Dexmedetomidine-Induced Aortic Contraction Involves Transactivation of the Epidermal Growth Factor Receptor in Rats
Abstract
:1. Introduction
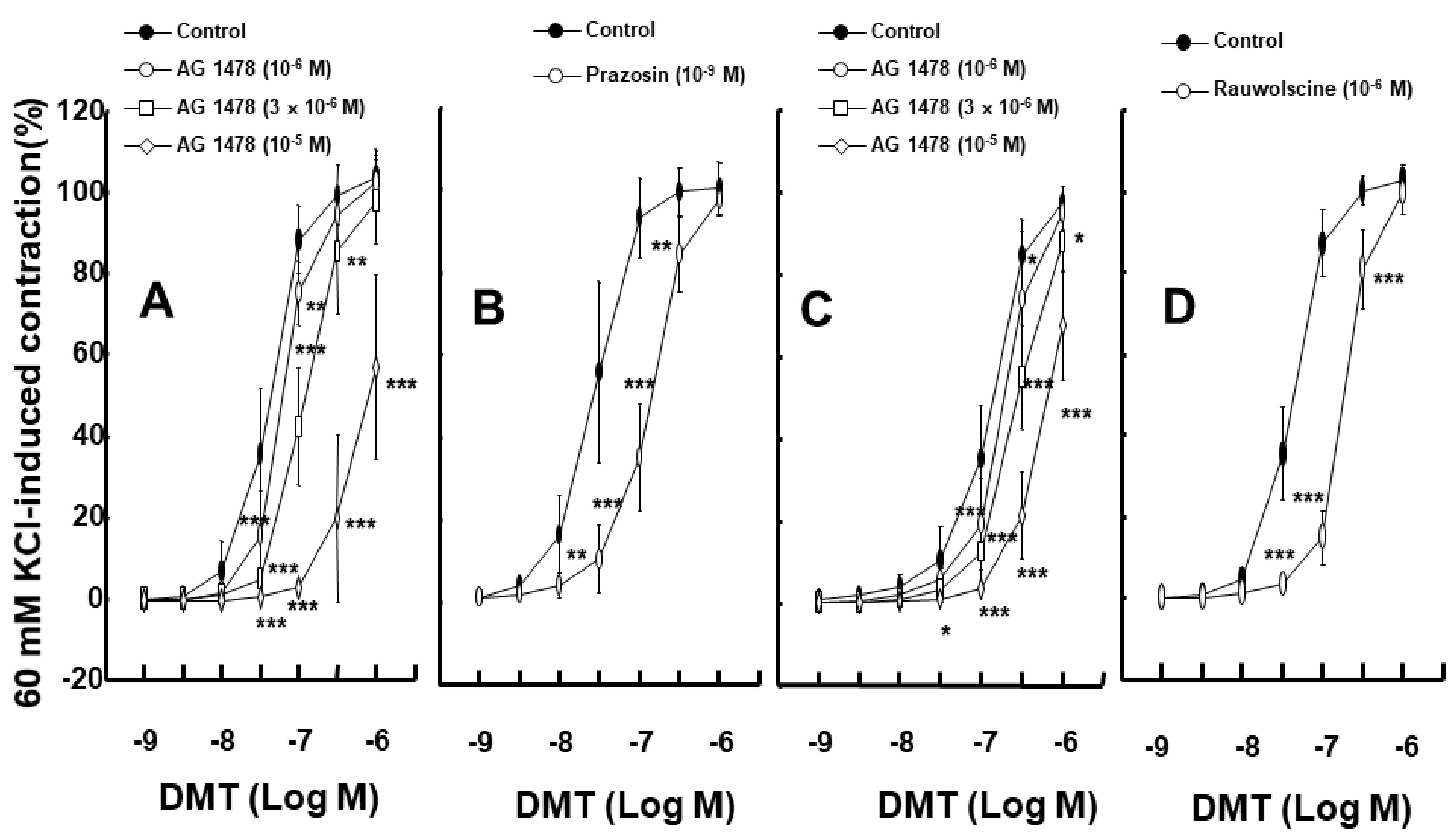
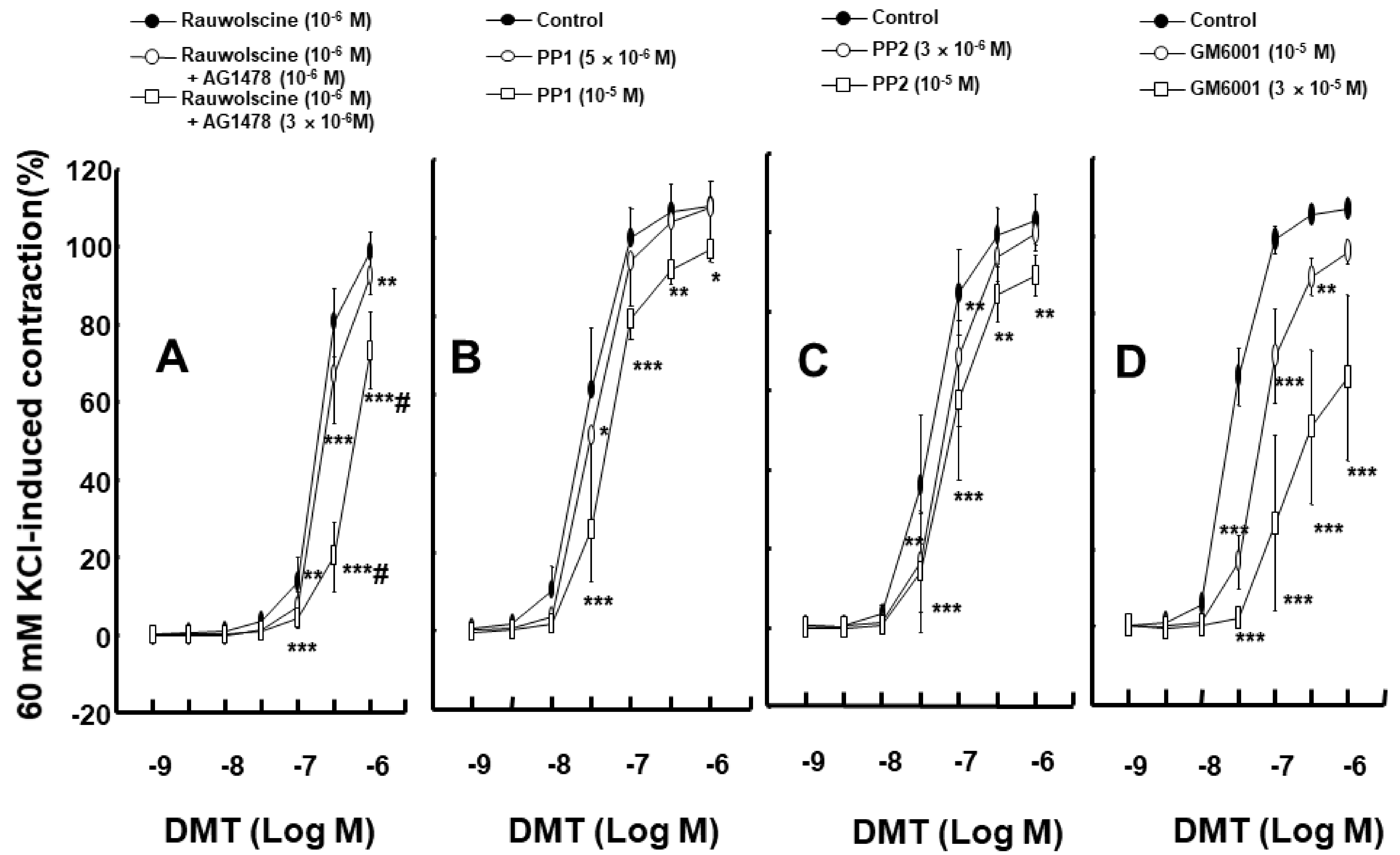
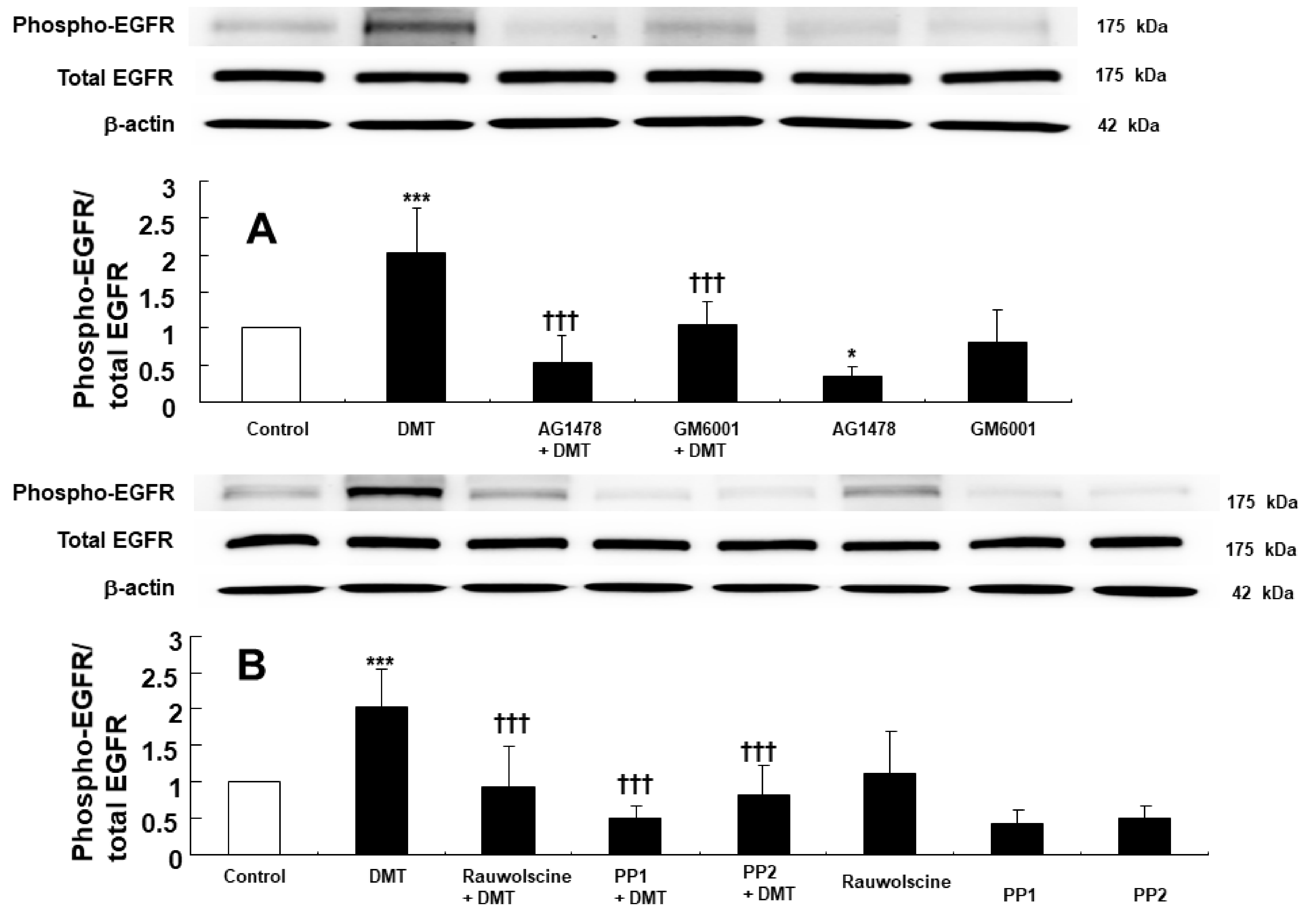
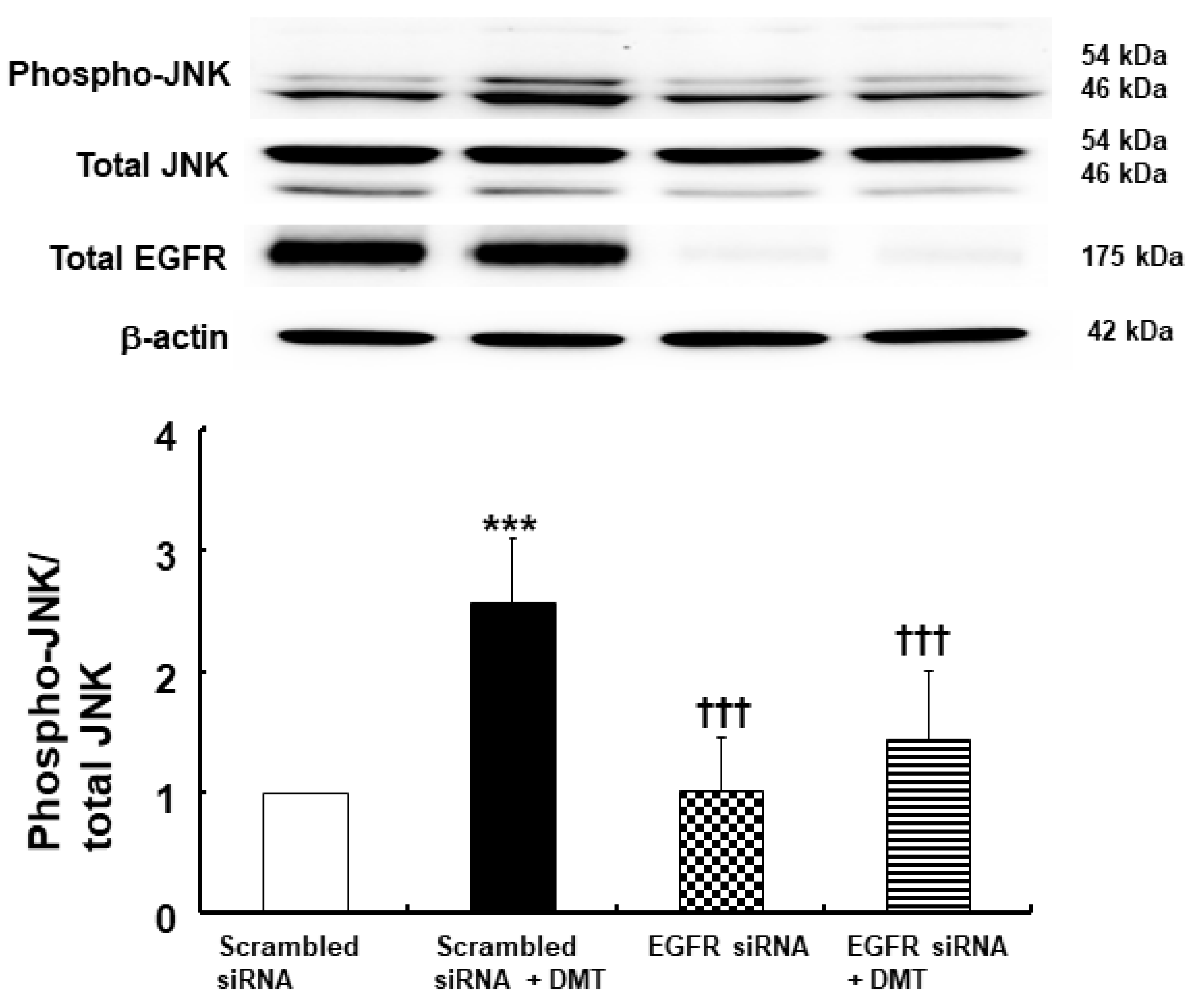
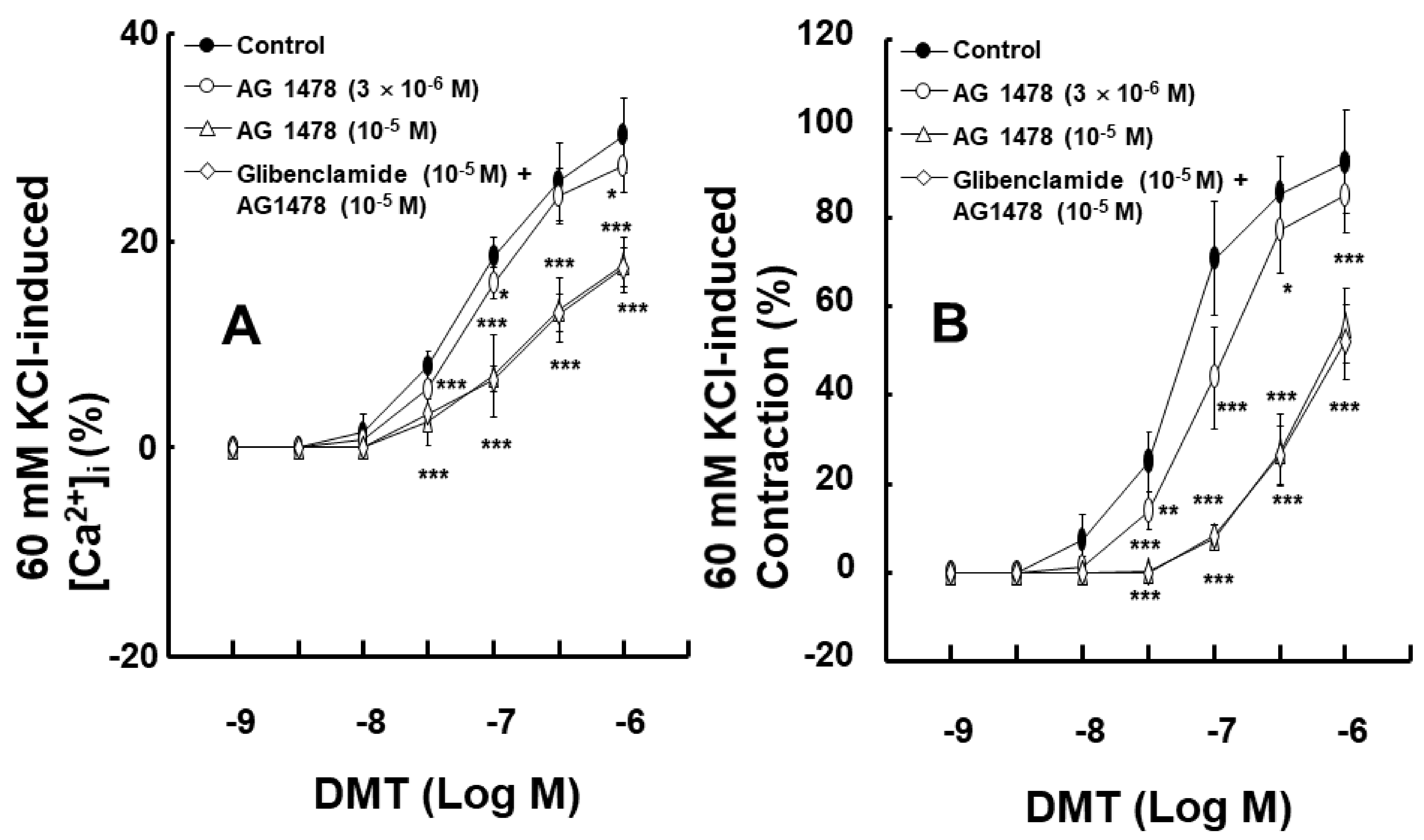
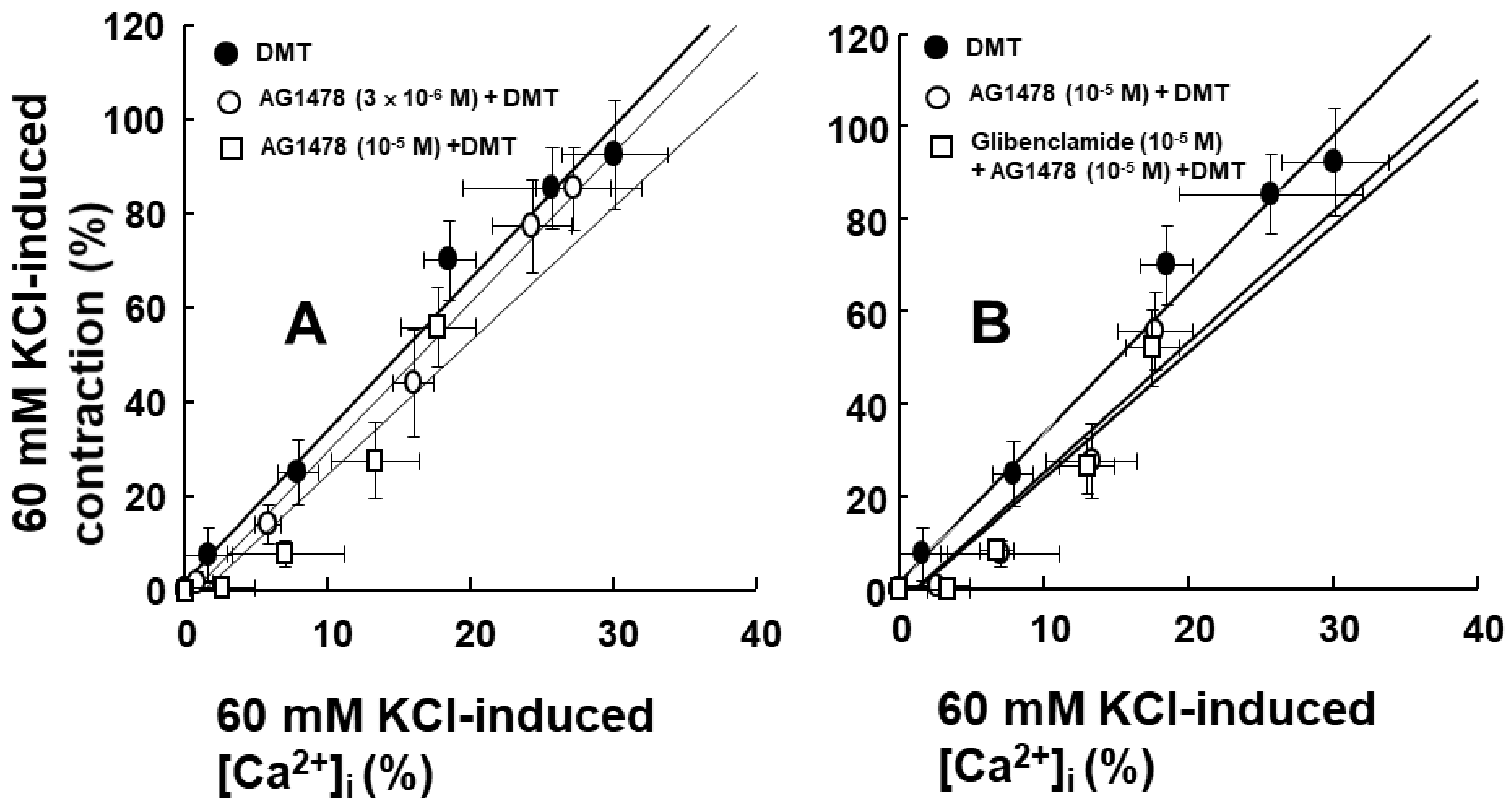
2. Results
3. Discussion
4. Materials and Methods
4.1. Preparation of Isolated Rat Aortas and Isometric Tension Measurement
4.2. Detection of EGFR and JNK Phosphorylation in Rat Aortic Smooth Muscle
4.3. Cell Culture
4.4. Gene Silencing Experiments Using Small Interfering RNA (siRNA)
4.5. Simultaneous Calcium Level ([Ca2+]i) and Tension Measurement Using Fura-2-Loaded Aortic Strips
4.6. Chemicals
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gertler, R.; Brown, H.C.; Mitchell, D.H.; Silvius, E.N. Dexmedetomidine: A novel sedative-analgesic agent. Proc. (Bayl. Univ. Med. Cent.) 2001, 14, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Kim, J.S.; Choi, E.Y.; Chang, Y.; Choi, W.I.; Hwang, J.J.; Moon, J.Y.; Lee, K.; Kim, S.W.; Kang, H.K.; et al. Korean NIV Study Group. Utilization of pain and sedation therapy on noninvasive mechanical ventilation in Korean intensive care units: A multi-center prospective observational study. Acute Crit. Care 2020, 35, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Bloor, B.C.; Ward, D.S.; Belleville, J.P.; Maze, M. Effects of intravenous dexmedetomidine in humans. II. Hemodynamic changes. Anesthesiology 1992, 77, 1134–1142. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, S.; Moura, D. Vascular adrenoceptors: An update. Pharmacol. Rev. 2001, 53, 319–356. [Google Scholar]
- Ebert, T.J.; Hall, J.E.; Barney, J.A.; Uhrich, T.D.; Colinco, M.D. The effects of increasing plasma concentrations of dexmedetomidine in humans. Anesthesiology 2000, 93, 382–394. [Google Scholar] [CrossRef]
- Erkonen, G.; Lamb, F.; Tobias, J.D. High-dose dexmedetomidine-induced hypertension in a child with traumatic brain injury. Neurocrit. Care 2008, 9, 366–369. [Google Scholar] [CrossRef]
- Mason, K.P.; Zurakowski, D.; Zgleszewski, S.; Prescilla, R.; Fontaine, P.J.; Dinardo, J.A. Incidence and predictors of hypertension during high-dose dexmedetomidine sedation for pediatric MRI. Paediatr. Anaesth. 2010, 20, 516–523. [Google Scholar] [CrossRef]
- Mizobe, T. Adrenergic receptor and knockout mouse: 2). Alpha adrenergic receptor knockout mouse. Masui 2001, 50, 12–19. [Google Scholar]
- Makki, N.; Thiel, K.W.; Miller, F.J., Jr. The epidermal growth factor receptor and its ligands in cardiovascular disease. Int. J. Mol. Sci. 2013, 14, 20597–20613. [Google Scholar] [CrossRef] [Green Version]
- Berk, B.C.; Brock, T.A.; Webb, R.C.; Taubman, M.B.; Atkinson, W.J.; Gimbrone, M.A., Jr.; Alexander, R.W. Epidermal growth factor, a vascular smooth muscle mitogen, induces rat aortic contraction. J. Clin. Investig. 1985, 75, 1083–1086. [Google Scholar] [CrossRef] [Green Version]
- Ok, S.H.; Jeong, Y.S.; Kim, J.G.; Lee, S.M.; Sung, H.J.; Kim, H.J.; Chang, K.C.; Kwon, S.C.; Sohn, J.T. c-Jun NH₂-terminal kinase contributes to dexmedetomidine-induced contraction in isolated rat aortic smooth muscle. Yonsei Med. J. 2011, 52, 420–428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ok, S.H.; Bae, S.I.; Shim, H.S.; Sohn, J.T. Dexmedetomidine-induced contraction of isolated rat aorta is dependent on extracellular calcium concentration. Korean J. Anesthesiol. 2012, 63, 253–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cussac, D.; Schaak, S.; Denis, C.; Paris, H. alpha 2B-adrenergic receptor activates MAPK via a pathway involving arachidonic acid metabolism, matrix metalloproteinases, and epidermal growth factor receptor transactivation. J. Biol. Chem. 2002, 277, 19882–19888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, B.; Du, T.; Li, H.; Gu, L.; Zhang, H.; Huang, J.; Hertz, L.; Peng, L. Signalling pathways for transactivation by dexmedetomidine of epidermal growth factor receptors in astrocytes and its paracrine effect on neurons. Br. J. Pharmacol. 2008, 154, 191–203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mifune, M.; Ohtsu, H.; Suzuki, H.; Nakashima, H.; Brailoiu, E.; Dun, N.J.; Frank, G.D.; Inagami, T.; Higashiyama, S.; Thomas, W.G.; et al. G protein coupling and second messenger generation are indispensable for metalloprotease-dependent, heparin-binding epidermal growth factor shedding through angiotensin II type-1 receptor. J. Biol. Chem. 2005, 280, 26592–26599. [Google Scholar] [CrossRef] [Green Version]
- Hao, L.; Du, M.; Lopez-Campistrous, A.; Fernandez-Patron, C. Agonist-induced activation of matrix metalloproteinase-7 promotes vasoconstriction through the epidermal growth factor-receptor pathway. Circ. Res. 2004, 94, 68–76. [Google Scholar] [CrossRef] [Green Version]
- Virtanen, R.; Savola, J.M.; Saano, V.; Nyman, L. Characterization of the selectivity, specificity and potency of medetomidine as an alpha 2-adrenoceptor agonist. Eur. J. Pharmacol. 1988, 150, 9–14. [Google Scholar] [CrossRef]
- Ulu, N.; Gurdal, H.; Landheer, S.W.; Duin, M.; Guc, M.O.; Buikema, H.; Henning, R.H. α1-Adrenoceptor-mediated contraction of rat aorta is partly mediated via transactivation of the epidermal growth factor receptor. Br. J. Pharmacol. 2010, 161, 1301–1310. [Google Scholar] [CrossRef] [Green Version]
- Schreier, B.; Döhler, M.; Rabe, S.; Schneider, B.; Schwerdt, G.; Ruhs, S.; Sibilia, M.; Gotthardt, M.; Gekle, M.; Grossmann, C. Consequences of epidermal growth factor receptor (ErbB1) loss for vascular smooth muscle cells from mice with targeted deletion of ErbB1. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 1643–1652. [Google Scholar] [CrossRef] [Green Version]
- Lambert, D.G. Drugs and receptors. Contin. Educ. Anaesth. Crit. Care Pain 2004, 4, 181–184. [Google Scholar] [CrossRef]
- Yan, Y.; Shirakabe, K.; Werb, Z. The metalloprotease Kuzbanian (ADAM10) mediates the transactivation of EGF receptor by G protein-coupled receptors. J. Cell. Biol. 2002, 158, 221–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eguchi, S.; Dempsey, P.J.; Frank, G.D.; Motley, E.D.; Inagami, T. Activation of MAPKs by angiotensin II in vascular smooth muscle cells. Metalloprotease-dependent EGF receptor activation is required for activation of ERK and p38 MAPK but not for JNK. J. Biol. Chem. 2001, 276, 7957–7962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reynolds, C.M.; Eguchi, S.; Frank, G.D.; Motley, E.D. Signaling mechanisms of heparin-binding epidermal growth factor-like growth factor in vascular smooth muscle cells. Hypertension 2002, 39 Pt 2, 525–529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trinh, N.T.; Privé, A.; Kheir, L.; Bourret, J.C.; Hijazi, T.; Amraei, M.G.; Noël, J.; Brochiero, E. Involvement of KATP and KvLQT1 K+ channels in EGF-stimulated alveolar epithelial cell repair processes. Am. J. Physiol. Lung. Cell. Mol. Physiol. 2007, 293, L870–L882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, S.; Sangari, T.; Qasim, M.; Martin, T. Severe hypertension and bradycardia after dexmedetomidine for radiology sedation in a patient with acute transverse myelitis. Paediatr. Anaesth. 2008, 18, 681–682. [Google Scholar] [CrossRef]
- Clifford, P.S. Local control of blood flow. Adv. Physiol. Educ. 2011, 35, 5–15. [Google Scholar] [CrossRef]
- Lee, S.H.; Kwon, S.C.; Ok, S.H.; Hong, J.M.; Kim, J.Y.; Ahn, S.H.; Bae, S.I.; Shin, Y.; Sohn, J.T. Levobupivacaine-induced vasoconstriction involves caldesmon phosphorylation mediated by tyrosine kinase-induced ERK phosphorylation. Eur. J. Pharmacol. 2019, 842, 167–176. [Google Scholar] [CrossRef]
- Yu, J.; Tokinaga, Y.; Kuriyama, T.; Uematsu, N.; Mizumoto, K.; Hatano, Y. Involvement of Ca2+ sensitization in ropivacaine-induced contraction of rat aortic smooth muscle. Anesthesiology 2005, 103, 548–555. [Google Scholar] [CrossRef]
- Satpathy, M.; Mezencev, R.; Wang, L.; McDonald, J.F. Targeted in vivo delivery of EGFR siRNA inhibits ovarian cancer growth and enhances drug sensitivity. Sci. Rep. 2016, 6, 36518. [Google Scholar] [CrossRef]
- Lavergne, C.; Martinez, M.J.; Trottier, C. Empirical model selection in generalized linear mixed effects models. Comput. Stat. 2008, 23, 99–109. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.H.; Kwon, S.-C.; Ok, S.-H.; Ahn, S.H.; Bae, S.I.; Kim, J.-Y.; Hwang, Y.; Park, K.-E.; Kim, M.; Sohn, J.-T. Dexmedetomidine-Induced Aortic Contraction Involves Transactivation of the Epidermal Growth Factor Receptor in Rats. Int. J. Mol. Sci. 2022, 23, 4320. https://doi.org/10.3390/ijms23084320
Lee SH, Kwon S-C, Ok S-H, Ahn SH, Bae SI, Kim J-Y, Hwang Y, Park K-E, Kim M, Sohn J-T. Dexmedetomidine-Induced Aortic Contraction Involves Transactivation of the Epidermal Growth Factor Receptor in Rats. International Journal of Molecular Sciences. 2022; 23(8):4320. https://doi.org/10.3390/ijms23084320
Chicago/Turabian StyleLee, Soo Hee, Seong-Chun Kwon, Seong-Ho Ok, Seung Hyun Ahn, Sung Il Bae, Ji-Yoon Kim, Yeran Hwang, Kyeong-Eon Park, Mingu Kim, and Ju-Tae Sohn. 2022. "Dexmedetomidine-Induced Aortic Contraction Involves Transactivation of the Epidermal Growth Factor Receptor in Rats" International Journal of Molecular Sciences 23, no. 8: 4320. https://doi.org/10.3390/ijms23084320
APA StyleLee, S. H., Kwon, S.-C., Ok, S.-H., Ahn, S. H., Bae, S. I., Kim, J.-Y., Hwang, Y., Park, K.-E., Kim, M., & Sohn, J.-T. (2022). Dexmedetomidine-Induced Aortic Contraction Involves Transactivation of the Epidermal Growth Factor Receptor in Rats. International Journal of Molecular Sciences, 23(8), 4320. https://doi.org/10.3390/ijms23084320





