Quadruped Gait and Regulation of Apoptotic Factors in Tibiofemoral Joints following Intra-Articular rhPRG4 Injection in Prg4 Null Mice
Abstract
:1. Introduction
2. Results
2.1. Gait and Posture Indices of Prg4−/− and Prg4+/+ Mice
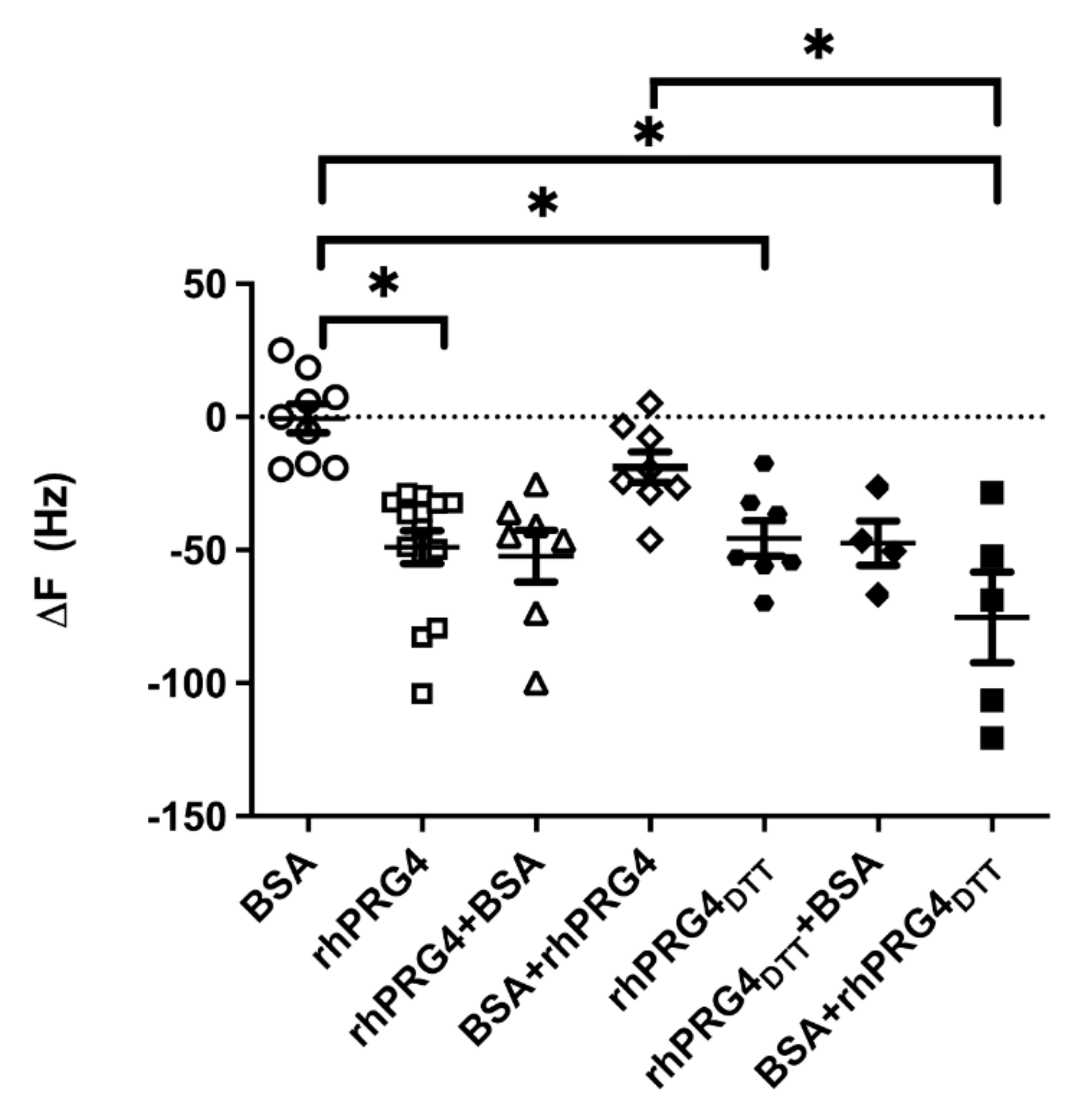
Changes in Gait and Posture Indices following rhPRG4 Injection in Prg4−/− Mice
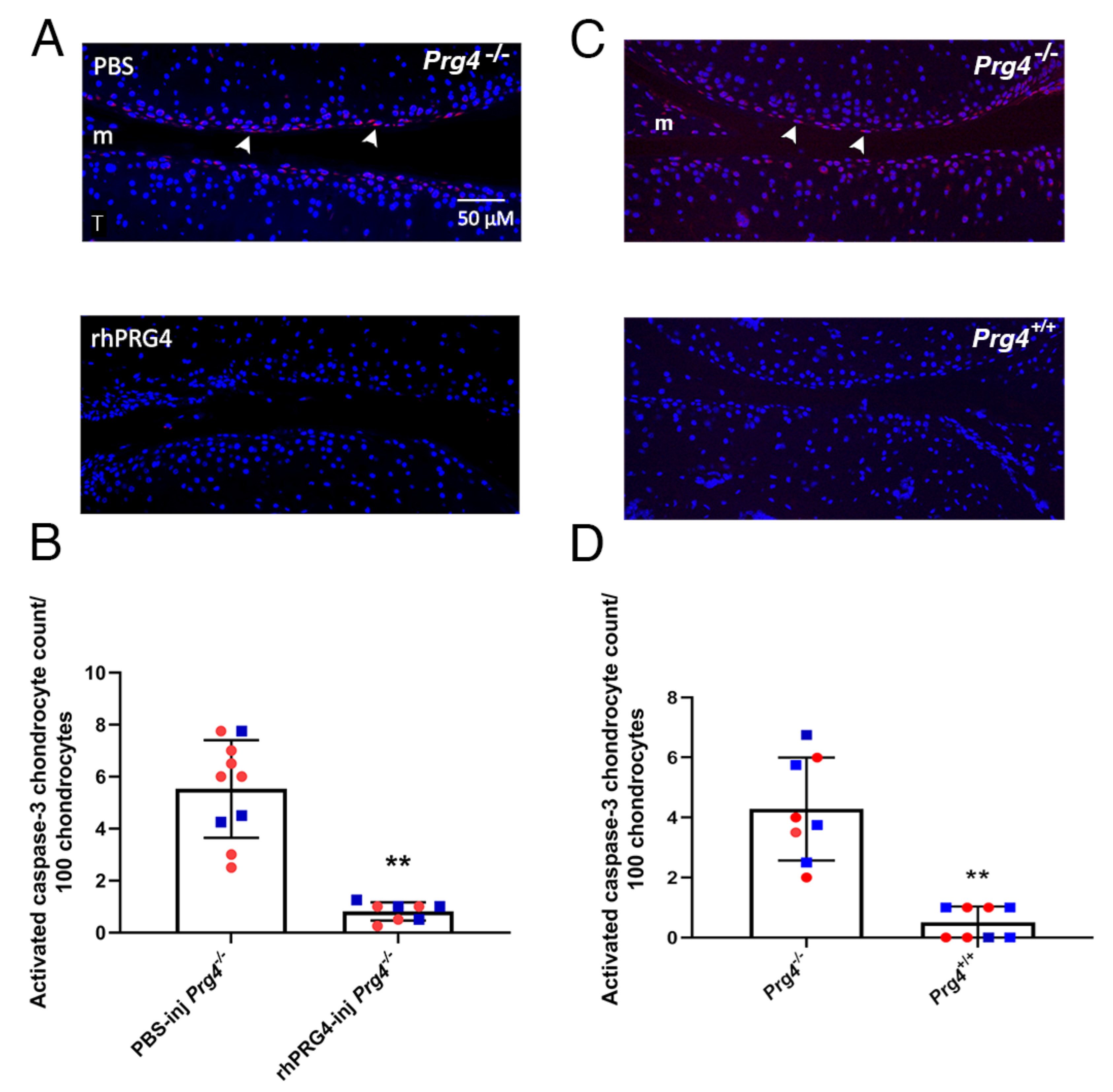
2.2. Caspase-3 Activation in Superficial Zone Chondrocytes
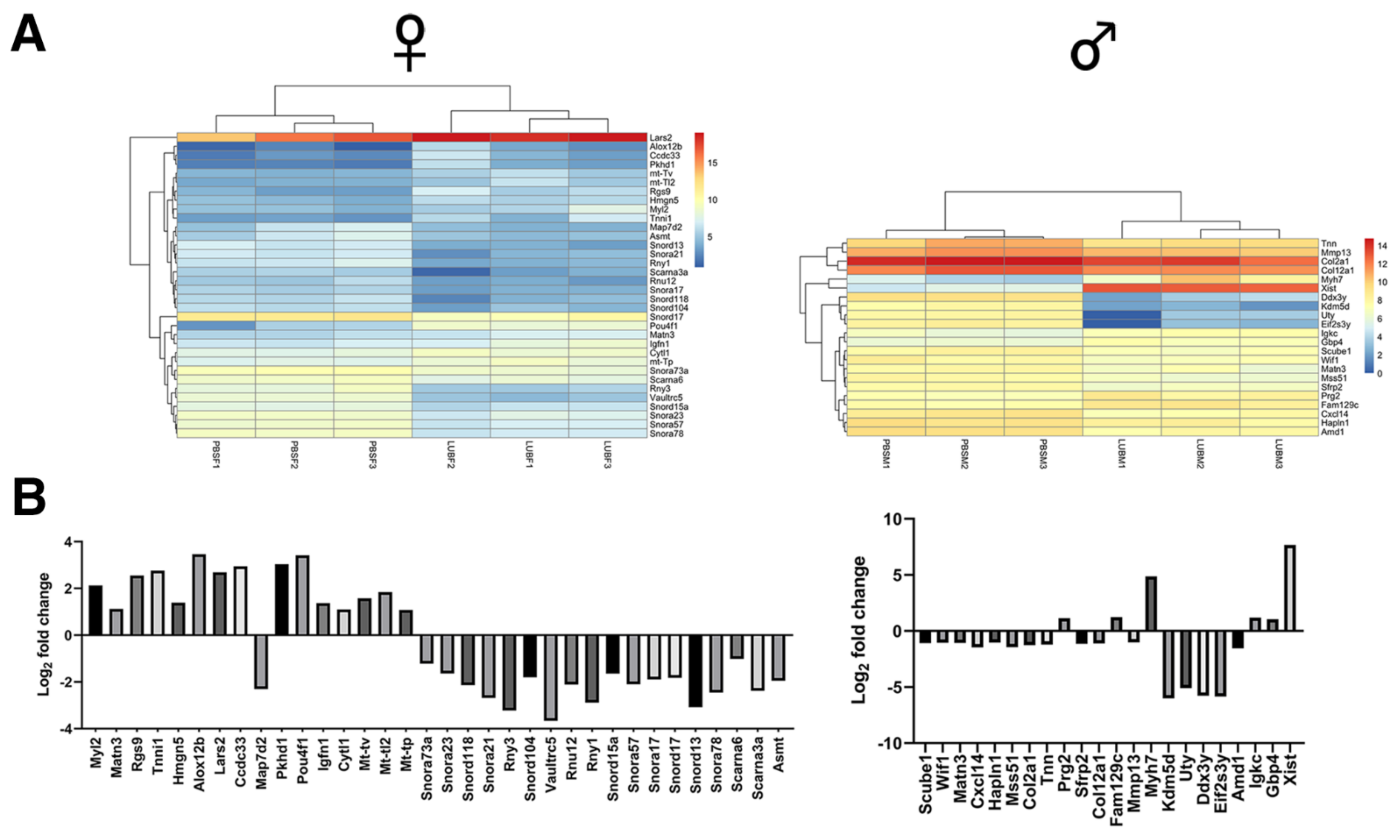
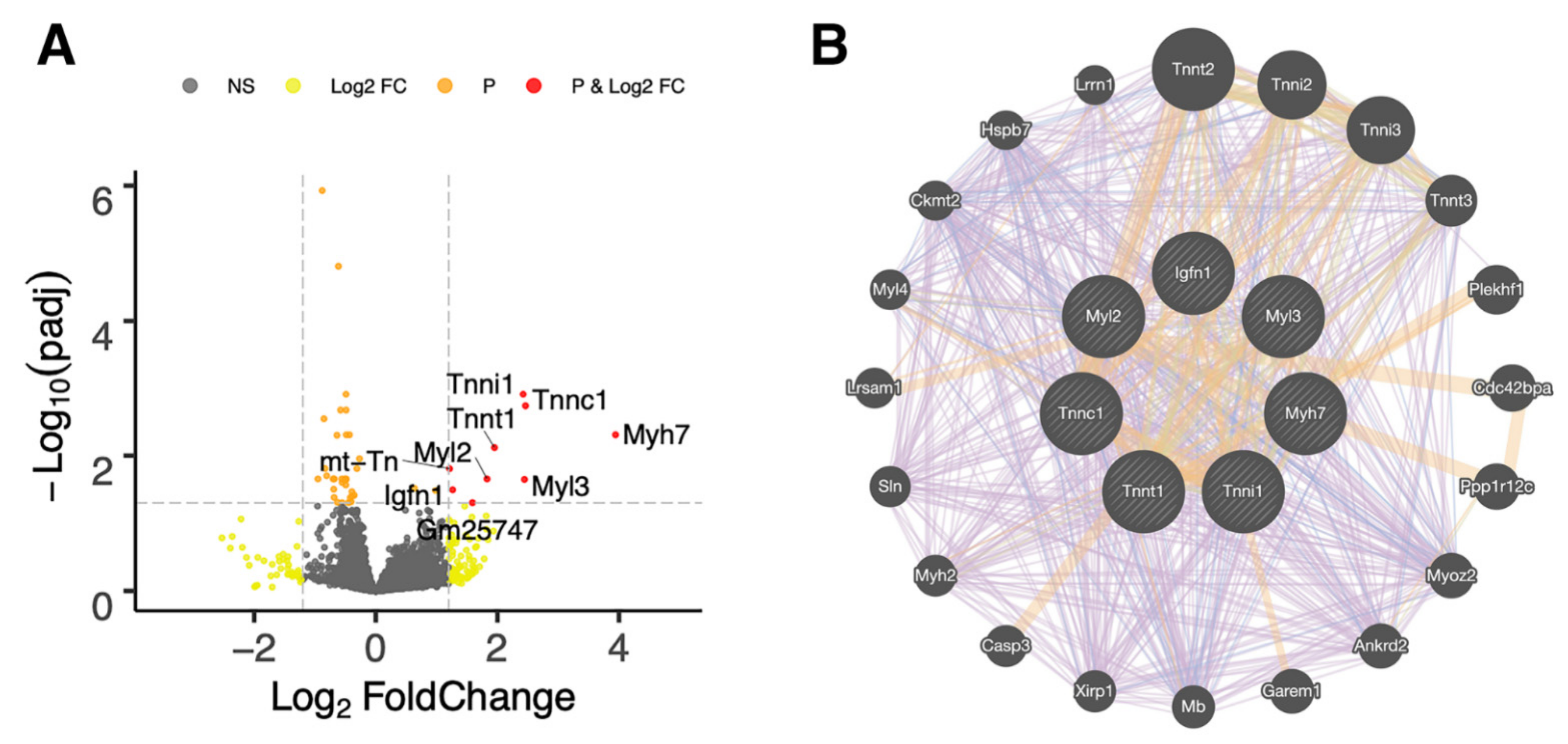
2.3. RNA-Seq Analysis of Recovered Articular Cartilage in Male and Female Prg4−/− Mice following rhPRG4 Injection
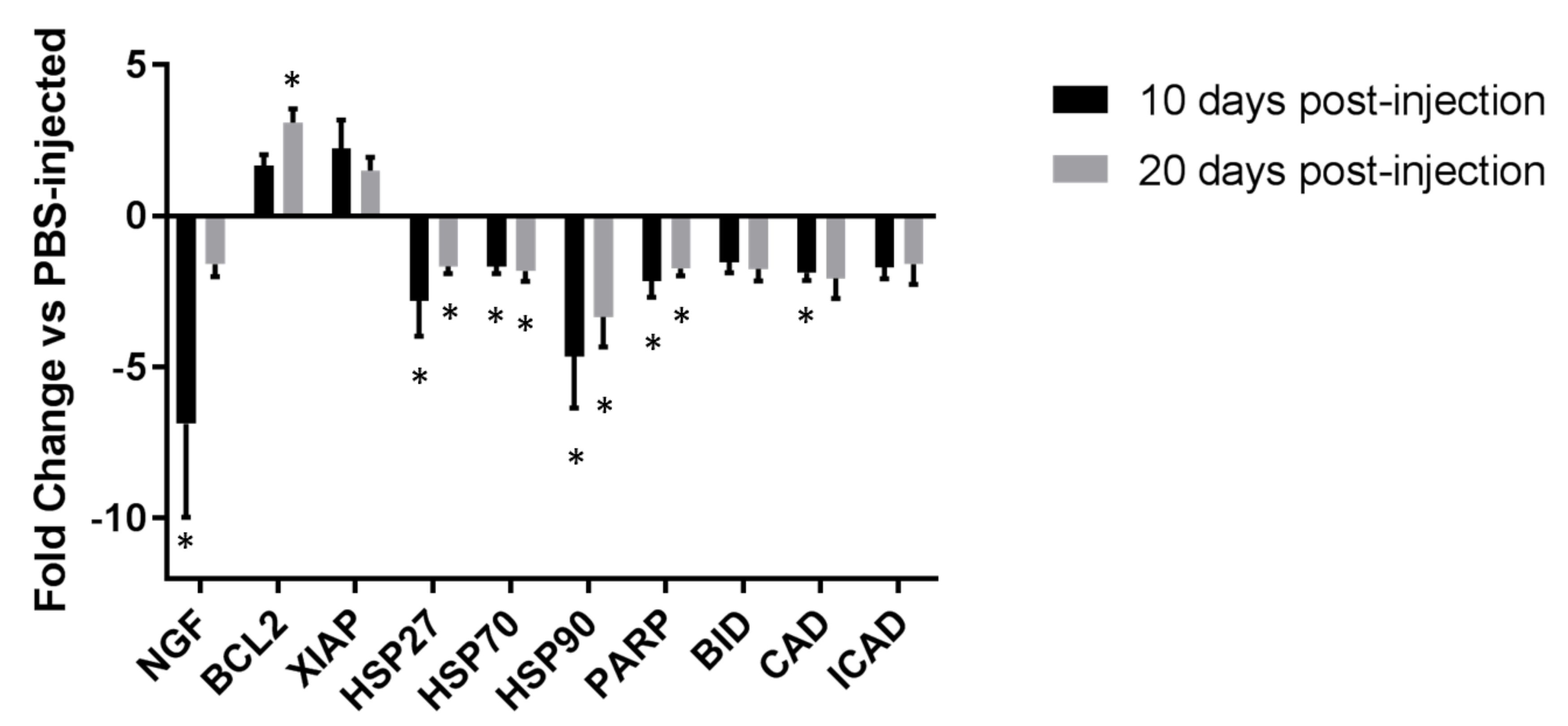
2.4. qRT-PCR of Nerve Growth Factor and Apoptotic Genes from Recovered Synovium
2.5. Accumulation of Collagen-Binding Protein in the Superficial Zone
2.6. Quartz Microbalance Measurements for Adsorption and Anti-Adhesion
3. Discussion
4. Materials and Methods
4.1. Prg4−/− Mice
4.2. Manufacture and Purification of rhPRG4
4.3. In Vivo Tribosupplementation of Prg4−/− Mouse Knees
4.4. Gait Analysis
4.5. Tissue Processing for Immunohistochemistry of Caspase-3 and Collagen Hybridizing Peptide
4.6. Quantitative Real-Time Polymerase Chain Reaction of Synovial RNA
4.7. RNA-Seq Analysis of Chondrocyte RNA
4.8. Gene Ontology Analysis
4.9. Adsorption and Anti-Adhesion by rhPRG4 Using Quartz Crystal Microbalance
4.10. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schaefer, D.B.; Wendt, D.; Moretti, M.; Jakob, M.; Jay, G.D.; Heberer, M.; Martin, I. Lubricin reduces cartilage—Cartilage integration. Biorheology 2004, 41, 503–508. [Google Scholar]
- Rhee, D.K.; Marcelino, J.; Al-Mayouf, S.; Schelling, D.K.; Bartels, C.F.; Cui, Y.; Laxer, R.; Goldbach-Mansky, R.; Warman, M.L. Consequences of Disease-causing Mutations on Lubricin Protein Synthesis, Secretion, and Post-translational Processing. J. Biol. Chem. 2005, 280, 31325–31332. [Google Scholar] [CrossRef] [Green Version]
- Hill, A.; Waller, K.A.; Cui, Y.; Allen, J.M.; Smits, P.; Zhang, L.X.; Ayturk, U.M.; Hann, S.; Lessard, S.G.; Zurakowski, D.; et al. Lubricin restoration in a mouse model of congenital deficiency. Arthritis Rheumatol. 2015, 67, 3070–3081. [Google Scholar] [CrossRef] [Green Version]
- Yilmaz, S.; Uludag Alkaya, D.; Kasapcopur, O.; Barut, K.; Akdemir, E.S.; Celen, C.; Youngblood, M.W.; Yasuno, K.; Bilguvar, K.; Gunel, M.; et al. Genotype-phenotype investigation of 35 patients from 11 unrelated families with camptodactyly-arthropathy-coxa vara-pericarditis (CACP) syndrome. Mol. Genet. Genom. Med. 2018, 6, 230–248. [Google Scholar] [CrossRef]
- Waller, K.A.; Chin, K.E.; Jay, G.D.; Zhang, L.X.; Teeple, E.; McAllister, S.; Badger, G.J.; Schmidt, T.A.; Fleming, B.C. Intra-articular Recombinant Human Proteoglycan 4 Mitigates Cartilage Damage After Destabilization of the Medial Meniscus in the Yucatan Minipig. Am. J. Sports Med. 2017, 45, 1512–1521. [Google Scholar] [CrossRef]
- Rhee, D.K.; Marcelino, J.; Baker, M.; Gong, Y.; Smits, P.; Lefebvre, V.; Jay, G.D.; Stewart, M.; Wang, H.; Warman, M.L.; et al. The secreted glycoprotein lubricin protects cartilage surfaces and inhibits synovial cell overgrowth. J. Clin. Investig. 2005, 115, 622–631. [Google Scholar] [CrossRef] [Green Version]
- Jay, G.D.; Torres, J.R.; Rhee, D.K.; Helminen, H.J.; Hytinnen, M.M.; Cha, C.J.; Elsaid, K.; Kim, K.S.; Cui, Y.; Warman, M.L. Association between friction and wear in diarthrodial joints lacking lubricin. Arthritis Rheum. 2007, 56, 3662–3669. [Google Scholar] [CrossRef] [Green Version]
- Waller, K.A.; Zhang, L.X.; Jay, G.D. Friction-Induced Mitochondrial Dysregulation Contributes to Joint Deterioration in Prg4 Knockout Mice. Int. J. Mol. Sci. 2017, 18, 1252. [Google Scholar] [CrossRef]
- Waller, K.A.; Zhang, L.X.; Elsaid, K.A.; Fleming, B.C.; Warman, M.L.; Jay, G.D. Role of lubricin and boundary lubrication in the prevention of chondrocyte apoptosis. Proc. Natl. Acad. Sci. USA 2013, 110, 5852–5857. [Google Scholar] [CrossRef] [Green Version]
- Bahabri, S.A.; Suwairi, W.M.; Laxer, R.M.; Polinkovsky, A.; Dalaan, A.A.; Warman, M.L. The camptodactyly-arthropathy-coxa vara-pericarditis syndrome: Clinical features and genetic mapping to human chromosome 1. Arthritis Rheum. 1998, 41, 730–735. [Google Scholar] [CrossRef]
- Marcelino, J.; Carpten, J.D.; Suwairi, W.M.; Gutierrez, O.M.; Schwartz, S.; Robbins, C.; Sood, R.; Makalowska, I.; Baxevanis, A.; Johnstone, B.; et al. CACP, encoding a secreted proteoglycan, is mutated in camptodactyly-arthropathy-coxa vara-pericarditis syndrome. Nat. Genet. 1999, 23, 319–322. [Google Scholar] [CrossRef]
- Bao, J.-P.; Chen, W.-P.; Wu, L.-D. Lubricin: A novel potential biotherapeutic approaches for the treatment of osteoarthritis. Mol. Biol. Rep. 2010, 38, 2879–2885. [Google Scholar] [CrossRef]
- Alazami, A.M.; Al-Mayouf, S.M.; Wyngaard, C.-A.; Meyer, B. Novel PRG4 mutations underlie CACP in Saudi families. Hum. Mutat. 2006, 27, 213. [Google Scholar] [CrossRef]
- Al-Mayouf, S.M.; Albuhairan, I. Camptodactyly-arthropathy-coxa vara-pericarditis syndrome: Clinical and molecular genetic findings. Bone Abstr. 2013, 2, 5. [Google Scholar]
- Drewniak, E.I.; Jay, G.D.; Fleming, B.C.; Zhang, L.; Warman, M.L.; Crisco, J.J. Cyclic loading increases friction and changes cartilage surface integrity in lubricin-mutant mouse knees. Arthritis Rheum. 2012, 64, 465–473. [Google Scholar] [CrossRef]
- Bonnevie, E.D.; Delco, M.L.; Bartell, L.R.; Jasty, N.; Cohen, I.; Fortier, L.A.; Bonassar, L.J. Microscale frictional strains determine chondrocyte fate in loaded cartilage. J. Biomech. 2018, 74, 72–78. [Google Scholar] [CrossRef]
- Ludwig, T.E.; McAllister, J.R.; Lun, V.; Wiley, J.P.; Schmidt, T.A. Diminished cartilage-lubricating ability of human osteoarthritic synovial fluid deficient in proteoglycan 4: Restoration through proteoglycan 4 supplementation. Arthritis Rheum. 2012, 64, 3963–3971. [Google Scholar] [CrossRef]
- Jay, G.D.; Elsaid, K.A.; Kelly, K.A.; Anderson, S.C.; Zhang, L.; Teeple, E.; Waller, K.; Fleming, B.C. Prevention of cartilage degeneration and gait asymmetry by lubricin tribosupplementation in the rat following anterior cruciate ligament transection. Arthritis Rheum. 2012, 64, 1162–1171. [Google Scholar] [CrossRef] [Green Version]
- Emad, Y.; Ragab, Y.; Khalifa, M.; Bassyouni, I.; EL-Shaarawy, N.; Rasker, J.J. Axial involvement with facet joint arthropathy and bony ankylosis in a case of camptodactyly, arthropathy, coxa vara, pericarditis (CACP) syndrome. Jt. Bone Spine 2013, 80, 520–522. [Google Scholar] [CrossRef]
- Jacobs, B.Y.; Kloefkorn, H.E.; Allen, K.D. Gait Analysis Methods for Rodent Models of Osteoarthritis. Curr. Pain Headache Rep. 2014, 18, 456. [Google Scholar] [CrossRef] [Green Version]
- Zitnay, J.L.; Li, Y.; Qin, Z.; San, B.H.; Depalle, B.; Reese, S.P.; Buehler, M.J.; Yu, S.M.; Weiss, J.A. Molecular level detection and localization of mechanical damage in collagen enabled by collagen hybridizing peptides. Nat. Commun. 2017, 8, 14913. [Google Scholar] [CrossRef]
- Waters, J.C. Accuracy and precision in quantitative fluorescence microscopy. J. Cell Biol. 2009, 185, 1135–1148. [Google Scholar] [CrossRef] [Green Version]
- Jay, G.D.; Tantravahi, U.; Britt, D.E.; Barrach, H.J.; Cha, C.-J. Homology of lubricin and superficial zone protein (SZP): Products of megakaryocyte stimulating factor (MSF) gene expression by human synovial fibroblasts and articular chondrocytes localized to chromosome 1q25. J. Orthop. Res. 2001, 19, 677–687. [Google Scholar] [CrossRef]
- Flannery, C.R.; Hughes, C.E.; Schumacher, B.L.; Tudor, D.; Aydelotte, M.B.; Kuettner, K.E.; Caterson, B. Articular cartilage superficial zone protein (SZP) is homologous to megakaryocyte stimulating factor precursor and Is a multifunctional proteoglycan with potential growth-promoting, cytoprotective, and lubricating properties in cartilage metabolism. Biochem. Biophys. Res. Commun. 1999, 254, 535–541. [Google Scholar] [CrossRef]
- Albuhairan, I.; Al-Mayouf, S.M. Camptodactyly-arthropathy-coxavara-pericarditis syndrome in Saudi Arabia: Clinical and molecular genetic findings in 22 patients. Semin. Arthritis Rheum. 2013, 43, 292–296. [Google Scholar] [CrossRef]
- D’Lima, D.; Hermida, J.; Hashimoto, S.; Colwell, C.; Lotz, M. Caspase inhibitors reduce severity of cartilage lesions in experimental osteoarthritis. Arthritis Rheum. 2006, 54, 1814–1821. [Google Scholar] [CrossRef]
- Bartell, L.R.; Fortier, L.A.; Bonassar, L.J.; Szeto, H.H.; Cohen, I.; Delco, M.L. Mitoprotective therapy prevents rapid, strain-dependent mitochondrial dysfunction after articular cartilage injury. J. Orthop. Res. 2020, 38, 1257–1267. [Google Scholar] [CrossRef]
- Wahyudi, H.; Reynolds, A.A.; Li, Y.; Owen, S.C.; Yu, S.M. Targeting collagen for diagnostic imaging and therapeutic delivery. J. Control. Release 2016, 240, 323–331. [Google Scholar] [CrossRef] [Green Version]
- Majd, S.E.; Kuijer, R.; Kowitsch, A.; Groth, T.; Schmidt, T.A.; Sharma, P.K. Both hyaluronan and collagen type II keep proteoglycan 4 (lubricin) at the cartilage surface in a condition that provides low friction during boundary lubrication. Langmuir 2014, 30, 14566–14572. [Google Scholar] [CrossRef]
- Chappuis, J.; Sherman, I.A.; Neumann, A.W. Surface tension of animal cartilage as it relates to friction in joints. Ann. Biomed. Eng. 1983, 11, 435–449. [Google Scholar] [CrossRef]
- Schlapakow, E.; Peeva, V.; Zsurka, G.; Jeub, M.; Wabbels, B.; Kornblum, C.; Kunz, W.S. Distinct segregation of the pathogenic m.5667G>A mitochondrial tRNA(Asn) mutation in extraocular and skeletal muscle in chronic progressive external ophthalmoplegia. Neuromuscul. Disord. 2019, 29, 358–367. [Google Scholar] [CrossRef]
- Reyes, A.; He, J.; Mao, C.C.; Bailey, L.J.; Di Re, M.; Sembongi, H.; Kazak, L.; Dzionek, K.; Holmes, J.B.; Cluett, T.J.; et al. Actin and myosin contribute to mammalian mitochondrial DNA maintenance. Nucleic Acids Res. 2011, 39, 5098–5108. [Google Scholar] [CrossRef]
- Johnston, J.R.; Chase, P.B.; Pinto, J.R. Troponin through the looking-glass: Emerging roles beyond regulation of striated muscle contraction. Oncotarget 2018, 9, 1461–1482. [Google Scholar] [CrossRef] [Green Version]
- GeneCards.TNNI1-TroponinI1, Slow Skeletal Type. Available online: https://www.genecards.org/cgi-bin/carddisppl?gene=TNNI1&keywords=Tnni1 (accessed on 30 March 2022).
- Franz, M.; Rodriguez, H.; Lopes, C.; Zuberi, K.; Montojo, J.; Bader, G.D.; Morris, Q. GeneMANIA update 2018. Nucleic Acids Res. 2018, 46, W60–W64. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Lin, J.L.; Erives, A.J.; Lin, C.I.; Lin, J.J. New insights into the roles of Xin repeat-containing proteins in cardiac development, function, and disease. Int. Rev. Cell Mol. Biol. 2014, 310, 89–128. [Google Scholar]
- Maurya, S.K.; Herrera, J.L.; Sahoo, S.K.; Reis, F.C.G.; Vega, R.B.; Kelly, D.P.; Periasamy, M. Sarcolipin Signaling Promotes Mitochondrial Biogenesis and Oxidative Metabolism in Skeletal Muscle. Cell Rep. 2018, 24, 2919–2931. [Google Scholar] [CrossRef] [Green Version]
- Bean, C.; Verma, N.K.; Yamamoto, D.L.; Chemello, F.; Cenni, V.; Filomena, M.C.; Chen, J.; Bang, M.L.; Lanfranchi, G. Ankrd2 is a modulator of NF-κB-mediated inflammatory responses during muscle differentiation. Cell Death Dis. 2014, 5, e1002. [Google Scholar] [CrossRef]
- Park, N.; Marquez, J.; Garcia, M.V.F.; Shimizu, I.; Lee, S.R.; Kim, H.K.; Han, J. Phosphorylation in Novel Mitochondrial Creatine Kinase Tyrosine Residues Render Cardioprotection against Hypoxia/Reoxygenation Injury. J. Lipid Atheroscler. 2021, 10, 223–239. [Google Scholar] [CrossRef]
- Niemann, A.; Huber, N.; Wagner, K.M.; Somandin, C.; Horn, M.; Lebrun-Julien, F.; Angst, B.; Pereira, J.A.; Halfter, H.; Welzl, H.; et al. The Gdap1 knockout mouse mechanistically links redox control to Charcot–Marie–Tooth disease. Brain 2014, 137, 668–682. [Google Scholar] [CrossRef]
- Chen, W.; Li, N.; Chen, T.; Han, Y.; Li, C.; Wang, Y.; He, W.; Zhang, L.; Wan, T.; Cao, X. The lysosome-associated apoptosis-inducing protein containing the pleckstrin homology (PH) and FYVE domains (LAPF), representative of a novel family of PH and FYVE domain-containing proteins, induces caspase-independent apoptosis via the lysosomal-mitochondrial pathway. J. Biol. Chem. 2005, 280, 40985–40995. [Google Scholar]
- Tiosano, D.; Mears, J.A.; Buchner, D.A. Mitochondrial Dysfunction in Primary Ovarian Insufficiency. Endocrinology 2019, 160, 2353–2366. [Google Scholar] [CrossRef] [PubMed]
- Plaas, A.; Li, J.; Riesco, J.; Das, R.; Sandy, J.D.; Harrison, A. Intraarticular injection of hyaluronan prevents cartilage erosion, periarticular fibrosis and mechanical allodynia and normalizes stance time in murine knee osteoarthritis. Arthritis Res. Ther. 2011, 13, R46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vincelette, J.; Xu, Y.; Zhang, L.-N.; Schaefer, C.J.; Vergona, R.; Sullivan, M.E.; Hampton, T.G.; Wang, Y.-X. Gait analysis in a murine model of collagen-induced arthritis. Arthritis Res. Ther. 2007, 9, R123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feldman, G.; Offemaria, A.; Sawan, H.; Parvizi, J.; Freeman, T.A. A murine model for developmental dysplasia of the hip: Ablation of CX3CR1 affects acetabular morphology and gait. J. Transl. Med. 2017, 15, 233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berryman, E.R.; Harris, R.L.; Moalli, M.; Bagi, C.M. Digigait quantitation of gait dynamics in rat rheumatoid arthritis model. J. Musculoskelet. Neuronal Interact. 2009, 9, 89–98. [Google Scholar]
- Elsaid, K.A.; Zhang, L.; Waller, K.; Tofte, J.; Teeple, E.; Fleming, B.C.; Jay, G.D. The impact of forced joint exercise on lubricin biosynthesis from articular cartilage following ACL transection and intra-articular lubricin’s effect in exercised joints following ACL transection. Osteoarthr. Cartil. 2012, 20, 940–948. [Google Scholar] [CrossRef] [Green Version]
- Qadri, M.; Jay, G.D.; Zhang, L.X.; Wong, W.; Reginato, A.M.; Sun, C.; Schmidt, T.A.; Elsaid, K.A. Recombinant human proteoglycan-4 reduces phagocytosis of urate crystals and downstream nuclear factor kappa B and inflammasome activation and production of cytokines and chemokines in human and murine macrophages. Arthritis Res. Ther. 2018, 20, 192. [Google Scholar] [CrossRef] [Green Version]
- Malfait, A.M.; Miller, R.E. Why we should study osteoarthritis pain in experimental models in both sexes. Osteoarthr. Cartil. 2020, 28, 397–399. [Google Scholar] [CrossRef]
- Ma, H.-L.; Blanchet, T.J.; Peluso, D.; Hopkins, B.; Morris, E.A.; Glasson, S.S. Osteoarthritis severity is sex dependent in a surgical mouse model. Osteoarthr. Cartil. 2007, 15, 695–700. [Google Scholar] [CrossRef] [Green Version]
- Griffin, T.M.; Scanzello, C.R. Innate inflammation and synovial macrophages in osteoarthritis pathophysiology. Clin. Exp. Rheumatol. 2019, 37 (Suppl. 120), 57–63. [Google Scholar]
- Miller, R.J.; Malfait, A.-M.; Miller, R.E. The innate immune response as a mediator of osteoarthritis pain. Osteoarthr. Cartil. 2020, 28, 562–571. [Google Scholar] [CrossRef] [PubMed]
- Sorge, R.E.; Mapplebeck, J.C.S.; Rosen, S.; Beggs, S.; Taves, S.; Alexander, J.K.; Martin, L.J.; Austin, J.-S.; Sotocinal, S.G.; Chen, D.; et al. Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nat. Neurosci. 2015, 18, 1081–1083. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, R.E.; Ishihara, S.; Tran, P.B.; Golub, S.B.; Last, K.; Miller, R.J.; Fosang, A.J.; Malfait, A.M. An aggrecan fragment drives osteoarthritis pain through Toll-like receptor 2. JCI Insight 2018, 3, e95704. [Google Scholar] [CrossRef] [PubMed]
- Alquraini, A.; Garguilo, S.; D’Souza, G.; Zhang, L.X.; Schmidt, T.A.; Jay, G.D.; Elsaid, K.A. The interaction of lubricin/proteoglycan 4 (PRG4) with toll-like receptors 2 and 4: An anti-inflammatory role of PRG4 in synovial fluid. Arthritis Res. Ther. 2015, 17, 353. [Google Scholar] [CrossRef] [Green Version]
- Iqbal, S.M.; Leonard, C.; Regmi, S.C.; De Rantere, D.; Tailor, P.; Ren, G.; Ishida, H.; Hsu, C.; Abubacker, S.; Pang, D.S.; et al. Lubricin/Proteoglycan 4 binds to and regulates the activity of Toll-Like Receptors In Vitro. Sci. Rep. 2016, 6, 18910. [Google Scholar] [CrossRef] [Green Version]
- Qadri, M.; Jay, G.D.; Zhang, L.X.; Schmidt, T.A.; Totonchy, J.; Elsaid, K.A. Proteoglycan-4 is an essential regulator of synovial macrophage polarization and inflammatory macrophage joint infiltration. Arthritis Res. Ther. 2021, 23, 241. [Google Scholar] [CrossRef]
- Tang, H.M.; Tang, H.L. Anastasis: Recovery from the brink of cell death. R. Soc. Open Sci. 2018, 5, 180442. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Zhang, D.; Mi, X.; Xia, Q.; Yu, Y.; Zuo, Z.; Guo, W.; Zhao, X.; Cao, J.; Yang, Q.; et al. p27 suppresses arsenite-induced Hsp27/Hsp70 expression through inhibiting JNK2/c-Jun- and HSF-1-dependent pathways. J. Biol. Chem. 2010, 285, 26058–26065. [Google Scholar] [CrossRef] [Green Version]
- Hwang, H.S.; Kim, H.A. Chondrocyte Apoptosis in the Pathogenesis of Osteoarthritis. Int. J. Mol. Sci. 2015, 16, 26035–26054. [Google Scholar] [CrossRef]
- Lee, S.W.; Lee, H.J.; Chung, W.T.; Choi, S.M.; Rhyu, S.H.; Kim, D.K.; Kim, K.T.; Kim, J.Y.; Kim, J.-M.; Yoo, Y.H. TRAIL induces apoptosis of chondrocytes and influences the pathogenesis of experimentally induced rat osteoarthritis. Arthritis Rheum. 2004, 50, 534–542. [Google Scholar] [CrossRef]
- Pettersen, I.; Figenschau, Y.; Olsen, E.; Bakkelund, W.; Smedsrod, B.; Sveinbjornsson, B. Tumor necrosis factor-related apoptosis-inducing ligand induces apoptosis in human articular chondrocytes in vitro. Biochem. Biophys. Res. Commun. 2002, 296, 671–676. [Google Scholar] [CrossRef]
- Ruiz-Romero, C.; Calamia, V.; Mateos, J.; Carreira, V.; Martinez-Gomariz, M.; Fernandez, M.; Blanco, F.J. Mitochondrial dysregulation of osteoarthritic human articular chondrocytes analyzed by proteomics: A decrease in mitochondrial superoxide dismutase points to a redox imbalance. Mol. Cell. Proteom. MCP 2009, 8, 172–189. [Google Scholar] [CrossRef] [Green Version]
- Al-Sharif, A.; Jamal, M.; Zhang, L.X.; Larson, K.; Schmidt, T.A.; Jay, G.; Elsaid, K.A. Lubricin/Proteoglycan 4 Binding to CD44 Receptor: A Mechanism of the Suppression of Proinflammatory Cytokine-Induced Synoviocyte Proliferation by Lubricin. Arthritis Rheumatol. 2015, 67, 1503–1513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Del Carlo, M., Jr.; Loeser, R.F. Nitric oxide-mediated chondrocyte cell death requires the generation of additional reactive oxygen species. Arthritis Rheum. 2002, 46, 394–403. [Google Scholar] [CrossRef]
- Kühn, K.; D’Lima, D.; Hashimoto, S.; Lotz, M. Cell death in cartilage. Osteoarthr. Cartil. 2004, 12, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Higuchi, M.; Honda, T.; Proske, R.J.; Yeh, E.T. Regulation of reactive oxygen species-induced apoptosis and necrosis by caspase 3-like proteases. Oncogene 1998, 17, 2753–2760. [Google Scholar] [CrossRef] [Green Version]
- Larson, K.M.; Zhang, L.; Badger, G.J.; Jay, G.D. Early genetic restoration of lubricin expression in transgenic mice mitigates chondrocyte peroxynitrite release and caspase-3 activation. Osteoarthr. Cartil. 2017, 25, 1488–1495. [Google Scholar] [CrossRef] [Green Version]
- Grishko, V.; Xu, M.; Ho, R.; Mates, A.; Watson, S.; Kim, J.T.; Wilson, G.L.; Pearsall, A.W. Effects of hyaluronic acid on mitochondrial function and mitochondria-driven apoptosis following oxidative stress in human chondrocytes. J. Biol. Chem. 2009, 284, 9132–9139. [Google Scholar] [CrossRef] [Green Version]
- Blanco, F.J.; Rego, I.; Ruiz-Romero, C. The role of mitochondria in osteoarthritis. Nat. Rev. Rheumatol. 2011, 7, 161–169. [Google Scholar] [CrossRef]
- Matsuzaki, T.; Alvarez-Garcia, O.; Mokuda, S.; Nagira, K.; Olmer, M.; Gamini, R.; Miyata, K.; Akasaki, Y.; Su, A.I.; Asahara, H.; et al. FoxO transcription factors modulate autophagy and proteoglycan 4 in cartilage homeostasis and osteoarthritis. Sci. Transl. Med. 2018, 10, eaan0746. [Google Scholar] [CrossRef] [Green Version]
- Akula, S.K.; McCullough, K.B.; Weichselbaum, C.; Dougherty, J.D.; Maloney, S.E. The trajectory of gait development in mice. Brain Behav. 2020, 10, e01636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dorman, C.W.; Krug, H.E.; Frizelle, S.P.; Funkenbusch, S.; Mahowald, M.L. A comparison of DigiGait and TreadScan imaging systems: Assessment of pain using gait analysis in murine monoarthritis. J. Pain Res. 2013, 7, 25–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hurtig, M.; Zaghoul, I.; Sheardown, H.; Schmidt, T.A.; Liu, L.; Zhang, L.; Elsaid, K.A.; Jay, G.D. Two compartment pharmacokinetic model describes the intra-articular delivery and retention of rhprg4 following ACL transection in the Yucatan mini pig. J. Orthop. Res. 2019, 37, 386–396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ai, M.; Cui, Y.; Sy, M.S.; Lee, D.M.; Zhang, L.X.; Larson, K.M.; Kurek, K.C.; Jay, G.D.; Warman, M.L. Anti-lubricin monoclonal antibodies created using lubricin-knockout mice immunodetect lubricin in several species and in patients with healthy and diseased joints. PLoS ONE 2015, 10, e0116237. [Google Scholar] [CrossRef] [Green Version]
- Mao, X.; Fu, P.; Wang, L.; Xiang, C. Mitochondria: Potential Targets for Osteoarthritis. Front. Med. 2020, 7, 581402. [Google Scholar] [CrossRef] [PubMed]
- Ruan, M.Z.; Erez, A.; Guse, K.; Dawson, B.; Bertin, T.; Chen, Y.; Jiang, M.M.; Yustein, J.; Gannon, F.; Lee, B.H. Proteoglycan 4 expression protects against the development of osteoarthritis. Sci. Transl. Med. 2013, 5, 176ra134. [Google Scholar] [CrossRef] [Green Version]
- Seol, D.; Choe, H.H.; Zheng, H.; Brouillette, M.J.; Fredericks, D.C.; Petersen, E.B.; Song, I.; Jaidev, L.R.; Salem, A.; Martin, J.A. Intra-Articular Adeno-Associated Virus-Mediated Proteoglycan 4 Gene Therapy for Preventing Post-Traumatic Osteoarthritis. Hum. Gene Ther. 2022. [Google Scholar] [CrossRef]
- Lambiase, A.; Sullivan, B.D.; Schmidt, T.A.; Sullivan, D.A.; Jay, G.D.; Truitt, E.R., 3rd; Bruscolini, A.; Sacchetti, M.; Mantelli, F. A Two-Week, Randomized, Double-masked Study to Evaluate Safety and Efficacy of Lubricin (150 mug/mL) Eye Drops Versus Sodium Hyaluronate (HA) 0.18% Eye Drops (Vismed(R)) in Patients with Moderate Dry Eye Disease. Ocul. Surf. 2017, 15, 77–87. [Google Scholar] [CrossRef]
- Abubacker, S.; Dorosz, S.G.; Ponjevic, D.; Jay, G.; Matyas, J.R.; Schmidt, T.A. Full-Length Recombinant Human Proteoglycan 4 Interacts with Hyaluronan to Provide Cartilage Boundary Lubrication. Ann. Biomed. Eng. 2016, 44, 1128–1137. [Google Scholar] [CrossRef]
- Samsom, M.L.; Morrison, S.; Masala, N.; Sullivan, B.D.; Sullivan, D.A.; Sheardown, H.; Schmidt, T.A. Characterization of full-length recombinant human Proteoglycan 4 as an ocular surface boundary lubricant. Exp. Eye Res. 2014, 127, 14–19. [Google Scholar] [CrossRef]
- Xu, Y.; Tian, N.X.; Bai, Q.-Y.; Chen, Q.; Sun, X.-H.; Wang, Y. Gait Assessment of Pain and Analgesics: Comparison of the DigiGait and CatWalk Gait Imaging Systems. Neurosci. Bull. 2019, 35, 401–418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, J.; Huang, Y.; Burwell, T.J.; Peterson, N.C.; Connor, J.; Weiss, S.J.; Yu, S.M.; Li, Y. In Situ Imaging of Tissue Remodeling with Collagen Hybridizing Peptides. ACS Nano 2017, 11, 9825–9835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qadri, M.; Jay, G.D.; Zhang, L.X.; Richendrfer, H.; Schmidt, T.A.; Elsaid, K.A. Proteoglycan-4 regulates fibroblast to myofibro-blast transition and expression of fibrotic genes in the synovium. Arthritis Res. 2020, 22, 113. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Liu, D.; Kaminski, H.J. Myosin Heavy Chain Expression in Mouse Extraocular Muscle: More Complex than Expected. Investig. Opthalmology Vis. Sci. 2010, 51, 6355–6363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greene, G.W.; Martin, L.; Tabor, R.; Michalczyk, A.; Ackland, L.; Horn, R. Lubricin: A versatile, biological anti-adhesive with properties comparable to polyethylene glycol. Biomaterials 2015, 53, 127–136. [Google Scholar] [CrossRef] [PubMed]
| Male Mean (SD) | Female Mean (SD) | |||
|---|---|---|---|---|
| PSwingStride (%) | ||||
| Percent of the stride in the swing phase | ||||
| Prg4−/− | 37.1 | (2.1) | 38.0 | (1.3) |
| Prg4+/+ | 36.1 | (1.3) | 39.7 | (1.4) |
| p-value (Group differences within Sex) | 0.198 | 0.098 | ||
| p-value (Group by Sex Interaction) | 0.038 | |||
| PBrakeStride (%) | ||||
| Percent of the stride in the brake phase | ||||
| Prg4−/− | 12.5 | (2.5) | 13.4 | (1.8) |
| Prg4+/+ | 19.5 | (3.9) | 15.4 | (1.6) |
| p-value (Group differences within Sex) | <0.001 | 0.107 | ||
| p-value (Group by Sex Interaction) | 0.003 | |||
| PPropelStride (%) | ||||
| Percent of the stride in the propel phase | ||||
| Prg4−/− | 50.4 | (2.5) | 48.6 | (2.3) |
| Prg4+/+ | 44.4 | (4.1) | 44.9 | (1.1) |
| p-value (Group differences within Sex) | <0.001 | 0.007 | ||
| p-value (Group by Sex Interaction) | 0.184 | |||
| Stance/Swing | ||||
| Stance time divided by swing time | ||||
| Prg4−/− | 1.71 | (0.15) | 1.65 | (0.16) |
| Prg4+/+ | 1.78 | (0.10) | 1.51 | (0.08) |
| p-value (Group differences within Sex) | 0.247 | 0.059 | ||
| p-value (Group by Sex Interaction) | 0.029 | |||
| Swing Time (s) | ||||
| The time for forward portion of the stride in which the paw is not in contact with the belt | ||||
| Prg4−/− | 0.077 | (0.015) | 0.089 | (0.004) |
| Prg4+/+ | 0.072 | (0.006) | 0.072 | (0.006) |
| p-value (Group differences within Sex) | 0.048 | <0.001 | ||
| p-value (Group by Sex Interaction) | <0.001 | |||
| Stance Time (s) | ||||
| The time for portion of stride where the paw remains in contact with the belt | ||||
| Prg4−/− | 0.131 | (0.008) | 0.118 | (0.008) |
| Prg4+/+ | 0.127 | (0.007) | 0.134 | (0.005) |
| p-value (Group differences within Sex) | 0.185 | <0.001 | ||
| p-value (Group by Sex Interaction) | <0.001 | |||
| Stride Time (s) | ||||
| The amount of time needed to complete one full stride for one limb | ||||
| Prg4−/− | 0.208 | (0.016) | 0.190 | (0.011) |
| Prg4+/+ | 0.198 | (0.012) | 0.223 | (0.006) |
| p-value (Group differences within Sex) | 0.058 | <0.001 | ||
| p-value (Group by Sex Interaction) | <0.001 | |||
| Brake Time (s) | ||||
| The time between initial paw contact with the belt and the maximal paw contact | ||||
| Prg4−/− | 0.026 | (0.004) | 0.025 | (0.003) |
| Prg4+/+ | 0.039 | (0.008) | 0.034 | (0.004) |
| p-value (Group differences within Sex) | <0.001 | <0.001 | ||
| p-value (Group by Sex Interaction) | 0.186 | |||
| Propel Time (s) | ||||
| Time between maximal paw contact and the end of the stance, just before swing | ||||
| Prg4−/− | 0.105 | (0.010) | 0.093 | (0.009) |
| Prg4+/+ | 0.088 | (0.009) | 0.100 | (0.003) |
| p-value (Group differences within Sex) | <0.001 | 0.106 | ||
| p-value (Group by Sex Interaction) | <0.001 | |||
| (a) | ||||||
| Males | ||||||
| Baseline Mean (SD) | Day 3 Mean (SD) | Day 6 Mean (SD) | ||||
| PSwingStride (%) | ||||||
| rhPRG4 Injected | 37.8 | 2.2 | 36.6 | 2.6 | 36.6 | 2.2 |
| PBS Injected | 36.4 | 1.8 | 36.5 | 1.2 | 36.8 | 1.8 |
| p-value | 0.134 | 0.077 | ||||
| PBrakeStride (%) | ||||||
| rhPRG4 Injected | 12.4 | 2.1 | 12.2 | 1.7 | 12.3 | 2.8 |
| PBS Injected | 12.6 | 2.9 | 13.1 | 2.4 | 12.0 | 2.2 |
| p-value | 0.544 | 0.726 | ||||
| PPropelStride (%) | ||||||
| rhPRG4 Injected | 49.8 | 2.2 | 51.3 | 3.2 | 51.1 | 2.4 |
| PBS Injected | 51.0 | 2.7 | 50.4 | 1.5 | 51.2 | 2.9 |
| p-value | 0.152 | 0.441 | ||||
| Stance/Swing | ||||||
| rhPRG4 Injected | 1.66 | 0.15 | 1.76 | 0.23 | 1.74 | 0.16 |
| PBS Injected | 1.76 | 0.14 | 1.75 | 0.10 | 1.73 | 0.13 |
| p-value | 0.099 | 0.099 | ||||
| Swing Time (s) | ||||||
| rhPRG4 Injected | 0.079 | 0.009 | 0.078 | 0.009 | 0.074 | 0.007 |
| PBS Injected | 0.076 | 0.010 | 0.073 | 0.005 | 0.074 | 0.010 |
| p-value | 0.742 | 0.204 | ||||
| Stance Time (s) | ||||||
| rhPRG4 Injected | 0.130 | 0.006 | 0.134 | 0.007 | 0.127 | 0.004 |
| PBS Injected | 0.131 | 0.010 | 0.127 | 0.006 | 0.127 | 0.007 |
| p-value | 0.005 | 0.756 | ||||
| Stride Time (s) | ||||||
| rhPRG4 Injected | 0.210 | 0.013 | 0.212 | 0.013 | 0.200 | 0.010 |
| PBS Injected | 0.206 | 0.019 | 0.200 | 0.010 | 0.200 | 0.017 |
| p-value | 0.080 | 0.517 | ||||
| Brake Time (s) | ||||||
| rhPRG4 Injected | 0.026 | 0.004 | 0.026 | 0.004 | 0.025 | 0.005 |
| PBS Injected | 0.026 | 0.005 | 0.026 | 0.004 | 0.024 | 0.005 |
| p-value | 0.835 | 0.876 | ||||
| Propel Time (s) | ||||||
| rhPRG4 Injected | 0.104 | 0.008 | 0.109 | 0.008 | 0.102 | 0.006 |
| PBS Injected | 0.105 | 0.012 | 0.101 | 0.006 | 0.103 | 0.007 |
| p-value | 0.020 | 0.848 | ||||
| (b) | ||||||
| Females | ||||||
| Baseline Mean (SD) | Day 3 Mean (SD) | Day 6 Mean (SD) | ||||
| PSwingStride (%) | ||||||
| rhPRG4 Injected | 38.3 | 2.6 | 37.5 | 1.3 | 37.9 | 2.0 |
| PBS Injected | 37.8 | 2.1 | 38.5 | 1.3 | 38.2 | 2.4 |
| p-value | 0.154 | 0.401 | ||||
| PBrakeStride (%) | ||||||
| rhPRG4 Injected | 13.0 | 1.8 | 13.8 | 3.1 | 14.6 | 2.4 |
| PBS Injected | 13.5 | 1.8 | 12.5 | 3.1 | 13.1 | 1.9 |
| p-value | 0.240 | 0.203 | ||||
| PPropelStride (%) | ||||||
| rhPRG4 Injected | 48.7 | 2.5 | 48.7 | 2.4 | 47.5 | 2.2 |
| PBS Injected | 48.7 | 2.2 | 49.0 | 3.1 | 48.6 | 2.6 |
| p-value | 0.860 | 0.412 | ||||
| Stance/Swing | ||||||
| rhPRG4 Injected | 1.63 | 0.18 | 1.67 | 0.09 | 1.65 | 0.15 |
| PBS Injected | 1.66 | 0.15 | 1.60 | 0.09 | 1.64 | 0.17 |
| p-value | 0.202 | 0.591 | ||||
| Swing Time (s) | ||||||
| rhPRG4 Injected | 0.072 | 0.006 | 0.072 | 0.004 | 0.071 | 0.009 |
| PBS Injected | 0.073 | 0.006 | 0.076 | 0.007 | 0.074 | 0.008 |
| p-value | 0.388 | 0.476 | ||||
| Stance Time (s) | ||||||
| rhPRG4 Injected | 0.116 | 0.008 | 0.120 | 0.004 | 0.116 | 0.011 |
| PBS Injected | 0.120 | 0.009 | 0.121 | 0.007 | 0.119 | 0.009 |
| p-value | 0.522 | 0.884 | ||||
| Stride Time (s) | ||||||
| rhPRG4 Injected | 0.189 | 0.010 | 0.192 | 0.007 | 0.187 | 0.019 |
| PBS Injected | 0.193 | 0.012 | 0.196 | 0.013 | 0.193 | 0.013 |
| p-value | 0.968 | 0.770 | ||||
| Brake Time (s) | ||||||
| rhPRG4 Injected | 0.025 | 0.003 | 0.026 | 0.005 | 0.027 | 0.004 |
| PBS Injected | 0.026 | 0.002 | 0.024 | 0.005 | 0.025 | 0.003 |
| p-value | 0.189 | 0.207 | ||||
| Propel Time (s) | ||||||
| rhPRG4 Injected | 0.092 | 0.008 | 0.093 | 0.007 | 0.089 | 0.010 |
| PBS Injected | 0.094 | 0.009 | 0.096 | 0.010 | 0.094 | 0.009 |
| p-value | 0.904 | 0.544 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, D.S.; Dickerson, E.E.; Zhang, L.X.; Richendrfer, H.; Karamchedu, P.N.; Badger, G.J.; Schmidt, T.A.; Fredericks, A.M.; Elsaid, K.A.; Jay, G.D. Quadruped Gait and Regulation of Apoptotic Factors in Tibiofemoral Joints following Intra-Articular rhPRG4 Injection in Prg4 Null Mice. Int. J. Mol. Sci. 2022, 23, 4245. https://doi.org/10.3390/ijms23084245
Yang DS, Dickerson EE, Zhang LX, Richendrfer H, Karamchedu PN, Badger GJ, Schmidt TA, Fredericks AM, Elsaid KA, Jay GD. Quadruped Gait and Regulation of Apoptotic Factors in Tibiofemoral Joints following Intra-Articular rhPRG4 Injection in Prg4 Null Mice. International Journal of Molecular Sciences. 2022; 23(8):4245. https://doi.org/10.3390/ijms23084245
Chicago/Turabian StyleYang, Daniel S., Edward E. Dickerson, Ling X. Zhang, Holly Richendrfer, Padmini N. Karamchedu, Gary J. Badger, Tannin A. Schmidt, Alger M. Fredericks, Khaled A. Elsaid, and Gregory D. Jay. 2022. "Quadruped Gait and Regulation of Apoptotic Factors in Tibiofemoral Joints following Intra-Articular rhPRG4 Injection in Prg4 Null Mice" International Journal of Molecular Sciences 23, no. 8: 4245. https://doi.org/10.3390/ijms23084245
APA StyleYang, D. S., Dickerson, E. E., Zhang, L. X., Richendrfer, H., Karamchedu, P. N., Badger, G. J., Schmidt, T. A., Fredericks, A. M., Elsaid, K. A., & Jay, G. D. (2022). Quadruped Gait and Regulation of Apoptotic Factors in Tibiofemoral Joints following Intra-Articular rhPRG4 Injection in Prg4 Null Mice. International Journal of Molecular Sciences, 23(8), 4245. https://doi.org/10.3390/ijms23084245








