Natural Products for the Prevention and Treatment of Oral Mucositis—A Review
Abstract
:1. Introduction
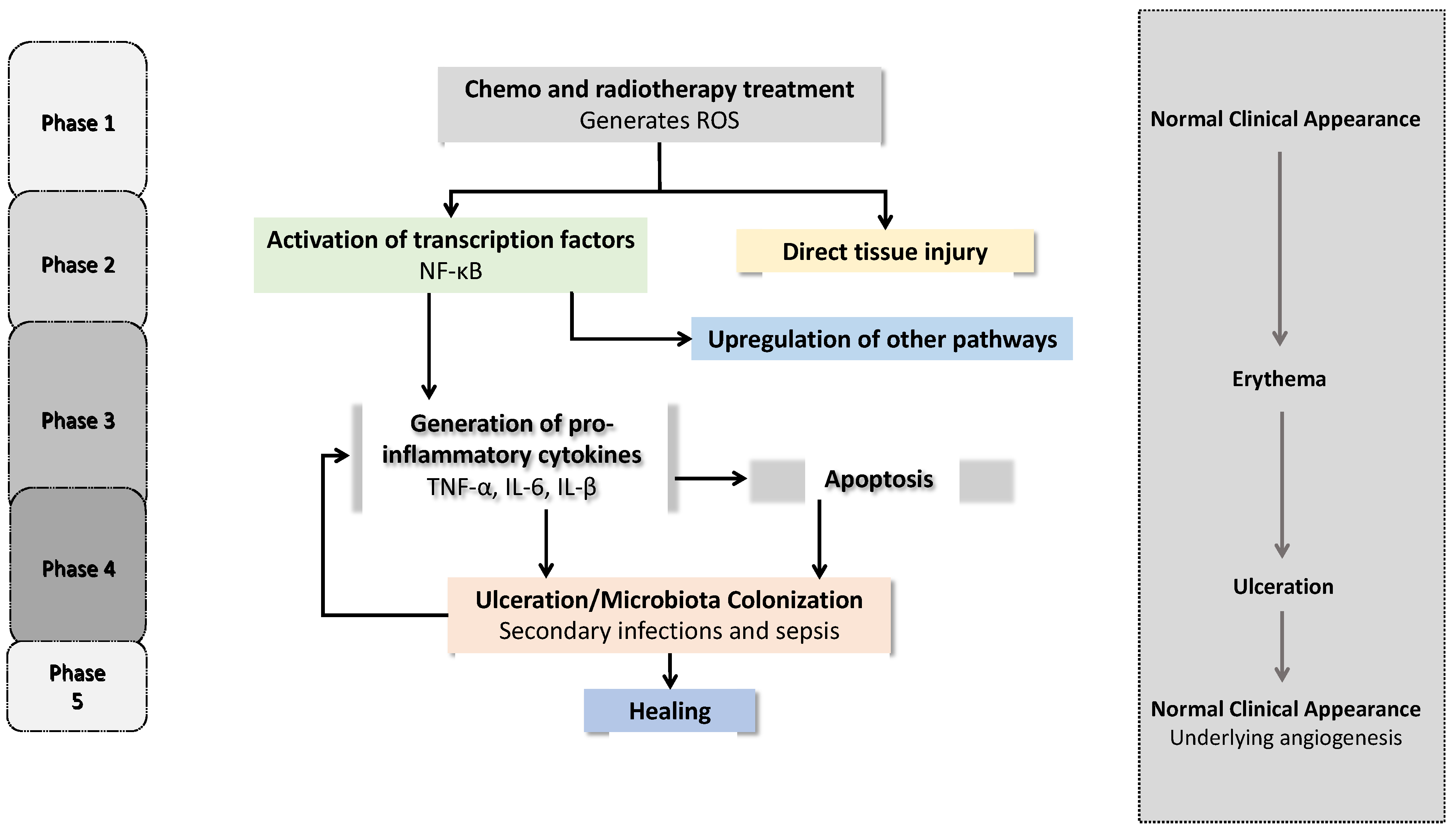
2. Oral Mucositis
2.1. Physiopathology of OM
2.2. Risk Factors
2.3. Prevention and Management of OM
3. Natural Compounds and Their Properties for Preventing/Treating OM
3.1. Bee Products
3.1.1. Propolis
3.1.2. Royal Jelly
3.2. Spondias Mombin
3.3. Camellia sinensis
3.4. Plantago Major
3.5. Aloe vera
3.6. Curcuma Longa
3.7. Olea Europaea
3.8. Glycyrrhiza glabra
3.9. Matricaria Recutita
3.10. Calendula officinalis
3.11. Other Compounds
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Cinausero, M.; Aprile, G.; Ermacora, P.; Basile, D.; Vitale, M.G.; Fanotto, V.; Parisi, G.; Calvetti, L.; Sonis, S.T. New Frontiers in the Pathobiology and Treatment of Cancer Regimen-Related Mucosal Injury. Front. Pharmacol. 2017, 8, 354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sonis, S.T. Mucositis: The impact, biology and therapeutic opportunities of oral mucositis. Oral Oncol. 2009, 45, 1015–1020. [Google Scholar] [CrossRef]
- Oronsky, B.; Goyal, S.; Kim, M.M.; Cabrales, P.; Lybeck, M.; Caroen, S.; Oronsky, N.; Burbano, E.; Carter, C.; Oronsky, A. A Review of Clinical Radioprotection and Chemoprotection for Oral Mucositis. Transl. Oncol. 2018, 11, 771–778. [Google Scholar] [CrossRef] [PubMed]
- Shetty, S.S.; Maruthi, M.; Dhara, V.; de Arruda, J.A.A.; Abreu, L.G.; Mesquita, R.A.; Teixeira, A.L.; Silva, T.A.; Merchant, Y. Oral mucositis: Current knowledge and future directions. Dis. Mon. 2021; 101300, in press. [Google Scholar] [CrossRef] [PubMed]
- Sonis, S.T. A hypothesis for the pathogenesis of radiation-induced oral mucositis: When biological challenges exceed physiologic protective mechanisms. Implications for pharmacological prevention and treatment. Support. Care Cancer 2021, 29, 4939–4947. [Google Scholar] [CrossRef]
- Kawashita, Y.; Soutome, S.; Umeda, M.; Saito, T. Oral management strategies for radiotherapy of head and neck cancer. Jpn. Dent. Sci. Rev. 2020, 56, 62–67. [Google Scholar] [CrossRef]
- Lalla, R.V.; Saunders, D.P.; Peterson, D.E. Chemotherapy or radiation-induced oral mucositis. Dent. Clin. N. Am. 2014, 58, 341–349. [Google Scholar] [CrossRef]
- Singh, V.; Singh, A.K. Oral mucositis. Natl. J. Maxillofac. Surg. 2020, 11, 159–168. [Google Scholar] [CrossRef]
- Pulito, C.; Cristaudo, A.; Porta, C.; Zapperi, S.; Blandino, G.; Morrone, A.; Strano, S. Oral mucositis: The hidden side of cancer therapy. J. Exp. Clin. Cancer Res. 2020, 39, 210. [Google Scholar] [CrossRef]
- Daugelaite, G.; Uzkuraityte, K.; Jagelaviciene, E.; Filipauskas, A. Prevention and Treatment of Chemotherapy and Radiotherapy Induced Oral Mucositis. Medicina 2019, 55, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.C.; Lai, Y.H.; Huang, B.S.; Lin, C.Y.; Fan, K.H.; Chang, J.T. Changes and predictors of radiation-induced oral mucositis in patients with oral cavity cancer during active treatment. Eur. J. Oncol. Nurs. 2015, 19, 214–219. [Google Scholar] [CrossRef] [PubMed]
- De Sanctis, V.; Bossi, P.; Sanguineti, G.; Trippa, F.; Ferrari, D.; Bacigalupo, A.; Ripamonti, C.I.; Buglione, M.; Pergolizzi, S.; Langendjik, J.A.; et al. Mucositis in head and neck cancer patients treated with radiotherapy and systemic therapies: Literature review and consensus statements. Crit. Rev. Oncol. Hematol. 2016, 100, 147–166. [Google Scholar] [CrossRef] [PubMed]
- Fidan, O.; Arslan, S. Development and Validation of the Oral Mucositis Risk Assessment Scale in Hematology Patients. Semin. Oncol. Nurs. 2021, 37, 151159. [Google Scholar] [CrossRef]
- Hearnden, V.; Sankar, V.; Hull, K.; Juras, D.V.; Greenberg, M.; Kerr, A.R.; Lockhart, P.B.; Patton, L.L.; Porter, S.; Thornhill, M.H. New developments and opportunities in oral mucosal drug delivery for local and systemic disease. Adv. Drug Deliv. Rev. 2012, 64, 16–28. [Google Scholar] [CrossRef]
- Elad, S.; Yarom, N.; Zadik, Y.; Kuten-Shorrer, M.; Sonis, S.T. The broadening scope of oral mucositis and oral ulcerative mucosal toxicities of anticancer therapies. CA Cancer J. Clin. 2022, 72, 57–77. [Google Scholar] [CrossRef]
- Kawashita, Y.; Koyama, Y.; Kurita, H.; Otsuru, M.; Ota, Y.; Okura, M.; Horie, A.; Sekiya, H.; Umeda, M. Effectiveness of a comprehensive oral management protocol for the prevention of severe oral mucositis in patients receiving radiotherapy with or without chemotherapy for oral cancer: A multicentre, phase II, randomized controlled trial. Int. J. Oral Maxillofac. Surg. 2019, 48, 857–864. [Google Scholar] [CrossRef]
- Basile, D.; Di Nardo, P.; Corvaja, C.; Garattini, S.K.; Pelizzari, G.; Lisanti, C.; Bortot, L.; Da Ros, L.; Bartoletti, M.; Borghi, M.; et al. Mucosal Injury during Anti-Cancer Treatment: From Pathobiology to Bedside. Cancers 2019, 11, 857. [Google Scholar] [CrossRef] [Green Version]
- Elting, L.S.; Chang, Y.C. Costs of Oral Complications of Cancer Therapies: Estimates and a Blueprint for Future Study. J. Natl. Cancer Inst. Monogr. 2019, 2019, lgz010. [Google Scholar] [CrossRef]
- Rodrigues-Oliveira, L.; Kowalski, L.P.; Santos, M.; Marta, G.N.; Bensadoun, R.J.; Martins, M.D.; Lopes, M.A.; Castro, G., Jr.; William, W.N., Jr.; Chaves, A.L.F.; et al. Direct costs associated with the management of mucositis: A systematic review. Oral Oncol. 2021, 118, 105296. [Google Scholar] [CrossRef]
- Sampson, M.M.; Nanjappa, S.; Greene, J.N. Mucositis and oral infections secondary to gram negative rods in patients with prolonged neutropenia. IDCases 2017, 9, 101–103. [Google Scholar] [CrossRef] [PubMed]
- Alkhouli, M.; Laflouf, M.; Comisi, J.C. Assessing the topical application efficiency of two biological agents in managing chemotherapy-induced oral mucositis in children: A randomized clinical trial. J. Oral Biol. Craniofac. Res. 2021, 11, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Kusiak, A.; Jereczek-Fossa, B.A.; Cichonska, D.; Alterio, D. Oncological-Therapy Related Oral Mucositis as an Interdisciplinary Problem-Literature Review. Int. J. Environ. Res. Public Health 2020, 17, 2464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tran, P.H.L.; Duan, W.; Tran, T.T.D. Recent developments of nanoparticle-delivered dosage forms for buccal delivery. Int. J. Pharm. 2019, 571, 118697. [Google Scholar] [CrossRef] [PubMed]
- Moslemi, D.; Nokhandani, A.M.; Otaghsaraei, M.T.; Moghadamnia, Y.; Kazemi, S.; Moghadamnia, A.A. Management of chemo/radiation-induced oral mucositis in patients with head and neck cancer: A review of the current literature. Radiother. Oncol. 2016, 120, 13–20. [Google Scholar] [CrossRef]
- Patel, P.; Robinson, P.D.; Baggott, C.; Gibson, P.; Ljungman, G.; Massey, N.; Ottaviani, G.; Phillips, R.; Revon-Riviere, G.; Treister, N.; et al. Clinical practice guideline for the prevention of oral and oropharyngeal mucositis in pediatric cancer and hematopoietic stem cell transplant patients: 2021 update. Eur. J. Cancer 2021, 154, 92–101. [Google Scholar] [CrossRef]
- Ayago Flores, D.; Ferriols Lisart, R. Effectiveness of palifermin in the prevention of oral mucositis in patients with haematological cancers. Farm. Hosp. 2010, 34, 163–169. [Google Scholar] [CrossRef]
- de Sousa Melo, A.; de Lima Dantas, J.B.; Medrado, A.; Lima, H.R.; Martins, G.B.; Carrera, M. Nutritional supplements in the management of oral mucositis in patients with head and neck cancer: Narrative literary review. Clin. Nutr. ESPEN 2021, 43, 31–38. [Google Scholar] [CrossRef]
- Yarom, N.; Ariyawardana, A.; Hovan, A.; Barasch, A.; Jarvis, V.; Jensen, S.B.; Zadik, Y.; Elad, S.; Bowen, J.; Lalla, R.V. Systematic review of natural agents for the management of oral mucositis in cancer patients. Support. Care Cancer 2013, 21, 3209–3221. [Google Scholar] [CrossRef]
- Yarom, N.; Hovan, A.; Bossi, P.; Ariyawardana, A.; Jensen, S.B.; Gobbo, M.; Saca-Hazboun, H.; Kandwal, A.; Majorana, A.; Ottaviani, G.; et al. Correction to: Systematic review of natural and miscellaneous agents, for the management of oral mucositis in cancer patients and Clinical Practice Guidelines—Part 1: Vitamins, minerals and nutritional supplements. Support. Care Cancer 2021, 29, 4175–4176. [Google Scholar] [CrossRef]
- Konuk Sener, D.; Aydin, M.; Cangur, S.; Guven, E. The Effect of Oral Care with Chlorhexidine, Vitamin E and Honey on Mucositis in Pediatric Intensive Care Patients: A Randomized Controlled Trial. J. Pediatric Nurs. 2019, 45, e95–e101. [Google Scholar] [CrossRef] [PubMed]
- Lima, I.; de Fatima Souto Maior, L.; Gueiros, L.A.M.; Leao, J.C.; Higino, J.S.; Carvalho, A.A.T. Clinical applicability of natural products for prevention and treatment of oral mucositis: A systematic review and meta-analysis. Clin. Oral Investig. 2021, 25, 4115–4124. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Tang, H.; Wang, L.; Peng, R.; Bai, F.; Shan, Y.; Yu, Z.; Zhou, P.; Cong, Y. Dimethyl Sulfoxide Prevents Radiation-Induced Oral Mucositis through Facilitating DNA Double-Strand Break Repair in Epithelial Stem Cells. Int. J. Radiat. Oncol. Biol. Phys. 2018, 102, 1577–1589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, P.M.; Lalla, R.V. Glutamine for Amelioration of Radiation and Chemotherapy Associated Mucositis during Cancer Therapy. Nutrients 2020, 12, 1675. [Google Scholar] [CrossRef]
- Charalambous, M.; Raftopoulos, V.; Paikousis, L.; Katodritis, N.; Lambrinou, E.; Vomvas, D.; Georgiou, M.; Charalambous, A. The effect of the use of thyme honey in minimizing radiation-induced oral mucositis in head and neck cancer patients: A randomized controlled trial. Eur. J. Oncol. Nurs. 2018, 34, 89–97, (clinicaltrials.gov identifier NCT01465308). [Google Scholar] [CrossRef]
- Charalambous, M.; Raftopoulos, V.; Lambrinou, E.; Charalambous, A. The effectiveness of honey for the management of radiotherapy-induced oral mucositis in head and neck cancer patients: A systematic review of clinical trials. Eur. J. Integr. Med. 2013, 5, 217–225. [Google Scholar] [CrossRef]
- Ramsay, E.I.; Rao, S.; Madathil, L.; Hegde, S.K.; Baliga-Rao, M.P.; George, T.; Baliga, M.S. Honey in oral health and care: A mini review. J. Oral Biosci. 2019, 61, 32–36. [Google Scholar] [CrossRef]
- Munstedt, K.; Momm, F.; Hubner, J. Honey in the management of side effects of radiotherapy- or radio/chemotherapy-induced oral mucositis. A systematic review. Complement. Ther. Clin. Pract. 2019, 34, 145–152. [Google Scholar] [CrossRef]
- Pires Marques, E.C.; Piccolo Lopes, F.; Nascimento, I.C.; Morelli, J.; Pereira, M.V.; Machado Meiken, V.M.; Pinheiro, S.L. Photobiomodulation and photodynamic therapy for the treatment of oral mucositis in patients with cancer. Photodiagn. Photodyn. Ther. 2020, 29, 101621. [Google Scholar] [CrossRef]
- de Carvalho, P.A.G.; Lessa, R.C.; Carraro, D.M.; Assis Pellizzon, A.C.; Jaguar, G.C.; Alves, F.A. Three photobiomodulation protocols in the prevention/treatment of radiotherapy-induced oral mucositis. Photodiagn. Photodyn. Ther. 2020, 31, 101906. [Google Scholar] [CrossRef]
- Cotomacio, C.C.; Calarga, C.C.; Yshikawa, B.K.; Arana-Chavez, V.E.; Simoes, A. Wound healing process with different photobiomodulation therapy protocols to treat 5-FU-induced oral mucositis in hamsters. Arch. Oral Biol. 2021, 131, 105250. [Google Scholar] [CrossRef] [PubMed]
- Blakaj, A.; Bonomi, M.; Gamez, M.E.; Blakaj, D.M. Oral mucositis in head and neck cancer: Evidence-based management and review of clinical trial data. Oral Oncol. 2019, 95, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Campos, M.I.; Campos, C.N.; Aarestrup, F.M.; Aarestrup, B.J. Oral mucositis in cancer treatment: Natural history, prevention and treatment. Mol. Clin. Oncol. 2014, 2, 337–340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abt, E. Probiotics May Lower the Risk of Oral Mucositis in Cancer Patients. J. Evid. Based Dent. Pract. 2021, 21, 101639. [Google Scholar] [CrossRef]
- Wardill, H.R.; Sonis, S.T.; Blijlevens, N.M.A.; Van Sebille, Y.Z.A.; Ciorba, M.A.; Loeffen, E.A.H.; Cheng, K.K.F.; Bossi, P.; Porcello, L.; Castillo, D.A.; et al. Prediction of mucositis risk secondary to cancer therapy: A systematic review of current evidence and call to action. Support. Care Cancer 2020, 28, 5059–5073. [Google Scholar] [CrossRef]
- Elad, S.; Cheng, K.K.F.; Lalla, R.V.; Yarom, N.; Hong, C.; Logan, R.M.; Bowen, J.; Gibson, R.; Saunders, D.P.; Zadik, Y.; et al. MASCC/ISOO clinical practice guidelines for the management of mucositis secondary to cancer therapy. Cancer 2020, 126, 4423–4431. [Google Scholar] [CrossRef]
- Logan, R.M.; Stringer, A.M.; Bowen, J.M.; Yeoh, A.S.; Gibson, R.J.; Sonis, S.T.; Keefe, D.M. The role of pro-inflammatory cytokines in cancer treatment-induced alimentary tract mucositis: Pathobiology, animal models and cytotoxic drugs. Cancer Treat. Rev. 2007, 33, 448–460. [Google Scholar] [CrossRef]
- Bertolini, M.; Sobue, T.; Thompson, A.; Dongari-Bagtzoglou, A. Chemotherapy Induces Oral Mucositis in Mice without Additional Noxious Stimuli. Transl. Oncol. 2017, 10, 612–620. [Google Scholar] [CrossRef]
- Raber-Durlacher, J.E.; Elad, S.; Barasch, A. Oral mucositis. Oral Oncol. 2010, 46, 452–456. [Google Scholar] [CrossRef]
- Bailly, C. Potential use of edaravone to reduce specific side effects of chemo-, radio- and immuno-therapy of cancers. Int. Immunopharmacol. 2019, 77, 105967. [Google Scholar] [CrossRef]
- Vigarios, E.; Epstein, J.B.; Sibaud, V. Oral mucosal changes induced by anticancer targeted therapies and immune checkpoint inhibitors. Support. Care Cancer 2017, 25, 1713–1739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kudrimoti, M.; Curtis, A.; Azawi, S.; Worden, F.; Katz, S.; Adkins, D.; Bonomi, M.; Elder, J.; Sonis, S.T.; Straube, R.; et al. Dusquetide: A novel innate defense regulator demonstrating a significant and consistent reduction in the duration of oral mucositis in preclinical data and a randomized, placebo-controlled phase 2a clinical study. J. Biotechnol. 2016, 239, 115–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ariyawardana, A.; Cheng, K.K.F.; Kandwal, A.; Tilly, V.; Al-Azri, A.R.; Galiti, D.; Chiang, K.; Vaddi, A.; Ranna, V.; Nicolatou-Galitis, O.; et al. Systematic review of anti-inflammatory agents for the management of oral mucositis in cancer patients and clinical practice guidelines. Support. Care Cancer 2019, 27, 3985–3995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davy, C.; Heathcote, S. A systematic review of interventions to mitigate radiotherapy-induced oral mucositis in head and neck cancer patients. Support. Care Cancer 2021, 29, 2187–2202. [Google Scholar] [CrossRef]
- Peterson, D.E.; Lalla, R.V. Oral mucositis: The new paradigms. Curr. Opin. Oncol. 2010, 22, 318–322. [Google Scholar] [CrossRef]
- Satheeshkumar, P.S.; El-Dallal, M.; Mohan, M.P. Feature selection and predicting chemotherapy-induced ulcerative mucositis using machine learning methods. Int. J. Med. Inform. 2021, 154, 104563. [Google Scholar] [CrossRef]
- Pratiwi, E.S.; Ismawati, N.D.S.; Ruslin, M. Prevalence and risk factors of oral mucositis in children with acute lymphoblastic leukemia in Dr. Soetomo Hospital Surabaya Indonesia. Enfermería Clínica 2020, 30, 289–292. [Google Scholar] [CrossRef]
- Soutome, S.; Yanamoto, S.; Nishii, M.; Kojima, Y.; Hasegawa, T.; Funahara, M.; Akashi, M.; Saito, T.; Umeda, M. Risk factors for severe radiation-induced oral mucositis in patients with oral cancer. J. Dent. Sci. 2021, 16, 1241–1246. [Google Scholar] [CrossRef]
- Parkhideh, S.; Zeraatkar, M.; Moradi, O.; Hajifathali, A.; Mehdizadeh, M.; Tavakoli-Ardakani, M. Azithromycin oral suspension in prevention and management of oral mucositis in patients undergoing hematopoietic stem cell transplantation: A randomized controlled trial. Support. Care Cancer 2022, 30, 251–257. [Google Scholar] [CrossRef]
- Aspinall, S.R.; Parker, J.K.; Khutoryanskiy, V.V. Oral care product formulations, properties and challenges. Colloids Surf. B 2021, 200, 111567. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Makiguchi, T.; Nakamura, H.; Yamatsu, Y.; Hirai, Y.; Shoda, K.; Suzuki, K.; Kim, M.; Kurozumi, S.; Motegi, S.I.; et al. Impact of muscle volume loss on acute oral mucositis in patients undergoing concurrent chemoradiotherapy after oral cancer resection. Int. J. Oral Maxillofac. Surg. 2021, 50, 1195–1202. [Google Scholar] [CrossRef] [PubMed]
- Orlandi, E.; Iacovelli, N.A.; Rancati, T.; Cicchetti, A.; Bossi, P.; Pignoli, E.; Bergamini, C.; Licitra, L.; Fallai, C.; Valdagni, R.; et al. Multivariable model for predicting acute oral mucositis during combined IMRT and chemotherapy for locally advanced nasopharyngeal cancer patients. Oral Oncol. 2018, 86, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.H.L.; Gueiros, L.A.; Fulton, J.S.; Cheng, K.K.F.; Kandwal, A.; Galiti, D.; Fall-Dickson, J.M.; Johansen, J.; Ameringer, S.; Kataoka, T.; et al. Systematic review of basic oral care for the management of oral mucositis in cancer patients and clinical practice guidelines. Support. Care Cancer 2019, 27, 3949–3967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inoue, Y.; Yamagata, K.; Nakamura, M.; Ohnishi, K.; Tabuchi, K.; Bukawa, H. Are Intraoral Stents Effective for Reducing the Severity of Oral Mucositis during Radiotherapy for Maxillary and Nasal Cavity Cancer? J. Oral Maxillofac. Surg. 2020, 78, 1214.e1–1214.e8. [Google Scholar] [CrossRef]
- Duzkaya, D.S.; Uysal, G.; Bozkurt, G.; Yakut, T. The Effect of Oral Care Using an Oral Health Care Guide on Preventing Mucositis in Pediatric Intensive Care. J. Pediatr. Nurs. 2017, 36, 98–102. [Google Scholar] [CrossRef]
- Edmans, J.G.; Murdoch, C.; Santocildes-Romero, M.E.; Hatton, P.V.; Colley, H.E.; Spain, S.G. Incorporation of lysozyme into a mucoadhesive electrospun patch for rapid protein delivery to the oral mucosa. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 112, 110917. [Google Scholar] [CrossRef]
- Liao, Y.C.; Hsu, L.F.; Hsieh, L.Y.; Luo, Y.Y. Effectiveness of green tea mouthwash for improving oral health status in oral cancer patients: A single-blind randomized controlled trial. Int. J. Nurs. Stud. 2021, 121, 103985, (clinicaltrials.gov identifier NCT04615780). [Google Scholar] [CrossRef]
- Freires, I.A.; Rosalen, P.L. How Natural Product Research has Contributed to Oral Care Product Development? A Critical View. Pharm. Res. 2016, 33, 1311–1317. [Google Scholar] [CrossRef]
- Yen, S.H.; Wang, L.W.; Lin, Y.H.; Jen, Y.M.; Chung, Y.L. Phenylbutyrate mouthwash mitigates oral mucositis during radiotherapy or chemoradiotherapy in patients with head-and-neck cancer. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, 1463–1470. [Google Scholar] [CrossRef]
- Nigro, O.; Tuzi, A.; Tartaro, T.; Giaquinto, A.; Vallini, I.; Pinotti, G. Biological effects of verbascoside and its anti-inflammatory activity on oral mucositis: A review of the literature. Anticancer Drugs 2020, 31, 1–5. [Google Scholar] [CrossRef]
- Baysal, E.; Sari, D.; Vural, F.; Cagirgan, S.; Saydam, G.; Tobu, M.; Sahin, F.; Soyer, N.; Gediz, F.; Acarlar, C.; et al. The Effect of Cryotherapy on the Prevention of Oral Mucositis and on the Oral pH Value in Multiple Myeloma Patients Undergoing Autologous Stem Cell Transplantation. Semin. Oncol. Nurs. 2021, 37, 151146. [Google Scholar] [CrossRef] [PubMed]
- Askarifar, M.; Lakdizaji, S.; Ramzi, M.; Rahmani, A.; Jabbarzadeh, F. The Effects of Oral Cryotherapy on Chemotherapy-Induced Oral Mucositis in Patients Undergoing Autologous Transplantation of Blood Stem Cells: A Clinical Trial. Iran. Red Crescent Med. J. 2016, 18, e24775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Migliorati, C.A.; Oberle-Edwards, L.; Schubert, M. The role of alternative and natural agents, cryotherapy, and/or laser for management of alimentary mucositis. Support. Care Cancer 2006, 14, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Sun, D.; Qin, N.; Liu, M.; Zhang, J.; Li, X. Comparative prevention potential of 10 mouthwashes on intolerable oral mucositis in cancer patients: A Bayesian network analysis. Oral Oncol. 2020, 107, 104751. [Google Scholar] [CrossRef]
- Karavana Hizarcioglu, S.Y.; Sezer, B.; Guneri, P.; Veral, A.; Boyacioglu, H.; Ertan, G.; Epstein, J.B. Efficacy of topical benzydamine hydrochloride gel on oral mucosal ulcers: An in vivo animal study. Int. J. Oral Maxillofac. Surg. 2011, 40, 973–978. [Google Scholar] [CrossRef]
- El-Salamouni, N.S.; Hanafy, A.S. Hyaluronic-benzydamine oromucosal films outperform conventional mouth rinse in ulcer healing. J. Drug Deliv. Sci. Technol. 2021, 65, 102690. [Google Scholar] [CrossRef]
- Yang, C.; Gong, G.; Jin, E.; Han, X.; Zhuo, Y.; Yang, S.; Song, B.; Zhang, Y.; Piao, C. Topical application of honey in the management of chemo/radiotherapy-induced oral mucositis: A systematic review and network meta-analysis. Int. J. Nurs. Stud. 2019, 89, 80–87. [Google Scholar] [CrossRef]
- Saunders, D.P.; Epstein, J.B.; Elad, S.; Allemano, J.; Bossi, P.; van de Wetering, M.D.; Rao, N.G.; Potting, C.; Cheng, K.K.; Freidank, A.; et al. Systematic review of antimicrobials, mucosal coating agents, anesthetics, and analgesics for the management of oral mucositis in cancer patients. Support. Care Cancer 2013, 21, 3191–3207. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, B.N.; Aagaard, G.; Henneberg, S.W.; Schmiegelow, K.; Hansen, S.H.; Romsing, J. Topical morphine for oral mucositis in children: Dose finding and absorption. J. Pain Symptom Manag. 2012, 44, 117–123. [Google Scholar] [CrossRef]
- Sanz, R.; Calpena, A.C.; Mallandrich, M.; Gimeno, A.; Halbaut, L.; Clares, B. Development of a buccal doxepin platform for pain in oral mucositis derived from head and neck cancer treatment. Eur. J. Pharm. Biopharm. 2017, 117, 203–211. [Google Scholar] [CrossRef]
- Chung, M.K.; Wang, S.; Oh, S.L.; Kim, Y.S. Acute and Chronic Pain from Facial Skin and Oral Mucosa: Unique Neurobiology and Challenging Treatment. Int. J. Mol. Sci. 2021, 22, 5810. [Google Scholar] [CrossRef] [PubMed]
- Miranzadeh, S.; Adib-Hajbaghery, M.; Soleymanpoor, L.; Ehsani, M. Effect of adding the herb Achillea millefolium on mouthwash on chemotherapy induced oral mucositis in cancer patients: A double-blind randomized controlled trial. Eur. J. Oncol. Nurs. 2015, 19, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Santos, H.T.C.; Coimbra, M.C.; Meri Junior, A.E.; Gomes, A.J.P.S. Effectiveness of topically applied chamomile in the treatment of oral mucositis: A literature review. Res. Soc. Dev. 2021, 10, e433101422081. [Google Scholar] [CrossRef]
- Honda, H.; Onda, T.; Hayashi, K.; Shibahara, T.; Takano, M. Comparison of topical agents that are effective against oral mucositis associated with chemotherapy using a rat anticancer agent-induced oral mucositis model. J. Oral Maxillofac. Surg. Med. Pathol. 2021; in press. [Google Scholar] [CrossRef]
- da Silva, V.C.R.; da Motta Silveira, F.M.; Barbosa Monteiro, M.G.; da Cruz, M.M.D.; Caldas Junior, A.F.; Pina Godoy, G. Photodynamic therapy for treatment of oral mucositis: Pilot study with pediatric patients undergoing chemotherapy. Photodiagn. Photodyn. Ther. 2018, 21, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Simoes, A.; Benites, B.M.; Benassi, C.; Torres-Schroter, G.; de Castro, J.R.; Campos, L. Antimicrobial photodynamic therapy on treatment of infected radiation-induced oral mucositis: Report of two cases. Photodiagn. Photodyn. Ther. 2017, 20, 18–20. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, A.B.; Ferrisse, T.M.; Basso, F.G.; Fontana, C.R.; Giro, E.M.A.; Brighenti, F.L. A systematic review and meta-analysis of the effect of photodynamic therapy for the treatment of oral mucositis. Photodiagn. Photodyn. Ther. 2021, 34, 102316. [Google Scholar] [CrossRef]
- Yarom, N.; Hovan, A.; Bossi, P.; Ariyawardana, A.; Jensen, S.B.; Gobbo, M.; Saca-Hazboun, H.; Kandwal, A.; Majorana, A.; Ottaviani, G.; et al. Systematic review of natural and miscellaneous agents, for the management of oral mucositis in cancer patients and clinical practice guidelines—Part 2: Honey, herbal compounds, saliva stimulants, probiotics, and miscellaneous agents. Support. Care Cancer 2020, 28, 2457–2472. [Google Scholar] [CrossRef]
- Nagi, R.; Patil, D.J.; Rakesh, N.; Jain, S.; Sahu, S. Natural agents in the management of oral mucositis in cancer patients-systematic review. J. Oral Biol. Craniofac. Res. 2018, 8, 245–254. [Google Scholar] [CrossRef]
- Aghamohamamdi, A.; Hosseinimehr, S.J. Natural Products for Management of Oral Mucositis Induced by Radiotherapy and Chemotherapy. Integr. Cancer Ther. 2016, 15, 60–68. [Google Scholar] [CrossRef]
- Khanal, B.; Baliga, M.; Uppal, N. Effect of topical honey on limitation of radiation-induced oral mucositis: An intervention study. Int. J. Oral Maxillofac. Surg. 2010, 39, 1181–1185. [Google Scholar] [CrossRef] [PubMed]
- Amanat, A.; Ahmed, A.; Kazmi, A.; Aziz, B. The Effect of Honey on Radiation-induced Oral Mucositis in Head and Neck Cancer Patients. Indian J. Palliat. Care 2017, 23, 317–320. [Google Scholar] [CrossRef] [PubMed]
- Lusby, P.E.; Coombes, A.; Wilkinson, J.M. Honey: A potent agent for wound healing? J. Wound Ostomy Cont. Nurs. 2002, 29, 295–300. [Google Scholar] [CrossRef]
- Molan, P.C. Re-introducing honey in the management of wounds and ulcers-theory and practice. Ostomy Wound Manag. 2002, 48, 28–40. [Google Scholar]
- Motallebnejad, M.; Akram, S.; Moghadamnia, A.; Moulana, Z.; Omidi, S. The effect of topical application of pure honey on radiation-induced mucositis: A randomized clinical trial. J. Contemp. Dent. Pract. 2008, 9, 40–47. [Google Scholar] [CrossRef] [Green Version]
- Al-Waili, N.S. An alternative treatment for pityriasis versicolor, tinea cruris, tinea corporis and tinea faciei with topical application of honey, olive oil and beeswax mixture: An open pilot study. Complement. Ther. Med. 2004, 12, 45–47. [Google Scholar] [CrossRef]
- Khanjani Pour-Fard-Pachekenari, A.; Rahmani, A.; Ghahramanian, A.; Asghari Jafarabadi, M.; Onyeka, T.C.; Davoodi, A. The effect of an oral care protocol and honey mouthwash on mucositis in acute myeloid leukemia patients undergoing chemotherapy: A single-blind clinical trial. Clin. Oral Investig. 2019, 23, 1811–1821, (WHO Trial registration IRCT2015121419919N7). [Google Scholar] [CrossRef]
- Raeessi, M.A.; Raeessi, N.; Panahi, Y.; Gharaie, H.; Davoudi, S.M.; Saadat, A.; Karimi Zarchi, A.A.; Raeessi, F.; Ahmadi, S.M.; Jalalian, H. “Coffee plus honey” versus “topical steroid” in the treatment of chemotherapy-induced oral mucositis: A randomised controlled trial. BMC Complement. Altern. Med. 2014, 14, 293, (Iranian Registry of Clinical Trials IRCT: 201104074737N3). [Google Scholar] [CrossRef] [Green Version]
- Davis, J.K.; Green, J.M. Caffeine and anaerobic performance: Ergogenic value and mechanisms of action. Sports Med. 2009, 39, 813–832. [Google Scholar] [CrossRef]
- Miłek, M.; Młodecki, Ł.; Dżugan, M. Caffeine content and antioxidant activity of various brews of specialty grade coffee. Acta Sci. Pol. Technol. Aliment. 2021, 20, 179–188. [Google Scholar]
- Barcelos, R.P.; Lima, F.D.; Carvalho, N.R.; Bresciani, G.; Royes, L.F. Caffeine effects on systemic metabolism, oxidative-inflammatory pathways, and exercise performance. Nutr. Res. 2020, 80, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Sforcin, J.M. Biological Properties and Therapeutic Applications of Propolis. Phytother. Res. 2016, 30, 894–905. [Google Scholar] [CrossRef] [PubMed]
- Melliou, E.; Chinou, I. Chemical analysis and antimicrobial activity of Greek propolis. Planta Med. 2004, 70, 515–519. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.Y.; Guo, X.L.; Luo, H.L.; Fang, X.W.; Zhu, T.G.; Zhang, X.L.; Chen, H.W.; Luo, L.P. Fast Differential Analysis of Propolis Using Surface Desorption Atmospheric Pressure Chemical Ionization Mass Spectrometry. Int. J. Anal. Chem. 2015, 2015, 176475. [Google Scholar] [CrossRef] [Green Version]
- Franco, T. Chemical composition of propolis: Vitamins and aminoacids. Rev. Bras. Farmacogn. 1985, 1, 12–19. [Google Scholar]
- Kamburoğlu, K.; Özen, T. Analgesic effect of Anatolian propolis in mice. Agri 2011, 23, 47–50. [Google Scholar]
- Montero, J.C.; Mori, G.G. Assessment of ion diffusion from a calcium hydroxide-propolis paste through dentin. Braz. Oral Res. 2012, 26, 318–322. [Google Scholar] [CrossRef]
- Ozan, F.; Sümer, Z.; Polat, Z.A.; Er, K.; Ozan, U.; Deger, O. Effect of mouthrinse containing propolis on oral microorganisms and human gingival fibroblasts. Eur. J. Dent. 2007, 1, 195–201. [Google Scholar]
- Cavalcante, D.R.; Oliveira, P.S.; Góis, S.M.; Soares, A.F.; Cardoso, J.C.; Padilha, F.F.; Albuquerque, R.L., Jr. Effect of green propolis on oral epithelial dysplasia in rats. Braz. J. Otorhinolaryngol. 2011, 77, 278–284. [Google Scholar] [CrossRef] [Green Version]
- Mirzoeva, O.K.; Calder, P.C. The effect of propolis and its components on eicosanoid production during the inflammatory response. Prostaglandins Leukot. Essent. Fat. Acids 1996, 55, 441–449. [Google Scholar] [CrossRef]
- AkhavanKarbassi, M.H.; Yazdi, M.F.; Ahadian, H.; SadrAbad, M.J. Randomized DoubleBlind Placebo Controlled Trial of Propolis for Oral Mucositis in Patients Receiving Chemotherapy for Head and Neck Cancer. Asian Pac. J. Cancer Prev. 2016, 17, 3611–3614. [Google Scholar] [PubMed]
- Pavel, C.; Mărghitaş, A.L.; Bobis, O.; Dezmirean, D.; Şapcaliu, A.; Radoi, I.; Mădaş, M. Biological Activities of Royal Jelly-Review. Lucr. Stiintifice 2011, 44, 108–118. [Google Scholar]
- Nagai, T.; Inoue, R. Preparation and the functional properties of water extract and alkaline extract of royal jelly. Food Chem. 2004, 84, 181–186. [Google Scholar] [CrossRef]
- Nagai, T.; Inoue, R.; Suzuki, N.; Nagashima, T. Antioxidant properties of enzymatic hydrolysates from royal jelly. J. Med. Food 2006, 9, 363–367. [Google Scholar] [CrossRef] [Green Version]
- Izuta, H.; Chikaraishi, Y.; Shimazawa, M.; Mishima, S.; Hara, H. 10-Hydroxy-2-decenoic acid, a major fatty acid from royal jelly, inhibits VEGF-induced angiogenesis in human umbilical vein endothelial cells. Evid. Based Complement. Altern. Med. 2009, 6, 489–494. [Google Scholar] [CrossRef]
- Šimúth, J.; Bíliková, K.; Kováčová, E.; Kuzmová, Z.; Schroder, W. Immunochemical Approach to Detection of Adulteration in Honey: Physiologically Active Royal Jelly Protein Stimulating TNF-α Release is a Regular Component of Honey. J. Agric. Food Chem. 2004, 52, 2154–2158. [Google Scholar] [CrossRef]
- Matsui, T.; Yukiyoshi, A.; Doi, S.; Sugimoto, H.; Yamada, H.; Matsumoto, K. Gastrointestinal enzyme production of bioactive peptides from royal jelly protein and their antihypertensive ability in SHR. J. Nutr. Biochem. 2002, 13, 80–86. [Google Scholar] [CrossRef]
- Fujiwara, S.; Imai, J.; Fujiwara, M.; Yaeshima, T.; Kawashima, T.; Kobayashi, K. A potent antibacterial protein in royal jelly. Purification and determination of the primary structure of royalisin. J. Biol. Chem. 1990, 265, 11333–11337. [Google Scholar] [CrossRef]
- Kohno, K.; Okamoto, I.; Sano, O.; Arai, N.; Iwaki, K.; Ikeda, M.; Kurimoto, M. Royal jelly inhibits the production of proinflammatory cytokines by activated macrophages. Biosci. Biotechnol. Biochem. 2004, 68, 138–145. [Google Scholar] [CrossRef] [Green Version]
- Fujii, A.; Kobayashi, S.; Kuboyama, N.; Furukawa, Y.; Kaneko, Y.; Ishihama, S.; Yamamoto, H.; Tamura, T. Augmentation of wound healing by royal jelly (RJ) in streptozotocin-diabetic rats. Jpn. J. Pharmacol. 1990, 53, 331–337. [Google Scholar] [CrossRef] [Green Version]
- Xue, X.; Wu, L.; Wang, K. Chemical Composition of Royal Jelly. In Bee Products-Chemical and Biological Properties; Alvarez-Suarez, J.M., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 181–190. [Google Scholar]
- Lin, Y.; Shao, Q.; Zhang, M.; Lu, C.; Fleming, J.; Su, S. Royal jelly-derived proteins enhance proliferation and migration of human epidermal keratinocytes in an in vitro scratch wound model. BMC Complement. Altern. Med. 2019, 19, 175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inoue, S.; Koya-Miyata, S.; Ushio, S.; Iwaki, K.; Ikeda, M.; Kurimoto, M. Royal Jelly prolongs the life span of C3H/HeJ mice: Correlation with reduced DNA damage. Exp. Gerontol. 2003, 38, 965–969. [Google Scholar] [CrossRef]
- Suemaru, K.; Cui, R.; Li, B.; Watanabe, S.; Okihara, K.; Hashimoto, K.; Yamada, H.; Araki, H. Topical application of royal jelly has a healing effect for 5-fluorouracil-induced experimental oral mucositis in hamsters. Methods Find. Exp. Clin. Pharmacol. 2008, 30, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Suemaru, K.; Takechi, K.; Kaji, H.; Imai, K.; Araki, H. Oral mucosal adhesive films containing royal jelly accelerate recovery from 5-fluorouracil-induced oral mucositis. J. Pharmacol. Sci. 2013, 121, 110–118. [Google Scholar] [CrossRef] [Green Version]
- Erdem, O.; Güngörmüş, Z. The effect of royal jelly on oral mucositis in patients undergoing radiotherapy and chemotherapy. Holist. Nurs. Pract. 2014, 28, 242–246. [Google Scholar] [CrossRef]
- Yamauchi, K.; Kogashiwa, Y.; Moro, Y.; Kohno, N. The effect of topical application of royal jelly on chemoradiotherapy-induced mucositis in head and neck cancer: A preliminary study. Int. J. Otolaryngol. 2014, 2014, 974967. [Google Scholar] [CrossRef] [Green Version]
- Gomes, M.S.; Lins, R.D.A.U.; Langassner, S.M.Z.; da Silveira, E.J.D.; de Carvalho, T.G.; de Sousa Lopes, M.L.D.; de Souza Araujo, L.; de Medeiros, C.A.C.X.; de Carvalho Leitão, R.F.; Guerra, G.C.B.; et al. Anti-inflammatory and antioxidant activity of hydroethanolic extract of Spondias mombin leaf in an oral mucositis experimental model. Arch. Oral Biol. 2020, 111, 104664. [Google Scholar] [CrossRef]
- Cabral, B.; Siqueira, E.M.S.; Bitencourt, M.A.O.; Lima, M.C.J.S.; Lima, A.K.; Ortmann, C.F.; Chaves, V.C.; Fernandes-Pedrosa, M.F.; Rocha, H.A.O.; Scortecci, K.C.; et al. Phytochemical study and anti-inflammatory and antioxidant potential of Spondias mombin leaves. Rev. Bras. Farmacogn. 2016, 26, 304–311. [Google Scholar] [CrossRef] [Green Version]
- Nworu, C.S.; Akah, P.A.; Okoye, F.B.C.; Toukam, D.K.; Udeh, J.; Esimone, C.O. The leaf extract of Spondias mombin L. displays an anti-inflammatory effect and suppresses inducible formation of tumor necrosis factor-α and nitric oxide (NO). J. Immunotoxicol. 2011, 8, 10–16. [Google Scholar] [CrossRef]
- Khan, N.; Mukhtar, H. Tea polyphenols for health promotion. Life Sci. 2007, 81, 519–533. [Google Scholar] [CrossRef] [Green Version]
- Lin, J.K.; Liang, Y.C.; Lin-Shiau, S.Y. Cancer chemoprevention by tea polyphenols through mitotic signal transduction blockade. Biochem. Pharmacol. 1999, 58, 911–915. [Google Scholar] [CrossRef]
- Moyers, S.B.; Kumar, N.B. Green Tea Polyphenols and Cancer Chemoprevention: Multiple Mechanisms and Endpoints for Phase II Trials. Nutr. Rev. 2004, 62, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, R.R.; Neto, R.O.; dos Santos D’Almeida, C.T.; do Nascimento, T.P.; Pressete, C.G.; Azevedo, L.; Martino, H.S.D.; Cameron, L.C.; Ferreira, M.S.L.; de Barros, F.A.R. Kombuchas from green and black teas have different phenolic profile, which impacts their antioxidant capacities, antibacterial and antiproliferative activities. Food Res. Int. 2020, 128, 108782. [Google Scholar] [CrossRef] [PubMed]
- Steinmann, J.; Buer, J.; Pietschmann, T.; Steinmann, E. Anti-infective properties of epigallocatechin-3-gallate (EGCG), a component of green tea. Br. J. Pharmacol. 2013, 168, 1059–1073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Musial, C.; Kuban-Jankowska, A.; Gorska-Ponikowska, M. Beneficial Properties of Green Tea Catechins. Int. J. Mol. Sci. 2020, 21, 1744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okabe, S.; Ochiai, Y.; Aida, M.; Park, K.; Kim, S.J.; Nomura, T.; Suganuma, M.; Fujiki, H. Mechanistic aspects of green tea as a cancer preventive: Effect of components on human stomach cancer cell lines. Jpn. J. Cancer Res. 1999, 90, 733–739. [Google Scholar] [CrossRef]
- Pervin, M.; Unno, K.; Takagaki, A.; Isemura, M.; Nakamura, Y. Function of green tea catechins in the brain: Epigallocatechin gallate and its metabolites. Int. J. Mol. Sci. 2019, 20, 3630. [Google Scholar] [CrossRef] [Green Version]
- Carulli, G.; Rocco, M.; Panichi, A.; Chios, C.F.; Ciurli, E.; Mannucci, C.; Sordi, E.; Caracciolo, F.; Papineschi, F.; Benedetti, E.; et al. Treatment of oral mucositis in hematologic patients undergoing autologous or allogeneic transplantation of peripheral blood stem cells: A prospective, randomized study with a mouthwash containing camelia sinensis leaf extract. Hematol. Rep. 2013, 5, 21–25. [Google Scholar] [CrossRef] [Green Version]
- Cabrera-Jaime, S.; Martinez, C.; Ferro-Garcia, T.; Giner-Boya, P.; Icart-Isern, T.; Estrada-Masllorens, J.M.; Fernandez-Ortega, P. Efficacy of Plantago major, chlorhexidine 0.12% and sodium bicarbonate 5% solution in the treatment of oral mucositis in cancer patients with solid tumour: A feasibility randomised triple-blind phase III clinical trial. Eur. J. Oncol. Nurs. 2018, 32, 40–47. [Google Scholar] [CrossRef]
- Soltani, G.M.; Hemati, S.; Sarvizadeh, M.; Kamalinejad, M.; Tafazoli, V.; Latifi, S.A. Efficacy of the Plantago major L. syrup on radiation induced oral mucositis in head and neck cancer patients: A randomized, double blind, placebo-controlled clinical trial. Complement. Ther. Med. 2020, 51, 102397, (Iranian Registry of Clinical Trials IRCT: 20190312043027N1). [Google Scholar] [CrossRef]
- Yazdian, M.A.; Khodadoost, M.; Gheisari, M.; Kamalinejad, M.; Ehsani, A.; Barikbin, B. A Hypothesis on the Possible Potential of Plantago major in the Treatment of Urticaria. Galen Med. J. 2014, 3, 123–126. [Google Scholar] [CrossRef]
- Ringbom, T.; Segura, L.; Noreen, Y.; Perera, P.; Bohlin, L. Ursolic acid from Plantago major, a selective inhibitor of cyclooxygenase-2 catalyzed prostaglandin biosynthesis. J. Nat. Prod. 1998, 61, 1212–1215. [Google Scholar] [CrossRef] [PubMed]
- Stenholm, A.; Göransson, U.; Bohlin, L. Bioassay-guided supercritical fluid extraction of cyclooxygenase-2 inhibiting substances in Plantago major L. Phytochem. Anal. 2013, 24, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Pourmorad, F.; Hosseinimehr, S.J.; Shahabimajd, N. Antioxidant activity of phenol and flavonoid contents of some Iranian medicinal plants. Afr. J. Adv. Biotechnol. 2005, 5, 1684–5315. [Google Scholar]
- Samuelsen, A.B.; Paulsen, B.S.; Wold, J.K.; Otsuka, H.; Yamada, H.; Espevik, T. Isolation and partial characterization of biologically active polysaccharides from Plantago major L. Phytother. Res. 1995, 9, 211–218. [Google Scholar] [CrossRef]
- Baechler, B.J.; Nita, F.; Jones, L.; Frestedt, J.L. A novel liquid multi-phytonutrient supplement demonstrates DNA-protective effects. Plant Foods Hum. Nutr. 2009, 64, 81–85. [Google Scholar] [CrossRef] [Green Version]
- Langmead, L.; Makins, R.J.; Rampton, D.S. Anti-inflammatory effects of Aloe vera gel in human colorectal mucosa in vitro. Aliment. Pharmacol. Ther. 2004, 19, 521–527. [Google Scholar] [CrossRef]
- Heggers, J.; Pineless, G.; Robson, M. Dermaide Aloe vera gel-comparison of the anti-microbial effects. J. Am. Med. Inform. Assoc. 1979, 41, 293–294. [Google Scholar]
- Yagi, A.; Kabash, A.; Okamura, N.; Haraguchi, H.; Moustafa, S.M.; Khalifa, T.I. Antioxidant, free radical scavenging and anti-inflammatory effects of aloesin derivatives in Aloe vera. Planta Med. 2002, 68, 957–960. [Google Scholar] [CrossRef]
- Hu, Y.; Xu, J.; Hu, Q. Evaluation of antioxidant potential of Aloe vera (Aloe barbadensis miller) extracts. J. Agric. Food Chem. 2003, 51, 7788–7791. [Google Scholar] [CrossRef]
- Heggie, S.; Bryant, G.P.; Tripcony, L.; Keller, J.; Rose, P.; Glendenning, M.; Heath, J. A Phase III study on the efficacy of topical Aloe vera gel on irradiated breast tissue. Cancer Nurs. 2002, 25, 442–451. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.H.; Leitner, M.G.; Russo, J.M.; Byrne, M.E. Wound healing. Oral and topical activity of Aloe vera. J. Am. Podiatr. Med. Assoc. 1989, 79, 559–562. [Google Scholar] [PubMed]
- Davis, R.H.; Leitner, M.G.; Russo, J.M.; Byrne, M.E. Anti-inflammatory activity of Aloe vera against a spectrum of irritants. J. Am. Podiatr. Med. Assoc. 1989, 79, 263–276. [Google Scholar] [CrossRef] [PubMed]
- Meadows, T.P. Aloe as a humectant in new skin preparations. Cosmet. Toilet. 1980, 95, 51–56. [Google Scholar]
- Davis, R.H.; Donato, J.J.; Hartman, G.M.; Haas, R.C. Anti-inflammatory and wound healing activity of a growth substance in Aloe vera. J. Am. Podiatr. Med. Assoc. 1994, 84, 77–81. [Google Scholar] [PubMed]
- Vázquez, B.; Avila, G.; Segura, D.; Escalante, B. Anti-inflammatory activity of extracts from Aloe vera gel. J. Ethnopharmacol. 1996, 55, 69–75. [Google Scholar] [CrossRef]
- Wei, A.; Shibamoto, T. Antioxidant/lipoxygenase inhibitory activities and chemical compositions of selected essential oils. J. Agric. Food Chem. 2010, 58, 7218–7225. [Google Scholar] [CrossRef]
- Su, C.K.; Mehta, V.; Ravikumar, L.; Shah, R.; Pinto, H.; Halpern, J.; Koong, A.; Goffinet, D.; Le, Q.-T. Phase II double-blind randomized study comparing oral Aloe vera versus placebo to prevent radiation-related mucositis in patients with head-and-neck neoplasms. Int. J. Radiat. Oncol. Biol. Phys. 2004, 60, 171–177. [Google Scholar] [CrossRef]
- Mansouri, P.; Haghighi, M.; Beheshtipour, N.; Ramzi, M. The effect of Aloe vera solution on Chemotherapy-induced stomatitis in clients with lymphoma and leukemia: A randomized controlled Clinical Trial. Int. J. Community Based Nurs. Midwifery 2016, 4, 119–126, (Iranian Registry of Clinical Trials: 2014092819318N1). [Google Scholar]
- Karbasizade, S.; Ghorbani, F.; Ghasemi Darestani, N.; Mansouri-Tehrani, M.M.; Kazemi, A.H. Comparison of therapeutic effects of statins and Aloe vera mouthwash on chemotherapy induced oral mucositis. Int. J. Physiol. Pathophysiol. Pharmacol. 2021, 13, 110–116. [Google Scholar]
- Alkhouli, M.; Laflouf, M.; Alhaddad, M. Efficacy of Aloe-vera use for prevention of chemotherapy-induced oral mucositis in children with acute lymphoblastic leukemia: A randomized controlled clinical trial. Compr. Child Adolesc. Nurs. 2021, 44, 49–62, (Australian New Zealand Clinical Trials Registry: 12618001931268). [Google Scholar] [CrossRef] [PubMed]
- Sahebjamee, M.; Mansourian, A.; Hajimirzamohammad, M.; Zadeh, M.T.; Bekhradi, R.; Kazemian, A.; Manifar, S.; Ashnagar, S.; Doroudgar, K. Comparative efficacy of Aloe vera and benzydamine mouthwashes on radiation-induced oral mucositis: A triple-blind, randomised, controlled clinical trial. Oral Health Prev. Dent. 2015, 13, 309–315, (Iranian Registry of Clinical Trials: 2012072410377N1). [Google Scholar] [PubMed]
- Kocaadam, B.; Şanlier, N. Curcumin, an active component of turmeric (Curcuma longa), and its effects on health. Crit. Rev. Food Sci. Nutr. 2017, 57, 2889–2895. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.C.; Sung, B.; Kim, J.H.; Prasad, S.; Li, S.; Aggarwal, B.B. Multitargeting by turmeric, the golden spice: From kitchen to clinic. Mol. Nutr. Food Res. 2013, 57, 1510–1528. [Google Scholar] [CrossRef]
- Deogade, S.C.; Ghate, S. Curcumin: Therapeutic applications in systemic and oral health. Int. J. Biol. Pharm. Res. 2015, 6, 281–290. [Google Scholar]
- Li, S. Chemical composition and product quality control of turmeric (Curcuma longa L.). Pharm. Crops 2011, 5, 28–54. [Google Scholar] [CrossRef]
- Shehzad, A.; Lee, Y. Curcumin: Multiple molecular targets mediate multiple pharmacological actions: A review. Drugs Future 2010, 35, 113. [Google Scholar] [CrossRef]
- Srinivasan, M. Effect of curcumin on blood sugar as seen in a diabetic subject. Indian J. Med. Sci. 1972, 26, 269–270. [Google Scholar]
- Srimal, R.C.; Dhawan, B.N. Pharmacology of diferuloyl methane (curcumin), a non-steroidal anti-inflammatory agent. J. Pharm. Pharmacol. 1973, 25, 447–452. [Google Scholar] [CrossRef]
- Sharma, O.P. Antioxidant activity of curcumin and related compounds. Biochem. Pharmacol. 1976, 25, 1811–1812. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Harikumar, K.B. Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases. Int. J. Biochem. Cell Biol. 2009, 41, 40–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gandhy, S.U.; Kim, K.; Larsen, L.; Rosengren, R.J.; Safe, S. Curcumin and synthetic analogs induce reactive oxygen species and decreases specificity protein (Sp) transcription factors by targeting microRNAs. BMC Cancer 2012, 12, 564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.G.; Veena, M.S.; Basak, S.K.; Han, E.; Tajima, T.; Gjertson, D.W.; Starr, J.; Eidelman, O.; Pollard, H.B.; Srivastava, M.; et al. Curcumin treatment suppresses IKKβ kinase activity of salivary cells of patients with head and neck cancer: A pilot study. Clin. Cancer Res. 2011, 17, 5953–5961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, L.; Vinayak, M. Curcumin attenuates carcinogenesis by down regulating proinflammatory cytokine interleukin-1 (IL-1α and IL-1β) via modulation of AP-1 and NF-IL6 in lymphoma bearing mice. Int. Immunopharmacol. 2014, 20, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Rujirachotiwat, A.; Suttamanatwong, S. Curcumin upregulates transforming growth factor-β1, its receptors, and vascular endothelial growth factor expressions in an in vitro human gingival fibroblast wound healing model. BMC Oral Health 2021, 21, 535. [Google Scholar] [CrossRef]
- Mani, H.; Sidhu, G.S.; Kumari, R.; Gaddipati, J.P.; Seth, P.; Maheshwari, R.K. Curcumin differentially regulates TGF-beta1, its receptors and nitric oxide synthase during impaired wound healing. Biofactors 2002, 16, 29–43. [Google Scholar] [CrossRef]
- Panchatcharam, M.; Miriyala, S.; Gayathri, V.S.; Suguna, L. Curcumin improves wound healing by modulating collagen and decreasing reactive oxygen species. Mol. Cell. Biochem. 2006, 290, 87–96. [Google Scholar] [CrossRef]
- Elad, S.; Meidan, I.; Sellam, G.; Simaan, S.; Zeevi, I.; Waldman, E.; Weintraub, M.; Revel-Vilk, S. Topical curcumin for the prevention of oral mucositis in pediatric patients: Case series. Altern. Ther. Health Med. 2013, 19, 21–24. [Google Scholar]
- Patil, K.; Guledgud, M.V.; Kulkarni, P.K.; Keshari, D.; Tayal, S. Use of Curcumin mouthrinse in radio-chemotherapy induced Oral Mucositis patients: A pilot study. J. Clin. Diagn. Res. 2015, 9, ZC59–ZC62. [Google Scholar] [CrossRef]
- Delavarian, Z.; Pakfetrat, A.; Ghazi, A.; Jaafari, M.R.; Homaei Shandiz, F.; Dalirsani, Z.; Mohammadpour, A.H.; Rahimi, H.R. Oral administration of nanomicelle curcumin in the prevention of radiotherapy-induced mucositis in head and neck cancers. Spec. Care Dent. 2019, 39, 166–172. [Google Scholar] [CrossRef]
- Ahmed, K.M. The effect of olive leaf extract in decreasing the expression of two pro-inflammatory cytokines in patients receiving chemotherapy for cancer. A randomized clinical trial. Saudi Dent. J. 2013, 25, 141–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee-Huang, S.; Huang, P.L.; Zhang, D.; Lee, J.W.; Bao, J.; Sun, Y.; Chang, Y.T.; Zhang, J.; Huang, P.L. Discovery of small-molecule HIV-1 fusion and integrase inhibitors oleuropein and hydroxytyrosol: Part I. fusion inhibition. Biochem. Biophys. Res. Commun. 2007, 354, 872–878. [Google Scholar] [CrossRef] [PubMed]
- Markin, D.; Duek, L.; Berdicevsky, I. In vitro antimicrobial activity of olive leaves. Mycoses 2003, 46, 132–136. [Google Scholar] [CrossRef] [PubMed]
- Coni, E.; Di Benedetto, R.; Di Pasquale, M.; Masella, R.; Modesti, D.; Mattei, R.; Carlini, E.A. Protective effect of oleuropein, an olive oil biophenol, on low density lipoprotein oxidizability in rabbits. Lipids 2000, 35, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Singh, I.; Mok, M.; Christensen, A.M.; Turner, A.H.; Hawley, J.A. The effects of polyphenols in olive leaves on platelet function. Nutr. Metab. Cardiovasc. Dis. 2008, 18, 127–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campolo, M.; Di Paola, R.; Impellizzeri, D.; Crupi, R.; Morittu, V.M.; Procopio, A.; Perri, E.; Britti, D.; Peli, A.; Esposito, E.; et al. Effects of a polyphenol present in olive oil, oleuropein aglycone, in a murine model of intestinal ischemia/reperfusion injury. J. Leukoc. Biol. 2013, 93, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.; Gomes, L.; Leitão, F.; Coelho, A.; Boas, L.V. Phenolic compounds and antioxidant activity of Olea europaea L. fruits and leaves. Food Sci. Technol. Int. 2006, 12, 385–395. [Google Scholar] [CrossRef]
- Koca, U.; Süntar, I.; Akkol, E.K.; Yilmazer, D.; Alper, M. Wound repair potential of Olea europaea L. leaf extracts revealed by in vivo experimental models and comparative evaluation of the extracts’ antioxidant activity. J. Med. Food 2011, 14, 140–146. [Google Scholar] [CrossRef]
- Mehraein, F.; Sarbishegi, M.; Aslani, A. Evaluation of effect of oleuropein on skin wound healing in aged male BALB/c mice. Cell J. 2014, 16, 25–30. [Google Scholar]
- Ahmed, K. Olive leaf extract as a new topical management for oral mucositis following chemotherapy: A microbiological examination, experimental animal study and clinical trial. Pharm. Anal. Acta 2013, 4, 4–9. [Google Scholar]
- Kondo, K.; Shiba, M.; Nakamura, R.; Morota, T.; Shoyama, Y. Constituent properties of licorices derived from Glycyrrhiza uralensis, G. glabra, or G. inflata identified by genetic information. Biol. Pharm. Bull. 2007, 30, 1271–1277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, V.K.; Fatima, A.; Faridi, U.; Negi, A.S.; Shanker, K.; Kumar, J.K.; Rahuja, N.; Luqman, S.; Sisodia, B.S.; Saikia, D.; et al. Antimicrobial potential of Glycyrrhiza glabra roots. J. Ethnopharmacol. 2008, 116, 377–380. [Google Scholar] [CrossRef] [PubMed]
- Saxena, R.C.; Garg, K.C.; Bhargava, K.P.; Gupta, G.P. A clinical trial of glycyrrhitinic acid in allergic conditions of the eye. J. Indian Med. Prof. 1965, 12, 5487–5490. [Google Scholar] [PubMed]
- Lv, H.; Yang, H.; Wang, Z.; Feng, H.; Deng, X.; Cheng, G.; Ci, X. Nrf2 signaling and autophagy are complementary in protecting lipopolysaccharide/d-galactosamine-induced acute liver injury by licochalcone A. Cell Death Dis. 2019, 10, 313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, Y.; Mei, L.; Wang, M.; Huang, Q.; Huang, R. Anti-inflammatory and pro-apoptotic effects of 18beta-glycyrrhetinic acid in vitro and in vivo models of rheumatoid arthritis. Front. Pharmacol. 2021, 12, 681525. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Zhang, X.; Su, X.; Liu, X.; Ren, K.; Ning, C.; Zhang, Q.; Zhang, S. Daphnes Cortex and its licorice-processed products suppress inflammation via the TLR4/NF-κB/NLRP3 signaling pathway and regulation of the metabolic profile in the treatment of rheumatoid arthritis. J. Ethnopharmacol. 2022, 283, 114657. [Google Scholar] [CrossRef]
- Sadinpour, A.; Seyedi, Z.S.; Arabdolatabadi, A.; Razavi, Y.; Ajdary, M. The synergistic effect of Paeonia spp and Glycyrrhiza glabra on polycystic ovary induced in mice. Pak. J. Pharm. Sci. 2020, 33, 1665–1670. [Google Scholar]
- Hsu, Y.W.; Chen, H.Y.; Chiang, Y.F.; Chang, L.C.; Lin, P.H.; Hsia, S.M. The effects of isoliquiritigenin on endometriosis in vivo and in vitro study. Phytomedicine 2020, 77, 153214. [Google Scholar] [CrossRef]
- Zadeh, J.B.; Kor, Z.M.; Goftar, M.K. Licorice (Glycyrrhiza glabra Linn) as a valuable medicinal plant. Int. J. Adv. Biol. Biomed. Res. 2013, 1, 1281–1288. [Google Scholar]
- Pastorino, G.; Cornara, L.; Soares, S.; Rodrigues, F.; Oliveira, M. Liquorice (Glycyrrhiza glabra): A phytochemical and pharmacological review. Phytother. Res. 2018, 32, 2323–2339. [Google Scholar] [CrossRef]
- Vispute, S.; Khopade, A. Glycyrrhiza glabra Linn.-“Klitaka”: A Review. Int. J. Pharma Bio Sci. 2011, 2, 42–51. [Google Scholar]
- Damle, M. Glycyrrhiza glabra (Liquorice)-a potent medicinal herbInt. J. Herb. Med. 2014, 2, 132–136. [Google Scholar]
- Kaur, R.; Kaur, H.; Dhindsa, A.S. Glycyrrhiza glabra: A phytopharmacological review. Int. J. Pharm. Sci. Res. 2013, 4, 2470. [Google Scholar]
- Tewari, D.; Mocan, A.; Parvanov, E.D.; Sah, A.N.; Nabavi, S.M.; Huminiecki, L.; Ma, Z.F.; Lee, Y.Y.; Horbańczuk, J.O.; Atanasov, A.G. Ethnopharmacological approaches for therapy of jaundice: Part II. Highly used plant species from Acanthaceae, Euphorbiaceae, Asteraceae, Combretaceae, and Fabaceae families. Front. Pharmacol. 2017, 8, 519. [Google Scholar] [CrossRef] [Green Version]
- El-Saber Batiha, G.; Magdy Beshbishy, A.; El-Mleeh, A.; Abdel-Daim, M.M.; Prasad Devkota, H. Traditional uses, bioactive chemical constituents, and pharmacological and toxicological activities of Glycyrrhiza glabra L. (Fabaceae). Biomolecules 2020, 10, 352. [Google Scholar] [CrossRef] [Green Version]
- Najafi, S.; Koujan, S.E.; Manifar, S.; Kharazifard, M.J.; Kidi, S.; Hajheidary, S. Preventive effect of Glycyrrhiza glabra extract on oral mucositis in patients under head and neck radiotherapy: A randomized clinical trial. J. Dent. 2017, 14, 267–274. [Google Scholar]
- Lee, S.H.; Bae, I.H.; Choi, H.; Choi, H.W.; Oh, S.; Marinho, P.A.; Min, D.J.; Kim, D.Y.; Lee, T.R.; Lee, C.S.; et al. Ameliorating effect of dipotassium glycyrrhizinate on an IL-4- and IL-13-induced atopic dermatitis-like skin-equivalent model. Arch. Dermatol. Res. 2019, 311, 131–140. [Google Scholar] [CrossRef]
- Shim, J.Y.; Yim, S.B.; Chung, J.H.; Hong, K.S. Antiplaque and antigingivitis effects of a mouthrinse containing cetylpyridinium chloride, triclosan and dipotassium glycyrrhizinate. J. Periodontal Implant Sci. 2012, 42, 33–38. [Google Scholar] [CrossRef]
- Vitali, R.; Palone, F.; Cucchiara, S.; Negroni, A.; Cavone, L.; Costanzo, M.; Aloi, M.; Dilillo, A.; Stronati, L. Dipotassium glycyrrhizate inhibits hmgb1-dependent inflammation and ameliorates colitis in mice. PLoS ONE 2013, 8, e66527. [Google Scholar] [CrossRef] [Green Version]
- Okimasu, E.; Moromizato, Y.; Watanabe, S.; Sasaki, J.; Shiraishi, N.; Morimoto, Y.M.; Miyahara, M.; Utsumi, K. Inhibition of phospholipase A2 and platelet aggregation by glycyrrhizin, an antiinflammation drug. Acta Med. Okayama 1983, 37, 385–391. [Google Scholar]
- Leite, C.D.S.; Pires, O.C.; Tenis, D.G.; Ziegler, J.V.N.; Priolli, D.G.; Rocha, T. Effects of dipotassium glycyrrhizinate on wound healing. Acta Cir. Bras. 2021, 36, e360801. [Google Scholar] [CrossRef] [PubMed]
- Harwansh, R.; Patra, K. Pharmacological studies on Glycyrrhiza glabra. Pharmacologyonline 2011, 2, 1032–1038. [Google Scholar]
- Bhattacharjee, S.; Bhattacharjee, A.; Majumder, S.; Majumdar, S.B.; Majumdar, S. Glycyrrhizic acid suppresses Cox-2-mediated anti-inflammatory responses during Leishmania donovani infection. J. Antimicrob. Chemother. 2012, 67, 1905–1914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richard, S.A. Exploring the pivotal immunomodulatory and anti-inflammatory potentials of glycyrrhizic and glycyrrhetinic acids. Mediat. Inflamm. 2021, 2021, 6699560. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, T. The protective effect of glycyrrhizin on anti-Fas antibody-induced hepatitis in mice. Eur. J. Pharmacol. 2000, 387, 229–232. [Google Scholar] [CrossRef]
- Abe, N.; Ebina, T.; Ishida, N. Interferon induction by glycyrrhizin and glycyrrhetinic acid in mice. Microbiol. Immunol. 1982, 26, 535–539. [Google Scholar] [CrossRef] [Green Version]
- Pakravan, F.; Salehabad, N.H.; Karimi, F.; Isfahani, M. N, Comparative study of the effect of licorice muco-adhesive film on radiotherapy induced oral mucositis, a randomized controlled clinical trial. Gulf J. Oncol. 2021, 1, 42–47. [Google Scholar]
- Sattari, A.; Shariati, A.; Maram, N.; Ehsanpour, A.; Maraghi, E. Comparative study of the effect of licorice root extract mouthwash and combined mouthwash on the incidence and severity of chemotherapy-induced mucositis symptoms in colon cancer patients admitted to intensive care units. Jundishapur J. Health Sci. 2019; in press. [Google Scholar] [CrossRef]
- Martins, M.D.; Marques, M.M.; Bussadori, S.K.; Martins, M.A.; Pavesi, V.C.; Mesquita-Ferrari, R.A.; Fernandes, K.P. Comparative analysis between Chamomilla recutita and corticosteroids on wound healing. An in vitro and in vivo study. Phytother. Res. 2009, 23, 274–278. [Google Scholar] [CrossRef]
- Braga, F.T.M.M.; Santos, A.C.F.; Bueno, P.C.P.; Silveira, R.C.C.P.; Santos, C.B.; Bastos, J.K.; Carvalho, E.C. Use of Chamomilla recutita in the prevention and treatment of oral mucositis in patients undergoing hematopoietic stem cell transplantation: A randomized, controlled, phase ii clinical trial. Cancer Nurs. 2015, 38, 322–329. [Google Scholar] [CrossRef]
- Srivastava, J.K.; Shankar, E.; Gupta, S. Chamomile: A herbal medicine of the past with bright future. Mol. Med. Rep. 2010, 3, 895–901. [Google Scholar] [PubMed]
- Srivastava, J.K.; Pandey, M.; Gupta, S. Chamomile, a novel and selective COX-2 inhibitor with anti-inflammatory activity. Life Sci. 2009, 85, 663–669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smolinski, A.T.; Pestka, J.J. Modulation of lipopolysaccharide-induced proinflammatory cytokine production in vitro and in vivo by the herbal constituents apigenin (chamomile), ginsenoside Rb(1) (ginseng) and parthenolide (feverfew). Food Chem. Toxicol. 2003, 41, 1381–1390. [Google Scholar] [CrossRef]
- dos Reis, P.E.D.; Ciol, M.A.; de Melo, N.S.; de Souza Figueiredo, P.T.; Leite, A.F.; de Melo Manzi, N. Chamomile infusion cryotherapy to prevent oral mucositis induced by chemotherapy: A pilot study. Support. Care Cancer 2016, 24, 4393–4398. [Google Scholar] [CrossRef] [PubMed]
- Shabanloei, R.; Ahmadi, F.; Vaez, J.; Ansarin, K.; Hajizadeh, E.; Javadzadeh, Y.; Dolathkhah, R.; Gholchin, M. Alloporinol, chamomile and normal saline mouthwashes for the prevention of chemotherapy-induced stomatitis. J. Clin. Diagn. Res. 2009, 3, 1537–1542. [Google Scholar]
- Muley, B.; Khadabadi, S.S.; Banarase, N. Phytochemical constituents and pharmacological activities of Calendula officinalis Linn (Asteraceae): A review. Trop. J. Pharm. Res. 2009, 8, 455–465. [Google Scholar] [CrossRef] [Green Version]
- Hadfield, R.A.; Vlahovic, T.C.; Khan, M.T. The use of marigold therapy for podiatric skin conditions. Foot Ankle J. 2008, 1, 1–8. [Google Scholar] [CrossRef]
- Tanideh, N.; Tavakoli, P.; Saghiri, M.A.; Garcia-Godoy, F.; Amanat, D.; Tadbir, A.A.; Samani, S.M.; Tamadon, A. Healing acceleration in hamsters of oral mucositis induced by 5-fluorouracil with topical Calendula officinalis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2013, 115, 332–338. [Google Scholar] [CrossRef]
- Fronza, M.; Heinzmann, B.; Hamburger, M.; Laufer, S.; Merfort, I. Determination of the wound healing effect of Calendula extracts using the scratch assay with 3T3 fibroblasts. J. Ethnopharmacol. 2009, 126, 463–467. [Google Scholar] [CrossRef]
- Dzubak, P.; Hajduch, M.; Vydra, D.; Hustova, A.; Kvasnica, M.; Biedermann, D.; Markova, L.; Urban, M.; Sarek, J. Pharmacological activities of natural triterpenoids and their therapeutic implications. Nat. Prod. Rep. 2006, 23, 394–411. [Google Scholar] [CrossRef]
- Givol, O.; Kornhaber, R.; Visentin, D.; Cleary, M.; Haik, J.; Harats, M. A systematic review of Calendula officinalis extract for wound healing. Wound Repair Regen. 2019, 27, 548–561. [Google Scholar] [CrossRef] [PubMed]
- Middleton, E., Jr.; Kandaswami, C.; Theoharides, T.C. The effects of plant flavonoids on mammalian cells: Implications for inflammation, heart disease, and cancer. Pharmacol. Rev. 2000, 52, 673–751. [Google Scholar] [PubMed]
- Babaee, N.; Moslemi, D.; Khalilpour, M.; Vejdani, F.; Moghadamnia, Y.; Bijani, A.; Baradaran, M.; Kazemi, M.T.; Khalilpour, A.; Pouramir, M.; et al. Antioxidant capacity of Calendula officinalis flowers extract and prevention of radiation induced oropharyngeal mucositis in patients with head and neck cancers: A randomized controlled clinical study. Daru 2013, 21, 18, (Iranian Registry of Clinical Trials: 201106076734N1). [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maddocks-Jennings, W.; Wilkinson, J.M.; Cavanagh, H.M.; Shillington, D. Evaluating the effects of the essential oils Leptospermum scoparium (manuka) and Kunzea ericoides (kanuka) on radiotherapy induced mucositis: A randomized, placebo controlled feasibility study. Eur. J. Oncol. Nurs. 2009, 13, 87–93. [Google Scholar] [CrossRef]
- You, W.C.; Hsieh, C.C.; Huang, J.T. Effect of extracts from indigowood root (Isatis indigotica Fort.) on immune responses in radiation-induced mucositis. J. Altern. Complement. Med. 2009, 15, 771–778. [Google Scholar] [CrossRef]
- Loo, W.T.; Jin, L.J.; Chow, L.W.; Cheung, M.N.; Wang, M. Rhodiola algida improves chemotherapy-induced oral mucositis in breast cancer patients. Expert Opin. Investig. Drugs 2010, 19, S91–S100. [Google Scholar] [CrossRef] [PubMed]
- Mutluay Yayla, E.; Izgu, N.; Ozdemir, L.; Aslan Erdem, S.; Kartal, M. Sage tea-thyme-peppermint hydrosol oral rinse reduces chemotherapy-induced oral mucositis: A randomized controlled pilot study. Complement. Ther. Med. 2016, 27, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Tanideh, N.; Badie, A.D.; Habibagahi, R.; Koohi-Hosseinabadi, O.; Haghnegahdar, S.; Andisheh-tadbir, A. Effect of topical 2% eucalyptus extract on 5-fu-induced oral mucositis in male golden hamsters. Braz. Dent. J. 2020, 31, 310–318. [Google Scholar] [CrossRef]
- Koohi-Hosseinabadi, O.; Andisheh-Tadbir, A.; Bahadori, P.; Sepehrimanesh, M.; Mardani, M.; Tanideh, N. Comparison of the therapeutic effects of the dietary and topical forms of Zizyphus jujuba extract on oral mucositis induced by 5-fluorouracil: A golden hamster model. J. Clin. Exp. Dent. 2015, 7, e304–e309. [Google Scholar] [CrossRef] [Green Version]
- Aghamohammadi, A.; Moslemi, D.; Akbari, J.; Ghasemi, A.; Azadbakht, M.; Asgharpour, A.; Hosseinimehr, S.J. The effectiveness of Zataria extract mouthwash for the management of radiation-induced oral mucositis in patients: A randomized placebo-controlled double-blind study. Clin. Oral Investig. 2018, 22, 2263–2272. [Google Scholar] [CrossRef]
- Soares, A.D.S.; Wanzeler, A.M.V.; Cavalcante, G.H.S.; Barros, E.; Carneiro, R.C.M.; Tuji, F.M. Therapeutic effects of andiroba (Carapa guianensis Aubl) oil, compared to low power laser, on oral mucositis in children underwent chemotherapy: A clinical study. J. Ethnopharmacol. 2021, 264, 113365. [Google Scholar] [CrossRef] [PubMed]
- Wanzeler, A.M.V.; Júnior, S.M.A.; Gomes, J.T.; Gouveia, E.H.H.; Henriques, H.Y.B.; Chaves, R.H.; Soares, B.M.; Salgado, H.L.C.; Santos, A.S.; Tuji, F.M. Therapeutic effect of andiroba oil (Carapa guianensis Aubl.) against oral mucositis: An experimental study in golden Syrian hamsters. Clin. Oral Investig. 2018, 22, 2069–2079. [Google Scholar] [CrossRef] [PubMed]
- Hasheminasab, F.S.; Hashemi, S.M.; Dehghan, A.; Sharififar, F.; Setayesh, M.; Sasanpour, P.; Tasbandi, M.; Raeiszadeh, M. Effects of a Plantago ovata-based herbal compound in prevention and treatment of oral mucositis in patients with breast cancer receiving chemotherapy: A double-blind, randomized, controlled crossover trial. J. Integr. Med. 2020, 18, 214–221, (Iranian Registry of Clinical Trials IRCT: 20180923041093N1). [Google Scholar] [CrossRef] [PubMed]
- Bertoglio, J.C.; Folatre, I.; Bombardelli, E.; Riva, A.; Morazzoni, P.; Ronchi, M.; Petrangolini, G. Management of gastrointestinal mucositis due to cancer therapies in pediatric patients: Results of a case series with SAMITAL®. Future Oncol. 2012, 8, 1481–1486. [Google Scholar] [CrossRef] [PubMed]
- Davarmanesh, M.; Miri, R.; Haghnegahdar, S.; Tadbir, A.A.; Tanideh, N.; Saghiri, M.A.; Garcia-Godoy, F.; Asatourian, A. Protective effect of bilberry extract as a pretreatment on induced oral mucositis in hamsters. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2013, 116, 702–708. [Google Scholar] [CrossRef]
- Morazzoni, P.; Petrangolini, G.; Bombardelli, E.; Ronchi, M.; Cabri, W.; Riva, A. SAMITAL®: A new botanical drug for the treatment of mucositis induced by oncological therapies. Future Oncol. 2013, 9, 1717–1725. [Google Scholar] [CrossRef]
- Mardani, M.; Afra, S.M.; Tanideh, N.; Tadbir, A.A.; Modarresi, F.; Koohi-Hosseinabadi, O.; Iraji, A.; Sepehrimanesh, M. Hydroalcoholic extract of Carum carvi L. in oral mucositis: A clinical trial in male golden hamsters. Oral Dis. 2016, 22, 39–45. [Google Scholar] [CrossRef]
- Tanideh, N.; Davarmanesh, M.; Andisheh-Tadbir, A.; Ranjbar, Z.; Mehriar, P.; Koohi-Hosseinabadi, O. Healing acceleration of oral mucositis induced by 5-fluorouracil with Pistacia atlantica (bene) essential oil in hamsters. J. Oral Pathol. Med. 2017, 46, 725–730. [Google Scholar] [CrossRef]
- Tanideh, N.; Zareh, A.A.; Fani, M.M.; Mardani, M.; Farrokhi, F.; Talati, A.; Koohi Hosseinabadi, O.; Kamali, M. Evaluation of the effect of a topical gel form of Pistacia atlantica and Trachyspermum ammi on induced oral mucositis in male golden hamsters by bio-marker indices and stereological assessment. J. Dent. 2019, 20, 240–248. [Google Scholar]
- Tanideh, N.; Namazi, F.; Andisheh Tadbir, A.; Ebrahimi, H.; Koohi-Hosseinabadi, O. Comparative assessment of the therapeutic effects of the topical and systemic forms of Hypericum perforatum extract on induced oral mucositis in golden hamsters. Int. J. Oral Maxillofac. Surg. 2014, 43, 1286–1292. [Google Scholar] [CrossRef]
- Koohi-Hosseinabadi, O.; Ranjbar, Z.; Sepehrimanesh, M.; AndisheTadbir, A.; Poorbaghi, S.L.; Bahranifard, H.; Tanideh, N.; Koohi-Hosseinabadi, M.; Iraji, A. Biochemical, hematological, and pathological related healing effects of Elaeagnus angustifolia hydroalcoholic extract in 5-fluorouracil-induced oral mucositis in male golden hamster. Environ. Sci. Pollut. Res. Int. 2017, 24, 24447–24453. [Google Scholar] [CrossRef] [PubMed]
- Kuduban, O.; Mazlumoglu, M.R.; Kuduban, S.D.; Erhan, E.; Cetin, N.; Kukula, O.; Yarali, O.; Cimen, F.K.; Cankaya, M. The effect of Hippophae rhamnoides extract on oral mucositis induced in rats with methotrexate. J. Appl. Oral Sci. 2016, 24, 423–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erhan, E.; Terzi, S.; Celiker, M.; Yarali, O.; Cankaya, M.; Cimen, F.K.; Malkoc, I.; Suleyman, B. Effect of Hippophae rhamnoides extract on oxidative oropharyngeal mucosal damage induced in rats using methotrexate. Clin. Exp. Otorhinolaryngol. 2017, 10, 181–187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
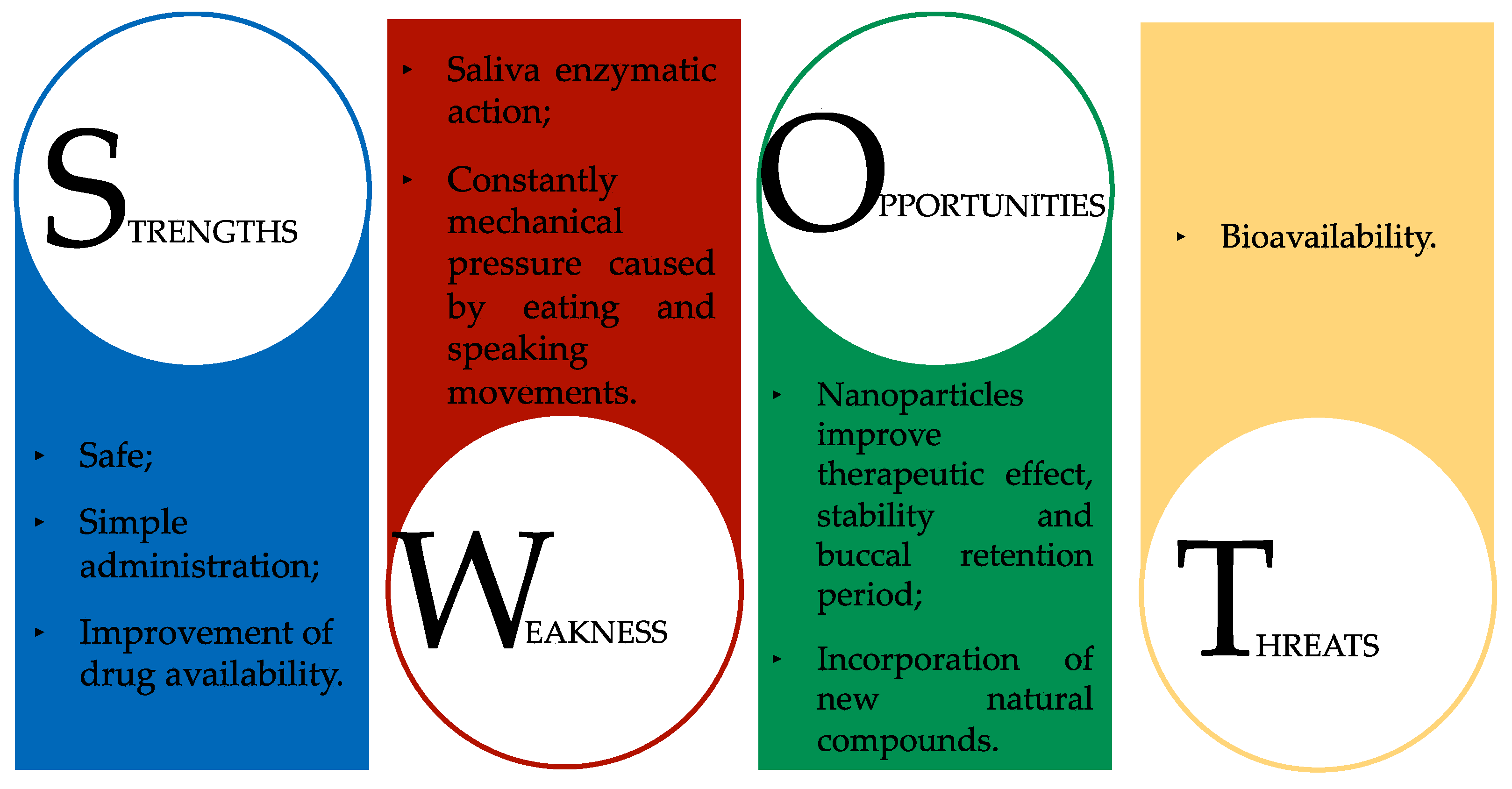
- Shahiwala, A. Applications of Polymers in Buccal Drug Delivery. In Applications of Polymers in Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2021; pp. 43–76. [Google Scholar]
- Sandri, G.; Ruggeri, M.; Rossi, S.; Bonferoni, M.C.; Vigani, B.; Ferrari, F. (Trans) buccal drug delivery. In Nanotechnology for Oral Drug Delivery; Academic Press: London, UK, 2020; pp. 225–250. [Google Scholar]
- Moroz, E.; Matoori, S.; Leroux, J.C. Oral delivery of macromolecular drugs: Where we are after almost 100 years of attempts. Adv. Drug Deliv. Rev. 2016, 101, 108–121. [Google Scholar] [CrossRef] [Green Version]
- Li, T.; Lalla, R.V.; Burgess, D.J. Enhanced drug loading of in situ forming gels for oral mucositis pain control. Int. J. Pharm. 2021, 595, 120225. [Google Scholar] [CrossRef]
- Paderni, C.; Compilato, D.; Giannola, L.I.; Campisi, G. Oral local drug delivery and new perspectives in oral drug formulation. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012, 114, e25–e34. [Google Scholar] [CrossRef]
- Tedesco, M.P.; Monaco-Lourenco, C.A.; Carvalho, R.A. Characterization of oral disintegrating film of peanut skin extract-Potential route for buccal delivery of phenolic compounds. Int. J. Biol. Macromol. 2017, 97, 418–425. [Google Scholar] [CrossRef]
- Aksungur, P.; Sungur, A.; Unal, S.; Iskit, A.B.; Squier, C.A.; Senel, S. Chitosan delivery systems for the treatment of oral mucositis: In Vitro and in vivo studies. J. Control. Release 2004, 98, 269–279. [Google Scholar] [CrossRef]
- Fonseca-Santos, B.; Chorilli, M. An overview of polymeric dosage forms in buccal drug delivery: State of art, design of formulations and their in vivo performance evaluation. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 86, 129–143. [Google Scholar] [CrossRef] [Green Version]
- Borges, J.G.; De Carvalho, R.A. Orally disintegrating films containing propolis: Properties and release profile. J. Pharm. Sci. 2015, 104, 1431–1439. [Google Scholar] [CrossRef]
- Agrawal, U.; Sharma, R.; Gupta, M.; Vyas, S.P. Is nanotechnology a boon for oral drug delivery? Drug Discov. Today 2014, 19, 1530–1546. [Google Scholar] [CrossRef] [PubMed]
- Morantes, S.J.; Buitrago, D.M.; Ibla, J.F.; García, Y.M.; Lafaurie, G.I.; Parraga, J.E. Composites of hydrogels and nanoparticles. In Biopolymer-Based Composites; Woodhead Publishing: Duxford, UK, 2017; pp. 107–138. [Google Scholar]
- Allaker, R.P.; Yuan, Z. Nanoparticles and the control of oral biofilms. In Nanobiomaterials in Clinical Dentistry; Elsevier: Amsterdam, The Netherlands, 2019; pp. 243–275. [Google Scholar]
- Campos, J.C.; Cunha, D.; Ferreira, D.C.; Reis, S.; Costa, P.J. Oromucosal precursors of in loco hydrogels for wound-dressing and drug delivery in oral mucositis: Retain, resist, and release. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 118, 111413. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.H.; Choi, J.S.; Park, E.; Eom, M.R.; Jo, S.; Lee, M.S.; Kwon, S.K.; Lee, H. Chitosan oral patches inspired by mussel adhesion. J. Control. Release 2020, 317, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, I.; Kamiki, Y.; Makino, K. Therapeutic efficacy of rebamipide-loaded PLGA nanoparticles coated with chitosan in a mouse model for oral mucositis induced by cancer chemotherapy. Colloids Surf. B 2018, 167, 468–473. [Google Scholar] [CrossRef]
| Scale | Grade 0 | Grade 1 (Mild) | Grade 2 (Moderate) | Grade 3 (Severe) | Grade 4 (Life-Threatening) | Grade 5 (Death) |
|---|---|---|---|---|---|---|
| WHO | No findings | Oral erythema and soreness; no ulcers | Oral erythema, ulcers; solid diet tolerated | Oral ulcers; liquid diet only | Oral alimentation impossible | NA |
| CTCAE | None | Asymptomatic or mild symptoms; intervention not indicated | Moderate pain or ulcer that does not interfere with oral intake; modified diet indicated | Severe pain, interfering with oral intake | Life-threatening consequences; urgent intervention indicated | Death |
| RTOG | No change over baseline | Irritation; may experience mild pain, not requiring analgesics | Patchy mucositis that may produce an inflammatory serosanguinous discharge; may experience moderate pain requiring analgesia | Confluent, fibrinous mucositis; may include severe pain requiring narcotics | Ulceration, hemorrhage, or necrosis | NA |
| Risk Factor | Criteria | References |
|---|---|---|
| Related to patient | ||
| Age | Extremities | [9,12,25,45,56,57,58] |
| Gender | Female | [2,3,10,23] |
| Body mass index (BMI) | Low and high body mass index | [2,3,10,23] |
| Dental prosthesis | Orthodontics and prosthesis | [9,11] |
| Education | Lack of health literacy | [7,55,59,60] |
| Oral hygiene | Oral hygiene less than 2 times/day Periodontal disease | [2,3,10,23] |
| Comorbidities | Diabetes mellitus, renal and hepatic dysfunction | [16,54,61] |
| Leucocytes | Neutropenic patients are immunocompromised | [2,18,45] |
| Alcohol | Use of alcohol prior to and during treatment | [2,3,18,45] |
| Smoking | Smoking prior to and during treatment increases the severity | [5,45] |
| Genetics | Genetic polymorphisms (e.g., TNF-α) | [10,45] |
| Mucosal trauma | Sharpened teeth | [5] |
| Related to tumor | ||
| Types of cancer | Solid tumors have higher risk, mainly those located near oral cavity | [12,45,47] |
| Related to treatment | ||
| Type of treatment | 5-fluorouracil, Doxorubicin, Methotrexate, Cisplatin, Vinblastine, Mitomycin, Transtuzumabe, Docetaxel, Melphalan | [10,16,54] |
| Dose | High doses over short periods and their extension | [10,16,54] |
| Type of administration | Intravenous | [2,10,16,45,54] |
| Microbiota | ||
| Intervention | Aim | MASCC/ISOO Guideline Category | Results | References |
|---|---|---|---|---|
| Oral care | Prevention | Suggestion | Increases patient’s awareness and enhances their compliance with treatment | [5,37,46,60,63,67,68,69] |
| Oral cryotherapy | Prevention | Recommendation | Local vasoconstriction that minimizes drug absorption | [11,46,70,71,72,73] |
| Photobiomodulation therapy | Prevention | Recommendation | Promotes wound healing and has an anti-inflammatory effect | [23,26,39,40,41,46] |
| Benzydamine mouthwash | Prevention | Recommendation | Anti-inflammatory properties by inhibiting the production of pro-inflammatory cytokines | [46,53,54,74,75,76] |
| Keratinocyte growth factor-1 (palifermin) | Prevention | Recommendation | Proliferation and restoration of epithelial cells | [26,27,46] |
| Glutamine | Prevention | Suggestion | It is used by cells of the immune system | [28,30,34,46] |
| Honey | Prevention | Suggestion | Inhibits bacterial growth and enhances healing rate | [32,35,37,38,46,77] |
| Patient-controlled analgesia (e.g., 0.2% morphine mouthwash) | Treatment | Recommendation | Pain management | [46,78,79] |
| Zinc supplements | Prevention | Suggestion | Prevents lipids peroxidation and replaces redox-reactive metals | [28,30,46] |
| Doxepin mouthwash | Treatment | Suggestion | In topical application, it has analgesic and anesthetic properties | [46,78,80] |
| Vitamin E | Prevention | Suggestion | Antioxidant that may protect tissue damage from free oxygen radicals | [28,30,31,46,73] |
| Amifostine | Prevention | Suggestion | Reduces DNA strand breaks, recruits ROS scavengers, and preserves salivary glands, endothelium, and connective tissue integrity | [4,33,46] |
| Name | Properties/Mechanisms | Application | Experimental Setting/Model | References |
|---|---|---|---|---|
| Manuka (Leptospermum scoparium) essential oil | Anti-inflammatory, analgesic, antimycotic, and antibacterial | Mouthwash | Randomized placebo-controlled trial | [235] |
| Kanuka (Kunzea ericoides) essential oil | Anti-inflammatory, analgesic, antimycotic, and antibacterial | Mouthwash | Randomized placebo-controlled trial | [235] |
| Indigo root (Isatis indigotica) | Anti-inflammatory and antiviral | Mouthwash | Randomized clinical trial | [236] |
| Rhodiola algida | Immunomodulatory effects | Mouthwash | Randomized clinical trial | [237] |
| Thymus spp. L | Antiseptic, anti-inflammatory, antimicrobial, and antimycotic | Mouthwash | Randomized pilot study | [238] |
| Eucalyptus | Antibacterial, antiviral, antifungal, anti-inflammatory, analgesic, and antioxidant | Topical gel | Hamsters | [239] |
| Zizyphus jujuba | Anti-inflammatory, analgesic, and wound healing | Topical gel and dietary | Hamsters | [240] |
| Zataria multiflora | Carminative, stimulant, diaphoretic, diuretic, antiseptic, anesthetic, antispasmodic, anti-hermitic, antidiarrheal, and analgesic | Mouthwash | Randomized clinical trial | [241] |
| Carapa guianensis oil | Anti-inflammatory, analgesic, and antimicrobial | Topical gel/swab | Controlled and randomized clinical trial/ hamsters | [242,243] |
| Plantago ovata | Antioxidant, anti-inflammatory, and antibacterial | Mouthwash | Randomized cross-over clinical trial | [244] |
| Achillea millefolium | Antimicrobial and anti-inflammatory | Mouthwash | Double-blind, randomized, controlled trial | [82] |
| Vaccinium myrtillus | Antioxidant, cardioprotective, neuroprotective, anti-inflammatory, and anticarcinogenic | Topical application, gavage administration, mouthwash | Clinical trials, Hamsters | [245,246,247] |
| Carum carvi | Antioxidant, antidiabetic, antifungal, and antimicrobial | Topical gel | Hamsters | [248] |
| Pistacia atlantica | Antioxidant and anti-inflammatory | Topical gel | Hamsters | [249,250] |
| Hypericum perforatum | Antioxidant and anti-inflammatory | Topical gel | Hamsters | [251] |
| Elaeagnus angustifolia | Anti-inflammatory, analgesic, and wound healing | Topical gel | Hamster | [252] |
| Trachyspermum ammi | Anti-inflammatory, antiviral, antifungal, antioxidant, and analgesic | Topical gel | Hamsters | [250] |
| Hippophae rhamnoides | Antioxidant, anti-inflammatory, antimicrobial, and anti-ulcerogenic | Gavage administration | Rats | [253,254] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira, A.S.; Macedo, C.; Silva, A.M.; Delerue-Matos, C.; Costa, P.; Rodrigues, F. Natural Products for the Prevention and Treatment of Oral Mucositis—A Review. Int. J. Mol. Sci. 2022, 23, 4385. https://doi.org/10.3390/ijms23084385
Ferreira AS, Macedo C, Silva AM, Delerue-Matos C, Costa P, Rodrigues F. Natural Products for the Prevention and Treatment of Oral Mucositis—A Review. International Journal of Molecular Sciences. 2022; 23(8):4385. https://doi.org/10.3390/ijms23084385
Chicago/Turabian StyleFerreira, Ana Sofia, Catarina Macedo, Ana Margarida Silva, Cristina Delerue-Matos, Paulo Costa, and Francisca Rodrigues. 2022. "Natural Products for the Prevention and Treatment of Oral Mucositis—A Review" International Journal of Molecular Sciences 23, no. 8: 4385. https://doi.org/10.3390/ijms23084385
APA StyleFerreira, A. S., Macedo, C., Silva, A. M., Delerue-Matos, C., Costa, P., & Rodrigues, F. (2022). Natural Products for the Prevention and Treatment of Oral Mucositis—A Review. International Journal of Molecular Sciences, 23(8), 4385. https://doi.org/10.3390/ijms23084385









