
- Foretz, M.; Guigas, B.; Bertrand, L.; Pollak, M.; Viollet, B. Metformin: From Mechanisms of Action to Therapies. Cell Metab. 2014, 20, 953–966. [Google Scholar] [CrossRef]
- Markowicz-Piasecka, M.; Huttunen, J.; Zajda, A.; Sikora, J.; Huttunen, K.M. Sulfonamide metformin derivatives induce mitochondrial-associated apoptosis and cell cycle arrest in breast cancer cells. Chem.-Biol. Interact. 2021, 352, 109795. [Google Scholar] [CrossRef] [PubMed]
- Pernicova, I.; Korbonits, M. Metformin—Mode of action and clinical implications for diabetes and cancer. Nat. Rev. Endocrinol. 2014, 10, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Polianskyte-Prause, Z.; Tolvanen, T.A.; Lindfors, S.; Dumont, V.; Van, M.; Wang, H.; Dash, S.N.; Berg, M.; Naams, J.-B.; Hautala, L.C.; et al. Metformin increases glucose uptake and acts renoprotectively by reducing SHIP2 activity. FASEB J. 2018, 33, 2858–2869. [Google Scholar] [CrossRef]
- Avogaro, A.; Albiero, M.; Menegazzo, L.; de Kreutzenberg, S.; Fadini, G.P. Endothelial Dysfunction in Diabetes: The role of reparatory mechanisms. Diabetes Care 2011, 34, S285–S290. [Google Scholar] [CrossRef] [PubMed]
- Brandes, R.P. Endothelial Dysfunction and Hypertension. Hypertension 2014, 64, 924–928. [Google Scholar] [CrossRef] [PubMed]
- Oosterhoff, L.A.; Kruitwagen, H.S.; Van Wolferen, M.E.; Van Balkom, B.W.; Mokry, M.; Lansu, N.; Dungen, N.A.V.D.; Penning, L.C.; Spanjersberg, T.C.; De Graaf, J.W.; et al. Characterization of Endothelial and Smooth Muscle Cells from Different Canine Vessels. Front. Physiol. 2019, 10, 101. [Google Scholar] [CrossRef] [PubMed]
- Zhuge, Y.; Zhang, J.; Qian, F.; Wen, Z.; Niu, C.; Xu, K.; Ji, H.; Rong, X.; Chu, M.; Jia, C. Role of smooth muscle cells in Cardiovascular Disease. Int. J. Biol. Sci. 2020, 16, 2741–2751. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Qu, J.; He, L.; Zhang, F.; Zhou, Z.; Yang, S.; Zhou, Y. Calcium in Vascular Smooth Muscle Cell Elasticity and Adhesion: Novel Insights Into the Mechanism of Action. Front. Physiol. 2019, 10, 852. [Google Scholar] [CrossRef]
- ICELLigence. Available online: https://www.aceabio.com (accessed on 10 September 2021).
- Obońska, K.; Grąbczewska, Z.; Fisz, J.; Kubica, J. Cukrzyca i dysfunkcja śródbłonka—Krótkie spojrzenie na złożony problem. Folia Cardiol. 2011, 6, 109–116. [Google Scholar]
- Sedding, D.G.; Boyle, E.C.; Demandt, J.A.F.; Sluimer, J.C.; Dutzmann, J.; Haverich, A.; Bauersachs, J. Vasa Vasorum Angiogenesis: Key Player in the Initiation and Progression of Atherosclerosis and Potential Target for the Treatment of Cardiovascular Disease. Front. Immunol. 2018, 9, 706. [Google Scholar] [CrossRef]
- Xu, Z.; Han, Y.; Liu, J.; Jiang, F.; Hu, H.; Wang, Y.; Liu, Q.; Gong, Y.; Li, X. MiR-135b-5p and MiR-499a-3p Promote Cell Proliferation and Migration in Atherosclerosis by Directly Targeting MEF2C. Sci. Rep. 2015, 5, 12276. [Google Scholar] [CrossRef]
- Napieralski, R.; Wagner, E.; Gebhard, H.; Schmitt, M.; Zimmermann, A.; Eckstein, H.-H.; Greißel, A.; Culmes, M.; Zernecke, A.; Pelisek, J. Alternation of histone and DNA methylation in human atherosclerotic carotid plaques. Thromb. Haemost. 2015, 114, 390–402. [Google Scholar] [CrossRef]
- Rorbach-Dolata, A.; Kubis, A.; Piwowar, A. Epigenetic modifications: An important mechanism in diabetic disturbances. Postępy Hig. I Med. Doświadczalnej 2017, 71, 960–974. [Google Scholar] [CrossRef]
- Markowicz-Piasecka, M.; Sadkowska, A.; Huttunen, K.M.; Podsiedlik, M.; Mikiciuk-Olasik, E.; Sikora, J. An investigation into the pleiotropic activity of metformin. A glimpse of haemostasis. Eur. J. Pharmacol. 2020, 872, 172984. [Google Scholar] [CrossRef]
- Roszkowski, K.; Ziółkowska, E. Fibrinolysis in neoplastic process. Contemp. Oncol. 2005, 9, 196–198. [Google Scholar]
- Wannamethee, S.G.; Sattar, N.; Rumley, A.; Whincup, P.H.; Lennon, L.; Lowe, G.D. Tissue Plasminogen Activator, von Willebrand Factor, and Risk of Type 2 Diabetes in Older Men. Diabetes Care 2008, 31, 995–1000. [Google Scholar] [CrossRef][Green Version]
- Chojnowski, K.; Podolak-Dawidziak, M.; Windyga, J. Diagnosis of the prolonged activated partial thromboplastin time (aPTT). Hematologia 2010, 1, 81–86. [Google Scholar]
- Lippi, G.; Franchini, M.; Targher, G.; Montagnana, M.; Salvagno, G.L.; Guidi, G.C.; Favaloro, E.J. Epidemiological association between fasting plasma glucose and shortened APTT. Clin. Biochem. 2008, 42, 118–120. [Google Scholar] [CrossRef]
- Tripodi, A.; Chantarangkul, V.; Martinelli, I.; Bucciarelli, P.; Mannucci, P.M. A shortened activated partial thromboplastin time is associated with the risk of venous thromboembolism. Blood 2004, 104, 3631–3634. [Google Scholar] [CrossRef]
- Pretorius, E.; Bester, J.; Vermeulen, N.; Alummoottil, S.; Soma, P.; Buys, A.V.; Kell, D.B. Poorly controlled type 2 diabetes is accompanied by significant morphological and ultrastructural changes in both erythrocytes and in thrombin-generated fibrin: Implications for diagnostics. Cardiovasc. Diabetol. 2015, 14, 30. [Google Scholar] [CrossRef]
- Skrypnik, D.; Bogdański, P.; Pupek-Musialik, D.; Rypiński, B. Leczenie przeciwzakrzepowe u pacjentów z cukrzycą. Forum Zaburzeń Metab. 2013, 4, 19–28. [Google Scholar]
- Sobczak, A.I.S.; Stewart, A.J. Coagulatory Defects in Type-1 and Type-2 Diabetes. Int. J. Mol. Sci. 2019, 20, 6345. [Google Scholar] [CrossRef]
- Sikora, J.; Markowicz-Piasecka, M.; Broncel, M.; Mikiciuk-Olasik, E. Extract of Aronia melanocarpa-modified hemostasis: In vitro studies. Eur. J. Nutr. 2014, 53, 1493–1502. [Google Scholar] [CrossRef]
- Markowicz-Piasecka, M.; Huttunen, J.; Sikora, J.; Huttunen, K.M. Sulfenamide derivatives can improve transporter-mediated cellular uptake of metformin and induce cytotoxicity in human breast adenocarcinoma cell lines. Bioorg. Chem. 2019, 87, 321–334. [Google Scholar] [CrossRef]
- Chalubinski, M.; Zemanek, K.; Skowron, W.; Wojdan, K.; Gorzelak-Pabiś, P.; Broncel, M. The effect of 7-ketocholesterol and 25-hydroxycholesterol on the integrity of the human aortic endothelial and intestinal epithelial barriers. Infamm. Res. 2013, 62, 1015–1023. [Google Scholar] [CrossRef]
- Markowicz-Piasecka, M.; Sadkowska, A.; Sikora, J.; Broncel, M.; Huttunen, K.M. Novel Sulfonamide-Based Analogs of Metformin Exert Promising Anti-Coagulant Effects without Compromising Glucose-Lowering Activity. Pharmaceuticals 2020, 13, 323. [Google Scholar] [CrossRef]
- Markowicz-Piasecka, M.; Huttunen, K.M.; Mateusiak, Ł.; Mikiciuk-Olasik, E.; Sikora, J. Sulfenamide and sulfonamide derivatives of metformin can exert anticoagulant and profibrinolytic properties. Chem. Biol. Interact. 2018, 284, 126–136. [Google Scholar] [CrossRef]
- Brown, D.L.; Kouides, P.A. Diagnosis and treatment of inherited factor X deficiency. Haemophilia 2008, 14, 1176–1182. [Google Scholar] [CrossRef]
- Markowicz-Piasecka, M.; Huttunen, K.M.; Sadkowska, A.; Sikora, J. Pleiotropic Activity of Metformin and Its Sulfonamide Derivatives on Vascular and Platelet Haemostasis. Molecules 2019, 25, 125. [Google Scholar] [CrossRef]
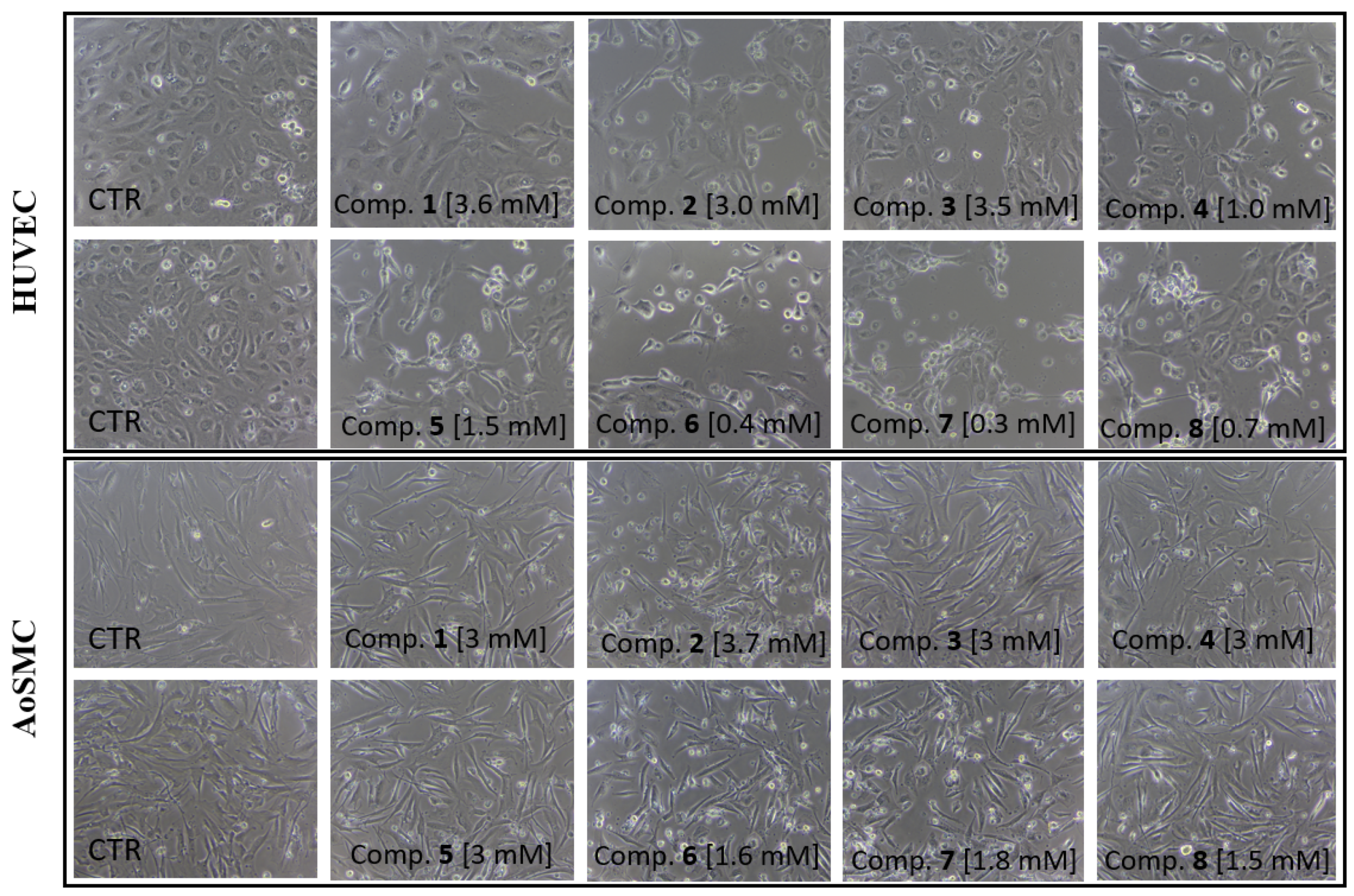

| Viability-IC50 [mM] | ||
|---|---|---|
| Compound | HUVEC cells | AoSMC cells |
| 1 | 3.643 ± 0.26 | >59% at 3 mM |
| 2 | n.d. | 3.710 ± 0.54 |
| 3 | 3.506 ± 0.24 | >76% at 3 mM |
| 4 | 1.038 ± 0.15 | >56% at 3 mM |
| 5 | 1.589 ± 0.13 | >62% at 3 mM |
| 6 | 0.461 ± 0.13 | 1.685 ± 0.21 |
| 7 | 0.304 ± 0.12 | 1.891 ± 0.16 |
| 8 | 0.791 ± 0.13 | 1.573 ± 0.14 |
| Compound | Concentration (mM) | Released t-PA (pg/mL) |
|---|---|---|
| CTR | - | 2895.4 ± 208.7 |
| 1 | 0.3 | 2427.2 ± 246.0 ** |
| 1.0 | 1709.5 ± 219.1 *** | |
| 2 | 0.3 | 1633.9 ± 263.2 *** |
| 1.0 | 990.0 ± 158.5 *** | |
| 3 | 0.3 | 1550.9 ± 135.2 *** |
| 1.0 | 1267.1 ± 350.6 *** | |
| 4 | 0.3 | 1958.3 ± 175.3 *** |
| 1.0 | 1481.8 ± 134.0 *** | |
| 5 | 0.3 | 1947.0 ± 207.3 *** |
| 1.0 | 1326.2 ± 149.4 *** |
| Compound | Concentr. (mM) | PT (s) | INR | APTT (s) | TT (s) |
|---|---|---|---|---|---|
| 1 | 0 (CTR) | 12.46 ± 1.49 | 1.00 ± 0.12 | 29.36 ± 2.23 | 15.50 ± 0.92 |
| 0.006 | 13.04 ± 2.41 | 1.04 ± 0.19 | 35.12 ± 4.75 | 14.03 ± 0.94 ** | |
| 0.06 | 12.78 ± 1.86 | 1.11 ± 0.23 | 33.36 ± 1.78 ** | 12.98 ± 1.08 ** | |
| 0.3 | 13.46 ± 2.71 | 1.08 ± 0.22 | 33.76 ± 2.53 * | 13.90 ± 1.24 * | |
| 0.6 | 13.08 ± 2.36 | 1.05 ± 0.19 | 34.68 ± 3.31 * | 12.64 ± 1.31 ** | |
| 1.5 | 13.48 ± 2.93 | 1.08 ± 0.23 | 34.68 ± 5.21 | 13.03 ± 0.86 ** | |
| 2 | 0 (CTR) | 12.38 ± 1.68 | 0.99 ± 0.13 | 30.77 ± 4.03 | 15.76 ± 0.36 |
| 0.006 | 13.30 ± 1.81 | 1.07 ± 0.15 | 30.79 ± 5.87 | 14.14 ± 0.59 *** | |
| 0.06 | 12.82 ± 1.33 | 1.03 ± 0.10 | 31.33 ± 5.53 | 13.86 ± 0.80 ** | |
| 0.3 | 13.10 ± 1.37 | 1.05 ± 0.11 | 33.49 ± 4.85 | 13.48 ± 2.10 ** | |
| 0.6 | 12.96 ± 1.42 | 1.04 ± 0.11 | 33.46 ± 5.59 | 14.40 ± 2.35 | |
| 1.5 | 13.20 ± 1.23 | 1.06 ± 0.10 | 34.83 ± 6.22 | 16.32 ± 2.21 | |
| 3 | 0 (CTR) | 12.60 ± 2.54 | 1.01 ± 0.20 | 30.90 ± 2.92 | 15.58 ± 1.07 |
| 0.006 | 12.52 ± 1.42 | 1.00 ± 0.11 | 32.97 ± 5.06 | 13.18 ± 1.97 * | |
| 0.06 | 13.02 ± 1.90 | 1.04 ± 0.15 | 34.50 ± 4.52 | 13.14 ± 2.01 * | |
| 0.3 | 13.60 ± 1.39 | 1.09 ± 0.11 | 33.00 ± 5.70 | 13.46 ± 1.97 ** | |
| 0.6 | 13.66 ± 2.27 | 1.09 ± 0.18 | 33.17 ± 5.34 | 13.50 ± 1.74 ** | |
| 1.5 | 13.30 ± 2.30 | 1.06 ± 0.18 | 32.49 ± 7.50 | 14.06 ± 1.60 ** | |
| 4 | 0 (CTR) | 13.26 ± 2.06 | 1.06 ± 0.14 | 37.30 ± 6.38 | 16.00 ± 0.78 |
| 0.006 | 12.18 ± 2.09 | 1.00 ± 0.15 | 33.04 ± 4.10 | 16.55 ± 1.11 | |
| 0.06 | 11.80 ± 1.24 | 0.93 ± 0.10 | 35.22 ± 2.68 | 14.83 ± 1.11 | |
| 0.3 | 11.38 ± 1.26 | 0.90 ± 0.10 | 34.14 ± 3.41 | 17.88 ± 3.58 | |
| 0.6 | 11.80 ± 0.39 | 0.95 ± 0.03 | 34.40 ± 4.46 | 17.78 ± 2.40 | |
| 1.5 | 12.02 ± 0.79 | 0.96 ± 0.07 | 43.85 ± 6.20 | 22.03 ± 2.29 ** | |
| 5 | 0 (CTR) | 12.90 ± 1.80 | 1.06 ± 0.13 | 34.80 ± 3.08 | 16.30 ± 1.72 |
| 0.006 | 13.70 ± 3.41 | 1.09 ± 0.27 | 35.06 ± 5.82 | 16.55 ± 1.57 | |
| 0.06 | 11.52 ± 0.86 | 0.92 ± 0.07 | 32.66 ± 3.72 | 16.70 ± 2.48 | |
| 0.3 | 11.88 ± 1.08 | 0.95 ± 0.09 | 32.38 ± 2.48 | 18.48 ± 1.34 | |
| 0.6 | 11.98 ± 0.97 | 0.96 ± 0.08 | 36.10 ± 4.40 | 23.30 ± 2.25 * | |
| 1.5 | 13.44 ± 1.18 | 1.05 ± 0.09 | 77.52 ± 9.85 *** | 28.08 ± 1.49 *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zajda, A.; Sikora, J.; Huttunen, K.M.; Markowicz-Piasecka, M. Structural Comparison of Sulfonamide-Based Derivatives That Can Improve Anti-Coagulation Properties of Metformin. Int. J. Mol. Sci. 2022, 23, 4132. https://doi.org/10.3390/ijms23084132
Zajda A, Sikora J, Huttunen KM, Markowicz-Piasecka M. Structural Comparison of Sulfonamide-Based Derivatives That Can Improve Anti-Coagulation Properties of Metformin. International Journal of Molecular Sciences. 2022; 23(8):4132. https://doi.org/10.3390/ijms23084132
Chicago/Turabian StyleZajda, Agnieszka, Joanna Sikora, Kristiina M. Huttunen, and Magdalena Markowicz-Piasecka. 2022. "Structural Comparison of Sulfonamide-Based Derivatives That Can Improve Anti-Coagulation Properties of Metformin" International Journal of Molecular Sciences 23, no. 8: 4132. https://doi.org/10.3390/ijms23084132
APA StyleZajda, A., Sikora, J., Huttunen, K. M., & Markowicz-Piasecka, M. (2022). Structural Comparison of Sulfonamide-Based Derivatives That Can Improve Anti-Coagulation Properties of Metformin. International Journal of Molecular Sciences, 23(8), 4132. https://doi.org/10.3390/ijms23084132








