Expression Patterns of Sugar Transporter Genes in the Allocation of Assimilates and Abiotic Stress in Lily
Abstract
:1. Introduction
2. Results
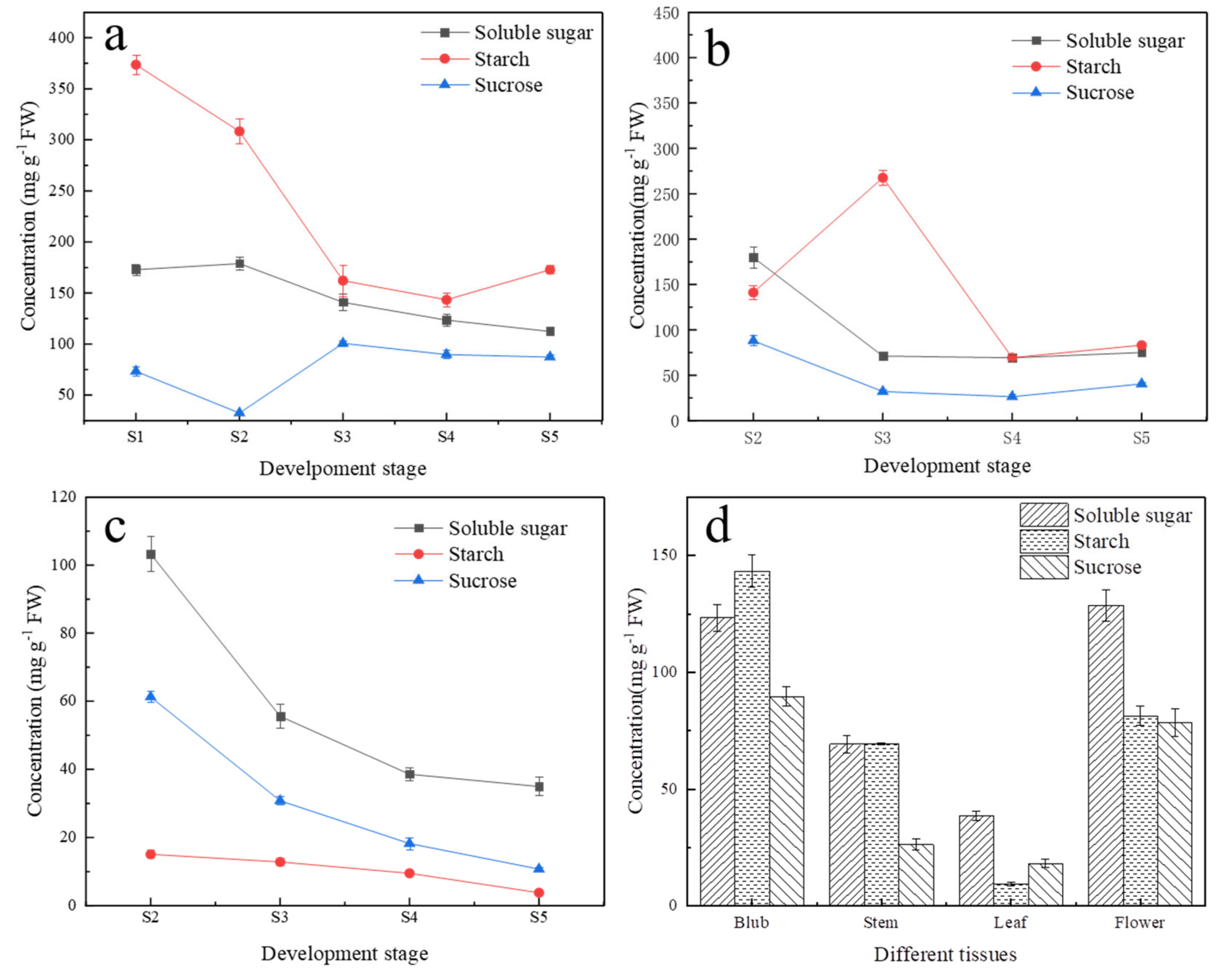
2.1. Variation of Carbohydrate Content during Lily Development in Different Tissues
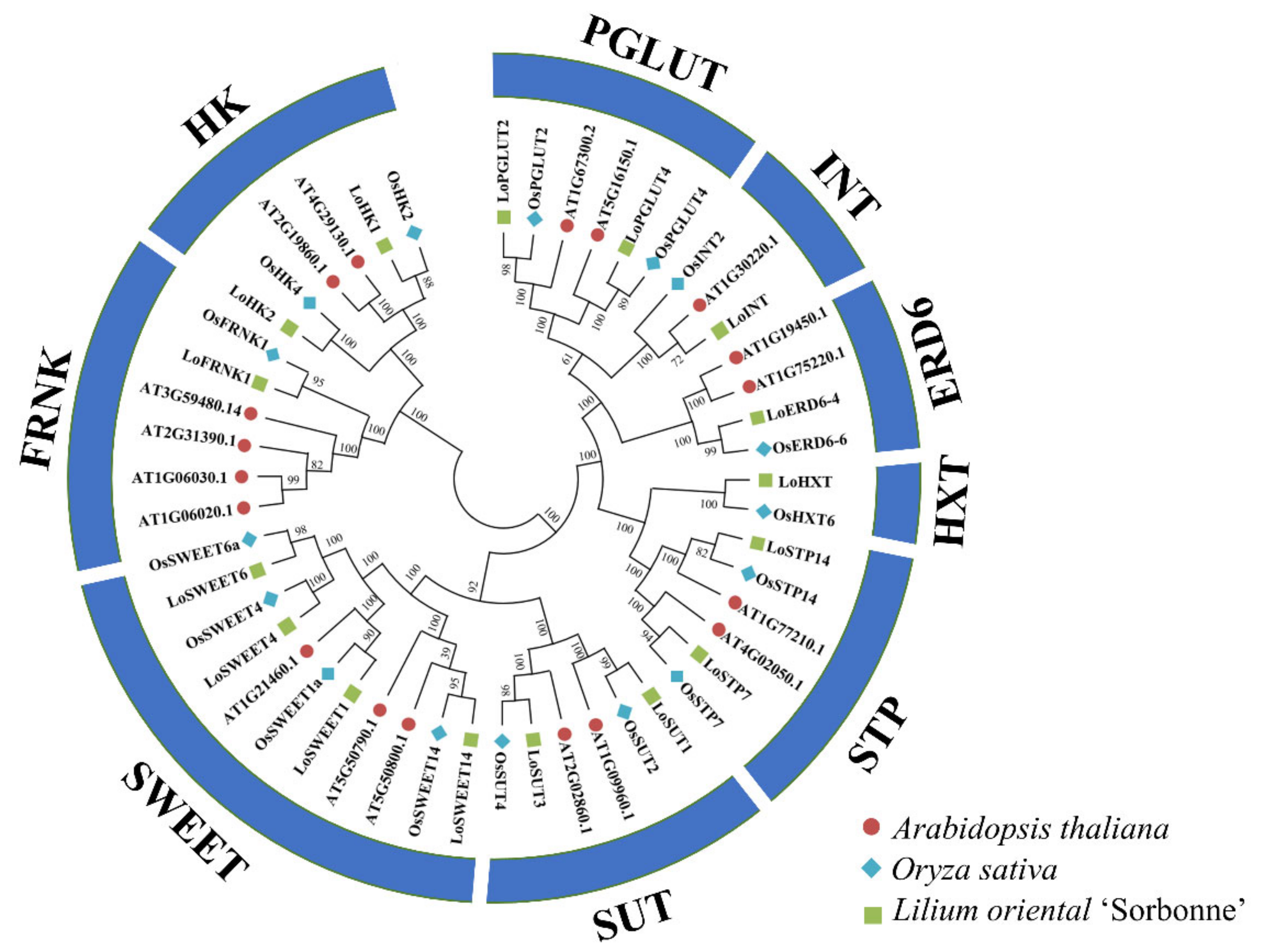
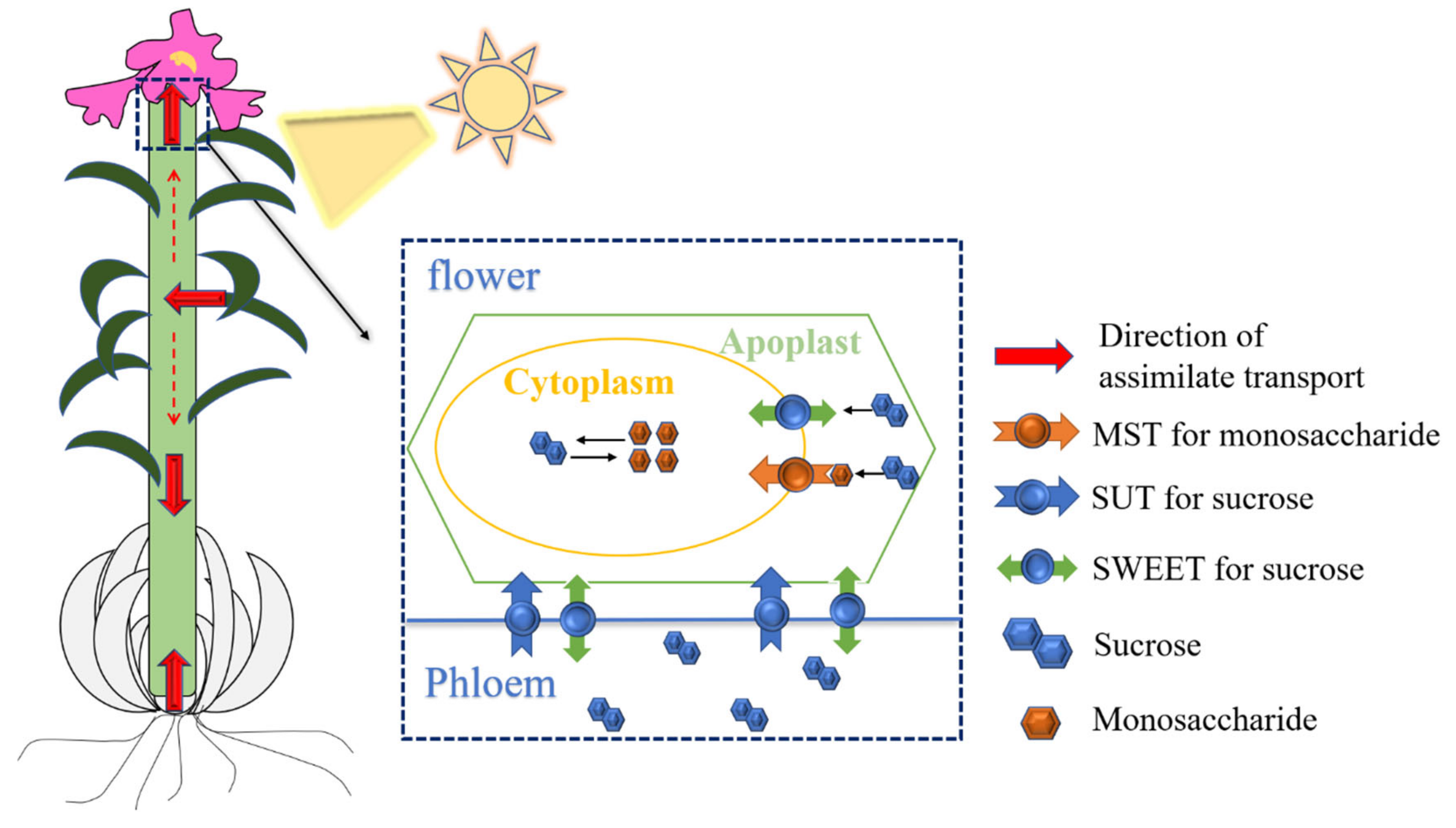
2.2. Identification and Phylogenetic Analysis of Key Sugar Transporter and Metabolism Related Genes during Allocation of Assimates
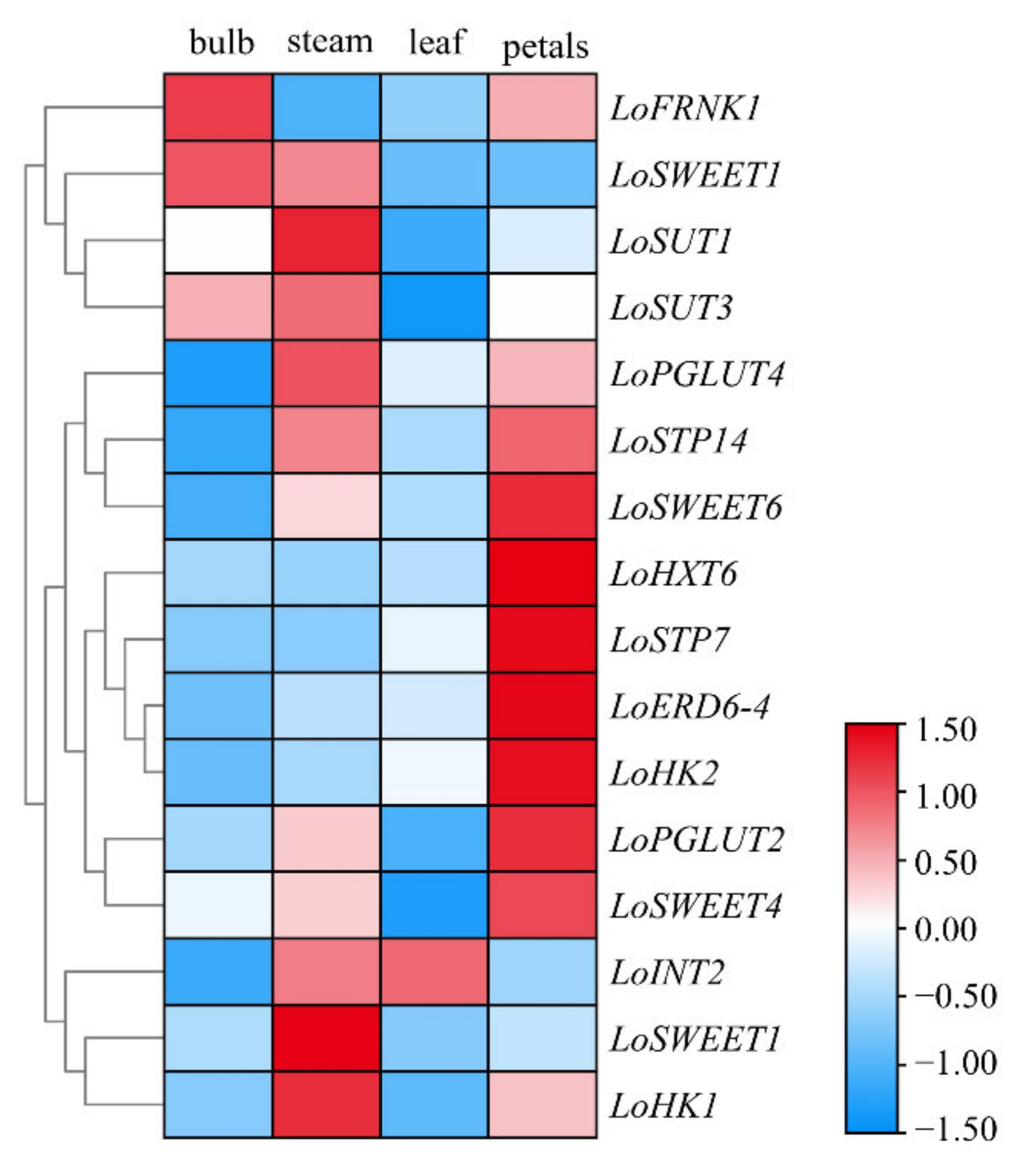
2.3. Tissue-Specific Expression Pattern of ST Genes
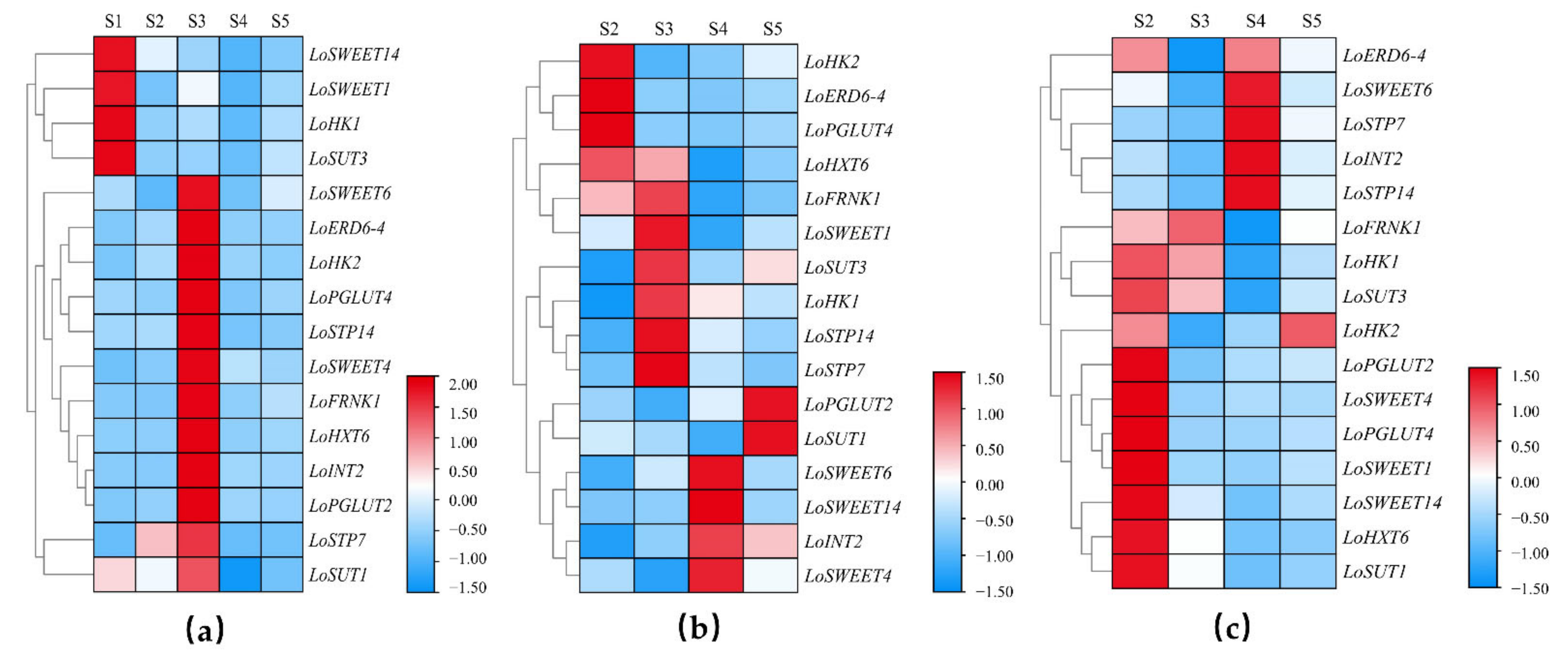
2.4. Spatial and Temporal Differential Expression Patterns of ST Genes during the Allocation of Assimilates
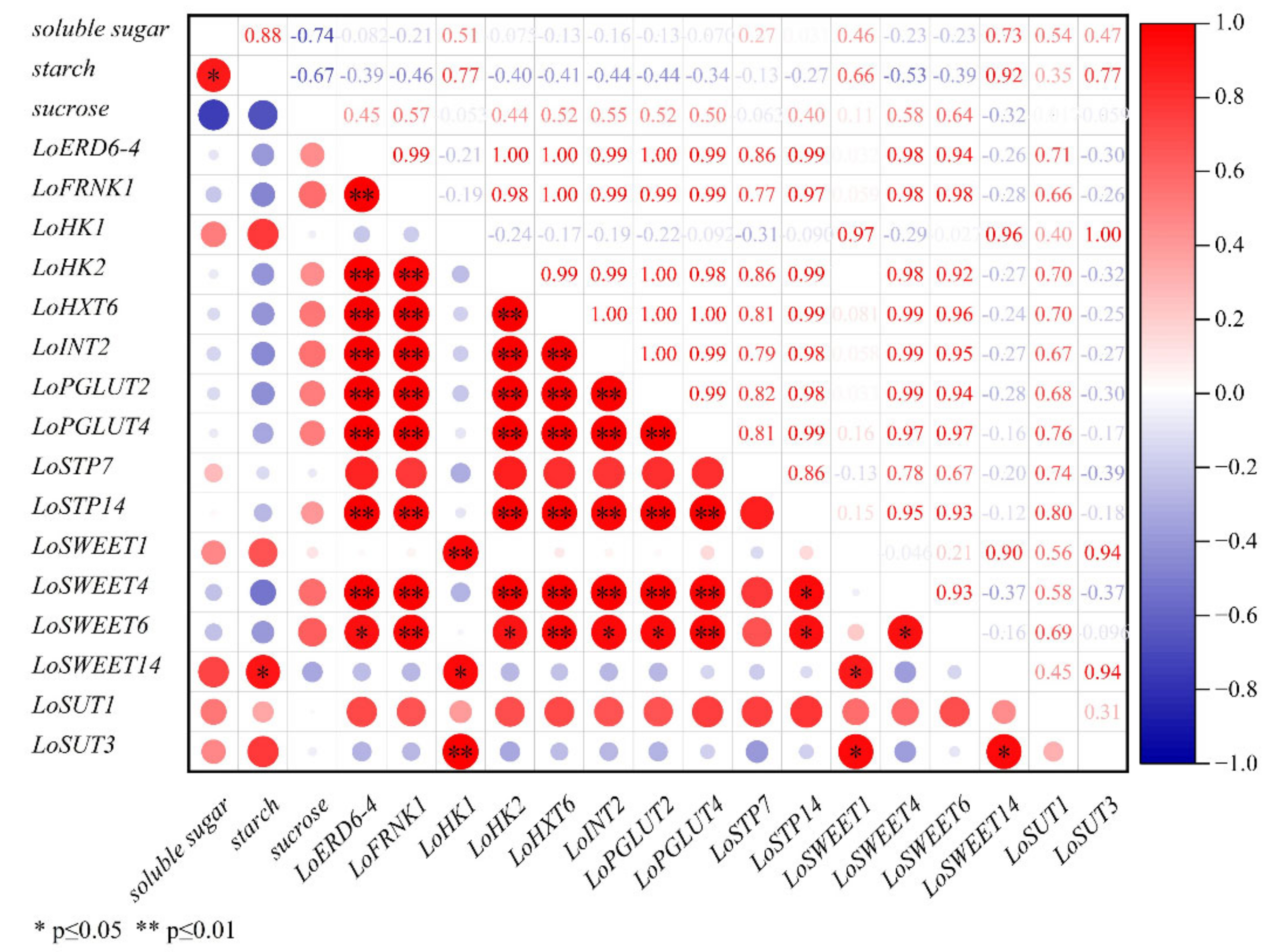
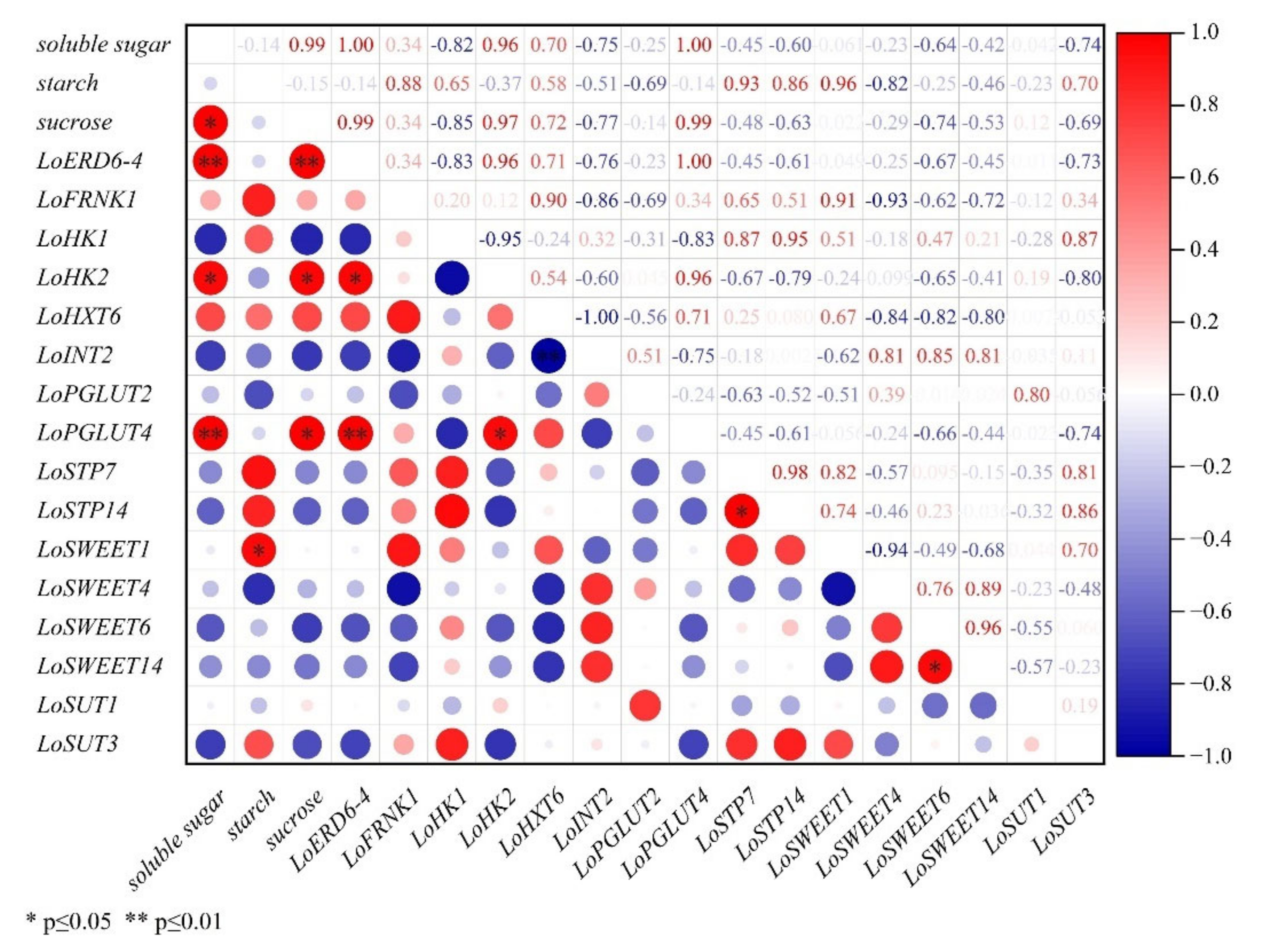
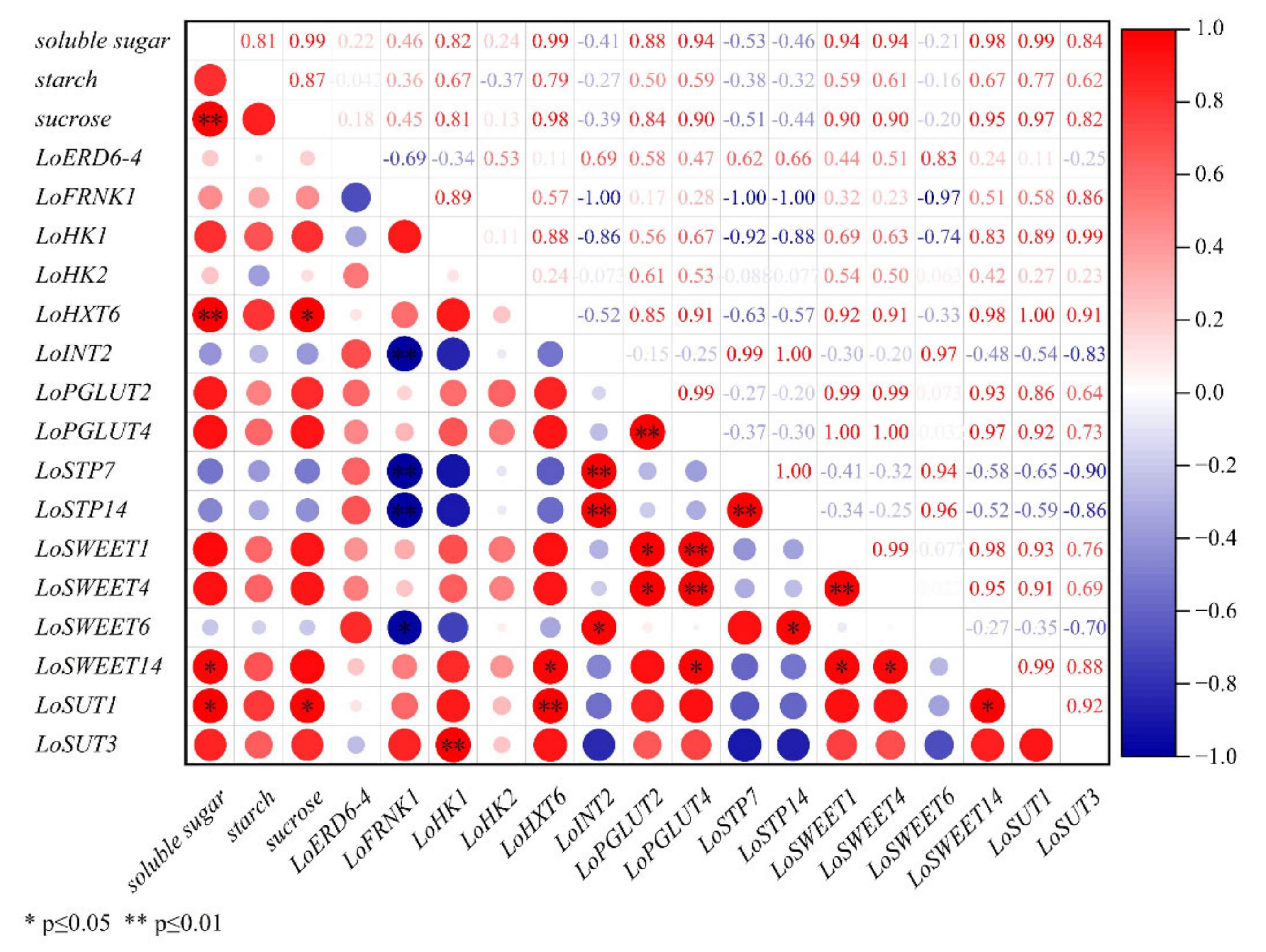
2.5. Correlation Analysis between Expression Levels of ST Genes and Carbohydrate Contents
2.6. Eexpression Patterns of ST Genes in Response to Abiotic Stress
3. Discussion
4. Materials and Methods
4.1. Plant Cultivation and Treatments
4.2. Determination of Carbohydrate Content
4.3. Identification and Phylogenetic Analysis of ST Genes
4.4. RNA Etraction and Quantitative Real-Time PCR
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
Abbreviations
| The BLAST | Basic local alignment search tool |
| NCBI | National Center for Biotechnology Information |
| qRT-PCR | Real-time quantitative reverse transcription polymerase chain reaction |
| ABA | Abscisic acid |
| TMDs | Transmembrane domains MFS major facilitator |
References
- Liu, X.; Yang, G. Adventitious shoot regeneration of oriental lily (Lilium orientalis) and genetic stability evaluation based on ISSR marker variation. In Vitro Cell. Dev. Biol. Plant 2012, 48, 172–179. [Google Scholar] [CrossRef]
- Langens-Gerrits, M.M.; Miller, W.B.M.; Croes, A.F.; de Klerk, G.-J. Effect of low temperature on dormancy breaking and growth after planting in lily bulblets regenerated in vitro. Plant Growth Regul. 2003, 40, 267–275. [Google Scholar] [CrossRef]
- Chang, L.; Xiao, Y.M.; She, L.F.; Xia, Y.P. Analysis of gene expression and enzyme activities related to starch metabolism in Lycoris sprengeri bulbs of different sizes. Sci. Hortic. 2013, 161, 118–124. [Google Scholar] [CrossRef]
- Wu, S.-S.; Wu, J.-D.; Jiao, X.-H.; Zhang, Q.-X.; Lv, Y.-M. The Dynamics of Changes in Starch and Lipid Droplets and Sub-Cellular Localization of β-Amylase During the Growth of Lily Bulbs. J. Integr. Agric. 2012, 11, 585–592. [Google Scholar] [CrossRef]
- Zheng, R.R.; Wu, Y.; Xia, Y.P. Chlorocholine chloride and paclobutrazol treatments promote carbohydrate accumulation in bulbs of Lilium Oriental hybrids ‘Sorbonne’. J. Zhejiang Univ. Sci. B 2012, 13, 136–144. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Ren, Z.; Gao, C.; Sun, M.; Li, S.; Min, R.; Wu, J.; Li, D.; Wang, X.; Wei, Y.; et al. Change in Sucrose Cleavage Pattern and Rapid Starch Accumulation Govern Lily Shoot-to-Bulblet Transition in vitro. Front. Plant Sci. 2020, 11, 564713. [Google Scholar] [CrossRef]
- Liu, D.D.; Chao, W.M.; Turgeon, R. Transport of sucrose, not hexose, in the phloem. J. Exp. Bot. 2012, 63, 4315–4320. [Google Scholar] [CrossRef] [Green Version]
- LE, W.; Lemoine, R.; Sauer, N. Sugar transporters in higher plants—A diversity of roles and complex regulation. Trends Plant Sci. 2000, 5, 283–290. [Google Scholar]
- Rolland, F.; Baena-Gonzalez, E.; Sheen, J. Sugar sensing and signaling in plants: Conserved and novel mechanisms. Annu. Rev. Plant Biol. 2006, 57, 675–709. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Wang, C.; Cheng, J.; Zhang, J.; da Silva, J.A.; Liu, X.; Duan, X.; Li, T.; Sun, H. Transcriptome analysis of carbohydrate metabolism during bulblet formation and development in Lilium davidii var. unicolor. BMC Plant Biol. 2014, 14, 358. [Google Scholar] [CrossRef] [Green Version]
- Julius, B.T.; Leach, K.A.; Tran, T.M.; Mertz, R.A.; Braun, D.M. Sugar Transporters in Plants: New Insights and Discoveries. Plant Cell Physiol. 2017, 58, 1442–1460. [Google Scholar] [CrossRef] [Green Version]
- Li, J.M.; Zheng, D.M.; Li, L.T.; Qiao, X.; Wei, S.W.; Bai, B.; Zhang, S.L.; Wu, J. Genome-Wide Function, Evolutionary Characterization and Expression Analysis of Sugar Transporter Family Genes in Pear (Pyrus bretschneideri Rehd). Plant Cell Physiol. 2015, 56, 1721–1737. [Google Scholar] [CrossRef] [Green Version]
- Buttner, M. The Arabidopsis sugar transporter (AtSTP) family: An update. Plant Biol. 2010, 12 (Suppl. S1), 35–41. [Google Scholar] [CrossRef]
- Buttner, M. The monosaccharide transporter(-like) gene family in Arabidopsis. FEBS Lett. 2007, 581, 2318–2324. [Google Scholar] [CrossRef] [Green Version]
- Gong, X.; Liu, M.; Zhang, L.; Ruan, Y.; Ding, R.; Ji, Y.; Zhang, N.; Zhang, S.; Farmer, J.; Wang, C. Arabidopsis AtSUC2 and AtSUC4, encoding sucrose transporters, are required for abiotic stress tolerance in an ABA-dependent pathway. Physiol. Plant 2015, 153, 119–136. [Google Scholar] [CrossRef]
- Feng, L.; Frommer, W.B. Structure and function of SemiSWEET and SWEET sugar transporters. Trends Biochem. Sci. 2015, 40, 480–486. [Google Scholar] [CrossRef] [Green Version]
- Reinders, A.; Sivitz, A.B.; Ward, J.M. Evolution of plant sucrose uptake transporters. Front. Plant Sci. 2012, 3, 22. [Google Scholar] [CrossRef] [Green Version]
- Iftikhar, J.; Lyu, M.; Liu, Z.; Mehmood, N.; Munir, N.; Ahmed, M.A.A.; Batool, W.; Aslam, M.M.; Yuan, Y.; Wu, B. Sugar and Hormone Dynamics and the Expression Profiles of SUT/SUC and SWEET Sweet Sugar Transporters during Flower Development in Petunia axillaris. Plants 2020, 9, 1770. [Google Scholar] [CrossRef]
- Chen, L.Q.; Hou, B.H.; Lalonde, S.; Takanaga, H.; Hartung, M.L.; Qu, X.Q.; Guo, W.J.; Kim, J.G.; Underwood, W.; Chaudhuri, B.; et al. Sugar transporters for intercellular exchange and nutrition of pathogens. Nature 2010, 468, 527–532. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.Q. SWEET sugar transporters for phloem transport and pathogen nutrition. New Phytol. 2014, 201, 1150–1155. [Google Scholar] [CrossRef]
- Afoufa-Bastien, D.; Medici, A.; Jeauffre, J.; Coutos-Thévenot, P.; Lemoine, R.; Atanassova, R.; Laloi1, M. The Vitis vinifera sugar transporter gene family: Phylogenetic overview and macroarray expression profiling. BMC Plant Biol. 2010, 10, 245. [Google Scholar] [CrossRef] [Green Version]
- Reuscher, S.; Akiyama, M.; Yasuda, T.; Makino, H.; Aoki, K.; Shibata, D.; Shiratake, K. The sugar transporter inventory of tomato: Genome-wide identification and expression analysis. Plant Cell Physiol. 2014, 55, 1123–1141. [Google Scholar] [CrossRef] [Green Version]
- Aslam, M.M.; Deng, L.; Wang, X.; Wang, Y.; Pan, L.; Liu, H.; Niu, L.; Lu, Z.; Cui, G.; Zeng, W.; et al. Expression patterns of genes involved in sugar metabolism and accumulation during peach fruit development and ripening. Sci. Hortic. 2019, 257, 108633. [Google Scholar] [CrossRef]
- Iqbal, S.; Ni, X.; Bilal, M.S.; Shi, T.; Khalil-Ur-Rehman, M.; Zhenpeng, P.; Jie, G.; Usman, M.; Gao, Z. Identification and expression profiling of sugar transporter genes during sugar accumulation at different stages of fruit development in apricot. Gene 2020, 742, 144584. [Google Scholar] [CrossRef]
- Kumawat, S.; Sharma, Y.; Vats, S.; Sudhakaran, S.; Sharma, S.; Mandlik, R.; Raturi, G.; Kumar, V.; Rana, N.; Kumar, A.; et al. Understanding the role of SWEET genes in fruit development and abiotic stress in pomegranate (Punica granatum L.). Mol. Biol. Rep. 2021, 49, 1329–1339. [Google Scholar] [CrossRef]
- Chen, L.Q.; Cheung, L.S.; Feng, L.; Tanner, W.; Frommer, W.B. Transport of sugars. Annu. Rev. Biochem. 2015, 84, 865–894. [Google Scholar] [CrossRef]
- Ruan, Y.L. Sucrose metabolism: Gateway to diverse carbon use and sugar signaling. Annu. Rev. Plant Biol. 2014, 65, 33–67. [Google Scholar] [CrossRef]
- Yamada, K.; Osakabe, Y.; Mizoi, J.; Nakashima, K.; Fujita, Y.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Functional analysis of an Arabidopsis thaliana abiotic stress-inducible facilitated diffusion transporter for monosaccharides. J. Biol. Chem. 2010, 285, 1138–1146. [Google Scholar] [CrossRef] [Green Version]
- Sambe, M.A.; He, X.; Tu, Q.; Guo, Z. A cold-induced myo-inositol transporter-like gene confers tolerance to multiple abiotic stresses in transgenic tobacco plants. Physiol. Plant 2015, 153, 355–364. [Google Scholar] [CrossRef]
- Ma, Q.J.; Sun, M.H.; Liu, Y.J.; Lu, J.; Hu, D.G.; Hao, Y.J. Molecular cloning and functional characterization of the apple sucrose transporter gene MdSUT2. Plant Physiol. Biochem. 2016, 109, 442–451. [Google Scholar] [CrossRef]
- Fernie, A.R.; Bachem, C.W.B.; Helariutta, Y.; Neuhaus, H.E.; Prat, S.; Ruan, Y.L.; Stitt, M.; Sweetlove, L.J.; Tegeder, M.; Wahl, V.; et al. Synchronization of developmental, molecular and metabolic aspects of source-sink interactions. Nat. Plants 2020, 6, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Hong-mei, S.; Tian-lai, L.; Yun-fei, L. Physiology Mechanism of Metabolisms in the Middle Scales of Lilium davidii var. unicolor Bulbs Stored at Low Temperature for Dormancy-Release. Agric. Sci. China 2005, 7, 521–527. [Google Scholar]
- Eveland, A.L.; Jackson, D.P. Sugars, signalling, and plant development. J. Exp. Bot. 2012, 63, 3367–3377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsoukas, I.G. Interplay between sugar and hormone signaling pathways modulate floral signal transduction. Front. Genet. 2014, 5, 218. [Google Scholar] [CrossRef] [Green Version]
- Hu, Z.; Tang, Z.; Zhang, Y.; Niu, L.; Yang, F.; Zhang, D.; Hu, Y. Rice SUT and SWEET Transporters. Int. J. Mol. Sci. 2021, 22, 11198. [Google Scholar] [CrossRef]
- Johnson, D.A.; Thomas, M.A. The monosaccharide transporter gene family in Arabidopsis and rice: A history of duplications, adaptive evolution, and functional divergence. Mol. Biol. Evol. 2007, 24, 2412–2423. [Google Scholar] [CrossRef]
- Karve, A.; Rauh, B.L.; Xia, X.; Kandasamy, M.; Meagher, R.B.; Sheen, J.; Moore, B.D. Expression and evolutionary features of the hexokinase gene family in Arabidopsis. Planta 2008, 228, 411–425. [Google Scholar] [CrossRef] [Green Version]
- He, L.; Zhang, S.; Qiu, Z.; Zhao, J.; Nie, W.; Lin, H.; Zhu, Z.; Zeng, D.; Qian, Q.; Zhu, L. Fructokinase-Like Protein 1 interacts with TRXz to regulate chloroplast development in rice. J. Integr. Plant Biol. 2018, 60, 94–111. [Google Scholar] [CrossRef] [Green Version]
- Riggs, J.W.; Cavales, P.C.; Chapiro, S.M.; Callis, J. Identification and biochemical characterization of the fructokinase gene family in Arabidopsis thaliana. BMC Plant Biol. 2017, 17, 83. [Google Scholar] [CrossRef] [Green Version]
- Xuan, C.; Lan, G.; Si, F.; Zeng, Z.; Wang, C.; Yadav, V.; Wei, C.; Zhang, X. Systematic Genome-Wide Study and Expression Analysis of SWEET Gene Family: Sugar Transporter Family Contributes to Biotic and Abiotic Stimuli in Watermelon. Int. J. Mol. Sci. 2021, 22, 8407. [Google Scholar] [CrossRef]
- Yuan, M.; Wang, S. Rice MtN3/saliva/SWEET family genes and their homologs in cellular organisms. Mol. Plant 2013, 6, 665–674. [Google Scholar] [CrossRef] [Green Version]
- Patil, G.; Valliyodan, B.; Deshmukh, R.; Prince, S.; Nicander, B.; Zhao, M.; Sonah, H.; Song, L.; Lin, L.; Chaudhary, J.; et al. Soybean (Glycine max) SWEET gene family: Insights through comparative genomics, transcriptome profiling and whole genome re-sequence analysis. BMC Genom. 2015, 16, 520. [Google Scholar] [CrossRef] [Green Version]
- Jia, B.; Zhu, X.F.; Pu, Z.J.; Duan, Y.X.; Hao, L.J.; Zhang, J.; Chen, L.Q.; Jeon, C.O.; Xuan, Y.H. Integrative View of the Diversity and Evolution of SWEET and SemiSWEET Sugar Transporters. Front. Plant Sci. 2017, 8, 2178. [Google Scholar] [CrossRef] [Green Version]
- Reddy, V.S.; Shlykov, M.A.; Castillo, R.; Sun, E.I.; Saier, M.H., Jr. The Major Facilitator Superfamily (MFS) Revisited. FEBS J. 2012, 279, 2022–2035. [Google Scholar] [CrossRef]
- Borghi, M.; Fernie, A.R. Floral Metabolism of Sugars and Amino Acids: Implications for Pollinators’ Preferences and Seed and Fruit Set. Plant Physiol. 2017, 175, 1510–1524. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.; Sui, X.; Lucas, W.J.; Li, Y.; Feng, S.; Ma, S.; Fan, J.; Gao, L.; Zhang, Z. Down-regulation of the Sucrose Transporter CsSUT1 Causes Male Sterility by Altering Carbohydrate Supply. Plant Physiol. 2019, 180, 986–997. [Google Scholar] [CrossRef] [Green Version]
- Lin, I.W.; Sosso, D.; Chen, L.Q.; Gase, K.; Kim, S.G.; Kessler, D.; Klinkenberg, P.M.; Gorder, M.K.; Hou, B.H.; Qu, X.Q.; et al. Nectar secretion requires sucrose phosphate synthases and the sugar transporter SWEET9. Nature 2014, 508, 546–549. [Google Scholar] [CrossRef]
- Nativ, N.; Hacham, Y.; Hershenhorn, J.; Dor, E.; Amir, R. Metabolic Investigation of Phelipanche aegyptiaca Reveals Significant Changes during Developmental Stages and in Its Different Organs. Front. Plant Sci. 2017, 8, 491. [Google Scholar] [CrossRef] [Green Version]
- Doidy, J.; Vidal, U.; Lemoine, R. Sugar transporters in Fabaceae, featuring SUT MST and SWEET families of the model plant Medicago truncatula and the agricultural crop Pisum sativum. PLoS ONE 2019, 14, e0223173. [Google Scholar] [CrossRef] [Green Version]
- Qin, J.-X.; Jiang, Y.-J.; Lu, Y.-Z.; Zhao, P.; Wu, B.-J.; Li, H.-X.; Wang, Y.; Xu, S.-B.; Sun, Q.-X.; Liu, Z.-S. Genome-wide identification and transcriptome profiling reveal great expansion of SWEET gene family and their wide-spread responses to abiotic stress in wheat (Triticum aestivum L.). J. Integr. Agric. 2020, 19, 1704–1720. [Google Scholar] [CrossRef]
- Miao, H.; Sun, P.; Liu, Q.; Miao, Y.; Liu, J.; Zhang, K.; Hu, W.; Zhang, J.; Wang, J.; Wang, Z.; et al. Genome-wide analyses of SWEET family proteins reveal involvement in fruit development and abiotic/biotic stress responses in banana. Sci. Rep. 2017, 7, 3536. [Google Scholar] [CrossRef] [Green Version]
- Morii, M.; Sugihara, A.; Takehara, S.; Kanno, Y.; Kawai, K.; Hobo, T.; Hattori, M.; Yoshimura, H.; Seo, M.; Ueguchi-Tanaka, M. The Dual Function of OsSWEET3a as a Gibberellin and Glucose Transporter Is Important for Young Shoot Development in Rice. Plant Cell Physiol. 2020, 61, 1935–1945. [Google Scholar] [CrossRef]
- Mathan, J.; Singh, A.; Ranjan, A. Sucrose transport in response to drought and salt stress involves ABA-mediated induction of OsSWEET13 and OsSWEET15 in rice. Physiol. Plant 2021, 171, 620–637. [Google Scholar] [CrossRef]
- Wu, Y.; Sun, M.; Zhang, J.; Zhang, L.; Ren, Z.; Min, R.; Wang, X.; Xia, Y. Differential Effects of Paclobutrazol on the Bulblet Growth of Oriental Lily Cultured In Vitro: Growth Behavior, Carbohydrate Metabolism, and Antioxidant Capacity. J. Plant Growth Regul. 2018, 38, 359–372. [Google Scholar] [CrossRef]
- Lu, X.H.; Huang, J.; Wang, J.M.; Lu, Y.M. RNA-Seq analysis reveals genetic bases of the flowering process in oriental hybrid lily cv. Sorbonne. Russ. J. Plant Physiol. 2014, 48, 523–544. [Google Scholar]
- Wang, J.; Wang, Q.; Yang, Y.; Liu, X.; Gu, J.; Li, W.; Ma, S.; Lu, Y. De Novo assembly and characterization of stress transcriptome and regulatory networks under temperature, salt and hormone stresses in Lilium lancifolium. Mol. Biol. Rep. 2014, 41, 8231–8245. [Google Scholar] [CrossRef]
- Gu, J.; Zeng, Z.; Wang, Y.; Lyu, Y. Transcriptome Analysis of Carbohydrate Metabolism Genes and Molecular Regulation of Sucrose Transport Gene LoSUT on the Flowering Process of Developing Oriental Hybrid Lily ‘Sorbonne’ Bulb. Int. J. Mol. Sci. 2020, 21, 3092. [Google Scholar] [CrossRef]
- Zhang, Y.; Zeng, Z.; Yong, Y.; Lyu, Y. Hormonal Regulatory Patterns of LaKNOXs and LaBEL1 Transcription Factors Reveal Their Potential Role in Stem Bulblet Formation in LA Hybrid Lily. Int. J. Mol. Sci. 2021, 22, 13502. [Google Scholar] [CrossRef]
- Yong, Y.B.; Li, W.Q.; Wang, J.M.; Zhang, Y.; Lu, Y.M. Identification of gene co-expression networks involved in cold resistance of Lilium lancifolium. Biol. Plant. 2018, 62, 287–298. [Google Scholar] [CrossRef]

| Gene ID | Name | CDS (bp) | Protein Length (aa) | TMDs | pI | Instability Index | Molecular Weight (kDa) | Subcellular Localization |
|---|---|---|---|---|---|---|---|---|
| Isoform0001530 | LoERD6-4 | 1488 | 495 | 12 | 9.15 | 40.07/N | 53.38 | PM |
| Isoform0001773 | LoSUT1 | 1495 | 497 | 12 | 9.2 | 33.71/S | 53.17 | PM |
| Isoform0002195 | LoHK2 | 1482 | 493 | 0 | 5.76 | 41.1/N | 53.05 | Chlo |
| Isoform0003167 | LoHK1 | 1503 | 500 | 0 | 6.34 | 33.93/S | 54.05 | Chlo |
| Isoform0003989 | LoHXT6 | 1497 | 498 | 12 | 9.16 | 38.99/S | 54.2 | PM |
| Isoform0008566 | LoSWEET14 | 837 | 278 | 7 | 8.49 | 24.01/S | 31.37 | PM |
| Isoform0008671 | LoFRNK1 | 999 | 332 | 0 | 5.21 | 27.58/S | 35.82 | Cy |
| Isoform0012368 | LoSWEET4 | 777 | 258 | 7 | 9.26 | 38.49/S | 28.64 | PM |
| Isoform0012812 | LoSWEET6 | 705 | 234 | 7 | 8.9 | 40.91/N | 25.97 | PM |
| Isoform0015959 | LoSWEET1 | 633 | 206 | 5 | 9.41 | 34.47/S | 22.82 | PM |
| Isoform0031035 | LoINT2 | 1728 | 575 | 12 | 8.91 | 40.22/N | 62.75 | PM |
| Isoform0031287 | LoSTP7 | 1524 | 507 | 12 | 9.37 | 36.62/S | 55.24 | PM |
| Isoform0031586 | LoPGLUT4 | 1605 | 534 | 10 | 9.31 | 35.66/S | 56.31 | PM |
| Isoform0031646 | LoSUT3 | 1773 | 590 | 12 | 6.86 | 34.39/S | 63.32 | PM |
| Isoform0031860 | LoSTP14 | 1533 | 510 | 10 | 8.04 | 41.18/N | 55.96 | PM |
| Isoform0031918 | LoPGLUT2 | 1521 | 506 | 10 | 8.29 | 41.12/N | 54.43 | PM |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, Z.; Lyu, T.; Jia, X.; Chen, Y.; Lyu, Y. Expression Patterns of Sugar Transporter Genes in the Allocation of Assimilates and Abiotic Stress in Lily. Int. J. Mol. Sci. 2022, 23, 4319. https://doi.org/10.3390/ijms23084319
Zeng Z, Lyu T, Jia X, Chen Y, Lyu Y. Expression Patterns of Sugar Transporter Genes in the Allocation of Assimilates and Abiotic Stress in Lily. International Journal of Molecular Sciences. 2022; 23(8):4319. https://doi.org/10.3390/ijms23084319
Chicago/Turabian StyleZeng, Zhen, Tong Lyu, Xin Jia, Yue Chen, and Yingmin Lyu. 2022. "Expression Patterns of Sugar Transporter Genes in the Allocation of Assimilates and Abiotic Stress in Lily" International Journal of Molecular Sciences 23, no. 8: 4319. https://doi.org/10.3390/ijms23084319





