Conductive Supramolecular Polymer Nanocomposites with Tunable Properties to Manipulate Cell Growth and Functions
Abstract
:1. Introduction
2. Results and Discussion
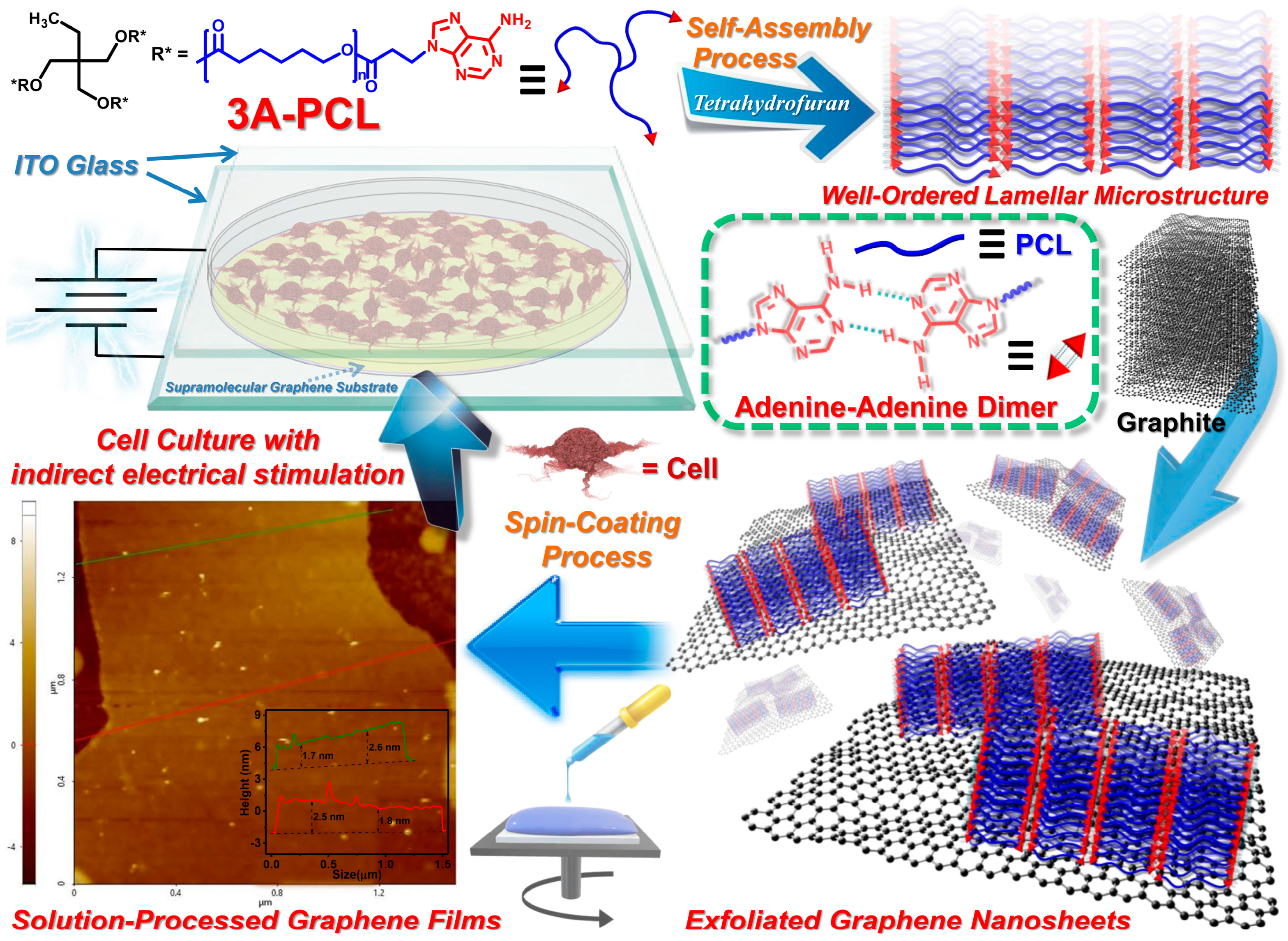
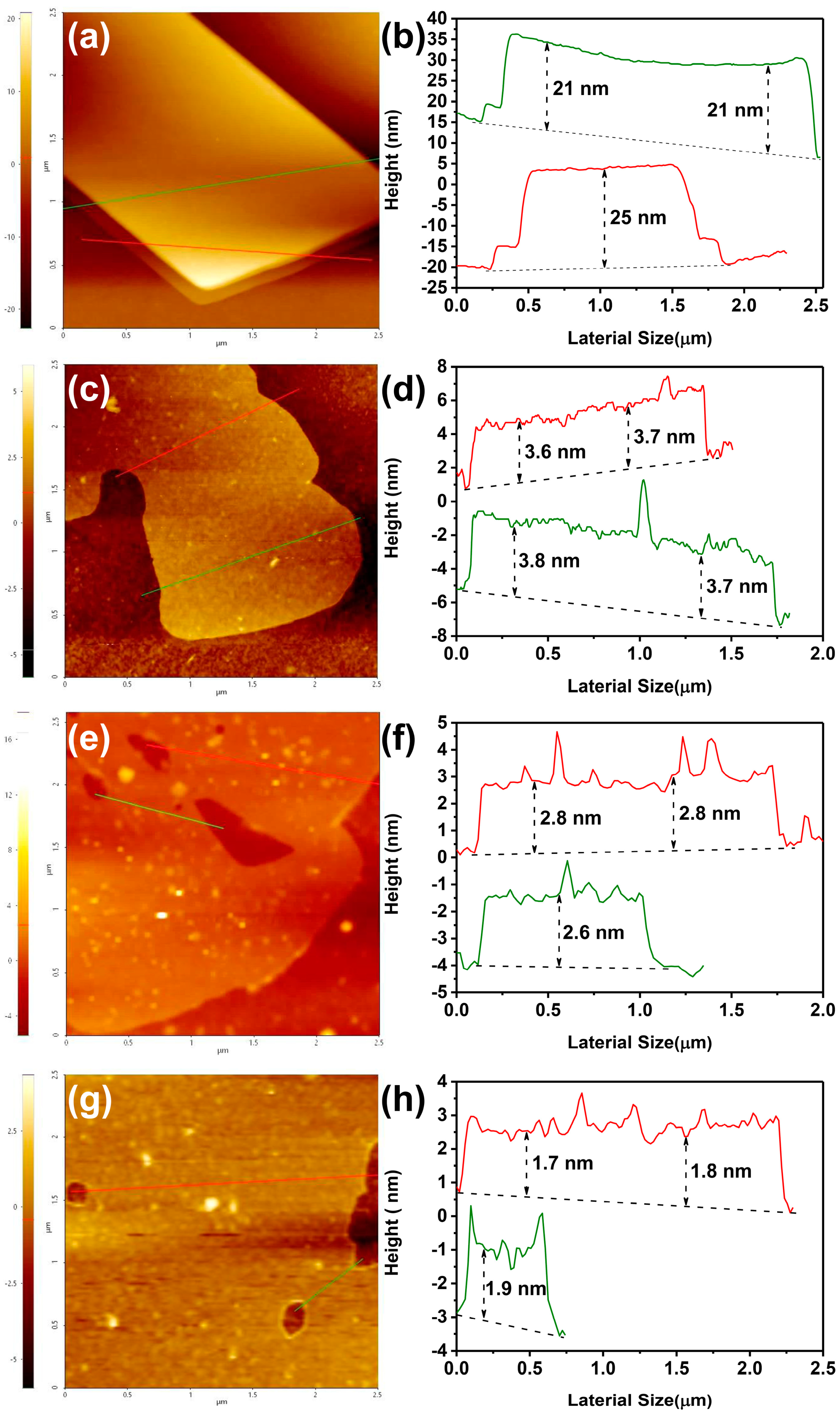
2.1. Physical Properties of Graphite/3A-PCL Composites
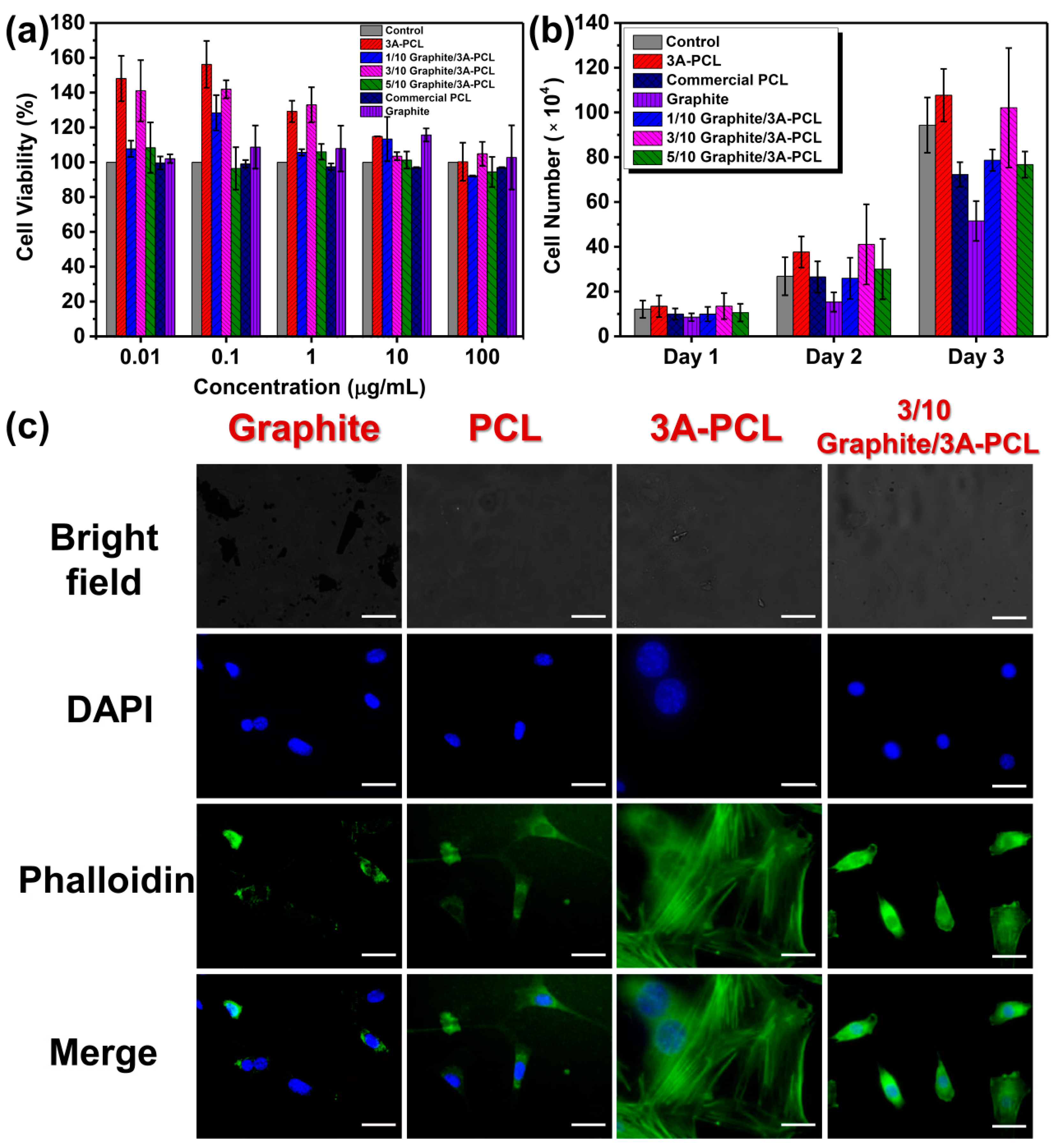
2.2. Graphite/3A-PCL Composites for Cell Culture Applications
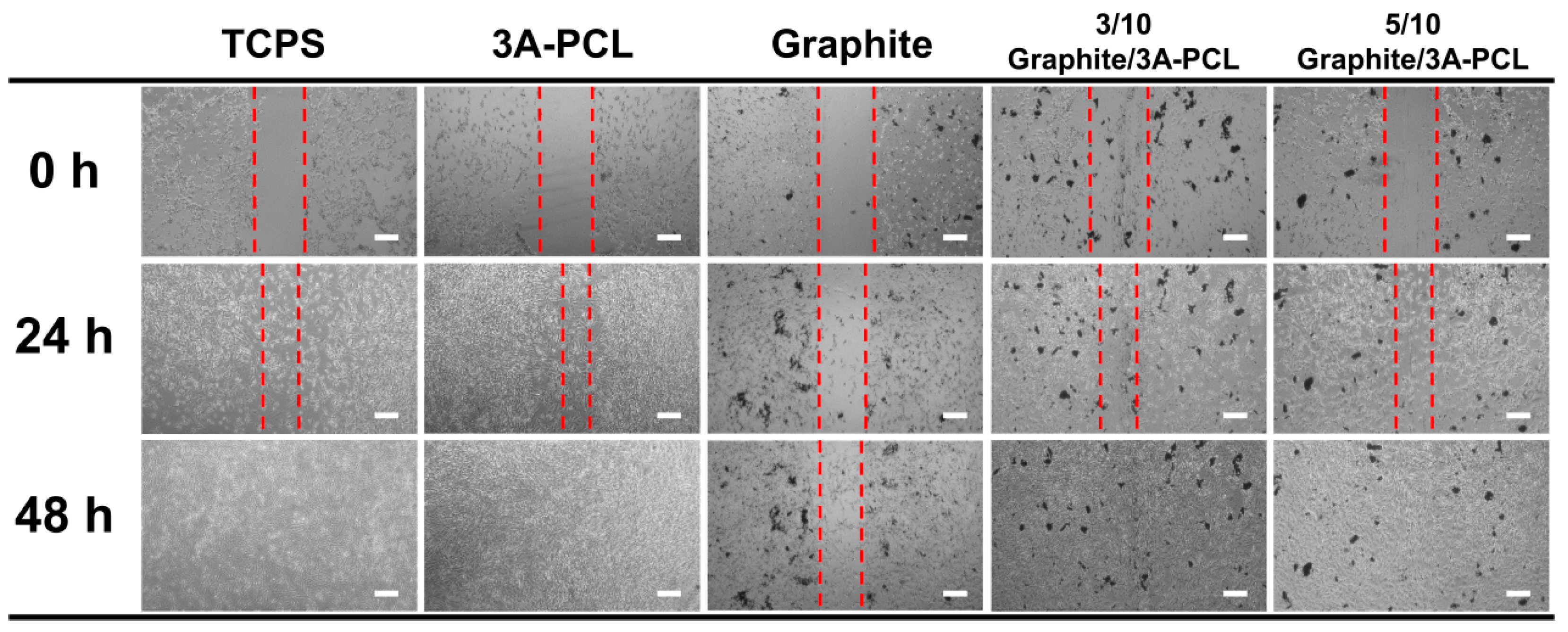
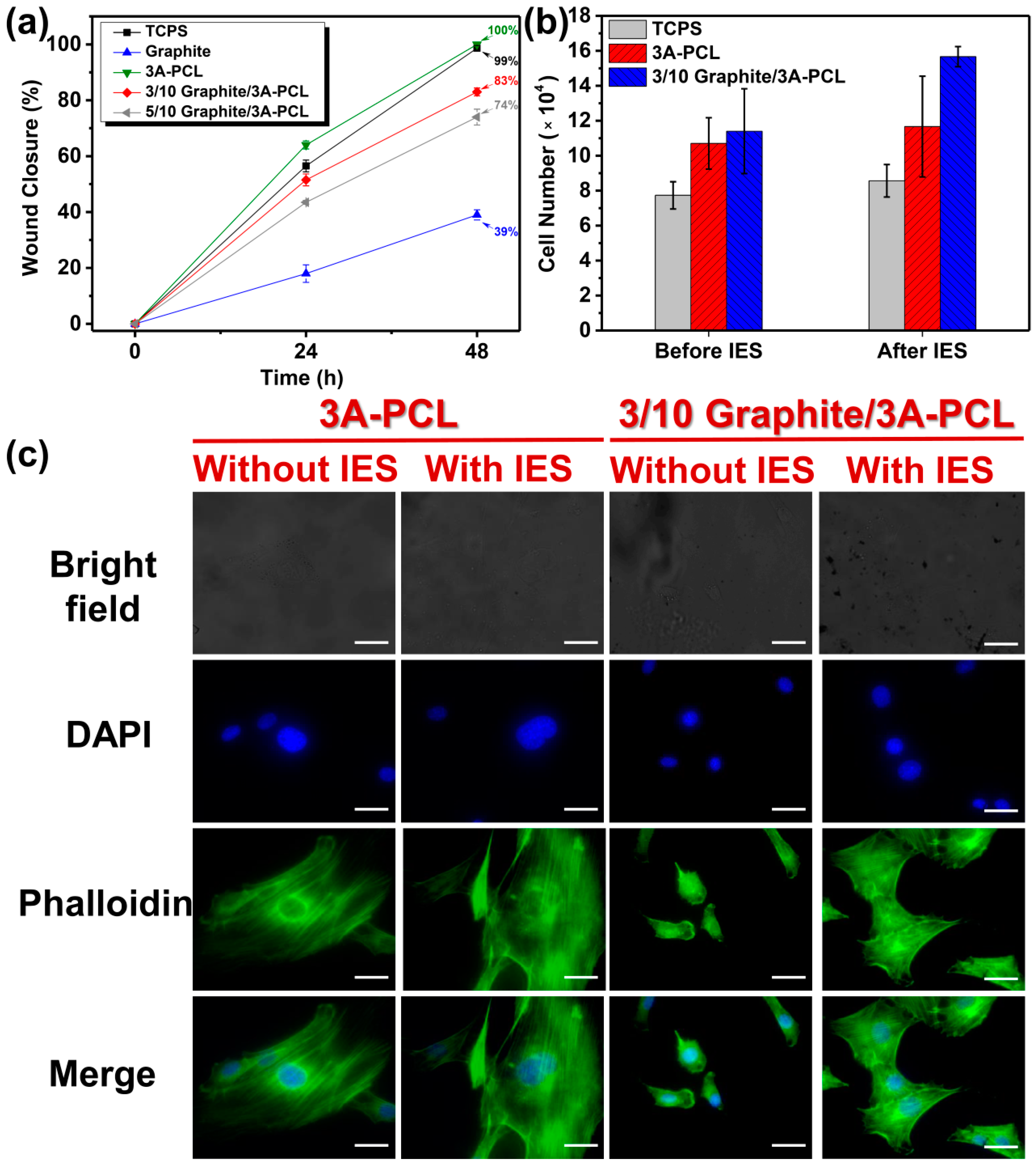
2.3. Evaluation of the In Vitro Wound-Healing Activity on Conductive Bioactive Substrates
2.4. Assessment of Cell Growth on Conductive Bioactive Substrates with IES
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liang, Y.; Kiick, K.L. Heparin-functionalized polymeric biomaterials in tissue engineering and drug delivery applications. Acta Biomater. 2014, 10, 1588–1600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Liu, K.; Müllen, K.; Yin, M. Self-assemblies of amphiphilic homopolymers: Synthesis, morphology studies and biomedical applications. Chem. Commun. 2015, 51, 11541–11555. [Google Scholar] [CrossRef] [PubMed]
- Suh, J.K.F.; Matthew, H.W.T. Application of chitosan-based polysaccharide biomaterials in cartilage tissue engineering: A review. Biomaterials 2000, 21, 2589–2598. [Google Scholar] [PubMed]
- Cao, L.; Cao, B.; Lu, C.; Wang, G.; Yu, L.; Ding, J. An injectable hydrogel formed by in situ cross-linking of glycol chitosan and multi-benzaldehyde functionalized PEG analogues for cartilage tissue engineering. J. Mater. Chem. B 2015, 3, 1268–1280. [Google Scholar] [CrossRef]
- Aizawa, Y.; Owen, S.C.; Shoichet, M.S. Polymers used to influence cell fate in 3D geometry: New trends. Prog. Polym. Sci. 2012, 37, 645–658. [Google Scholar] [CrossRef]
- Seo, J.-H.; Yui, N. The effect of molecular mobility of supramolecular polymer surfaces on fibroblast adhesion. Biomaterials 2013, 34, 55–63. [Google Scholar] [CrossRef]
- Dou, X.-Q.; Zhang, D.; Feng, C.; Jiang, L. Bioinspired hierarchical surface structures with tunable wettability for regulating bacteria adhesion. ACS Nano 2015, 9, 10664–10672. [Google Scholar] [CrossRef]
- Galbraith, C.G.; Sheetz, M.P. Forces on adhesive contacts affect cell function. Curr. Opin. Cell Biol. 1998, 10, 566–571. [Google Scholar] [CrossRef]
- Gumbiner, B.M. Cell adhesion: The molecular basis of tissue architecture and morphogenesis. Cell 1996, 84, 345–357. [Google Scholar] [CrossRef] [Green Version]
- Zhao, K.; Deng, Y.; Chen, J.C.; Chen, G.-Q. Polyhydroxyalkanoate (PHA) scaffolds with good mechanical properties and biocompatibility. Biomaterials 2003, 24, 1041–1045. [Google Scholar] [CrossRef]
- Qi, W.; Xue, Z.; Yuan, W.; Wang, H. Layer-by-layer assembled graphene oxide composite films for enhanced mechanical properties and fibroblast cell affinity. J. Mater. Chem. B 2014, 2, 325–331. [Google Scholar] [CrossRef]
- Yu, G.; Zhao, X.; Zhou, J.; Mao, Z.; Huang, X.; Wang, Z.; Hua, B.; Liu, Y.; Zhang, F.; He, Z.; et al. Supramolecular polymer-based nanomedicine: High therapeutic performance and negligible long-term immunotoxicity. J. Am. Chem. Soc. 2018, 140, 8005–8019. [Google Scholar] [CrossRef]
- Torchilin, V.P. Multifunctional, stimuli-sensitive nanoparticulate systems for drug delivery. Nat. Rev. Drug Discov. 2014, 13, 813–827. [Google Scholar] [CrossRef] [Green Version]
- Hillewaere, X.K.D.; Du Prez, F.E. Fifteen chemistries for autonomous external self-healing polymers and composites. Prog. Polym. Sci. 2015, 49–50, 121–153. [Google Scholar] [CrossRef]
- Roy, N.; Bruchmann, B.; Lehn, J.-M. Dynamers: Dynamic polymers as self-healing materials. Chem. Soc. Rev. 2015, 44, 3786–3807. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.-Y.; Zhang, Y.; Zhang, C.-W.; Chen, L.-J.; Wang, C.; Tan, H.; Yu, Y.; Li, X.; Yang, H.-B. Cross-linked supramolecular polymer gels constructed from discrete multi-pillar[5]arene metallacycles and their multiple stimuli-responsive behavior. J. Am. Chem. Soc. 2014, 136, 8577–8589. [Google Scholar] [CrossRef]
- Liu, J.; Tan, C.S.Y.; Yu, Z.; Li, N.; Abell, C.; Scherman, O.A. Tough supramolecular polymer networks with extreme stretchability and fast room-temperature self-healing. Adv. Mater. 2017, 29, 1605325. [Google Scholar] [CrossRef] [Green Version]
- Cheng, C.C.; Lee, D.J.; Chen, J.K. Self-assembled supramolecular polymers with tailorable properties that enhance cell attachment and proliferation. Acta Biomater. 2017, 50, 476–483. [Google Scholar] [CrossRef]
- Cheng, C.C.; Yang, X.J.; Fan, W.L.; Lee, A.W.; Lai, J.Y. Cytosine-functionalized supramolecular polymer-mediated cellular behavior and wound healing. Biomacromolecules 2020, 21, 3857–3866. [Google Scholar] [CrossRef]
- Zalatan, J.G.; Lee, M.E.; Almeida, R.; Gilbert, L.A.; Whitehead, E.H.; La Russa, M.; Tsai, J.C.; Weissman, J.S.; Dueber, J.E.; Qi, L.S.; et al. Engineering complex synthetic transcriptional programs with CRISPR RNA scaffolds. Cell 2015, 160, 339–350. [Google Scholar] [CrossRef] [Green Version]
- Huo, Y.; He, Z.; Wang, C.; Zhang, L.; Xuan, Q.; Wei, S.; Wang, Y.; Pan, D.; Dong, B.; Wei, R.; et al. The recent progress of synergistic supramolecular polymers: Preparation, properties and applications. Chem. Commun. 2021, 57, 1413–1429. [Google Scholar] [CrossRef] [PubMed]
- Cordier, P.; Tournilhac, F.; Soulié-Ziakovic, C.; Leibler, L. Self-healing and thermoreversible rubber from supramolecular assembly. Nature 2008, 451, 977–980. [Google Scholar] [CrossRef] [PubMed]
- Mollet, B.B.; Bogaerts, I.L.J.; van Almen, G.C.; Dankers, P.Y.W. A bioartificial environment for kidney epithelial cells based on a supramolecular polymer basement membrane mimic and an organotypical culture system. J. Tissue Eng. Regen. Med. 2017, 11, 1820–1834. [Google Scholar] [CrossRef] [PubMed]
- Mollet, B.B.; Comellas-Aragonès, M.; Spiering, A.J.H.; Söntjens, S.H.M.; Meijer, E.W.; Dankers, P.Y.W. A modular approach to easily processable supramolecular bilayered scaffolds with tailorable properties. J. Mater. Chem. B 2014, 2, 2483–2493. [Google Scholar] [CrossRef] [Green Version]
- Liao, G.; Hu, J.; Chen, Z.; Zhang, R.; Wang, G.; Kuang, T. Preparation, properties, and applications of graphene-based hydrogels. Front. Chem. 2018, 6, 450. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Zhou, M.; Jin, L.; Liu, L.; Mo, Y.; Li, X.; Mo, Z.; Liu, Z.; You, S.; Zhu, H. Research progress of the liquid-phase exfoliation and stable dispersion mechanism and method of grapheme. Front. Mater. 2019, 6, 325. [Google Scholar] [CrossRef]
- Marinho, B.; Ghislandi, M.; Tkalya, E.; Koning, C.E.; de With, G. Electrical conductivity of compacts of graphene, multi-wall carbon nanotubes, carbon black, and graphite powder. Powder Technol. 2012, 221, 351–358. [Google Scholar] [CrossRef]
- Rajendran, S.B.; Challen, K.; Wright, K.L.; Hardy, J.G. Electrical stimulation to enhance wound healing. J. Funct. Biomater. 2021, 12, 40. [Google Scholar] [CrossRef]
- Huang, Z.; Guo, Z.; Sun, M.; Fang, S.; Li, H. A study on graphene composites for peripheral nerve injury repair under electrical stimulation. RSC Adv. 2019, 9, 28627–28635. [Google Scholar] [CrossRef] [Green Version]
- Shin, S.R.; Li, Y.-C.; Jang, H.L.; Khoshakhlagh, P.; Akbari, M.; Nasajpour, A.; Zhang, Y.S.; Tamayol, A.; Khademhosseini, A. Graphene-based materials for tissue engineering. Adv. Drug Deliv. Rev. 2016, 105, 255–274. [Google Scholar] [CrossRef] [Green Version]
- Dybowska-Sarapuk, L.; Sosnowicz, W.; Krzeminski, J.; Grzeczkowicz, A.; Granicka, L.H.; Kotela, A.; Jakubowska, M. Printed graphene layer as a base for cell electrostimulation—preliminary results. Int. J. Mol. Sci. 2020, 21, 7865. [Google Scholar] [CrossRef] [PubMed]
- Aydin, T.; Gurcan, C.; Taheri, H.; Yilmazer, A. Graphene based materials in neural tissue regeneration. Cell Biol. Transl. Med. 2018, 3, 129–142. [Google Scholar]
- Wang, W.; Hou, Y.; Martinez, D.; Kurniawan, D.; Chiang, W.-H.; Bartolo, P. Carbon nanomaterials for electro-active structures: A review. Polymers 2020, 12, 2946. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Gao, Z.; Xiao, L. The application of stimuli-sensitive actuators based on graphene materials. Front Chem. 2019, 7, 803. [Google Scholar] [CrossRef]
- Lovley, D.R. Bug juice: Harvesting electricity with microorganisms. Nat. Rev. Microbiol. 2006, 4, 497–508. [Google Scholar] [CrossRef]
- Cheah, Y.J.; Buyong, M.R.; Mohd Yunus, M.H. Wound healing with electrical stimulation technologies: A review. Polymers 2021, 13, 3790. [Google Scholar] [CrossRef]
- Thrash, J.C.; Coates, J.D. Review: Direct and indirect electrical stimulation of microbial metabolism. Environ. Sci. Technol. 2008, 42, 3921–3931. [Google Scholar] [CrossRef]
- Wu, C.Y.; Melaku, A.Z.; Chuang, W.T.; Cheng, C.C. Manipulating the self-assembly behavior of graphene nanosheets via adenine-functionalized biodegradable polymers. Appl. Surf. Sci. 2022, 572, 151437. [Google Scholar] [CrossRef]
- Choi, J.; Reipa, V.; Hitchins, V.M.; Goering, P.L.; Malinauskas, R.A. Physicochemical characterization and in vitro hemolysis evaluation of silver nanoparticles. Toxicol. Sci. 2011, 123, 133–143. [Google Scholar] [CrossRef] [Green Version]
- Hisey, B.; Ragogna, P.J.; Gillies, E.R. Phosphonium-functionalized polymer micelles with intrinsic antibacterial activity. Biomacromolecules 2017, 18, 914–923. [Google Scholar] [CrossRef] [Green Version]
- Lee, W.C.; Lim, C.H.Y.X.; Shi, H.; Tang, L.A.L.; Wang, Y.; Lim, C.T.; Loh, K.P. Origin of enhanced stem cell growth and differentiation on graphene and graphene oxide. ACS Nano 2011, 5, 7334–7341. [Google Scholar] [CrossRef] [PubMed]
- Kostarelos, K.; Novoselov, K.S. Exploring the interface of graphene and biology. Science 2014, 344, 261–263. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yan, C.; Zheng, Z. Functional polymer surfaces for controlling cell behaviors. Mater. Today 2018, 21, 38–59. [Google Scholar] [CrossRef]
- Jonkman, J.E.N.; Cathcart, J.A.; Xu, F.; Bartolini, M.E.; Amon, J.E.; Stevens, K.M.; Colarusso, P. An introduction to the wound healing assay Using live-cell microscopy. Cell Adhes. Migr. 2014, 8, 440–451. [Google Scholar] [CrossRef] [Green Version]
- Martinotti, S.; Ranzato, E. Scratch wound healing assay. Methods Mol. Biol. 2020, 2109, 225–229. [Google Scholar]
- Tehovnik, E.J.; Tolias, A.S.; Sultan, F.; Slocum, W.M.; Logothetis, N.K. Direct and indirect activation of cortical neurons by electrical microstimulation. J. Neurophysiol. 2006, 96, 512–521. [Google Scholar] [CrossRef] [Green Version]
- Park, H.-H.; Jo, S.; Seo, C.H.; Jeong, J.H.; Yoo, Y.-E.; Lee, D.H. An indirect electric field-induced control in directional migration of rat mesenchymal stem cells. Appl. Phys. Lett. 2014, 105, 244109. [Google Scholar] [CrossRef]
- Ning, C.; Zhou, Z.; Tan, G.; Zhu, Y.; Mao, C. Electroactive polymers for tissue regeneration: Developments andperspectives. Prog. Polym. Sci. 2018, 81, 144–162. [Google Scholar] [CrossRef]
- Onuma, E.K.; Hui, S.W. Electric field-directed cell shape changes, displacement, and cytoskeletal reorganization are calcium dependent. J. Cell Biol. 1988, 106, 2067–2075. [Google Scholar] [CrossRef]
- Balint, R.; Cassidy, N.J.; Cartmell, S.H. Electrical stimulation: A novel tool for tissue engineering. Tissue Eng. Part B Rev. 2013, 19, 48–57. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, C.-Y.; Melaku, A.Z.; Ilhami, F.B.; Chiu, C.-W.; Cheng, C.-C. Conductive Supramolecular Polymer Nanocomposites with Tunable Properties to Manipulate Cell Growth and Functions. Int. J. Mol. Sci. 2022, 23, 4332. https://doi.org/10.3390/ijms23084332
Wu C-Y, Melaku AZ, Ilhami FB, Chiu C-W, Cheng C-C. Conductive Supramolecular Polymer Nanocomposites with Tunable Properties to Manipulate Cell Growth and Functions. International Journal of Molecular Sciences. 2022; 23(8):4332. https://doi.org/10.3390/ijms23084332
Chicago/Turabian StyleWu, Cheng-You, Ashenafi Zeleke Melaku, Fasih Bintang Ilhami, Chih-Wei Chiu, and Chih-Chia Cheng. 2022. "Conductive Supramolecular Polymer Nanocomposites with Tunable Properties to Manipulate Cell Growth and Functions" International Journal of Molecular Sciences 23, no. 8: 4332. https://doi.org/10.3390/ijms23084332
APA StyleWu, C.-Y., Melaku, A. Z., Ilhami, F. B., Chiu, C.-W., & Cheng, C.-C. (2022). Conductive Supramolecular Polymer Nanocomposites with Tunable Properties to Manipulate Cell Growth and Functions. International Journal of Molecular Sciences, 23(8), 4332. https://doi.org/10.3390/ijms23084332







