The Role of Propranolol as a Repurposed Drug in Rare Vascular Diseases
Abstract
:1. Rare Diseases and Drug Repurposing Opportunities
2. Propranolol as a Repurposed Drug for Rare Diseases
2.1. Vasoconstriction
2.2. Inhibition of Angiogenesis
2.3. Induction of Apoptosis
3. Propranolol for a Benign Vascular Tumor (IH)
4. Propranolol in Vascular and Tumoral Rare Diseases
4.1. Hereditary Hemorrhagic Telangiectasia (HHT)
4.2. Von Hippel-Lindau Disease (VHL)
4.3. Paraganglioma Syndrome (PPGL)
| Trial ID | Study Title | Status | Conditions | Propranolol Compared with | Outcome Measures | Phase | N | Start Date | Results |
|---|---|---|---|---|---|---|---|---|---|
| NCT01058317 | Propranolol Administration in Pediatric Patients With Recurrent Respiratory Papillomatosis | W | Recurrent Respiratory Papillomatosis | - | Number of surgeries Improved voice quality | 2/3 | 0 | 2010 | [66] |
| 2014-003671-30 | Therapeutic effect of propranolol in a series of patients with von Hippel-Lindau disease and retinal hemangioblastomas in short, medium and long term treatment. | C | Retinal Hemangioma | - | Number and size of the retinal or CNS hemangioblastomas | 3 | 10 | 2014 | [25,28] |
| 2015-005177-21 | Dose-Finding of Propranolol in combination with metronomic fixed oral cyclophosphamide based on Bivariate efficacy-tolerability outcome in patients with locally advanced or metastatic angiosarcoma | O | Angiosarcoma | cyclophosphamide | Toxicity and Response rate: Progression. Free survival. Growth modulation index. Overall survival. Tolerability. | 1/2 | 24 | 2015 | - |
| NCT02732678 | Dose-Finding of Propranolol in Combination With Metronomic Fixed Oral Cyclophosphamide Based on Bivariate Efficacy-tolerability Outcome in Patients With Locally Advanced or Metastatic Angiosarcoma: A Collaborative and Innovative Phase I-II Sequential Trial by the French Sarcoma Group (GSF/GETO) | U | Angiosarcoma | propranolol | Toxicity of each tested propranolol dose level in association to cyclophosphamide assessed according to NCI-CTC AE Version 4.0. Non-progression rate. | 1/2 | 24 | 2016 | - |
| NCT03633747 | Efficacy Evaluation of Propranolol Treatment of Hepatic Hemangioma | R | Hemangioma Liver | - | Tumor size. Objective response rate. | 1/2 | 25 | 2018 | - |
| NCT03474614 | Effect of Oral Propranolol on mRNA Expresssion in Symptomatic Cavernous Malformation | NYR | Cerebral Cavernous Malformations | - | Global mRNA and miRNA expression in the blood and tissue. Adverse event. | 2 | 20 | 2018 | - |
| NCT03589014 | Treat_CCM: Propranolol in Cerebral Cavernous Malformation | R | Cerebral Cavernous Malformations | - | Adverse clinical events and outcomes. De novo CCM lesions depiction. CCM size. Micro-hemorrhages at MRI. | 2 | 70 | 2018 | [67] |
| NCT03523650 | Oral Propanolol for Surgically Inaccessible Cavernous Malformations | U | Cavernous Malformations Cerebral and/or Spinal | Propranolol placebo | Number of symptomatic and silent hemorrhages on MRI. Rate of de novo lesion formation; changes in rate of breakthrough seizures or other neurological deficits | 1 | 346 | 2018 | - |
| NCT04518124 | Propranolol in Angiosarcoma | R | Angiosarcoma | - | Clinical and Histological response. | 2 | 14 | 2019 | [68] |
| 2019-002947-41 | Neoadjuvant trial on the efficacy of propranolol monotherapy in angiosarcoma (PROPANGIO) | O | Angiosarcoma | - | Tumor size examination, according to RECIST criteria. Difference in proliferation index. | 2 | 28 | 2019 | [68] |
| NCT04406870 | Sirolimus in the Treatment for Infantile Hepatic Hemangioendothelioma(IEEH) | NYR | Hemangioendothelioma of Liver | sirolimus | Changes on tumor size, PIVKA-II and alpha-1 fetoprotein (AFP) | 4 | 36 | 2020 | [69] |
4.4. Cerebral Cavernous Malformations (CCMs)
4.5. Angiosarcoma
4.6. Tuberous Sclerosis
4.7. Other Rare Carcinomas
5. Conclusions
- -
- Propranolol (an antagonist of ADBR1-2) has emerged as a candidate repurposed drug for an increasing number of RDs. It is a well-characterized drug (both in vitro and in vivo), with a safety profile and therapeutic experience that sufficiently support its use in mono- or combination therapies, as a repurposing drug in spite of the well-known side effects mentioned earlier: bradycardia and hypotension of repurposing drug in clinical application [78];
- -
- Propranolol is involved in a range of physiological and molecular mechanisms that support its potential therapeutic value, including vasodilation, apoptosis of cells in active division by increase in Bax, and Caspases3/7, inhibition of MAPKs, downregulation of HIF in hypoxic or pseudohypoxic processes, antiangiogenic drug decreasing VEGF and EPO protein expression (both HIF targets), and inhibition of dedifferentiating genes such as SOX-2;
- -
- Subsequently to propranolol success for IH treatment, propranolol has been used in monotherapy and combination therapy in different clinical trials for different tumor types, including RDs;
- -
- In RDs, the designation of propranolol as an OD for the treatment of VHL was a remarkable fact. In angiosarcoma, propranolol has been successful in treating a small number of patients, and further trials are ongoing. In CCM, three parallel clinical trials are currently underway.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Haendel, M.; Vasilevsky, N.; Unni, D.; Bologa, C.; Harris, N.; Rehm, H.; Hamosh, A.; Baynam, G.; Groza, T.; McMurry, J.; et al. How many rare diseases are there? Nat. Rev. Drug Discov. 2020, 19, 77–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira, C.R. The burden of rare diseases. Am. J. Med. Genet. A 2019, 179, 885–892. [Google Scholar] [CrossRef] [PubMed]
- Masoudi-Sobhanzadeh, Y.; Omidi, Y.; Amanlou, M.; Masoudi-Nejad, A. Drug databases and their contributions to drug repurposing. Genomics 2020, 112, 1087–1095. [Google Scholar] [CrossRef] [PubMed]
- The Potential of Drug Repurposing in Orphan Drug Development. 2018. Available online: https://www.pharmaceutical-technology.com/comment/potential-drug-repurposing-orphan-drug-development/ (accessed on 24 March 2022).
- Oprea, T.I.; Overington, J.P. Computational and practical aspects of drug repositioning. Assay Drug Dev. Technol. 2015, 13, 299–306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Black, J.W.; Crowther, A.F.; Shanks, R.G.; Smith, L.H.; Dornhorst, A.C. A new adrenergic. Beta-receptor antagonist. Lancet 1964, 1, 1080–1081. [Google Scholar] [CrossRef]
- Sommers Smith, S.K.; Smith, D.M. Beta blockade induces apoptosis in cultured capillary endothelial cells. In Vitro Cell. Dev. Biol. Anim. 2002, 38, 298–304. [Google Scholar] [CrossRef]
- Léauté-Labrèze, C.; De La Roque, E.D.; Hubiche, T.; Boralevi, F.; Thambo, J.B.; Taïeb, A. Propranolol for severe hemangiomas of infancy. N. Engl. J. Med. 2008, 358, 2649–2651. [Google Scholar] [CrossRef]
- Storch, C.H.; Hoeger, P.H. Propranolol for infantile haemangiomas: Insights into the molecular mechanisms of action. Br. J. Dermatol. 2010, 163, 269–274. [Google Scholar] [CrossRef]
- Sánchez-Carpintero, I.; Ruiz-Rodriguez, R.; López-Gutiérrez, J.C. Propranolol in the treatment of infantile hemangioma: Clinical effectiveness, risks, and recommendations. Actas Dermo-Sifiliogr. 2011, 102, 766–779. [Google Scholar] [CrossRef]
- Léauté-Labrèze, C.; Hoeger, P.; Mazereeuw-Hautier, J.; Guibaud, L.; Baselga, E.; Posiunas, G.; Phillips, R.J.; Caceres, H.; Lopez Gutierrez, J.C.; Ballona, R.; et al. A Randomized, Controlled Trial of Oral Propranolol in Infantile Hemangioma. N. Engl. J. Med. 2015, 372, 735–746. [Google Scholar] [CrossRef] [Green Version]
- McDonald, J.; Bayrak-Toydemir, P.; Pyeritz, R.E. Hereditary hemorrhagic telangiectasia: An overview of diagnosis, management, and pathogenesis. Genet. Med. 2011, 13, 607–616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maher, E.R.; Iselius, L.; Yates, J.R.W.; Littler, M.; Benjamin, C.; Harris, R.; Sampson, J.; Williams, A.; Ferguson-Smith, M.A.; Morton, N. Von Hippel-Lindau disease: A genetic study. J. Med. Genet. 1991, 28, 443–447. [Google Scholar] [CrossRef] [Green Version]
- Young, R.J.; Brown, N.J.; Reed, M.W.; Hughes, D.; Woll, P.J. Angiosarcoma. Lancet Oncol. 2010, 11, 983–991. [Google Scholar] [CrossRef]
- Horne, M.A.; Flemming, K.D.; Su, I.C.; Stapf, C.; Jeon, J.P.; Li, D.; Maxwell, S.S.; White, P.; Christianson, T.J.; Agid, R.; et al. Clinical course of untreated cerebral cavernous malformations: A meta-analysis of individual patient data. Lancet Neurol. 2016, 15, 166–173. [Google Scholar] [CrossRef] [Green Version]
- Mollá, B.; Heredia, M.; Sanz, P. Modulators of Neuroinflammation Have a Beneficial Effect in a Lafora Disease Mouse Model. Mol. Neurobiol. 2021, 58, 2508–2522. [Google Scholar] [CrossRef]
- Olesen, J.; Hertz, M. Isoproterenol and propranolol: Ability to cross the blood-brain barrier and effects on cerebral circulation in man. Stroke 1978, 9, 344–349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Westfall, T.C.; Westfall, D.P. Neurotransmission: The autonomic and somatic motor nervous systems. In Goodman and Gilman’s the Pharmacological Basis of Therapeutics, 11th ed.; McGraw-Hill: New York, NY, USA, 2006. [Google Scholar]
- Westfall, T.C.; Westfall, D.P. Adrenergic agonists and antagonists. In Goodman and Gilman’s the Pharmacological Basis of Therapeutics, 11th ed.; McGraw-Hill: New York, NY, USA, 2006. [Google Scholar]
- Hickey, M.M.; Simon, M.C. Regulation of Angiogenesis by Hypoxia and Hypoxia-Inducible Factors. Curr. Top. Dev. Biol. 2006, 76, 217–257. [Google Scholar] [PubMed]
- Huang, Y.; Lin, D.; Taniguchi, C.M. Hypoxia inducible factor (HIF) in the tumor microenvironment: Friend or foe? Sci. China Life Sci. 2017, 60, 1114–1124. [Google Scholar] [CrossRef]
- Hagen, R.; Ghareeb, E.; Jalali, O.; Zinn, Z. Infantile hemangiomas: What have we learned from propranolol? Curr. Opin. Pediatr. 2018, 30, 499–504. [Google Scholar] [CrossRef]
- Rotter, A.; de Oliveira, Z.N.P. Infantile hemangioma: Pathogenesis and mechanisms of action of propranolol. J. Dtsch. Dermatol. Ges. 2017, 15, 1185–1190. [Google Scholar] [CrossRef] [Green Version]
- Albiñana, V.; Villar Gómez De Las Heras, K.; Serrano-Heras, G.; Segura, T.; Perona-Moratalla, A.B.; Mota-Pérez, M.; De Campos, J.M.; Botella, L.M. Propranolol reduces viability and induces apoptosis in hemangioblastoma cells from von Hippel-Lindau patients. Orphanet J. Rare Dis. 2015, 10, 118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albiñana, V.; Escribano, R.M.J.; Soler, I.; Padial, L.R.; Recio-Poveda, L.; Villar Gómez De Las Heras, K.; Botella, L.M. Repurposing propranolol as a drug for the treatment of retinal haemangioblastomas in von Hippel-Lindau disease. Orphanet J. Rare Dis. 2017, 12, 122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albiñana, V.; Gallardo-Vara, E.; de Rojas-P, I.; Recio-Poveda, L.; Aguado, T.; Canto-Cano, A.; Aguirre, D.T.; Serra, M.M.; González-Peramato, P.; Martínez-Piñeiro, L.; et al. Targeting β2-Adrenergic Receptors Shows Therapeutical Benefits in Clear Cell Renal Cell Carcinoma from Von Hippel–Lindau Disease. J. Clin. Med. 2020, 9, 2740. [Google Scholar] [CrossRef] [PubMed]
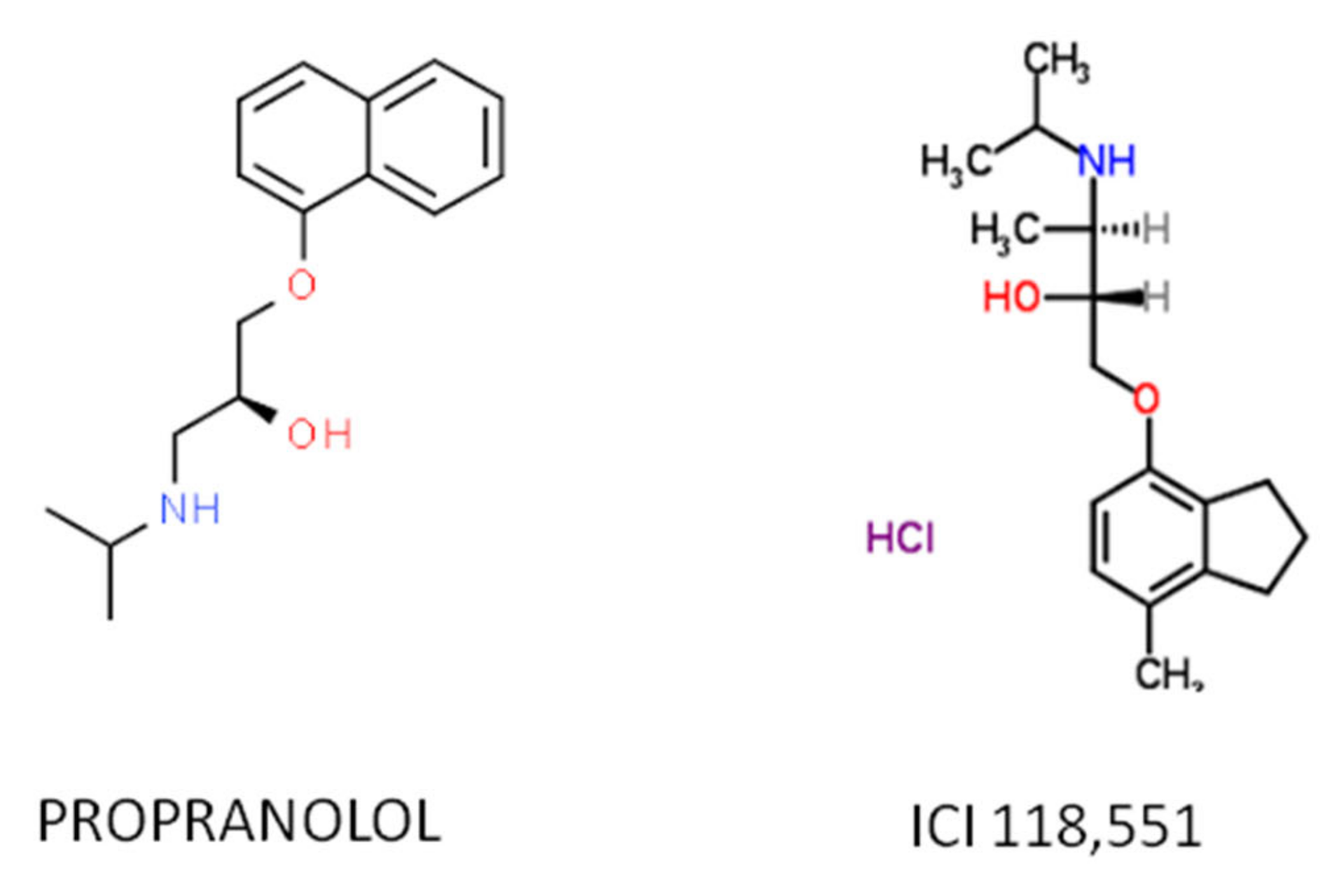
- Cuesta, A.M.; Albiñana, V.; Gallardo-Vara, E.; Recio-Poveda, L.; de Rojas-P, I.; de Las Heras, K.V.G.; Aguirre, D.T.; Botella, L.M. The β2-adrenergic receptor antagonist ICI-118,551 blocks the constitutively activated HIF signalling in hemangioblastomas from von Hippel-Lindau disease. Sci. Rep. 2019, 9, 10062. [Google Scholar] [CrossRef] [PubMed]
- González-Rodríguez, B.; De Las Heras, K.V.G.; Aguirre, D.T.; Rodríguez-Padial, L.; Albiñana, V.; Recio-Poveda, L.; Cuesta, A.M.; Botella, L.M.; Jiménez-Escribano, R.M. Evaluation of the safety and effectiveness of oral propranolol in patients with von Hippel-Lindau disease and retinal hemangioblastomas: Phase III clinical trial. BMJ Open Ophthalmol. 2019, 4, e000203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hogeling, M.; Adams, S.; Wargon, O. A randomized controlled trial of propranolol for infantile hemangiomas. Pediatrics 2011, 128, e259–e266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Léauté-Labrèze, C.; Dumas De La Roque, E.; Nacka, F.; Abouelfath, A.; Grenier, N.; Rebola, M.; Ezzedine, K.; Moore, N. Double-blind randomized pilot trial evaluating the efficacy of oral propranolol on infantile haemangiomas in infants <4 months of age. Br. J. Dermatol. 2013, 169, 181–183. [Google Scholar]
- Baselga, E.; Dembowska-Baginska, B.; Przewratil, P.; González-Enseñat, M.A.; Wyrzykowski, D.; Torrelo, A.; Gutiérrez, J.C.L.; Rychłowska-Pruszynska, M.; De Lucas-Laguna, R.; Esteve-Martinez, A.; et al. Efficacy of propranolol between 6 and 12 months of age in high-risk infantile hemangioma. Pediatrics 2018, 142, e20173866. [Google Scholar] [CrossRef] [Green Version]
- Zarrabeitia, R.; Albiñana, V.; Salcedo, M.; Señaris-Gonzalez, B.; Fernandez-Forcelledo, J.L.; Botella, L.M. A review on clinical management and pharmacological therapy on hereditary haemorrhagic telangiectasia (HHT). Curr. Vasc. Pharmacol. 2010, 8, 473–481. [Google Scholar] [CrossRef] [Green Version]
- Faughnan, M.E.; Mager, J.J.; Hetts, S.W.; Palda, V.A.; Lang-Robertson, K.; Buscarini, E.; Deslandres, E.; Kasthuri, R.S.; Lausman, A.; Poetker, D.; et al. Second International Guidelines for the Diagnosis and Management of Hereditary Hemorrhagic Telangiectasia. Ann. Intern. Med. 2020, 173, 989–1001. [Google Scholar] [CrossRef]
- Albiñana, V.; Cuesta, A.M.; de Rojas-P, I.; Gallardo-Vara, E.; Recio-Poveda, L.; Bernabéu, C.; Botella, L.M. Review of Pharmacological Strategies with Repurposed Drugs for Hereditary Hemorrhagic Telangiectasia Related Bleeding. J. Clin. Med. 2020, 9, 1766. [Google Scholar] [CrossRef] [PubMed]
- Albiñana, V.; Recio-Poveda, L.; Zarrabeitia, R.; Bernabéu, C.; Botella, L.M. Propranolol as antiangiogenic candidate for the therapy of hereditary haemorrhagic telangiectasia. Thromb. Haemost. 2012, 108, 41–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olitsky, S.E. Topical timolol for the treatment of epistaxis in hereditary hemorrhagic telangiectasia. Am. J. Otolaryngol. 2012, 33, 375–376. [Google Scholar] [CrossRef] [PubMed]
- Ichimura, K.; Kikuchi, H.; Imayoshi, S.; Dias, M.S. Topical application of timolol decreases the severity and frequency of epistaxis in patients who have previously undergone nasal dermoplasty for hereditary hemorrhagic telangiectasia. Auris Nasus Larynx 2016, 43, 429–432. [Google Scholar] [CrossRef] [PubMed]
- Botella-Cubells, L.M.; Zarrabeitia-Puente, R.; Albinana-Diaz, V.; Ojeda-Fernandez, M.L.; Diez-Gonzalez, V.; Parra-Blanco, J.A. Efficacy of topical timolol for the treatment of mucocutaneous telangiectasias in patients with hereditary haemorrhagic telangiectasia. Angiogenesis 2015, 18, 529. [Google Scholar]
- Mei-Zahav, M.; Blau, H.; Bruckheimer, E.; Zur, E.; Goldschmidt, N. Topical propranolol improves epistaxis in patients with hereditary hemorrhagic telangiectasia—A preliminary report. J. Otolaryngol. Head Neck Surg. 2017, 46, 58. [Google Scholar] [CrossRef] [Green Version]
- Mei-Zahav, M.; Gendler, Y.; Bruckheimer, E.; Prais, D.; Birk, E.; Watad, M.; Goldschmidt, N.; Soudry, E. Topical propranolol improves epistaxis control in hereditary hemorrhagic telangiectasia (HHT): A randomized double-blind placebo-controlled trial. J. Clin. Med. 2020, 9, 3130. [Google Scholar] [CrossRef]
- Esteban-Casado, S.; Martín de Rosales Cabrera, A.M.; Usarralde Pérez, A.; Martínez Simón, J.J.; Zhan Zhou, E.; Marcos Salazar, M.S.; Pérez Encinas, M.; Botella Cubells, L. Sclerotherapy and Topical Nasal Propranolol: An Effective and Safe Therapy for HHT-Epistaxis. Laryngoscope 2019, 129, 2216–2223. [Google Scholar] [CrossRef]
- Patier, J.L.; Camacho Aguirre, A.; Sirgo, N.; Gonzalez Nino, I.; Suárez Carantoña, C.; Gonzalez Garcia, A.; López Rodriguez, M.; Botella, L.M. Effectiveness and safety of the treatment with oral propranolol in patients with hereditary hemorrhagic telangiectasia and bloodhypertension or atrial fibrillation: A possible anti-angiogenictreatment in epistaxis. Angiogenesis 2019, 22, 628. [Google Scholar]
- Maher, E.R.; Neumann, H.P.H.; Richard, S. Von Hippel-Lindau disease: A clinical and scientific review. Eur. J. Hum. Genet. 2011, 19, 617–623. [Google Scholar] [CrossRef] [Green Version]
- Richard, S.; Gardie, B.; Couvé, S.; Gad, S. Von Hippel-Lindau: How a rare disease illuminates cancer biology. Semin. Cancer Biol. 2013, 23, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Gossage, L.; Eisen, T.; Maher, E.R. VHL, the story of a tumour suppressor gene. Nat. Rev. Cancer 2015, 15, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Bader, H.L.; Hsu, T. Systemic VHL gene functions and the VHL disease. FEBS Lett. 2012, 586, 1562–1569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haddad, N.M.N.; Cavallerano, J.D.; Silva, P.S. Von hippel-lindau disease: A genetic and clinical review. Semin. Ophthalmol. 2013, 28, 377–386. [Google Scholar] [CrossRef]
- Kassardjian, C.D.; Macdonald, R.L.; Munoz, D.G. Hemangioblastomas in the elderly: Epidemiology and clinical characteristics. J. Clin. Neurosci. 2014, 21, 1205–1208. [Google Scholar] [CrossRef]
- Kaelin, W.G. Molecular basis of the VHL hereditary cancer syndrome. Nat. Rev. Cancer 2002, 2, 673–682. [Google Scholar] [CrossRef]
- Vortmeyer, A.O.; Falke, E.A.; Gläsker, S.; Li, J.; Oldfield, E.H. Nervous system involvement in von Hippel-Lindau disease: Pathology and mechanisms. Acta Neuropathol. 2013, 125, 333–350. [Google Scholar] [CrossRef]
- Van Velthoven, V.; Reinacher, P.C.; Klisch, J.; Neumann, H.P.H.; Gläsker, S.; Bristol, R.E.; Spetzler, R.F.; Bricolo, A.; Benzel, E.C.; Lefranc, F.; et al. Treatment of Intramedullary Hemangioblastomas, with Special Attention to Von Hippel-Lindau Disease. Neurosurgery 2003, 53, 1306–1314. [Google Scholar] [CrossRef]
- Jonasch, E.; Donskov, F.; Iliopoulos, O.; Rathmell, W.K.; Narayan, V.; Maughan, B.L.; Oudard, S.; Else, T.; Maranchie, J.K.; Welsh, S.J.; et al. Phase II study of the oral HIF-2α inhibitor MK-6482 for Von Hippel-Lindau disease–associated renal cell carcinoma. J. Clin. Oncol. 2020, 38, 5003. [Google Scholar] [CrossRef]
- Choueiri, T.K.; Bauer, T.M.; Papadopoulos, K.P.; Plimack, E.R.; Merchan, J.R.; McDermott, D.F.; Michaelson, M.D.; Appleman, L.J.; Thamake, S.; Perini, R.F.; et al. Inhibition of hypoxia-inducible factor-2α in renal cell carcinoma with belzutifan: A phase 1 trial and biomarker analysis. Nat. Med. 2021, 27, 802–805. [Google Scholar] [CrossRef]
- Chang, P.Y.; Huang, W.Y.; Lin, C.L.; Huang, T.C.; Wu, Y.Y.; Chen, J.H.; Kao, C.H. Propranolol Reduces Cancer Risk: A Population-Based Cohort Study. Medicine 2015, 94, e1097. [Google Scholar] [CrossRef] [PubMed]
- Munabi, N.C.O.; England, R.W.; Edwards, A.K.; Kitajewski, A.A.; Tan, Q.K.; Weinstein, A.; Kung, J.E.; Wilcox, M.; Kitajewski, J.K.; Shawber, C.J.; et al. Propranolol Targets Hemangioma Stem Cells via cAMP and Mitogen-Activated Protein Kinase Regulation. Stem Cells Transl. Med. 2016, 5, 45–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martini, D.; Dal Monte, M.; Ristori, C.; Cupisti, E.; Mei, S.; Fiorini, P.; Filippi, L.; Bagnoli, P. Antiangiogenic effects of β 2-adrenergic receptor blockade in a mouse model of oxygen-induced retinopathy. J. Neurochem. 2011, 119, 1317–1329. [Google Scholar] [CrossRef]
- Sharifpanah, F.; Saliu, F.; Bekhite, M.M.; Wartenberg, M.; Sauer, H. β-adrenergic receptor antagonists inhibit vasculogenesis of embryonic stem cells by downregulation of nitric oxide generation and interference with VEGF signalling. Cell Tissue Res. 2014, 358, 443–452. [Google Scholar] [CrossRef]
- Yin, T.; Yu, S.; Xiao, L.; Zhang, J.; Liu, C.; Lu, Y.; Liu, C. Correlation between the expression of aquaporin 1 and hypoxia-inducible factor 1 in breast cancer tissues. J. Huazhong Univ. Sci. Technol. Med. Sci. 2008, 28, 346–348. [Google Scholar] [CrossRef]
- Abreu-Rodríguez, I.; Sánchez Silva, R.; Martins, A.P.; Soveral, G.; Toledo-Aral, J.J.; López-Barneo, J.; Echevarría, M. Functional and transcriptional induction of aquaporin-1 gene by hypoxia; analysis of promoter and role of HIF-1α. PLoS ONE 2011, 6, e28385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deb, P.; Pal, S.; Dutta, V.; Boruah, D.; Chandran, V.M.; Bhatoe, H.S. Correlation of expression pattern of aquaporin-1 in primary central nervous system tumors with tumor type, grade, proliferation, microvessel density, contrast-enhancement and perilesional edema. J. Cancer Res. Ther. 2012, 8, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Scott, P.; Anderson, K.; Singhania, M.; Cormier, R. Cystic fibrosis, CFTR, and colorectal cancer. Int. J. Mol. Sci. 2020, 21, 2891. [Google Scholar] [CrossRef] [Green Version]
- Mehta, N.; Rousslang, L.; Shokouh-Amiri, M.; Wiley, E.L.; Green, L. Complex solid and cystic breast cancer: A series of six case reports. J. Radiol. Case Rep. 2020, 14, 21–44. [Google Scholar] [CrossRef]
- Shi, Z.; Wei, J.; Na, R.; Resurreccion, W.K.; Zheng, S.L.; Hulick, P.J.; Helfand, B.T.; Talamonti, M.S.; Xu, J. Cystic fibrosis F508del carriers and cancer risk: Results from the UK Biobank. Int. J. Cancer 2021, 148, 1658–1664. [Google Scholar] [CrossRef]
- Lenders, J.W.M.; Kerstens, M.N.; Amar, L.; Prejbisz, A.; Robledo, M.; Taieb, D.; Pacak, K.; Crona, J.; Zelinka, T.; Mannelli, M.; et al. Genetics, diagnosis, management and future directions of research of phaeochromocytoma and paraganglioma: A position statement and consensus of the Working Group on Endocrine Hypertension of the European Society of Hypertension. J. Hypertens. 2020, 38, 1443–1456. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Castellanos, M.A.; Gómez de las Heras, K.V.; Díaz-Redondo, T.; González-Flores, E.; Albiñana, V.; Botella, L.M. Case Report: Propranolol increases the therapeutic response to temozolomide in a patient with metastatic paraganglioma. F1000Research 2017, 6, 2087. [Google Scholar] [CrossRef] [Green Version]
- Maturo, S.; Tse, S.M.; Kinane, T.B.; Hartnick, C.J. Initial experience using propranolol as an adjunctive treatment in children with aggressive recurrent respiratory papillomatosis. Ann. Otol. Rhinol. Laryngol. 2011, 120, 17–20. [Google Scholar] [CrossRef]
- Lanfranconi, S.; Scola, E.; Bertani, G.A.; Zarino, B.; Pallini, R.; D’Alessandris, G.; Mazzon, E.; Marino, S.; Carriero, M.R.; Scelzo, E.; et al. Propranolol for familial cerebral cavernous malformation (Treat_CCM): Study protocol for a randomized controlled pilot trial. Trials 2020, 21, 401. [Google Scholar] [CrossRef] [PubMed]
- Heinhuis, K.M.; Ijzerman, N.S.; Koenen, A.M.; Van Der Graaf, W.T.A.; Haas, R.L.; Beijnen, J.H.; Huitema, A.D.R.; Van Houdt, W.J.; Steeghs, N. PropAngio study protocol: A neoadjuvant trial on the efficacy of propranolol monotherapy in cutaneous angiosarcoma—A proof of principle study. BMJ Open 2020, 10, e03944. [Google Scholar] [CrossRef] [PubMed]
- Tian, R.; Liang, Y.; Wang, J.; Shan, Y.; Gao, H.; Zhang, L.; Xie, C.; Li, J.; Xu, M.; Gu, S. Propranolol for infantile hepatic hemangioendothelioma: Clinical evaluation of drug efficacy and safety using a single-center patient cohort. Ann. Hepatol. 2020, 19, 530–534. [Google Scholar] [CrossRef]
- Orsenigo, F.; Conze, L.L.; Jauhiainen, S.; Corada, M.; Lazzaroni, F.; Malinverno, M.; Sundell, V.; Cunha, S.I.; Brännström, J.; Globisch, M.A.; et al. Mapping endothelial-cell diversity in cerebral cavernous malformations at single-cell resolution. eLife 2020, 9, e61413. [Google Scholar] [CrossRef]
- Zabramski, J.M.; Kalani, M.Y.S.; Filippidis, A.S.; Spetzler, R.F. Propranolol Treatment of Cavernous Malformations with Symptomatic Hemorrhage. World Neurosurg. 2016, 88, 631–639. [Google Scholar] [CrossRef]
- Reinhard, M.; Schuchardt, F.; Meckel, S.; Heinz, J.; Felbor, U.; Sure, U.; Geisen, U. Propranolol stops progressive multiple cerebral cavernoma in an adult patient. J. Neurol. Sci. 2016, 367, 15–17. [Google Scholar] [CrossRef]
- Li, W.; Shenkar, R.; Detter, M.R.; Moore, T.; Benavides, C.; Lightle, R.; Girard, R.; Hobson, N.; Cao, Y.; Li, Y.; et al. Propranolol inhibits cavernous vascular malformations by β1 adrenergic receptor antagonism in animal models. J. Clin. Investig. 2021, 131, e154909. [Google Scholar] [CrossRef]
- Oldenburg, J.; Malinverno, M.; Globisch, M.A.; Maderna, C.; Corada, M.; Orsenigo, F.; Conze, L.L.; Rorsman, C.; Sundell, V.; Arce, M.; et al. Propranolol Reduces the Development of Lesions and Rescues Barrier Function in Cerebral Cavernous Malformations: A Preclinical Study. Stroke 2021, 52, 1418–1427. [Google Scholar] [CrossRef] [PubMed]
- NIH, US National Library of Medicine. Available online: https://ClinicalTrials.gov/show/NCT03523650 (accessed on 24 March 2022).
- NIH, US National Library of Medicine. Available online: https://ClinicalTrials.gov/show/NCT03474614 (accessed on 24 March 2022).
- NIH, US National Library of Medicine. Available online: https://ClinicalTrials.gov/show/NCT02104011 (accessed on 24 March 2022).
- Yang, L.; Agarwal, P. Systematic drug repositioning based on clinical side-effects. PLoS ONE 2011, 6, e28025. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cuesta, A.M.; Gallardo-Vara, E.; Casado-Vela, J.; Recio-Poveda, L.; Botella, L.-M.; Albiñana, V. The Role of Propranolol as a Repurposed Drug in Rare Vascular Diseases. Int. J. Mol. Sci. 2022, 23, 4217. https://doi.org/10.3390/ijms23084217
Cuesta AM, Gallardo-Vara E, Casado-Vela J, Recio-Poveda L, Botella L-M, Albiñana V. The Role of Propranolol as a Repurposed Drug in Rare Vascular Diseases. International Journal of Molecular Sciences. 2022; 23(8):4217. https://doi.org/10.3390/ijms23084217
Chicago/Turabian StyleCuesta, Angel M., Eunate Gallardo-Vara, Juan Casado-Vela, Lucía Recio-Poveda, Luisa-María Botella, and Virginia Albiñana. 2022. "The Role of Propranolol as a Repurposed Drug in Rare Vascular Diseases" International Journal of Molecular Sciences 23, no. 8: 4217. https://doi.org/10.3390/ijms23084217
APA StyleCuesta, A. M., Gallardo-Vara, E., Casado-Vela, J., Recio-Poveda, L., Botella, L.-M., & Albiñana, V. (2022). The Role of Propranolol as a Repurposed Drug in Rare Vascular Diseases. International Journal of Molecular Sciences, 23(8), 4217. https://doi.org/10.3390/ijms23084217








