Oxygen and Drug-Carrying Periodic Mesoporous Organosilicas for Enhanced Cell Viability under Normoxic and Hypoxic Conditions
Abstract
:1. Introduction
2. Results
2.1. Preparation and Characterization of Rutin-Coated Particles
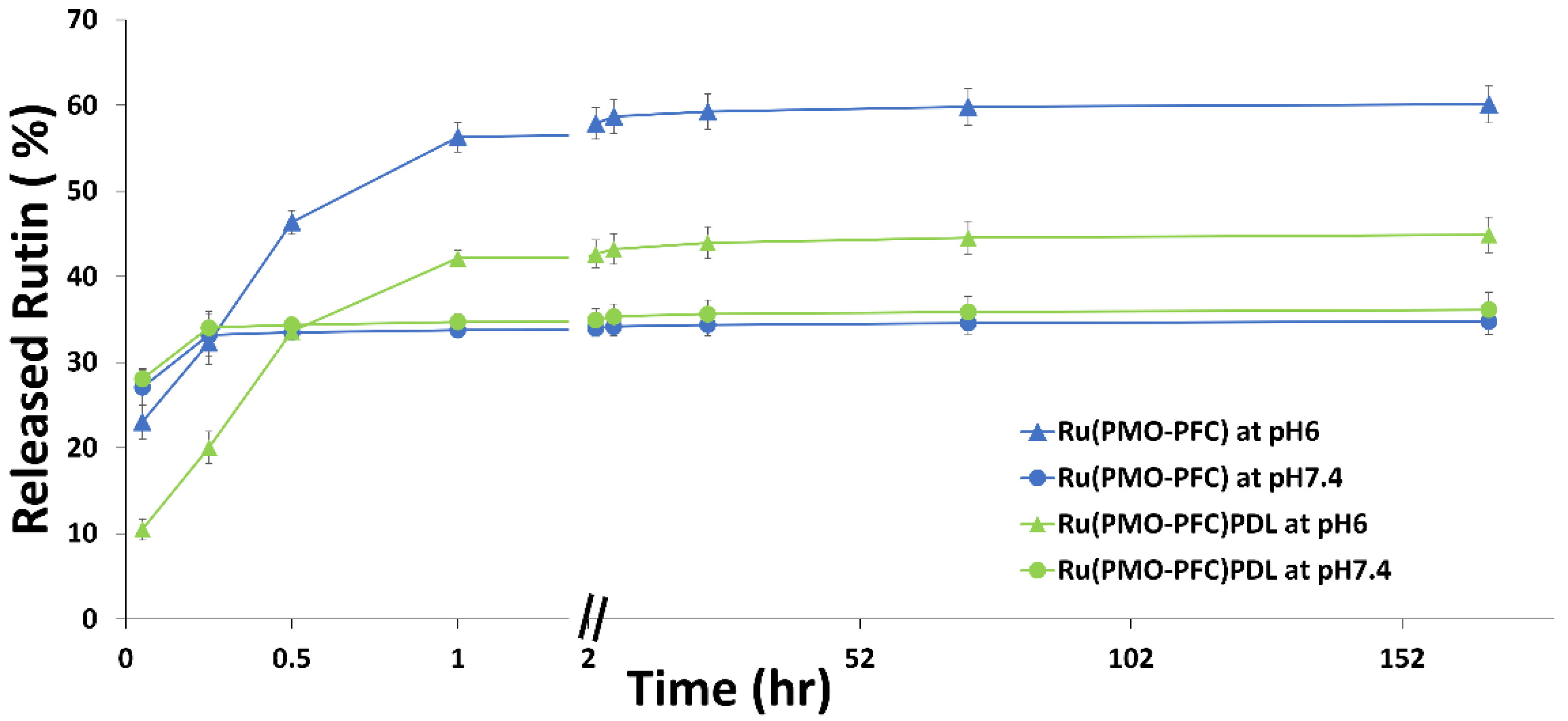
2.2. Rutin Release Experiment
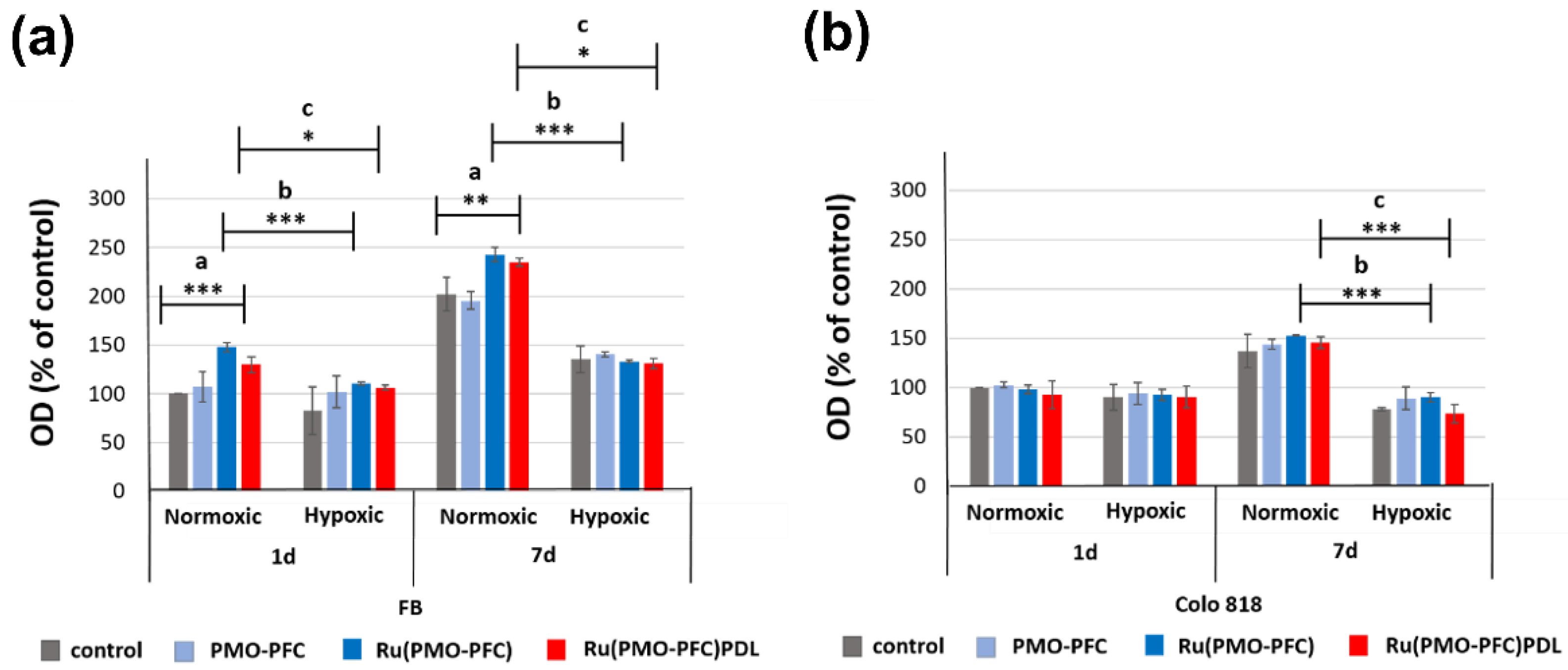
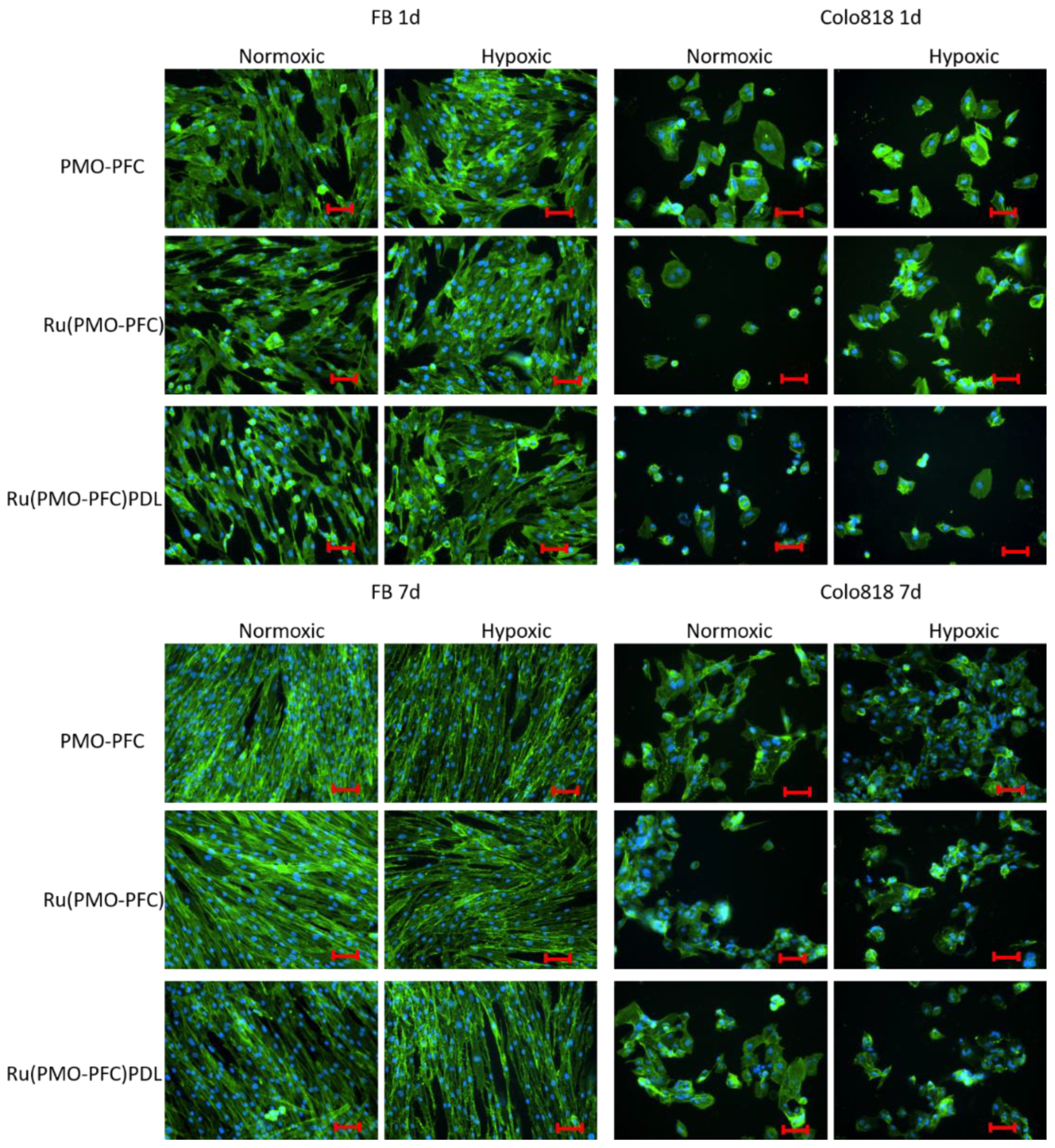
2.3. Cell Viability Experiment on PMO-PFC, Ru(PMO-PFC), and Ru(PMO-PFC)PDL
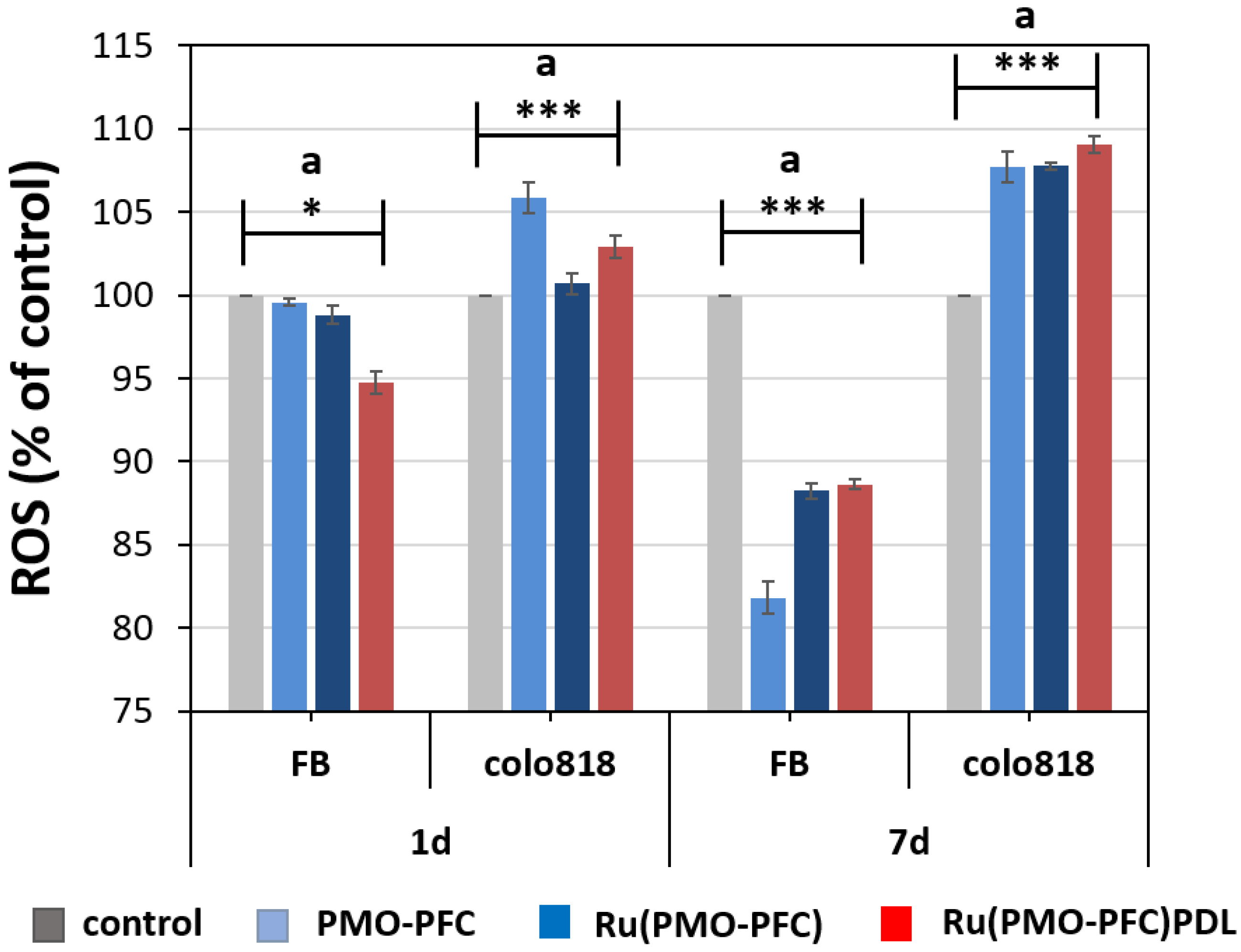
2.4. Reactive Oxygen Species (ROS)
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Periodic Mesoporous Organosilica (PMO-PFC)
3.3. Loading of Rutin to PMO-PFC Particles
3.4. Coating of Poly-D-Lysine (PDL) to Ru(PMO-PFC) Particles
3.5. Rutin Release in DMEM Media
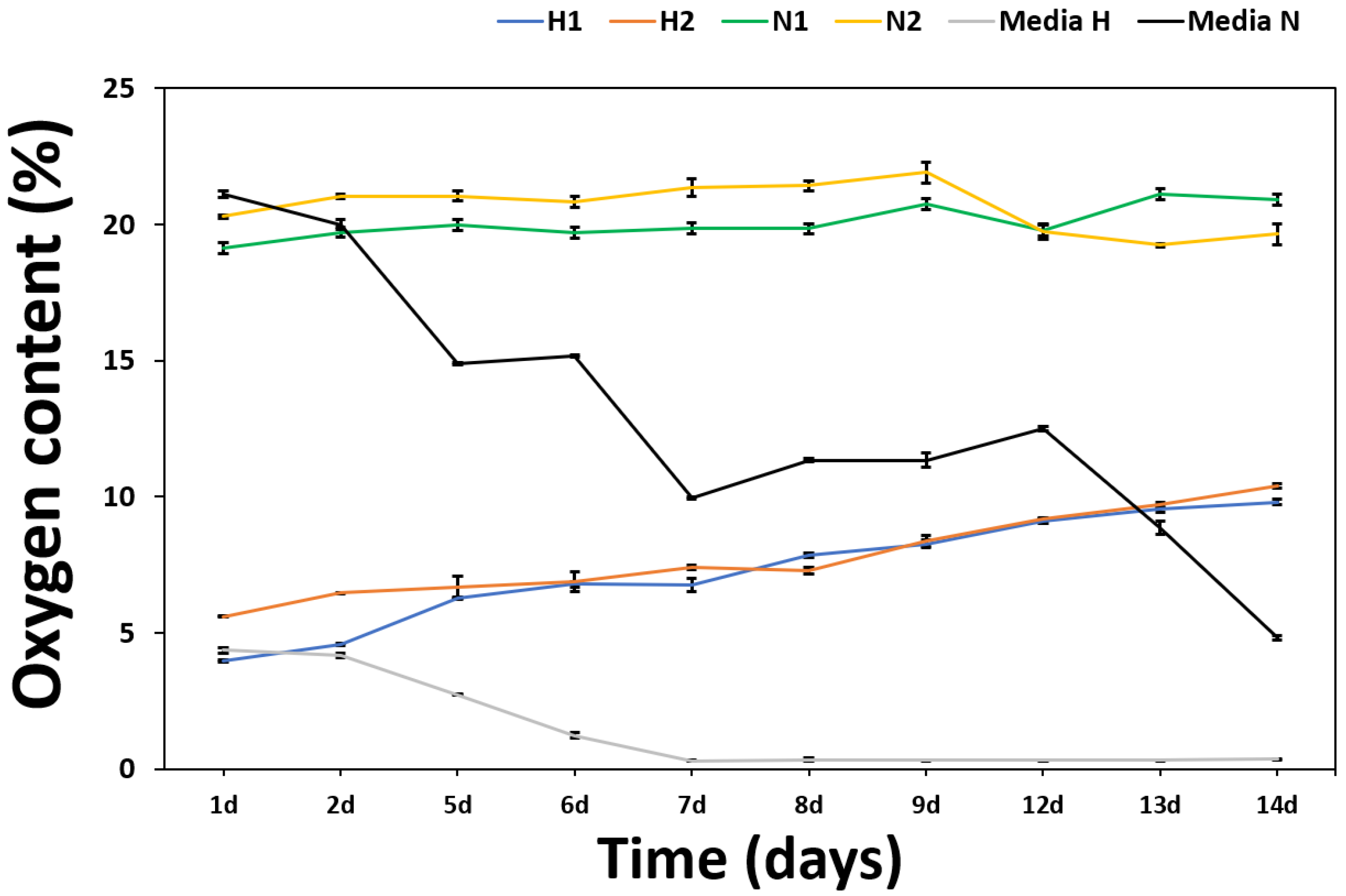
3.6. Measurement of O2 Content from PMO-PFC
3.7. Cell Viability of Samples on Cell Culture Plates
3.8. Co-Staining of Cells
3.9. Quantification of the Reactive Oxygen Species (ROS)
3.10. Characterization
3.11. Statistical Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Haque, S.T.; Chowdhury, E.H. Recent progress in delivery of therapeutic and imaging agents utilizing organic-inorganic hybrid nanoparticles. Curr. Drug Deliv. 2018, 15, 485–496. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Mattos, B.D.; Tardy, B.L.; Moody, V.M.; Xiao, G.; Ejima, H.; Cui, J.; Liang, K.; Richardson, J.J. Porous inorganic and hybrid systems for drug delivery: Future promise in combatting drug resistance and translation to botanical applications. Curr. Med. Chem. 2019, 26, 6107–6131. [Google Scholar] [CrossRef] [PubMed]
- Manatunga, D.C.; Godakanda, V.U.; Silva, R.M.; Silva, K.M.N. Recent developments in the use of organic-inorganic nanohybrids for drug delivery. WIREs Nanomed. Nanobiotechnol. 2020, 12, e1605. [Google Scholar] [CrossRef] [PubMed]
- Seaberg, J.; Montazerian, H.; Hossen, M.N.; Bhattacharya, R.; Khademhosseini, A.; Mukherjee, P. Hybrid nanosystems for biomedical applications. ACS Nano 2021, 15, 2099–2142. [Google Scholar] [CrossRef]
- Cai, A.Y.; Zhu, Y.J.; Qi, C. Biodegradable inorganic nanostructured biomaterials for drug delivery. Adv. Mater. Interfaces 2020, 7, 2000819. [Google Scholar] [CrossRef]
- Ilhan-Ayisigi, E.; Yesil-Celiktas, O. Silica-based organic-inorganic hybrid nanoparticles and nanoconjugates for improved anticancer drug delivery. Eng. Life Sci. 2018, 18, 882–892. [Google Scholar] [CrossRef]
- Liang, P.; Liu, C.J.; Zhuo, R.X.; Cheng, S.X. Self-assembled inorganic/organic hybrid nanoparticles with multi-functionalized surfaces for active targeting drug delivery. J. Mater. Chem. B 2013, 1, 4243–4250. [Google Scholar] [CrossRef]
- Mir, S.H.; Nagahara, L.A.; Thundat, T.; Mokarian-Tabari, P.; Furukawa, H.; Khosla, A. Review—Organic-inorganic hybrid functional materials: An integrated platform for applied technologies. J. Electrochem. Soc. 2018, 165, B3137. [Google Scholar] [CrossRef]
- Oshiro Junior, J.; Paiva Abuçafy, M.; Berbel Manaia, E.; Lallo da Silva, B.; Chiari-Andréo, B.; Aparecida Chiavacci, L. Drug delivery systems obtained from silica based organic-inorganic hybrids. Polymers 2016, 8, 91. [Google Scholar] [CrossRef] [Green Version]
- Motealleh, A.; Dorri, P.; Kehr, N.S. Self-assembled monolayers of chiral periodic mesoporous organosilica as a stimuli responsive local drug delivery system. J. Mater. Chem. B 2019, 7, 2362–2371. [Google Scholar] [CrossRef]
- Motealleh, A.; Kart, D.; Czieborowski, M.; Kehr, N.S. Functional nanomaterials and 3D-printable nanocomposite hydrogels for enhanced cell proliferation and for the reduction of bacterial biofilm formation. ACS Appl. Mater. Interfaces 2021, 13, 43755–43768. [Google Scholar] [CrossRef] [PubMed]
- Liechty, W.B.; Kryscio, D.R.; Slaughter, B.V.; Peppas, N.A. Polymers for drug delivery systems. Annu. Rev. Chem. Biomol. Eng. 2010, 1, 149–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gullón, B.; Lú-Chau, T.A.; Moreira, M.T.; Lema, J.M.; Eibes, G. Rutin: A review on extraction, identification and purification methods, biological activities and approaches to enhance its bioavailability. Trends Food Sci. Technol. 2017, 67, 220–235. [Google Scholar] [CrossRef]
- Ganeshpurkar, A.; Saluja, A.K. The pharmacological potential of rutin. Saudi Pharm. J. 2017, 25, 149–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, H.; Su, M.; Jin, H.; Li, X.; Wang, P.; Chen, J.; Chen, J. Rutin-loaded silver nanoparticles with antithrombotic function. Front. Bioeng. Biotechnol. 2020, 8, 1356. [Google Scholar] [CrossRef] [PubMed]
- Ghanbari-Movahed, M.; Mondal, A.; Farzaei, M.H.; Bishayee, A. Quercetin- and rutin-based nano-formulations for cancer treatment: A systematic review of improved efficacy and molecular mechanisms. Phytomedicine 2022, 97, 153909. [Google Scholar] [CrossRef]
- Negahdari, R.; Bohlouli, S.; Sharifi, S.; Maleki Dizaj, S.; Rahbar Saadat, Y.; Khezri, K.; Jafari, S.; Ahmadian, E.; Gorbani Jahandizi, N.; Raeesi, S. Therapeutic benefits of rutin and its nanoformulations. Phytother. Res. 2020, 35, 1719–1738. [Google Scholar] [CrossRef]
- Júlio, A.; Caparica, R.; Costa Lima, S.A.; Fernandes, A.S.; Rosado, C.; Prazeres, D.M.F.; Reis, S.; Santos de Almeida, T.; Fonte, P. Ionic liquid-polymer nanoparticle hybrid systems as new tools to deliver poorly soluble drugs. Nanomaterials 2019, 9, 1148. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Han, Y. Preparation, characterisation and antioxidant activities of rutin-loaded zein-sodium caseinate nanoparticles. PLoS ONE 2018, 13, e0194951. [Google Scholar] [CrossRef]
- Ahmad, N.; Ahmad, R.; Naqvi, A.A.; Alam, M.A.; Ashafaq, M.; Samim, M.; Iqbal, Z.; Ahmad, F.J. Rutin-encapsulated chitosan nanoparticles targeted to the brain in the treatment of Cerebral Ischemia. Int. J. Biol. Macromol. 2016, 91, 640–655. [Google Scholar] [CrossRef]
- Hu, B.; Dai, F.; Fan, Z.; Ma, G.; Tang, Q.; Zhang, X. Nanotheranostics: Congo red/Rutin-MNPs with enhanced magnetic resonance imaging and H2O2-responsive therapy of Alzheimer’s disease in APPswe/PS1dE9 transgenic mice. Adv. Mater. 2015, 27, 5499–5505. [Google Scholar] [CrossRef] [PubMed]
- Kunjiappan, S.; Panneerselvam, T.; Govindaraj, S.; Parasuraman, P.; Baskararaj, S.; Sankaranarayanan, M.; Arunachalam, S.; Babkiewicz, E.; Jeyakumar, A.; Lakshmanan, M. Design, in silico modelling, and functionality theory of novel folate receptor targeted rutin encapsulated folic acid conjugated keratin nanoparticles for effective cancer treatment. Anti-Cancer Agents Med. Chem. 2020, 19, 1966–1982. [Google Scholar] [CrossRef] [PubMed]
- Gul, A.; Kunwar, B.; Mazhar, M.; Faizi, S.; Ahmed, D.; Shah, M.R.; Simjee, S.U. Rutin and rutin-conjugated gold nanoparticles ameliorate collagen-induced arthritis in rats through inhibition of NF-κB and iNOS activation. Int. Immunopharmacol. 2018, 59, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Tian, R.; Long, X.; Yang, Z.; Lu, N.; Peng, Y.Y. Formation of a bovine serum albumin diligand complex with rutin and single-walled carbon nanotubes for the reduction of cytotoxicity. Biophys. Chem. 2020, 256, 106268. [Google Scholar] [CrossRef] [PubMed]
- Senthil Kumar, C.; Sivakumar, M.; Ruckmani, K. Microwave-assisted extraction of polysaccharides from Cyphomandra betacea and its biological activities. Int. J. Biol. Macromol. 2016, 92, 682–693. [Google Scholar] [CrossRef]
- Ravi, G.S.; Charyulu, R.N.; Dubey, A.; Prabhu, P.; Hebbar, S.; Mathias, A.C. Nano-lipid complex of rutin: Development, characterisation and in vivo investigation of hepatoprotective, antioxidant activity and bioavailability study in rats. AAPS PharmSciTech 2018, 19, 3631–3649. [Google Scholar] [CrossRef]
- Zeng, C.; Zheng, R.; Jiang, W.; He, C.; Li, J.; Xing, J. Chitosan coated chlorogenic acid and rutincomposite phospholipid liposomes: Preparation, characterizations, permeability and pharmacokinetic. Pak. J. Pharm. Sci. 2018, 8, 2095–2102. [Google Scholar]
- Kumar, V.; Chaudhary, H.; Kamboj, A. Development and evaluation of isradipine via rutin-loaded coated solid—Lipid nanoparticles. Interv. Med. Appl. Sci. 2018, 10, 236–246. [Google Scholar] [CrossRef]
- Wigerup, C.; Påhlman, S.; Bexell, D. Therapeutic targeting of hypoxia and hypoxia-inducible factors in cancer. Pharmacol. Ther. 2016, 164, 152–169. [Google Scholar] [CrossRef] [Green Version]
- Ashammakhi, N.; Darabi, M.A.; Kehr, N.S.; Erdem, A.; Hu, S.k.; Dokmeci, M.R.; Nasr, A.S.; Khademhosseini, A. Advances in controlled oxygen generating biomaterials for tissue engineering and regenerative therapy. Biomacromolecules 2019, 21, 56–72. [Google Scholar] [CrossRef]
- Chin, K.; Khattak, S.F.; Bhatia, S.R.; Roberts, S.C. Hydrogel-perfluorocarbon composite scaffold promotes oxygen transport to immobilized cells. Biotechnol. Prog. 2008, 24, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Goh, F.; Gross, J.D.; Simpson, N.E.; Sambanis, A. Limited beneficial effects of perfluorocarbon emulsions on encapsulated cells in culture: Experimental and modeling studies. J. Biotechnol. 2010, 150, 232–239. [Google Scholar] [CrossRef] [Green Version]
- Steg, H.; Buizer, A.T.; Woudstra, W.; Veldhuizen, A.G.; Bulstra, S.K.; Grijpma, D.W.; Kuijer, R. Control of oxygen release from peroxides using polymers. J. Mater. Sci. Mater. Med. 2015, 26, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Asakura, A.; Ward, C.L.; Corona, B.T.; Yoo, J.J.; Harrison, B.S.; Christ, G.J. Oxygen generating biomaterials preserve skeletal muscle homeostasis under hypoxic and ischemic conditions. PLoS ONE 2013, 8, e72485. [Google Scholar] [CrossRef]
- Gholipourmalekabadi, M.; Zhao, S.; Harrison, B.S.; Mozafari, M.; Seifalian, A.M. Oxygen-generating biomaterials: A new, viable paradigm for tissue engineering? Trends Biotechnol. 2016, 34, 1010–1021. [Google Scholar] [CrossRef] [Green Version]
- Camci-Unal, G.; Alemdar, N.; Annabi, N.; Khademhosseini, A. Oxygen-releasing biomaterials for tissue engineering. Polym. Int. 2013, 62, 843–848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ng, S.M.; Choi, J.Y.; Han, H.S.; Huh, J.S.; Lim, J.O. Novel microencapsulation of potential drugs with low molecular weight and high hydrophilicity: Hydrogen peroxide as a candidate compound. Int. J. Pharm. 2010, 384, 120–127. [Google Scholar] [CrossRef]
- Karimi, B.; Ganji, N.; Pourshiani, O.; Thiel, W.R. Periodic mesoporous organosilicas (PMOs): From synthesis strategies to applications. Prog. Mater. Sci. 2022, 125, 100896. [Google Scholar] [CrossRef]
- Guimarães, R.S.; Rodrigues, C.F.; Moreira, A.F.; Correia, I.J. Overview of stimuli-responsive mesoporous organosilica nanocarriers for drug delivery. Pharmacol. Res. 2020, 155, 104742. [Google Scholar] [CrossRef]
- Motealleh, A.; Schäfer, A.H.; Fromm, O.; Kehr, N.S. 3D-printed oxygen-carrying nanocomposite hydrogels for enhanced cell viability under hypoxic and normoxic conditions. Biomacromolecules 2021, 22, 4758–4769. [Google Scholar] [CrossRef]
- Guan, B.; Cui, Y.; Ren, Z.; Qiao, Z.A.; Wang, L.; Liu, Y.; Huo, Q. Highly ordered periodic mesoporous organosilica nanoparticles with controllable pore structures. Nanoscale 2012, 4, 6588–6596. [Google Scholar] [CrossRef] [PubMed]
- Park, S.S.; Shin, J.H.; Zhao, D.; Ha, C.S. Free-standing and bridged amine-functionalized periodic mesoporous organosilica films. J. Mater. Chem. 2010, 20, 7854–7858. [Google Scholar] [CrossRef]
- Riess, J.G. Understanding the fundamentals of perfluorocarbons and perfluorocarbon emulsions relevant to in vivo oxygen delivery. Artif. Cells Blood Substit. Biotechnol. 2009, 33, 47–63. [Google Scholar] [CrossRef] [PubMed]
- Lu, N.; Tian, Y.; Tian, W.; Huang, P.; Liu, Y.; Tang, Y.; Wang, C.; Wang, S.; Su, Y.; Zhang, Y.; et al. Smart cancer cell targeting imaging and drug delivery system by systematically engineering periodic mesoporous organosilica nanoparticles. ACS Appl. Mater. Interfaces 2016, 8, 2985–2993. [Google Scholar] [CrossRef]
- Fang, R.; Jing, H.; Chai, Z.; Zhao, G.; Stoll, S.; Ren, F.; Liu, F.; Leng, X. Study of the physicochemical properties of the BSA: Flavonoid nanoparticle. Eur. Food Res. Technol. 2011, 233, 275–283. [Google Scholar] [CrossRef]
- Al-Shabib, N.A.; Khan, J.M.; Malik, A.; Alsenaidy, M.A.; Rehman, M.T.; AlAjmi, M.F.; Alsenaidy, A.M.; Husain, F.M.; Khan, R.H. Molecular insight into binding behavior of polyphenol (rutin) with beta lactoglobulin: Spectroscopic, molecular docking and MD simulation studies. J. Mol. Liq. 2018, 269, 511–520. [Google Scholar] [CrossRef]
- Higuchi, T. Mechanism of sustained-action medication. Theoretical analysis of rate of release of solid drugs dispersed in solid matrices. J. Pharm. Sci. 1963, 52, 1145–1149. [Google Scholar] [CrossRef]
- Korsmeyer, R.W.; Gurny, R.; Doelker, E.; Buri, P.; Peppas, N.A. Mechanisms of solute release from porous hydrophilic polymers. Int. J. Pharm. 1983, 15, 25–35. [Google Scholar] [CrossRef]
- Juhász, Á.; Ungor, D.; Berta, K.; Seres, L.; Csapóa, E. Spreadsheet-based nonlinear analysis of in vitro release properties of a model drug from colloidal carriers. J. Mol. Liq. 2021, 328, 115405–115413. [Google Scholar] [CrossRef]
- Wang, J.; Yi, J. Cancer Cell killing via ROS: To Increase or Decrease, That Is the Question. Cancer Biol. Ther. 2014, 7, 1875–1884. [Google Scholar] [CrossRef]
- Zhou, D.; Shao, L.; Spitz, D.R. Chapter One—Reactive Oxygen Species in Normal and Tumor Stem Cells. In Advances in Cancer Research; Townsend, D.M., Tew, K.D., Eds.; Academic Press: San Diego, CA, USA, 2014; Volume 122, pp. 1–67. [Google Scholar]
- Halliwell, B. Oxidative stress and cancer: Have we moved forward? Biochem. J. 2007, 401, 1–11. [Google Scholar] [CrossRef] [PubMed]
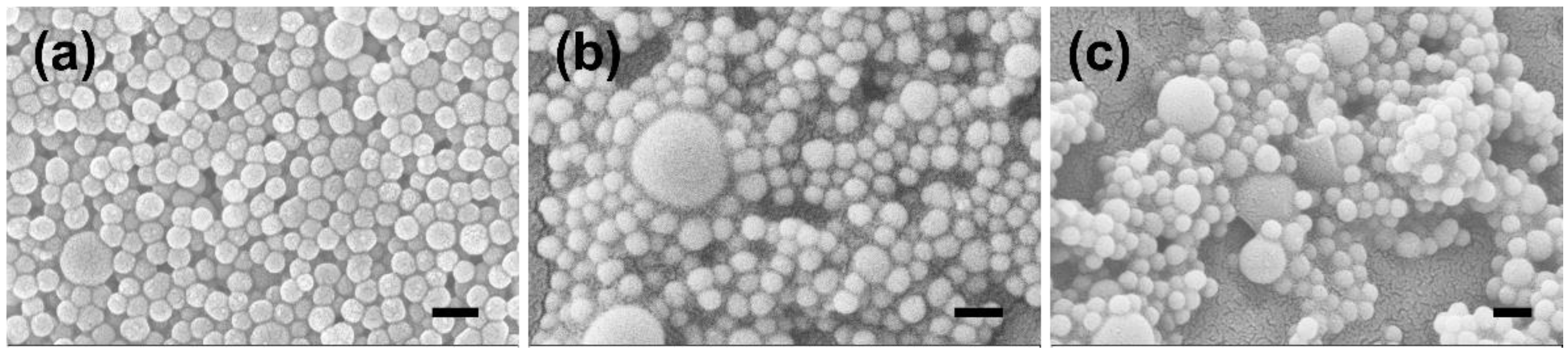
| Sample | Zeta Potential (mV) | Size (nm) |
|---|---|---|
| PMO-PFC | −9.93 ± 2.23 | 295 ± 50 |
| Ru(PMO-PFC) | −32.17 ± 1.02 | 334 ± 71 |
| Ru(PMO-PFC)PDL | 23.30 ± 0.79 | 485 ± 67 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, R.; Kehr, N.S. Oxygen and Drug-Carrying Periodic Mesoporous Organosilicas for Enhanced Cell Viability under Normoxic and Hypoxic Conditions. Int. J. Mol. Sci. 2022, 23, 4365. https://doi.org/10.3390/ijms23084365
Kumar R, Kehr NS. Oxygen and Drug-Carrying Periodic Mesoporous Organosilicas for Enhanced Cell Viability under Normoxic and Hypoxic Conditions. International Journal of Molecular Sciences. 2022; 23(8):4365. https://doi.org/10.3390/ijms23084365
Chicago/Turabian StyleKumar, Ravi, and Nermin Seda Kehr. 2022. "Oxygen and Drug-Carrying Periodic Mesoporous Organosilicas for Enhanced Cell Viability under Normoxic and Hypoxic Conditions" International Journal of Molecular Sciences 23, no. 8: 4365. https://doi.org/10.3390/ijms23084365
APA StyleKumar, R., & Kehr, N. S. (2022). Oxygen and Drug-Carrying Periodic Mesoporous Organosilicas for Enhanced Cell Viability under Normoxic and Hypoxic Conditions. International Journal of Molecular Sciences, 23(8), 4365. https://doi.org/10.3390/ijms23084365







