Magnetic Nanofibrous Scaffolds Accelerate the Regeneration of Muscle Tissue in Combination with Extra Magnetic Fields
Abstract
:1. Introduction
2. Results
2.1. Fabrication and Characterization of the Coaxial Electrospun Scaffolds
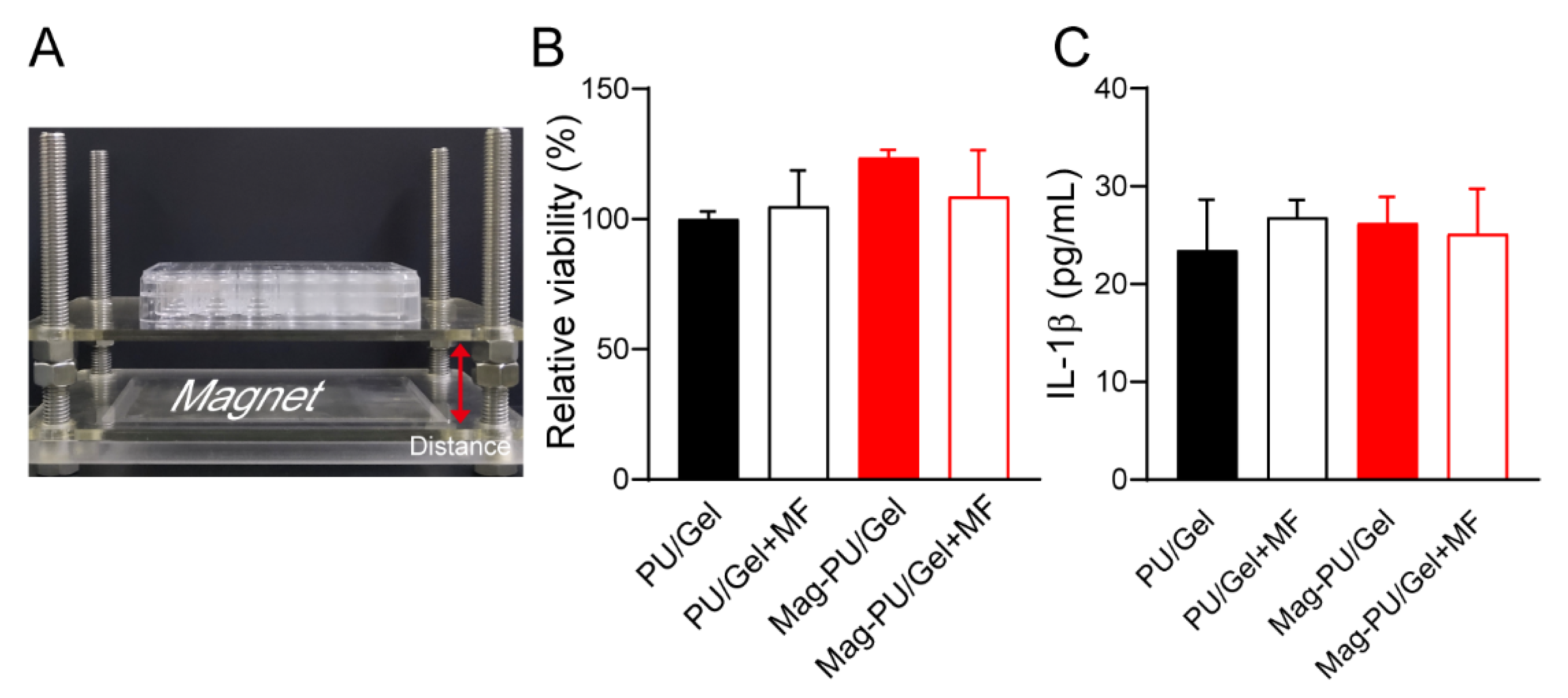
2.2. Composite Scaffolds Supported Cells Growth under the Magnetic Fields
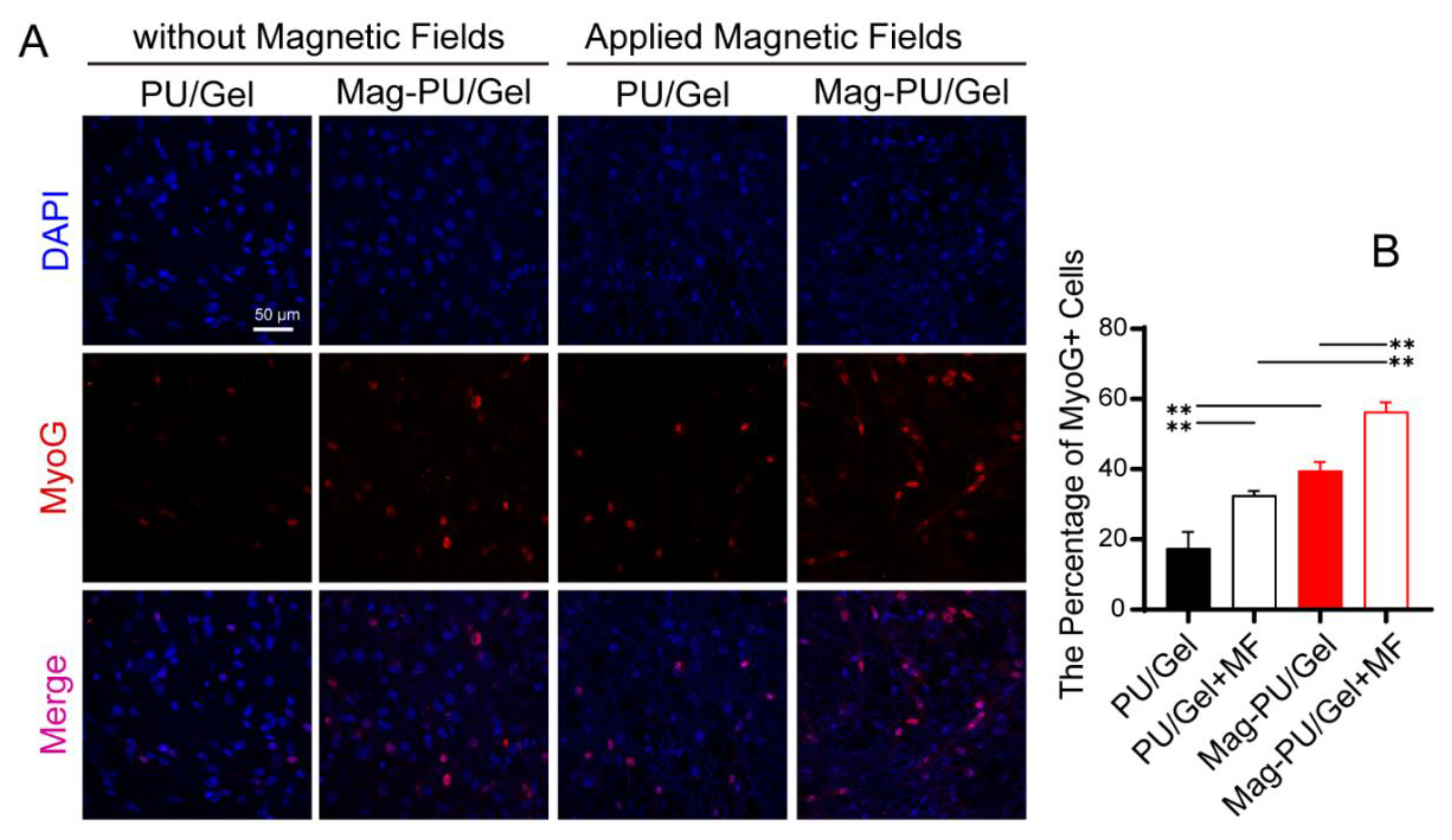
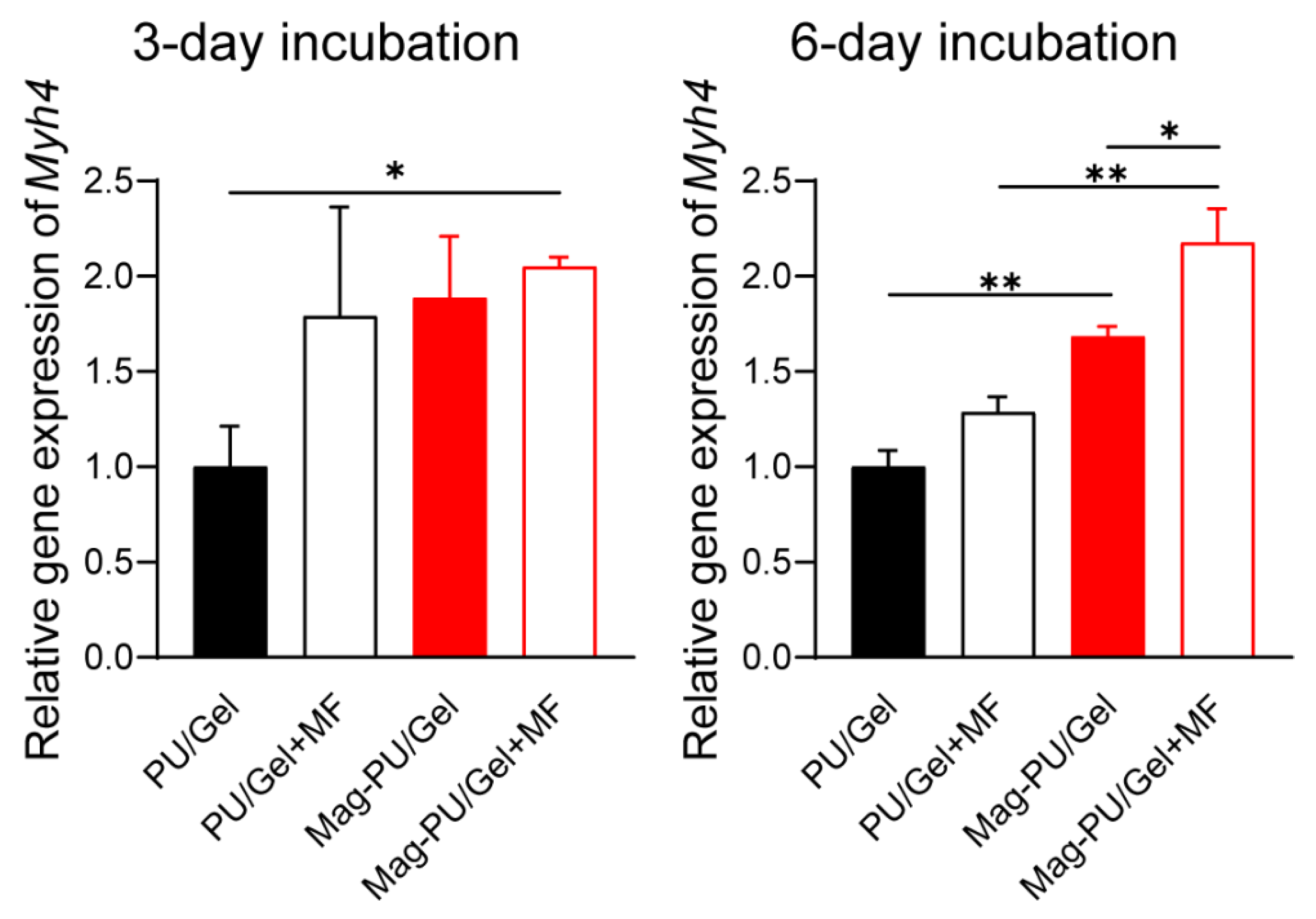
2.3. Magnetic Scaffolds Enhanced Cell Differentiation under the Magnetic Fields
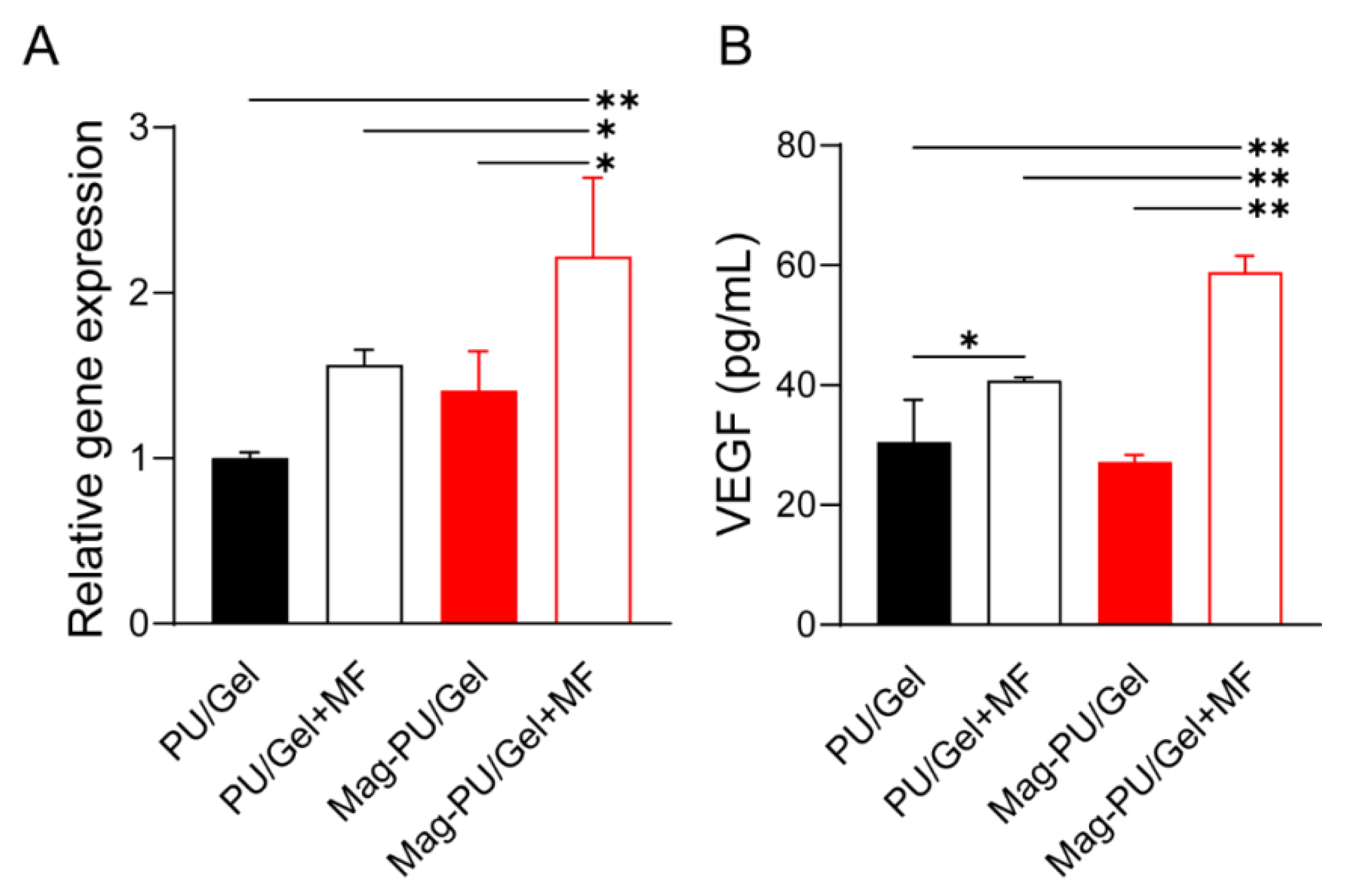
2.4. Magnetic Scaffolds Combined with the Magnetic Fields Promoted VEGF Production
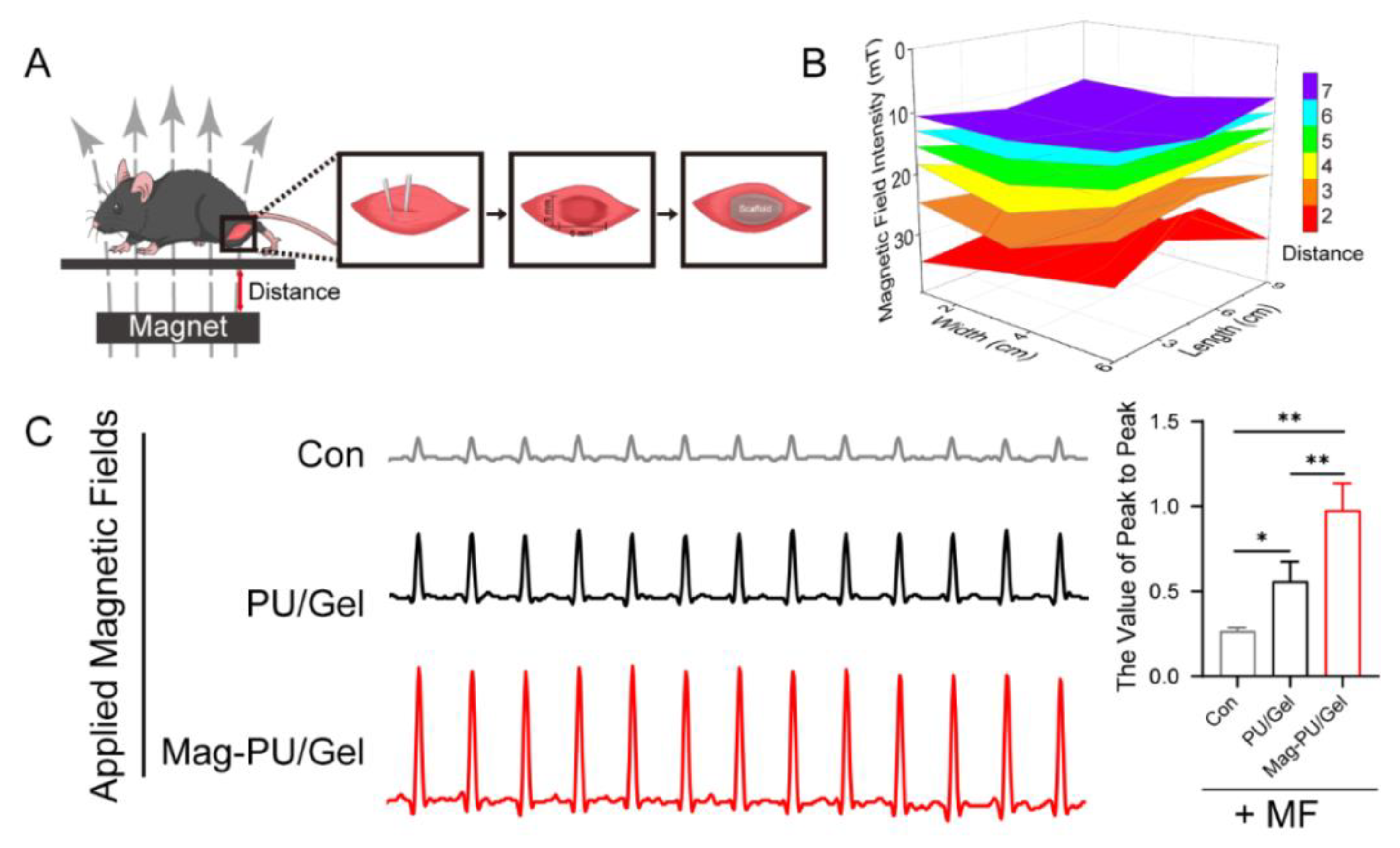
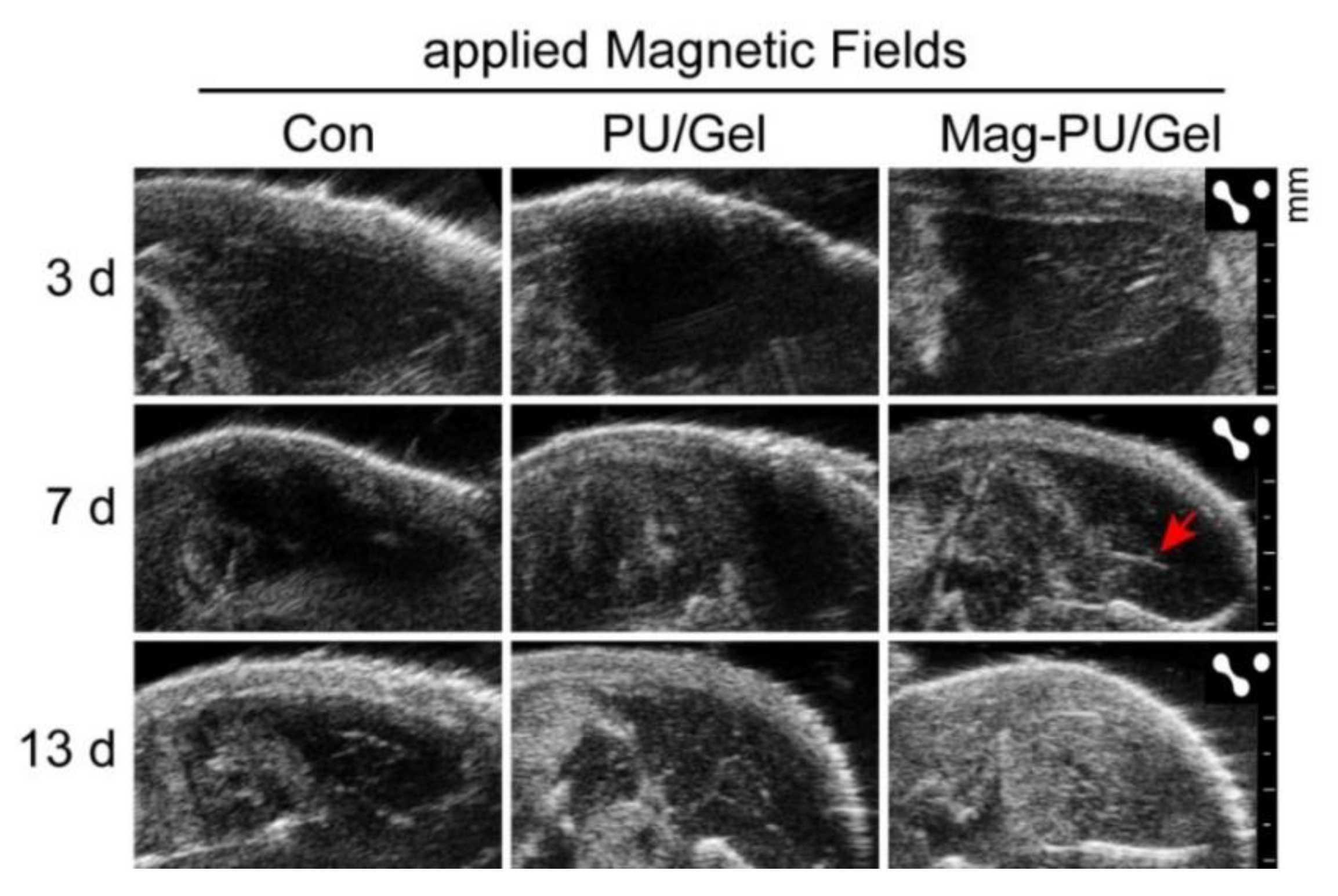
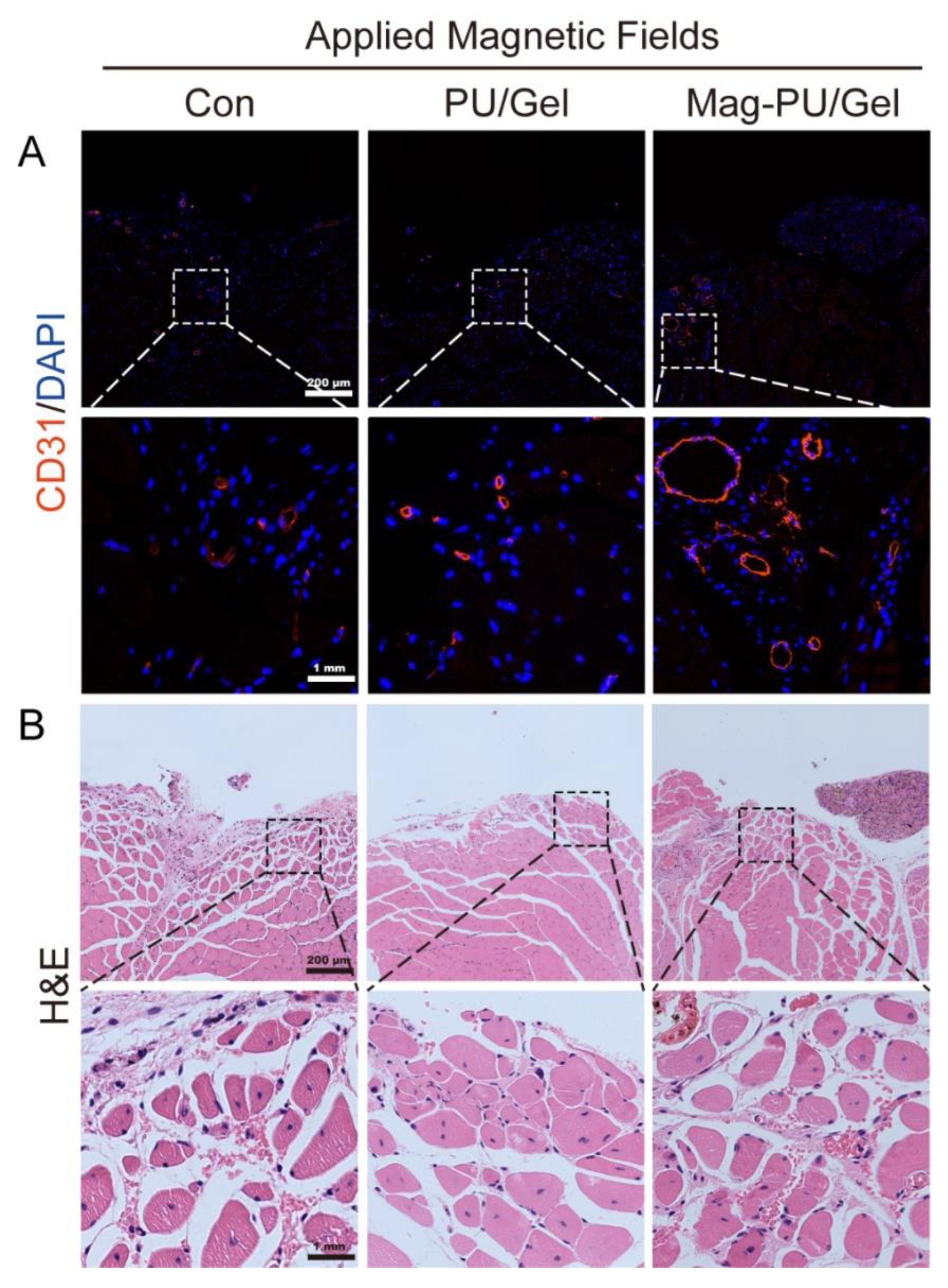
2.5. Magnetically Actuated Scaffold Accelerated the Regeneration of Muscle Tissue
3. Discussion
4. Materials and Methods
4.1. Preparation of Scaffolds
4.2. Physicochemical Characterizations
4.3. Cell Culture
4.4. Setup of Magnetic Fields for the Cell Culture System
4.5. Cell Viability Assay
4.6. Immunofluorescence Staining Assay
4.7. Quantitative Real-Time PCR (qRT-PCR) Analysis Assay
- Myh4 forward primer, 5′-TTGAAAAGACGAAGCAGCGAC-3′,
- Myh4 reverse primer, 5′-AGAGAGCGGGACTCCTTCTG-3′,
- Vegfa forward primer, 5′-GCACATAGAGAGAATGAGCTTCC-3′,
- Vegfa reverse primer, 5′-CTCCGCTCTGAACAAGGCT-3′,
- Gapdh forward primer, 5′-TGACCTCAACTACATGGTCTACA-3′,
- Gapdh reverse primer, 5′-CTTCCCATTCTCGGCCTTG-3′.
4.8. Cytokines Production Assay
4.9. Mice Skeletal Muscle Injury Model
4.10. Electrophysiological Signal Measurement
4.11. Ultrasound Imaging Assay
4.12. Hematoxylin-Eosin (H&E) Staining and Immunofluorescence Staining
4.13. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dong, R.; Ma, P.X.; Guo, B. Conductive biomaterials for muscle tissue engineering. Biomaterials 2020, 229, 119584. [Google Scholar] [CrossRef] [PubMed]
- Frontera, W.R.; Ochala, J. Skeletal muscle: A brief review of structure and function. Calcif. Tissue Int. 2015, 96, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Ma, P.X. Conducting Polymers for Tissue Engineering. Biomacromolecules 2018, 19, 1764–1782. [Google Scholar] [CrossRef] [PubMed]
- Dunn, A.; Talovic, M.; Patel, K.; Patel, A.; Marcinczyk, M.; Garg, K. Biomaterial and stem cell-based strategies for skeletal muscle regeneration. J. Orthop. Res. 2019, 37, 1246–1262. [Google Scholar] [CrossRef] [PubMed]
- Lev, R.; Seliktar, D. Hydrogel biomaterials and their therapeutic potential for muscle injuries and muscular dystrophies. J. R. Soc. Interface 2018, 15, 20170380. [Google Scholar] [CrossRef]
- Ahuja, N.; Awad, K.; Peper, S.; Brotto, M.; Varanasi, V. Mini review: Biomaterials in repair and regeneration of nerve in a volumetric muscle loss. Neurosci. Lett. 2021, 762, 136145. [Google Scholar] [CrossRef]
- Nii, T.; Katayama, Y. Biomaterial-Assisted Regenerative Medicine. Int. J. Mol. Sci. 2021, 22, 8657. [Google Scholar] [CrossRef]
- Nii, T.; Makino, K.; Tabata, Y. Three-Dimensional Culture System of Cancer Cells Combined with Biomaterials for Drug Screening. Cancers 2020, 12, 2754. [Google Scholar] [CrossRef]
- Yue, S.; He, H.; Li, B.; Hou, T. Hydrogel as a Biomaterial for Bone Tissue Engineering: A Review. Nanomaterials 2020, 10, 1511. [Google Scholar] [CrossRef]
- Groeber, F.; Holeiter, M.; Hampel, M.; Hinderer, S.; Schenke-Layland, K. Skin tissue engineering--in vivo and in vitro applications. Adv. Drug Deliv. Rev. 2011, 63, 352–366. [Google Scholar] [CrossRef]
- Kim, H.N.; Choi, N. Consideration of the Mechanical Properties of Hydrogels for Brain Tissue Engineering and Brain-on-a-chip. BioChip J. 2019, 13, 8–19. [Google Scholar] [CrossRef]
- Heher, P.; Maleiner, B.; Prüller, J.; Teuschl, A.H.; Kollmitzer, J.; Monforte, X.; Wolbank, S.; Redl, H.; Rünzler, D.; Fuchs, C. A novel bioreactor for the generation of highly aligned 3D skeletal muscle-like constructs through orientation of fibrin via application of static strain. Acta Biomater. 2015, 24, 251–265. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, T.; Hashimoto, A.; Kiyokawa, T.; Kikuchi, K.; Miyakoshi, J. Myotube orientation using strong static magnetic fields. Bioelectromagnetics 2012, 33, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Coletti, D.; Teodori, L.; Albertini, M.C.; Rocchi, M.; Pristerà, A.; Fini, M.; Molinaro, M.; Adamo, S. Static magnetic fields enhance skeletal muscle differentiation in vitro by improving myoblast alignment. Cytom. Part A 2007, 71, 846–856. [Google Scholar] [CrossRef] [PubMed]
- Jimena, I.; Tasset, I.; López-Martos, R.; Rubio, A.J.; Luque, E.; Montilla, P.; Peña, J.; Túnez, I. Effects of magnetic stimulation on oxidative stress and skeletal muscle regeneration induced by mepivacaine in rat. Med. Chem. 2009, 5, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Stölting, M.N.; Arnold, A.S.; Haralampieva, D.; Handschin, C.; Sulser, T.; Eberli, D. Magnetic stimulation supports muscle and nerve regeneration after trauma in mice. Muscle Nerve 2016, 53, 598–607. [Google Scholar] [CrossRef] [PubMed]
- Sapir-Lekhovitser, Y.; Rotenberg, M.Y.; Jopp, J.; Friedman, G.; Polyak, B.; Cohen, S. Magnetically actuated tissue engineered scaffold: Insights into mechanism of physical stimulation. Nanoscale 2016, 8, 3386–3399. [Google Scholar] [CrossRef]
- Bettini, S.; Bonfrate, V.; Valli, L.; Giancane, G. Paramagnetic Functionalization of Biocompatible Scaffolds for Biomedical Applications: A Perspective. Bioengineering 2020, 7, 153. [Google Scholar] [CrossRef]
- Meng, J.; Zhang, Y.; Qi, X.; Kong, H.; Wang, C.; Xu, Z.; Xie, S.; Gu, N.; Xu, H. Paramagnetic nanofibrous composite films enhance the osteogenic responses of pre-osteoblast cells. Nanoscale 2010, 2, 2565–2569. [Google Scholar] [CrossRef]
- Meng, J.; Xiao, B.; Zhang, Y.; Liu, J.; Xue, H.; Lei, J.; Kong, H.; Huang, Y.; Jin, Z.; Gu, N.; et al. Super-paramagnetic responsive nanofibrous scaffolds under static magnetic field enhance osteogenesis for bone repair in vivo. Sci. Rep. 2013, 3, 2655. [Google Scholar] [CrossRef] [Green Version]
- Filippi, M.; Dasen, B.; Guerrero, J.; Garello, F.; Isu, G.; Born, G.; Ehrbar, M.; Martin, I.; Scherberich, A. Magnetic nanocomposite hydrogels and static magnetic field stimulate the osteoblastic and vasculogenic profile of adipose-derived cells. Biomaterials 2019, 223, 119468. [Google Scholar] [CrossRef] [PubMed]
- Aldebs, A.I.; Zohora, F.T.; Nosoudi, N.; Singh, S.P.; Ramirez-Vick, J.E. Effect of Pulsed Electromagnetic Fields on Human Mesenchymal Stem Cells Using 3D Magnetic Scaffolds. Bioelectromagnetics 2020, 41, 175–187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandes, M.M.; Correia, D.M.; Ribeiro, C.; Castro, N.; Correia, V.; Lanceros-Mendez, S. Bioinspired Three-Dimensional Magnetoactive Scaffolds for Bone Tissue Engineering. ACS Appl. Mater. Interfaces 2019, 11, 45265–45275. [Google Scholar] [CrossRef]
- He, Y.; Yu, L.; Liu, J.; Li, Y.; Wu, Y.; Huang, Z.; Wu, D.; Wang, H.; Wu, Z.; Qiu, G. Enhanced osteogenic differentiation of human bone-derived mesenchymal stem cells in 3-dimensional printed porous titanium scaffolds by static magnetic field through up-regulating Smad4. FASEB J. 2019, 33, 6069–6081. [Google Scholar] [CrossRef] [PubMed]
- Kesse, X.; Adam, A.; Begin-Colin, S.; Mertz, D.; Larquet, E.; Gacoin, T.; Maurin, I.; Vichery, C.; Nedelec, J.M. Elaboration of Superparamagnetic and Bioactive Multicore-Shell Nanoparticles (γ-Fe2O3@SiO2-CaO): A Promising Material for Bone Cancer Treatment. ACS Appl. Mater. Interfaces 2020, 12, 47820–47830. [Google Scholar] [CrossRef] [PubMed]
- Meshkini, A.; Sistanipour, E.; Izadi, A. Mg.ATP-decorated ultrafine magnetic nanofibers: A bone scaffold with high osteogenic and antibacterial properties in the presence of an electromagnetic field. Colloids Surf. B Biointerfaces 2022, 210, 112256. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Chen, H.; Zhao, Y.; Zhang, F.; Li, X.; Wang, L.; Weir, M.D.; Ma, J.; Reynolds, M.A.; Gu, N.; et al. Novel magnetic calcium phosphate-stem cell construct with magnetic field enhances osteogenic differentiation and bone tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 98, 30–41. [Google Scholar] [CrossRef]
- Xia, Y.; Sun, J.; Zhao, L.; Zhang, F.; Liang, X.J.; Guo, Y.; Weir, M.D.; Reynolds, M.A.; Gu, N.; Xu, H.H.K. Magnetic field and nano-scaffolds with stem cells to enhance bone regeneration. Biomaterials 2018, 183, 151–170. [Google Scholar] [CrossRef]
- Zhao, Y.; Fan, T.; Chen, J.; Su, J.; Zhi, X.; Pan, P.; Zou, L.; Zhang, Q. Magnetic bioinspired micro/nanostructured composite scaffold for bone regeneration. Colloids Surf. B Biointerfaces 2019, 174, 70–79. [Google Scholar] [CrossRef]
- Zhang, C.; Cai, Y.Z.; Lin, X.J.; Wang, Y. Magnetically Actuated Manipulation and Its Applications for Cartilage Defects: Characteristics and Advanced Therapeutic Strategies. Front. Cell Dev. Biol. 2020, 8, 526. [Google Scholar] [CrossRef]
- Fuhrer, R.; Hofmann, S.; Hild, N.; Vetsch, J.R.; Herrmann, I.K.; Grass, R.N.; Stark, W.J. Pressureless mechanical induction of stem cell differentiation is dose and frequency dependent. PLoS ONE 2013, 8, e81362. [Google Scholar] [CrossRef] [PubMed]
- Sapir, Y.; Ruvinov, E.; Polyak, B.; Cohen, S. Magnetically actuated alginate scaffold: A novel platform for promoting tissue organization and vascularization. Methods Mol. Biol. 2014, 1181, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Goranov, V.; Shelyakova, T.; De Santis, R.; Haranava, Y.; Makhaniok, A.; Gloria, A.; Tampieri, A.; Russo, A.; Kon, E.; Marcacci, M.; et al. 3D Patterning of cells in Magnetic Scaffolds for Tissue Engineering. Sci. Rep. 2020, 10, 2289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sapir, Y.; Cohen, S.; Friedman, G.; Polyak, B. The promotion of in vitro vessel-like organization of endothelial cells in magnetically responsive alginate scaffolds. Biomaterials 2012, 33, 4100–4109. [Google Scholar] [CrossRef] [Green Version]
- Funnell, J.L.; Ziemba, A.M.; Nowak, J.F.; Awada, H.; Prokopiou, N.; Samuel, J.; Guari, Y.; Nottelet, B.; Gilbert, R.J. Assessing the combination of magnetic field stimulation, iron oxide nanoparticles, and aligned electrospun fibers for promoting neurite outgrowth from dorsal root ganglia in vitro. Acta Biomater. 2021, 131, 302–313. [Google Scholar] [CrossRef]
- Karimi, S.; Bagher, Z.; Najmoddin, N.; Simorgh, S.; Pezeshki-Modaress, M. Alginate-magnetic short nanofibers 3D composite hydrogel enhances the encapsulated human olfactory mucosa stem cells bioactivity for potential nerve regeneration application. Int. J. Biol. Macromol. 2021, 167, 796–806. [Google Scholar] [CrossRef]
- Liu, Z.; Zhu, S.; Liu, L.; Ge, J.; Huang, L.; Sun, Z.; Zeng, W.; Huang, J.; Luo, Z. A magnetically responsive nanocomposite scaffold combined with Schwann cells promotes sciatic nerve regeneration upon exposure to magnetic field. Int. J. Nanomed. 2017, 12, 7815–7832. [Google Scholar] [CrossRef] [Green Version]
- Tomás, A.R.; Gonçalves, A.I.; Paz, E.; Freitas, P.; Domingues, R.M.A.; Gomes, M.E. Magneto-mechanical actuation of magnetic responsive fibrous scaffolds boosts tenogenesis of human adipose stem cells. Nanoscale 2019, 11, 18255–18271. [Google Scholar] [CrossRef]
- Hao, S.; Zhang, Y.; Meng, J.; Liu, J.; Wen, T.; Gu, N.; Xu, H. Integration of a Superparamagnetic Scaffold and Magnetic Field To Enhance the Wound-Healing Phenotype of Fibroblasts. ACS Appl. Mater. Interfaces 2018, 10, 22913–22923. [Google Scholar] [CrossRef]
- Moradian, E.; Rabiee, S.M.; Haghighipour, N.; Salimi-Kenari, H. Fabrication and physicochemical characterization of a novel magnetic nanocomposite scaffold: Electromagnetic field effect on biological properties. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 116, 111222. [Google Scholar] [CrossRef]
- Wu, F.; Gao, A.; Liu, J.; Shen, Y.; Xu, P.; Meng, J.; Wen, T.; Xu, L.; Xu, H. High Modulus Conductive Hydrogels Enhance In Vitro Maturation and Contractile Function of Primary Cardiomyocytes for Uses in Drug Screening. Adv. Healthc. Mater. 2018, 7, e1800990. [Google Scholar] [CrossRef] [PubMed]
- Somers, S.M.; Spector, A.A.; DiGirolamo, D.J.; Grayson, W.L. Biophysical Stimulation for Engineering Functional Skeletal Muscle. Tissue Eng. Part B Rev. 2017, 23, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.P.; Meng, J.; Zhang, S.; Ma, X.; Wang, S. Amplified effect of surface charge on cell adhesion by nanostructures. Nanoscale 2016, 8, 12540–12543. [Google Scholar] [CrossRef] [PubMed]
- Hao, S.; Meng, J.; Zhang, Y.; Liu, J.; Nie, X.; Wu, F.; Yang, Y.; Wang, C.; Gu, N.; Xu, H. Macrophage phenotypic mechanomodulation of enhancing bone regeneration by superparamagnetic scaffold upon magnetization. Biomaterials 2017, 140, 16–25. [Google Scholar] [CrossRef]
- Grasman, J.M.; Zayas, M.J.; Page, R.L.; Pins, G.D. Biomimetic scaffolds for regeneration of volumetric muscle loss in skeletal muscle injuries. Acta Biomater. 2015, 25, 2–15. [Google Scholar] [CrossRef] [Green Version]
- Beldjilali-Labro, M.; Jellali, R.; Brown, A.D.; Garcia Garcia, A.; Lerebours, A.; Guenin, E.; Bedoui, F.; Dufresne, M.; Stewart, C.; Grosset, J.F.; et al. Multiscale-Engineered Muscle Constructs: PEG Hydrogel Micro-Patterning on an Electrospun PCL Mat Functionalized with Gold Nanoparticles. Int. J. Mol. Sci. 2021, 23, 260. [Google Scholar] [CrossRef]
- Zhao, C.; Andersen, H.; Ozyilmaz, B.; Ramaprabhu, S.; Pastorin, G.; Ho, H.K. Spontaneous and specific myogenic differentiation of human mesenchymal stem cells on polyethylene glycol-linked multi-walled carbon nanotube films for skeletal muscle engineering. Nanoscale 2015, 7, 18239–18249. [Google Scholar] [CrossRef]
- Blaauw, B.; Schiaffino, S.; Reggiani, C. Mechanisms modulating skeletal muscle phenotype. Compr. Physiol. 2013, 3, 1645–1687. [Google Scholar] [CrossRef]
- Gilbert-Honick, J.; Grayson, W. Vascularized and Innervated Skeletal Muscle Tissue Engineering. Adv. Healthc. Mater. 2020, 9, e1900626. [Google Scholar] [CrossRef]
- Latroche, C.; Weiss-Gayet, M.; Muller, L.; Gitiaux, C.; Leblanc, P.; Liot, S.; Ben-Larbi, S.; Abou-Khalil, R.; Verger, N.; Bardot, P.; et al. Coupling between Myogenesis and Angiogenesis during Skeletal Muscle Regeneration Is Stimulated by Restorative Macrophages. Stem Cell Rep. 2017, 9, 2018–2033. [Google Scholar] [CrossRef] [Green Version]
- Le Moal, E.; Pialoux, V.; Juban, G.; Groussard, C.; Zouhal, H.; Chazaud, B.; Mounier, R. Redox Control of Skeletal Muscle Regeneration. Antioxid. Redox Signal. 2017, 27, 276–310. [Google Scholar] [CrossRef] [PubMed]
- Gattazzo, F.; De Maria, C.; Rimessi, A.; Donà, S.; Braghetta, P.; Pinton, P.; Vozzi, G.; Bonaldo, P. Gelatin-genipin-based biomaterials for skeletal muscle tissue engineering. J. Biomed. Mater. Res. B Appl. Biomater. 2018, 106, 2763–2777. [Google Scholar] [CrossRef] [PubMed]
- Mitsui, R.; Matsukawa, M.; Nakagawa, K.; Isomura, E.; Kuwahara, T.; Nii, T.; Tanaka, S.; Tabata, Y. Efficient cell transplantation combining injectable hydrogels with control release of growth factors. Regen. Ther. 2021, 18, 372–383. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Chen, S.; Wang, L.; Zhang, X.; Gao, J.; Jiang, L.; Tang, D.; Zhang, L.; Midgley, A.; Kong, D.; et al. Rapid endothelialization and controlled smooth muscle regeneration by electrospun heparin-loaded polycaprolactone/gelatin hybrid vascular grafts. J. Biomed. Mater. Res. B Appl. Biomater. 2019, 107, 2040–2049. [Google Scholar] [CrossRef] [PubMed]
- Ergene, E.; Yagci, B.S.; Gokyer, S.; Eyidogan, A.; Aksoy, E.A.; Yilgor Huri, P. A novel polyurethane-based biodegradable elastomer as a promising material for skeletal muscle tissue engineering. Biomed. Mater. 2019, 14, 025014. [Google Scholar] [CrossRef]
- Park, H.; Kim, I.G.; Wu, Y.; Cho, H.; Shin, J.W.; Park, S.A.; Chung, E.J. Experimental investigation of esophageal reconstruction with electrospun polyurethane nanofiber and 3D printing polycaprolactone scaffolds using a rat model. Head Neck 2021, 43, 833–848. [Google Scholar] [CrossRef]
- Ergene, E.; Sezlev Bilecen, D.; Kaya, B.; Yilgor Huri, P.; Hasirci, V. 3D cellular alignment and biomimetic mechanical stimulation enhance human adipose-derived stem cell myogenesis. Biomed. Mater. 2020, 15, 055017. [Google Scholar] [CrossRef]
- Tajima, S.; Tabata, Y. Preparation and functional evaluation of cell aggregates incorporating gelatin microspheres with different degradabilities. J. Tissue Eng. Regen. Med. 2013, 7, 801–811. [Google Scholar] [CrossRef]
- Wosczyna, M.N.; Rando, T.A. A Muscle Stem Cell Support Group: Coordinated Cellular Responses in Muscle Regeneration. Dev. Cell 2018, 46, 135–143. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, X.; Liu, W.; Sun, L.; Xu, S.; Wang, T.; Meng, J.; Wen, T.; Liu, Q.; Liu, J.; Xu, H. Magnetic Nanofibrous Scaffolds Accelerate the Regeneration of Muscle Tissue in Combination with Extra Magnetic Fields. Int. J. Mol. Sci. 2022, 23, 4440. https://doi.org/10.3390/ijms23084440
Hu X, Liu W, Sun L, Xu S, Wang T, Meng J, Wen T, Liu Q, Liu J, Xu H. Magnetic Nanofibrous Scaffolds Accelerate the Regeneration of Muscle Tissue in Combination with Extra Magnetic Fields. International Journal of Molecular Sciences. 2022; 23(8):4440. https://doi.org/10.3390/ijms23084440
Chicago/Turabian StyleHu, Xuechun, Wenhao Liu, Lihong Sun, Shilin Xu, Tao Wang, Jie Meng, Tao Wen, Qingqiao Liu, Jian Liu, and Haiyan Xu. 2022. "Magnetic Nanofibrous Scaffolds Accelerate the Regeneration of Muscle Tissue in Combination with Extra Magnetic Fields" International Journal of Molecular Sciences 23, no. 8: 4440. https://doi.org/10.3390/ijms23084440





