Accelerated Generation of Extra-Islet Insulin-Producing Cells in Diabetic Rats, Treated with Sodium Phthalhydrazide
Abstract
:1. Introduction
2. Results
2.1. Confirmation of T2D Development
2.2. Extra-Islet IPCs in the Pancreas of Non-Diabetic Rats
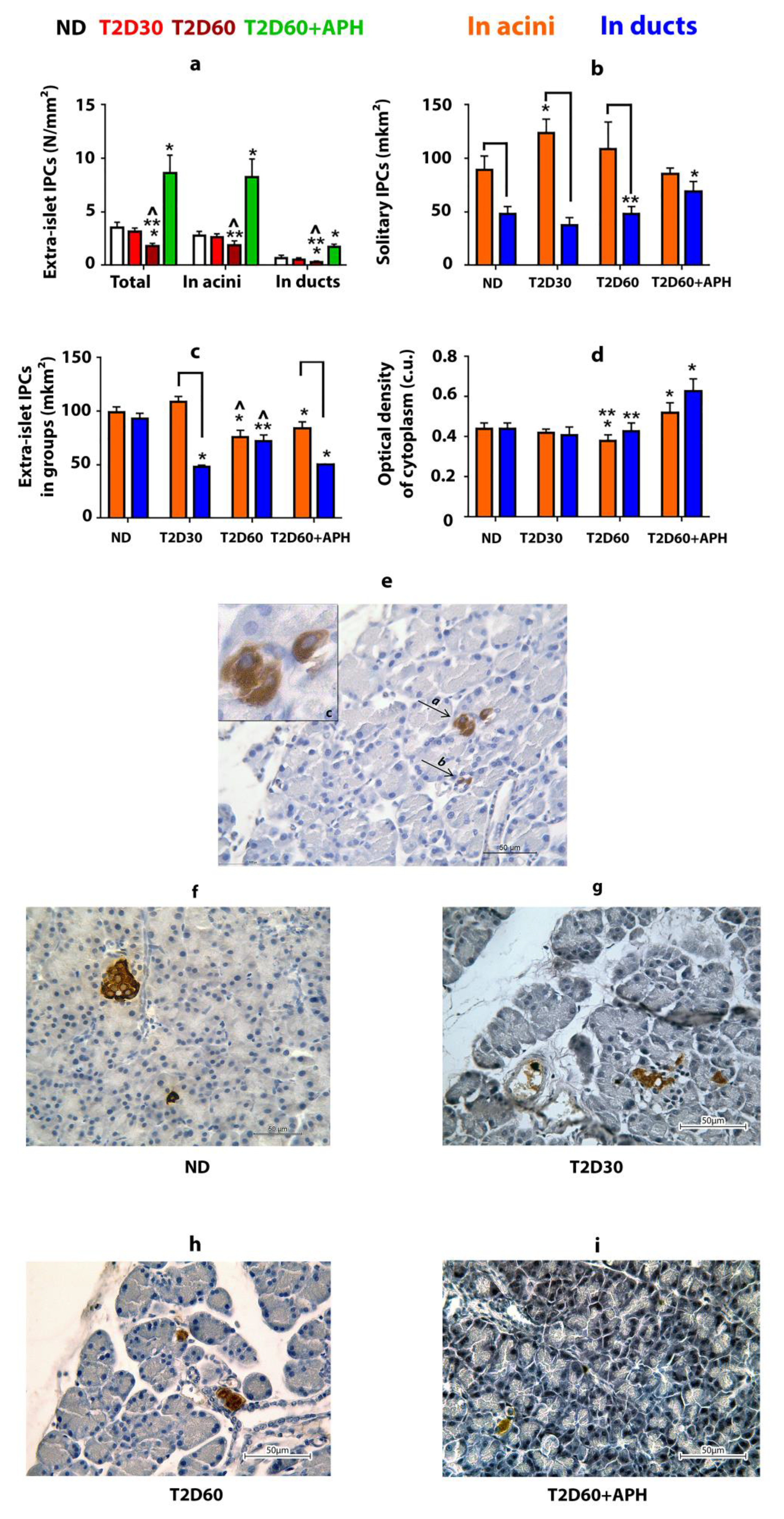
2.3. The Effect of Type 2 Diabetes on the Pancreatic Extra-Islet IPCs
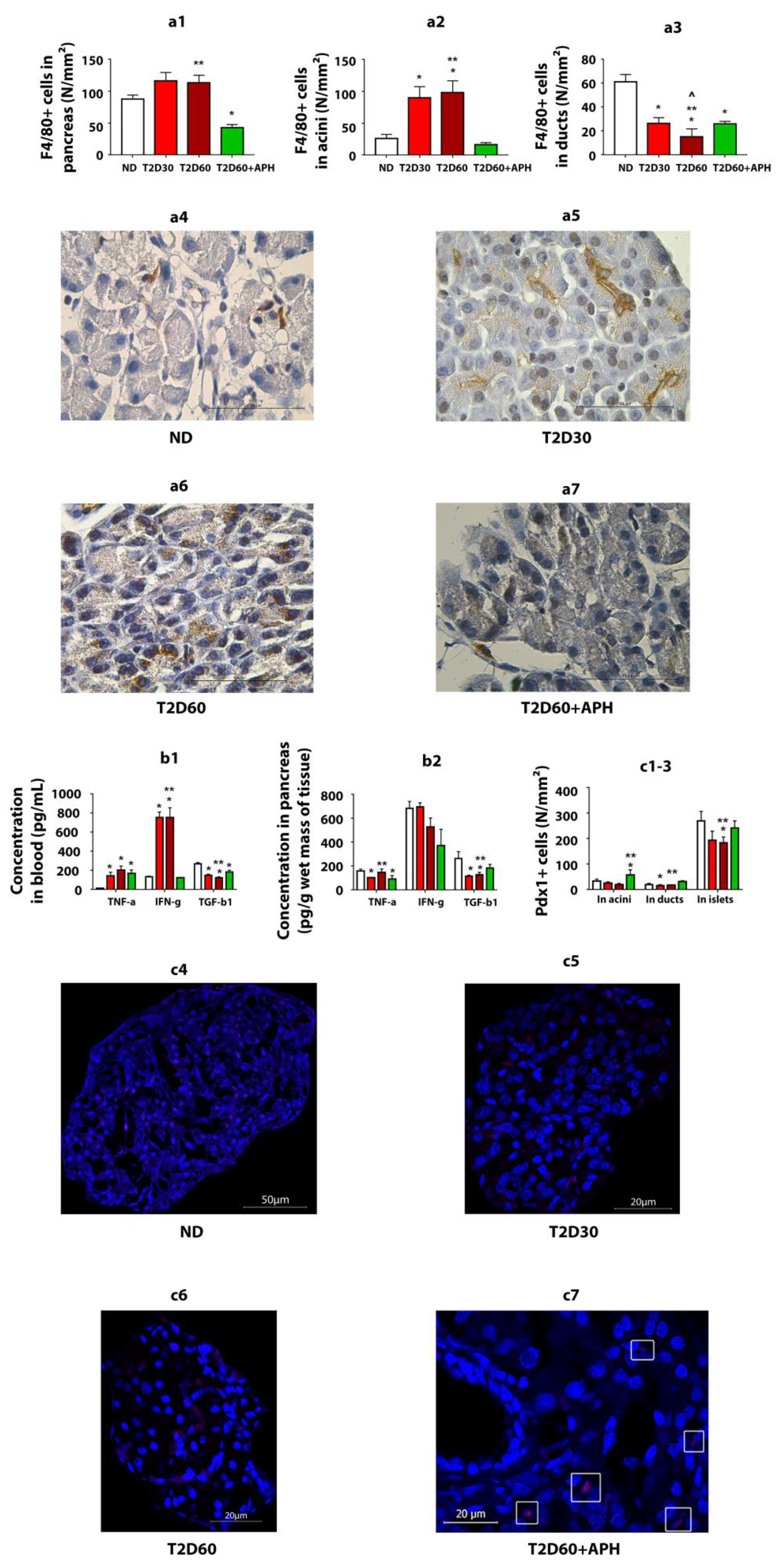
2.4. Pro- and Anti-Inflammatory Cytokines in Blood and Pancreas
2.5. Extra-Islet IPCs in the Pancreas of Diabetic APH-Treated Rats
2.6. Characteristics of Glucose Metabolism in Diabetic APH-Treated Rats
2.7. Expression of Pdx1 in Exocrine Pancreas
2.8. Quantity of F4/80+ Cells
3. Discussion
3.1. Extra-Islet IPS in the Healthy Pancreas
3.2. Changes in Extra-Islet IPCs, Promoted by Stz-NA Diabetes
3.3. The Effect of APH on Extra-Islet IPCs
4. Materials and Methods
4.1. Induction of Experimental Diabetes
4.2. Experimental Protocol
4.3. Fasting Blood Glucose (FBG) Level, Glycosylated Hemoglobin (HbA1c), and Oral Glucose Tolerance Test (OGTT)
4.4. Insulin Level and HOMA-Estimated Insulin Resistance
4.5. Preparation of Tissue Samples
4.6. Immunohistochemical Evaluation of Pancreatic Tissues
4.7. Morphometric Analysis
4.8. Microscopic Examination
4.9. Inflammatory Characteristics and Cytokines Content
4.10. Statistical Data Analysis
5. Conclusions
6. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- International Diabetes Federation. IDF Diabetes Atlas, 6th ed.; International Diabetes Federation: Brussels, Belgium, 2013; Available online: http://www.idf.org/diabetesatlas (accessed on 12 December 2021).
- Shaw, J.E.; Sicree, R.A.; Zimmet, P.Z. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res. Clin. Pract. 2010, 87, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Ashcroft, F.M.; Rorsman, P. Diabetes mellitus and the β cell: The last ten years. Cell 2012, 148, 1160–1171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwartz, S.S.; Epstein, S.; Corkey, B.E.; Grant, S.F.; Gavin, J.R., 3rd; Aguilar, R.B. The Time Is Right for a New Classification System for Diabetes: Rationale and Implications of the β-Cell-Centric Classification Schema. Diabetes Care 2016, 39, 179–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Butler, A.E.; Janson, J.; Bonner-Weir, S.; Ritzel, R.; Rizza, R.A.; Butler, P.C. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes 2003, 52, 102–110. [Google Scholar] [CrossRef] [Green Version]
- Beamish, C.A.; Strutt, B.J.; Arany, E.J.; Hill, D.J. Insulin-positive, Glut2-low cells present within mouse pancreas exhibit lineage plasticity and are enriched within extra-islet endocrine cell clusters. Islets 2016, 18, 65–82. [Google Scholar] [CrossRef] [Green Version]
- Bogdani, M.; Lefebvre, V.; Buelens, N.; Bock, T.; Pipeleers-Marichal, M.; Veld, P.; Pipeleers, D. Formation of insulin-positive cells in implants of human pancreatic duct cell preparations from young donors. Diabetologia 2003, 46, 830–838. [Google Scholar] [CrossRef] [Green Version]
- El-Gohary, Y.; Wiersch, J.; Tulachan, S.; Xiao, X.; Guo, P.; Rymer, C.; Fischbach, S.; Prasadan, K.; Shiota, C.; Gaffar, I.; et al. Intraislet Pancreatic Ducts Can Give Rise to Insulin-Positive Cells. Endocrinology 2016, 157, 166–175. [Google Scholar] [CrossRef] [Green Version]
- Sokolova, K.; Gette, I.; Danilova, I.; Abidov, M. Extra-islet insulin-producing cells in experimental diabetes mellitus and at modulation activity of macrophages. Virchows Archiv. 2018, 473, S68. [Google Scholar]
- Bouwens, L.; Pipeleers, D.G. Extra-insular beta cells associated with ductules are frequent in adult human pancreas. Diabetologia 1998, 41, 629–633. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Bank, S.; Ghosh, R.; Sinha, A.K. Extra pancreatic synthesis of insulin. Integr. Obes. Diabetes 2015, 2, 176–179. [Google Scholar] [CrossRef]
- Bornfeldt, K.E.; Tabas, I. Insulin resistance, hyperglycemia and atherosclerosis. Cell Metab. 2011, 14, 575–585. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, R.; Bank, S.; Bhattacharya, R.; Khan, N.N.; Sinha, A.K. Neutralization by insulin of the hypertensive effect of dermcidin isoform-2: An environmentally induced diabetogenic and hypertensive protein. Cardiol. Res. Pract. 2014, 2014, 412815. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Dorrell, C.; Naugler, W.E.; Heskett, M.; Spellman, P.; Li, B.; Galivo, F.; Haft, A.; Wakefield, L.; Grompe, M. Long-Term Correction of Diabetes in Mice by In Vivo Reprogramming of Pancreatic Ducts. Mol. Ther. 2018, 26, 1327–1342. [Google Scholar] [CrossRef] [Green Version]
- Kaya-Dagistanli, F.; Ozturk, M. Transdifferentiation of both intra- and extra-islet cells into beta cells in nicotinamide treated neonatal diabetic rats: An in situ hybridization and double immunohistochemical study. Acta Histochem. 2020, 122, 151612. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, Q.; Zhou, Z.; Ikeda, Y. PDX1, Neurogenin-3, and MAFA: Critical transcription regulators for beta cell development and regeneration. Stem Cell Res. Ther. 2017, 8, 240. [Google Scholar] [CrossRef] [Green Version]
- Akinci, E.; Banga, A.; Greder, L.V.; Dutton, J.R.; Slack, J.M.W. Reprogramming of pancreatic exocrine cells towards a beta (β) cell character using Pdx1, Ngn3 and MafA. Biochem. J. 2012, 442, 539–550. [Google Scholar] [CrossRef] [Green Version]
- Miyashita, K.; Miyatsuka, T.; Matsuoka, T.A.; Sasaki, S.; Takebe, S.; Yasuda, T.; Watada, H.; Kaneto, H.; Shimomura, I. Sequential introduction and dosage balance of defined transcription factors affect reprogramming efficiency from pancreatic duct cells into insulin-producing cells. Biochem. Biophys. Res. Commun. 2014, 444, 514–519. [Google Scholar] [CrossRef]
- Taniguchi, H.; Yamato, E.; Tashiro, F.; Ikegami, H.; Ogihara, T.; Miyazaki, J. β-Cell neogenesis induced by adenovirus-mediated gene delivery of transcription factor pdx-1 into mouse pancreas. Gene Ther. 2003, 10, 15–23. [Google Scholar] [CrossRef] [Green Version]
- Noguchi, H.; Kaneto, H.; Weir, G.C.; Bonner-Weir, S.; Noguchi, H. PDX-1 protein containing its own Antennapedia-like protein transduction domain can transduce pancreatic duct and islet cells. Diabetes 2003, 52, 1732–1737. [Google Scholar] [CrossRef] [Green Version]
- Gao, T.; McKenna, B.; Li, C.; Reichert, M.; Nguyen, J.; Singh, T.; Yang, C.; Pannikar, A.; Doliba, N.; Zhang, T.; et al. Pdx1 maintains β cell identity and function by repressing an α cell program. Cell Metab. 2014, 19, 259–271. [Google Scholar] [CrossRef] [Green Version]
- Spaeth, J.M.; Gupte, M.; Perelis, M.; Yang, Y.-P.; Cyphert, H.; Guo, S.; Liu, J.-H.; Guo, M.; Bass, J.; Magnuson, M.A.; et al. Defining a Novel Role for the Pdx1 Transcription Factor in Islet β-Cell Maturation and Proliferation During Weaning. Diabetes 2017, 66, 2830–2839. [Google Scholar] [CrossRef] [Green Version]
- Park, J.H.; Stoffers, D.A.; Nicholls, R.D.; Simmons, R.A. Development of type 2 diabetes following intrauterine growth retardation in rats is associated with progressive epigenetic silencing of Pdx1. J. Clin. Investig. 2008, 118, 2316–2324. [Google Scholar] [CrossRef] [Green Version]
- Yang, B.T.; Dayeh, T.A.; Volkov, P.A.; Kirkpatrick, C.L.; Malmgren, S.; Jing, X.; Renström, E.; Wollheim, C.B.; Nitert, M.D.; Ling, C. Increased DNA methylation and decreased expression of PDX-1 in pancreatic islets from patients with type 2 diabetes. Mol. Endocrinol. 2012, 26, 1203–1212. [Google Scholar] [CrossRef] [Green Version]
- Yao, X.; Li, K.; Liang, C.; Zhou, Z.; Wang, J.; Wang, S.; Liu, L.; Yu, C.-L.; Song, Z.-B.; Bao, Y.-L.; et al. Tectorigenin enhances PDX1 expression and protects pancreatic β-cells by activating ERK and reducing ER stress. J. Biol. Chem. 2020, 295, 12975–12992. [Google Scholar] [CrossRef]
- Del Poggetto, E.; Ho, I.L.; Balestrieri, C.; Yen, E.Y.; Zhang, S.; Citron, F.; Shah, R.; Corti, D.; Diaferia, G.R.; Li, C.Y.; et al. Epithelial memory of inflammation limits tissue damage while promoting pancreatic tumorigenesis. Science 2021, 373, eabj0486. [Google Scholar] [CrossRef]
- Clayton, H.W.; Osipovich, A.B.; Stancill, J.S.; Schneider, J.D.; Vianna, P.G.; Shanks, C.M.; Yuan, W.; Gu, G.; Manduchi, E.; Stoeckert, C.J.; et al. Pancreatic Inflammation Redirects Acinar to Beta Cell Reprogramming. Cell Rep. 2016, 17, 2028–2041. [Google Scholar] [CrossRef] [Green Version]
- Oishi, Y.; Manabe, I. Macrophages in inflammation, repair and regeneration. Int. Immunol. 2018, 30, 511–528. [Google Scholar] [CrossRef]
- Jensen, D.M.; Hendricks, K.V.; Mason, A.T.; Tessem, J.S. Good Cop, Bad Cop: The Opposing Effects of Macrophage Activation State on Maintaining or Damaging Functional β-Cell Mass. Metabolites 2020, 10, 485. [Google Scholar] [CrossRef]
- Abidov, M.T. New N-heterocyclic pharmaceuticals for humans. Almanach 2016, 11, 74–80. [Google Scholar]
- Jukic, T.; Abidov, M.T.; Ihan, A. A tetrahydrophthalazine derivative “sodium nucleinate” exerts a potent suppressive effect upon LPS-stimulated mononuclear cells in vitro and in vivo. Coll. Antropol. 2011, 35, 1219–1223. [Google Scholar]
- Abidov, A.M.; Ishmuratov, A.S.; Danilova, I.G. Method for Obtaining 5-amino 2.3-dihydrophthalazine-1.4-dione Alkali Metal Salts and their Use in Medicine. U.S. Patent US8536171B2, 17 September 2013. [Google Scholar]
- Pozdina, V.A.; Zvedeninova, U.V.; Ulitko, M.V.; Danilova, I.G.; Abidov, M.T. Immunophenotypic and Morphometric Evaluation of Bone Marrow Macrophage Culture Stimulated by Sodium Aminodihydrophthalazinedione In Vitro. Cell Tiss. Biol. 2021, 15, 594–603. [Google Scholar] [CrossRef]
- Danilova, I.G.; Bulavintceva, T.S.; Gette, I.F.; Medvedeva, S.Y.; Emelyanov, V.V.; Abidov, M.T. Partial recovery from alloxan-induced diabetes by sodium phthalhydrazide in rats. Biomed. Pharmacother. 2017, 95, 103–110. [Google Scholar] [CrossRef]
- Pokrywczynska, M.; Krzyzanowska, S.; Jundzill, A.; Adamowicz, J.; Drewa, T. Differentiation of Stem Cells into Insulin-Producing Cells: Current Status and Challenges. Arch. Immunol. Ther. Exp. 2013, 61, 149–158. [Google Scholar] [CrossRef] [Green Version]
- Hribal, M.L.; Perego, L.; Lovari, S.; Andreozzi, F.; Menghini, R.; Perego, C.; Finzi, G.; Usellini, L.; Placidi, C.; Capella, C.; et al. Chronic hyperglycemia impairs insulin secretion by affecting insulin receptor expression, splicing, and signaling in RIN β cell line and human islets of Langerhans. FASEB J. 2003, 17, 1340–1342. [Google Scholar] [CrossRef]
- El-Gohary, Y.; Tulachan, S.; Wiersch, J.; Guo, P.; Welsh, C.; Prasadan, K.; Paredes, J.; Shiota, C.; Xiao, X.; Wada, Y.; et al. A smad signaling network regulates islet cell proliferation. Diabetes 2014, 63, 224–236. [Google Scholar] [CrossRef] [Green Version]
- Xiao, X.; Wiersch, J.; El-Gohary, Y.; Guo, P.; Prasadan, K.; Paredes, J.; Welsh, C.; Shiota, C.; Gittes, G.K. TGFβ Receptor Signaling Is Essential for Inflammation-Induced but Not β-Cell Workload–Induced β-Cell Proliferation. Diabetes 2013, 62, 1217–1226. [Google Scholar] [CrossRef] [Green Version]
- Islam, M.S.; Wilson, R.D. Experimentally induced rodent models of type 2 diabetes. In Animal Models in Diabetes Research. Methods in Molecular Biology (Methods and Protocols); Joost, H.G., Al-Hasani, H., Schürmann, A., Eds.; Humana Press: Totowa, NJ, USA, 2012; Volume 933, pp. 161–174. [Google Scholar] [CrossRef]
- Danilova, I.; Medvedeva, S.; Shmakova, S.; Chereshneva, M.; Sarapultsev, A.; Sarapultsev, P. Pathological changes in the cellular structures of retina and choroidea in the early stages of alloxan-induced diabetes. World J. Diabetes 2018, 9, 239–251. [Google Scholar] [CrossRef]
- Sakaguchi, K.; Takeda, K.; Maeda, M.; Ogawa, W.; Sato, T.; Okada, S.; Ohnishi, Y.; Hiromu, N.; Atsunori, K. Glucose area under the curve during oral glucose tolerance test as an index of glucose intolerance. Diabetol. Int. 2016, 7, 53–58. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [Green Version]
- Hewitt, S.M.; Baskin, D.G.; Frevert, C.W.; Stahl, W.L.; Rosa-Molinar, E. Controls for immunohistochemistry: The Histochemical Society’s standards of practice for validation of immunohistochemical assays. J. Histochem. Cytochem. 2014, 62, 693–697. [Google Scholar] [CrossRef]
- Seeberger, K.L.; Anderson, S.J.; Ellis, C.E.; Yeung, T.Y.; Korbutt, G.S. Identification and differentiation of PDX1 β-cell progenitors within the human pancreatic epithelium. World J. Diabetes 2014, 5, 59–68. [Google Scholar] [CrossRef]
- Kumar, G.L.; Rudbeck, L. Immunohistochemical (IHC) Staining Methods, 5th ed.; Dako North America: Carpinteria, CA, USA, 2009. [Google Scholar]

| ND | T2D30 | T2D60 | T2D60 + APH | |
|---|---|---|---|---|
| IPCs/mm 2 | ||||
| Total IPCs mass | 169.82 ± 43.23 | 63.45 ± 12.97 1 | 51.52 ± 15.27 1 | 120.52 ± 27.03 3 |
| Islet IPCs (β-cells) | 166.32 ± 42.76 | 63.3 ± 15.88 1 | 49.72 ± 12.35 1 | 111.87 ± 20.71 3 |
| Extra-islet IPCs | 3.5 ± 0.54 | 3.15 ± 0.34 | 1.81 ± 0.24 1,2 | 8.65 ± 1.66 1,3 |
| Among them (IPCs in acini from all extra-islet IPCs, %): | ||||
| In acini | 2.8 ± 0.37 (81.05 ± 5.02) | 2.61 ± 0.33 (82.98 ± 5.39) | 1.86 ± 0.43 2 (78.28 ± 7.86) | 8.23 ± 1.71 1,3 (76.51 ± 6.89) |
| In ducts | 0.71 ± 0.24 | 0.54 ± 0.16 | 0.28 ± 0.12 1,2 | 1.74 ± 0.24 1,3 |
| Including: | ||||
| solitary IPCs: | ||||
| In acini | 0.28 ± 0.09 | 0.78 ± 0.14 1 | 0.28 ± 0.09 2 | 1.94 ± 0.36 1,3 |
| In ducts | 0.12 ± 0.05 | 0.11 ± 0.04 | 0.17 ± 0.07 | 0.52 ± 0.06 1,3 |
| IPCs in groups: | ||||
| In acini | 2.51 ± 0.34 | 1.83 ± 0.27 | 1.58 ± 0.42 1 | 6.29 ± 2.05 1,3 |
| In ducts | 0.59 ± 0.19 | 0.43 ± 0.15 | 0.09 ± 0.06 1 | 1.22 ± 0.28 1,3 |
| In acini | 81.05 ± 5.02 * | 82.98 ± 5.39 * | 78.28 ± 7.86 * | 76.51 ± 6.89 * |
| In ducts | 18.95 ± 5.02 | 17.02 ± 5.39 | 21.72 ± 7.86 | 23.49 ± 6.89 |
| Area of extra-islet IPCs, mkm2: | ||||
| Solitary IPCs: | ||||
| In acini | 89.23 ± 12.97 * | 123.49 ± 13.25 1,* | 109.03 ± 24.98 * | 85.8 ± 5.1 2 |
| In ducts | 47.65 ± 7.45 | 37.2 ± 7.45 | 47.8 ± 7.31 | 68.7 ± 9.7 1,2,3 |
| IPCs in groups: | ||||
| In acini | 98.69 ± 5.51 | 108.88 ± 4.98 | 76.03 ± 6.18 1,2 | 83.88 ± 6.24 1 |
| In ducts | 92.97 ± 5.26 | 47.88 ± 1.68 1,* | 72.34 ± 5.54 2 | 49.98 ± 0.04 1,3,* |
| Optical density of IPCs’ cytoplasm, conventional units: | ||||
| Solitary IPCs: | ||||
| In acini | 0.43 ± 0.04 | 0.41 ± 0.03 | 0.36 ± 0.02 | 0.5 ± 0.05 3 |
| In ducts | 0.44 ± 0.04 | 0.39 ± 0.02 | 0.35 ± 0.05 | 0.69 ± 0.07 1,2,3 |
| IPCs in groups: | ||||
| In acini | 0.45 ± 0.01 | 0.43 ± 0.02 | 0.36 ± 0.02 1,2 | 0.55 ± 0.03 1,2,3 |
| In ducts | 0.44 ± 0.02 | 0.44 ± 0.06 | 0.44 ± 0.03 | 0.58 ± 0.02 1,2,3 |
| Islet IPCs (β-cells) | 0.41 ± 0.02 | 0.38 ± 0.03 | 0.35 ± 0.02 1 | 0.53 ± 0.08 1,2,3 |
| Detected Antigen | Primary Antibodies: Reference, Supplier, Dilution | Secondary Antibodies: Reference, Supplier, Dilution |
|---|---|---|
| Proinsulin and insulin | Anti-Insulin/Proinsulin: clone INS04+INS05, MA5-12042, Invitrogen, Carlsbad, CA, USA, 1:200 | Biotin Goat anti-Mouse Ig (Multiple Absorption), BD Pharmingen, San Diego, USA, 1:500 |
| Pdx1 | Anti-PDX1: ab 227586, Abcam, Branford, CT, USA, 1:200 | Goat anti-Rabbit IgG (H+L) + Texas Red, Thermo Fisher Scientific, Waltham, MA, USA, 1:100 |
| F4/80 | Anti-F4/80 Polyclonal Antibody, PA5-21399, Thermo Fisher Scientific, Waltham, MA, USA, 1:200 | Biotin Goat Anti-Rabbit IgG, Thermo Fisher, Scientific, Waltham, MA, USA, 1:50 |
| Detectable protein | ELISA Kit, supplier | |
| Insulin | Rat Insulin ELISA Kit, Invitrogen-Thermo Fisher Scientific, Waltham, MA, USA | |
| Corticosterone | Corticosterone ELISA Kit, Abcam, Cambridge, Great Britain | |
| TNF-α | TNF alpha Rat ELISA Kit, Invitrogen-Thermo Fisher Scientific, Waltham, MA, USA | |
| IFN-γ | IFN gamma Rat ELISA Kit, Invitrogen-Thermo Fisher Scientific, Waltham, MA, USA | |
| TGF-β1 | TGF beta-1 Rat ELISA Kit, Invitrogen-Thermo Fisher Scientific, Waltham, MA, USA | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abidov, M.T.; Sokolova, K.V.; Gette, I.F.; Danilova, I.G. Accelerated Generation of Extra-Islet Insulin-Producing Cells in Diabetic Rats, Treated with Sodium Phthalhydrazide. Int. J. Mol. Sci. 2022, 23, 4286. https://doi.org/10.3390/ijms23084286
Abidov MT, Sokolova KV, Gette IF, Danilova IG. Accelerated Generation of Extra-Islet Insulin-Producing Cells in Diabetic Rats, Treated with Sodium Phthalhydrazide. International Journal of Molecular Sciences. 2022; 23(8):4286. https://doi.org/10.3390/ijms23084286
Chicago/Turabian StyleAbidov, Musa T., Ksenia V. Sokolova, Irina F. Gette, and Irina G. Danilova. 2022. "Accelerated Generation of Extra-Islet Insulin-Producing Cells in Diabetic Rats, Treated with Sodium Phthalhydrazide" International Journal of Molecular Sciences 23, no. 8: 4286. https://doi.org/10.3390/ijms23084286
APA StyleAbidov, M. T., Sokolova, K. V., Gette, I. F., & Danilova, I. G. (2022). Accelerated Generation of Extra-Islet Insulin-Producing Cells in Diabetic Rats, Treated with Sodium Phthalhydrazide. International Journal of Molecular Sciences, 23(8), 4286. https://doi.org/10.3390/ijms23084286






