A Role for Exchange of Extracellular Vesicles in Porcine Spermatogonial Co-Culture
Abstract
1. Introduction
2. Results
2.1. Establishment of Defined Feeder Cell and Spermatogonial Populations from Testis Tissue
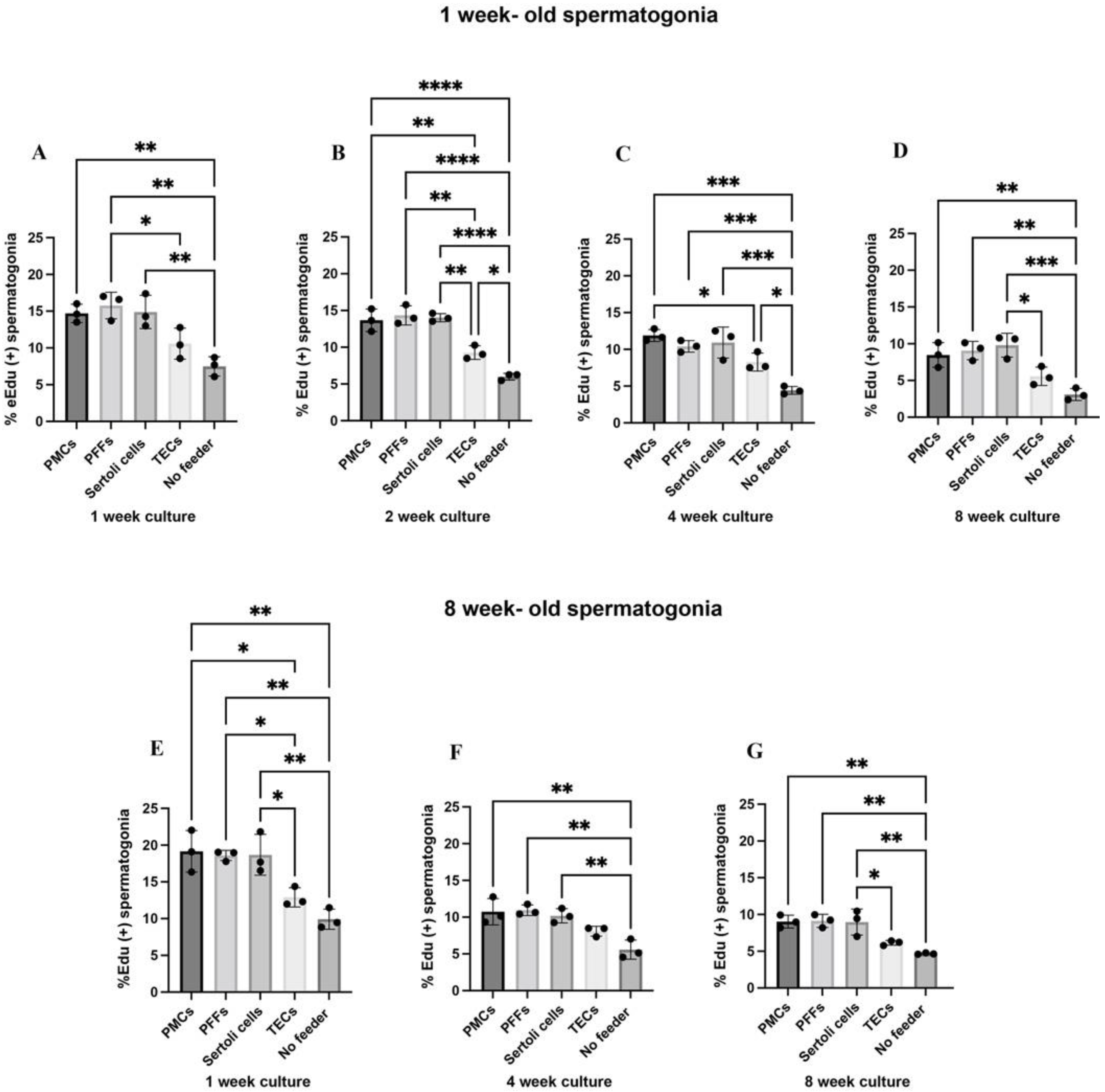
2.2. Co-Culture with PMC, PFF, and Sertoli Cell Feeder Cells Supports the In Vitro Maintenance of Spermatogonia
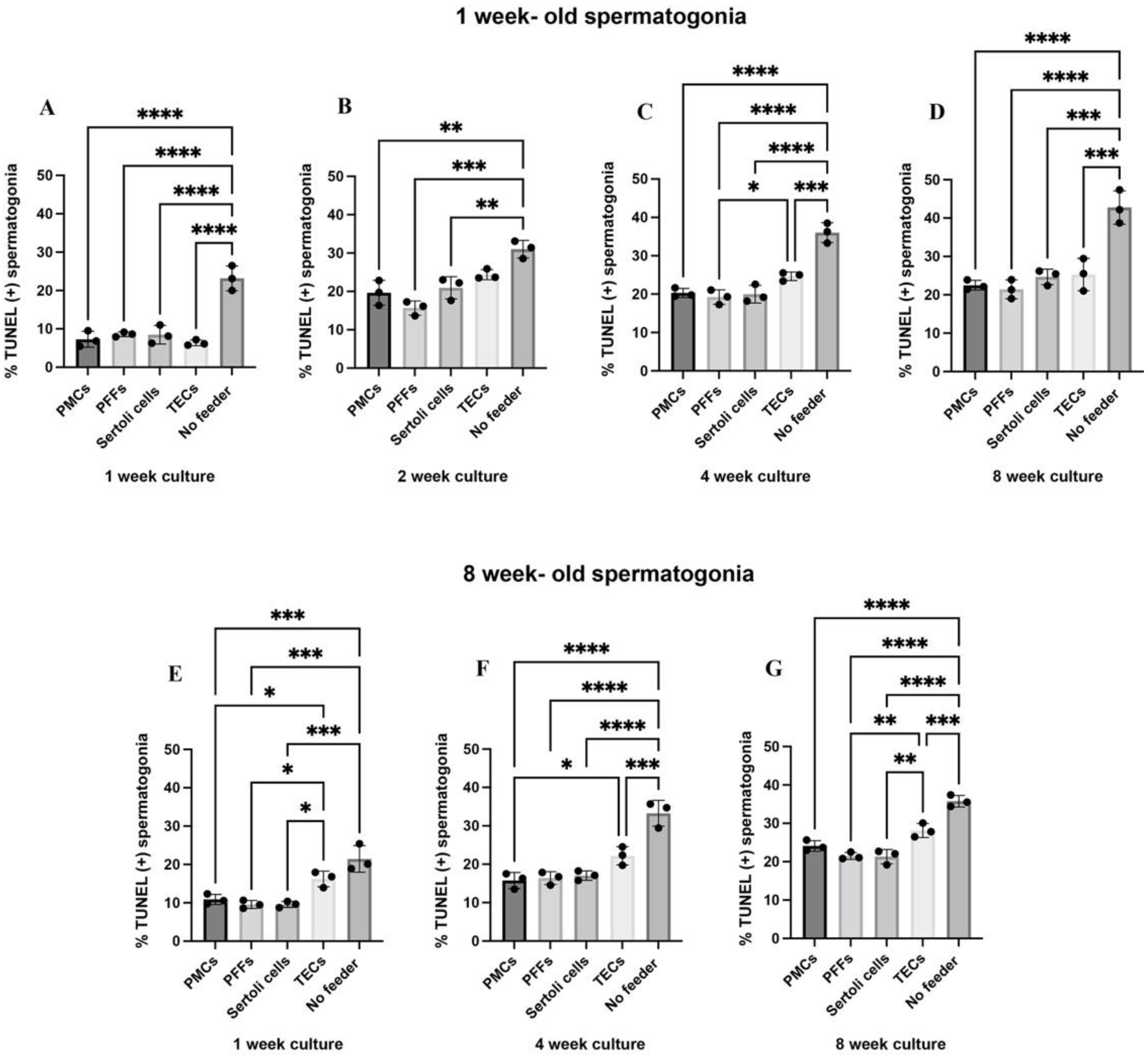
2.3. Co-Cultures with PMC, PFF, and Sertoli Cell Feeder Cells Reduce Rates of Apoptosis in Spermatogonia
2.4. Sertoli Cell Feeders Express the Highest Levels of GDNF, BMP4, and NELL2
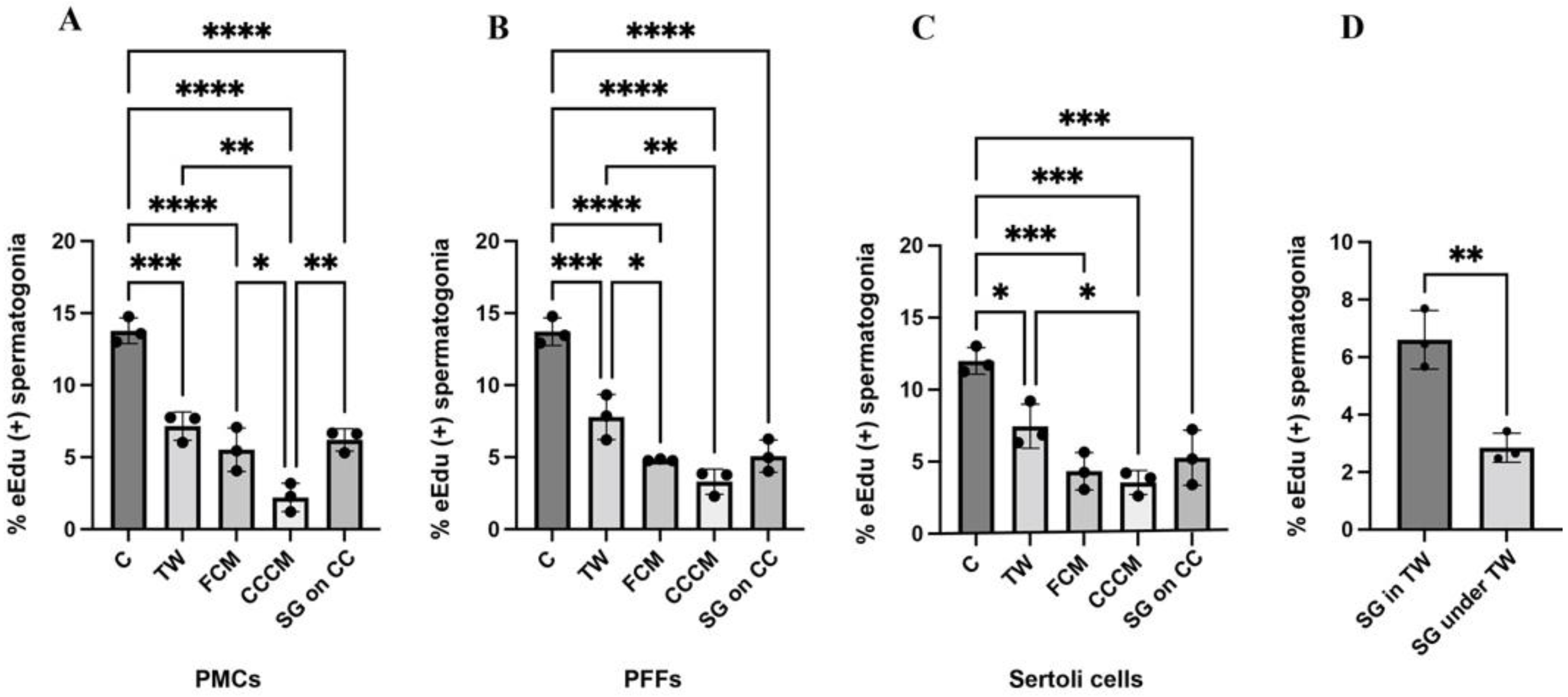
2.5. Contact Co-Cultures Are Beneficial for the Proliferation of Porcine Spermatogonia
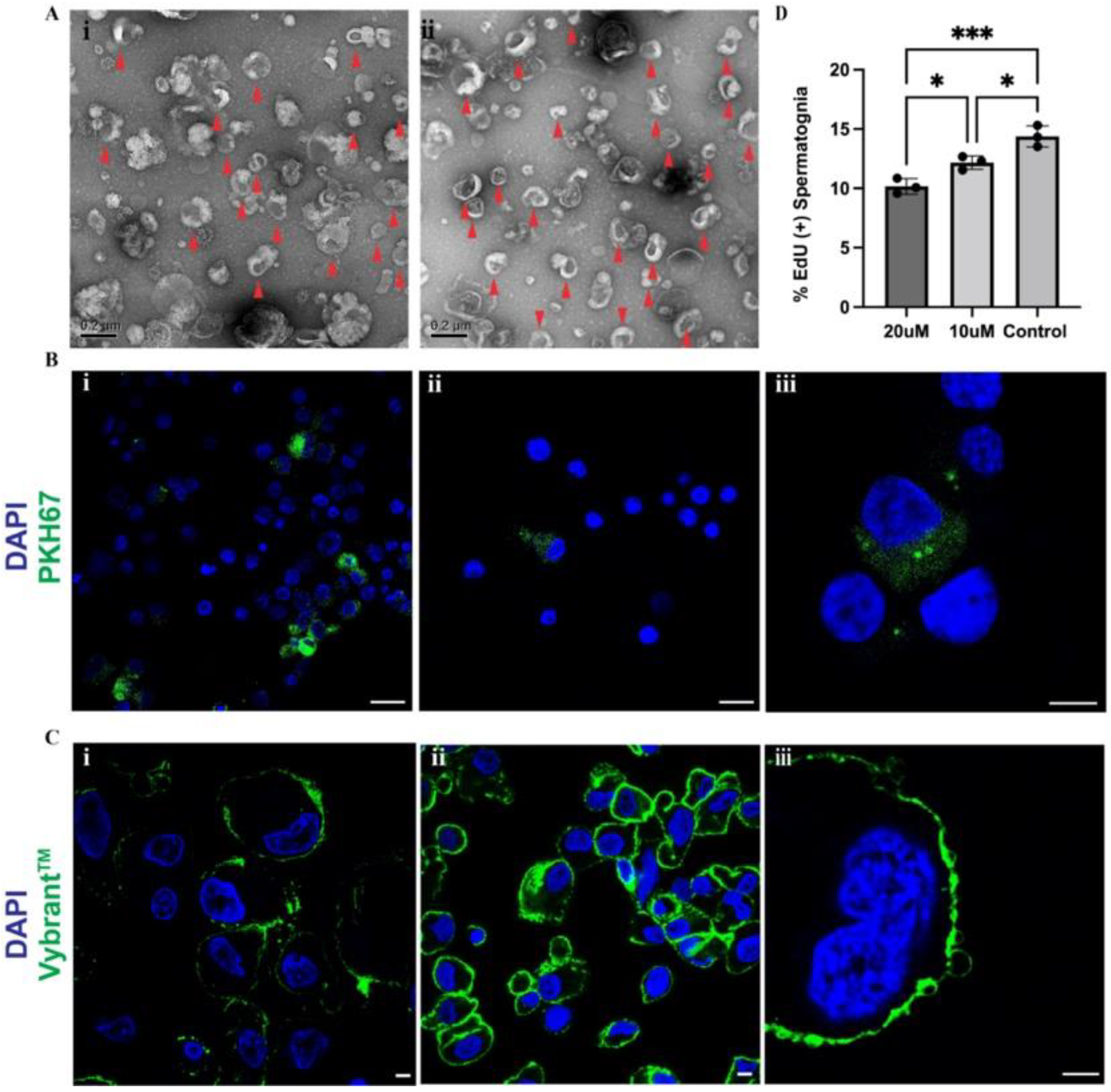
2.6. Exosomes Released by Sertoli Cells Are Taken up by Spermatogonia In Vitro
2.7. Sertoli Cell Exosome Biogenesis Involves Lipid Raft Formation
2.8. Inhibition of Sertoli Cell Exosome Release Reduces Spermatogonial Proliferation In Vitro
3. Discussion
4. Materials and Methods
4.1. Experimental Model and Sample Details
4.2. Feeder Cell and Spermatogonia Isolation and Enrichment
4.3. Immunofluorescence
4.4. Co-Culture Conditions
4.5. EdU Incorporation Assay and TUNEL Staining
4.6. qRT-PCR
4.7. Transwell Spermatogonia Co-Cultures
4.8. Extracellular Vesicle Isolation
4.9. TEM of Extracellular Vesicles
4.10. Extracellular Vesicle Labelling
4.11. Treatment of Sertoli Cells with GW4869
4.12. Lipid Raft Labelling
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kostereva, N.; Hofmann, M.C. Regulation of the Spermatogonial Stem Cell Niche. Reprod. Domest. Anim. 2008, 43 (Suppl. 2), 386–392. [Google Scholar] [CrossRef] [PubMed]
- Oatley, J.M.; Brinster, R.L. Regulation of Spermatogonial Stem Cell Self-Renewal in Mammals. Annu. Rev. Cell Dev. Biol. 2008, 24, 263–286. [Google Scholar] [CrossRef] [PubMed]
- Oatley, J.M.; Brinster, R.L. Spermatogonial Stem Cells. Methods Enzymol. 2006, 419, 259–282. [Google Scholar] [CrossRef] [PubMed]
- Morrison, S.J.; Spradling, A.C. Stem Cells and Niches: Mechanisms That Promote Stem Cell Maintenance throughout Life. Cell 2008, 132, 598–611. [Google Scholar] [CrossRef]
- Takashima, S.; Shinohara, T. Culture and transplantation of spermatogonial stem cells. Stem Cell Res. 2018, 29, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Kubota, H.; Brinster, R.L. Technology insight: In vitro culture of spermatogonial stem cells and their potential therapeutic uses. Nat. Clin. Pract. Endocrinol. Metab. 2006, 2, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Brinster, R.L.; Avarbock, M.R. Germline transmission of donor haplotype following spermatogonial transplantation. Proc. Natl. Acad. Sci. USA 1994, 91, 11303–11307. [Google Scholar] [CrossRef]
- Brinster, R.L.; Zimmermann, J.W. Spermatogenesis following male germ-cell transplantation. Proc. Natl. Acad. Sci. USA 1994, 91, 11298–11302. [Google Scholar] [CrossRef]
- Tagelenbosch, R.A.J.; de Rooij, D.G. A quantitative study of spermatogonial multiplication and stem cell renewal in the C3H/101 F1 hybrid mouse. Mutat. Res.-Fundam. Mol. Mech. Mutagene. 1993, 290, 193–200. [Google Scholar] [CrossRef]
- Hermann, B.P.; Phillips, B.T.; Orwig, K.E. The Elusive Spermatogonial Stem Cell Marker? Biol. Reprod. 2011, 85, 221–223. [Google Scholar] [CrossRef][Green Version]
- Aponte, P.M. Spermatogonial stem cells: Current biotechnological advances in reproduction and regenerative medicine. World J. Stem Cells 2015, 7, 669. [Google Scholar] [CrossRef] [PubMed]
- Kanatsu-Shinohara, M.; Ogonuki, N.; Inoue, K.; Miki, H.; Ogura, A.; Toyokuni, S.; Shinohara, T. Long-Term Proliferation in Culture and Germline Transmission of Mouse Male Germline Stem Cells. Biol. Reprod. 2003, 69, 612–616. [Google Scholar] [CrossRef] [PubMed]
- Kanatsu-Shinohara, M.; Muneto, T.; Lee, J.; Takenaka, M.; Chuma, S.; Nakatsuji, N.; Horiuchi, T.; Shinohara, T. Long-Term Culture of Male Germline Stem Cells From Hamster Testes. Biol. Reprod. 2008, 78, 611–617. [Google Scholar] [CrossRef] [PubMed]
- Kubota, H.; Brinster, R.L. Culture of Rodent Spermatogonial Stem Cells, Male Germline Stem Cells of the Postnatal Animal. Methods Cell Biol. 2008, 86, 59–84. [Google Scholar] [CrossRef]
- Ryu, B.Y.; Kubota, H.; Avarbock, M.R.; Brinster, R.L. Conservation of spermatogonial stem cell self-renewal signaling between mouse and rat. Proc. Natl. Acad. Sci. USA 2005, 102, 14302–14307. [Google Scholar] [CrossRef]
- Kubota, H.; Wu, X.; Goodyear, S.M.; Avarbock, M.R.; Brinster, R.L. Glial cell line-derived neurotrophic factor and endothelial cells promote self-renewal of rabbit germ cells with spermatogonial stem cell properties. FASEB J. 2011, 25, 2604–2614. [Google Scholar] [CrossRef][Green Version]
- Kanatsu-Shinohara, M.; Inoue, K.; Ogonuki, N.; Morimoto, H.; Ogura, A.; Shinohara, T. Serum- and Feeder-Free Culture of Mouse Germline Stem Cells. Biol. Reprod. 2011, 84, 97–105. [Google Scholar] [CrossRef]
- Kanatsu-Shinohara, M.; Miki, H.; Inoue, K.; Ogonuki, N.; Toyokuni, S.; Ogura, A.; Shinohara, T. Long-Term Culture of Mouse Male Germline Stem Cells Under Serum-or Feeder-Free Conditions. Biol. Reprod. 2005, 72, 985–991. [Google Scholar] [CrossRef]
- Choi, N.Y.; Park, Y.S.; Ryu, J.S.; Lee, H.J.; Araúzo-Bravo, M.J.; Ko, K.; Han, D.W.; Schöler, H.R.; Ko, K. A novel feeder-free culture system for expansion of mouse spermatogonial stem cells. Mol. Cells 2014, 37, 473–479. [Google Scholar] [CrossRef]
- Kanatsu-Shinohara, M.; Ogonuki, N.; Matoba, S.; Morimoto, H.; Ogura, A.; Shinohara, T. Improved Serum- and Feeder-Free Culture of Mouse Germline Stem Cells. Biol. Reprod. 2014, 91, 88. [Google Scholar] [CrossRef]
- Kanatsu-Shinohara, M.; Ogonuki, N.; Iwano, T.; Lee, J.; Kazuki, Y.; Inoue, K.; Miki, H.; Takehashi, M.; Toyokuni, S.; Shinkai, Y.; et al. Genetic and epigenetic properties of mouse male germline stem cells during long-term culture. Development 2005, 132, 4155–4163. [Google Scholar] [CrossRef] [PubMed]
- Aponte, P.M.; Soda, T.; Teerds, K.J.; Mizrak, S.C.; van de Kant, H.J.G.; de Rooij, D.G. Propagation of bovine spermatogonial stem cells in vitro. Reproduction 2008, 136, 543–557. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Qiu, L.; Guo, Q.; Chen, Y.; Zhang, X.; Chen, B.; Zhang, Y.; Chang, G. Long-term in vitro culture and preliminary establishment of chicken primordial germ cell lines. PLoS ONE 2018, 13, e0196459. [Google Scholar] [CrossRef]
- Suyatno; Kitamura, Y.; Ikeda, S.; Minami, N.; Yamada, M.; Imai, H. Long-term culture of undifferentiated spermatogonia isolated from immature and adult bovine testes. Mol. Reprod. Dev. 2018, 85, 236–249. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Chen, X.; Zheng, Y.; Zhu, J.; Qin, Y.; Lv, Y.; Zeng, W. Long-Term Propagation of Porcine Undifferentiated Spermatogonia. Stem Cells Dev. 2017, 26, 1121–1131. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Lee, W.Y.; Kim, J.H.; Park, C.K.; Do, J.T.; Kim, J.H.; Choi, Y.S.; Kim, N.H.; Song, H. Subculture of germ cell-derived colonies with gata4-positive feeder cells from neonatal pig testes. Stem Cells Int. 2016, 2016, 6029271. [Google Scholar] [CrossRef] [PubMed]
- Oatley, J.M.; Brinster, R.L. The Germline Stem Cell Niche Unit in Mammalian Testes. Physiol. Rev. 2012, 92, 577–595. [Google Scholar] [CrossRef]
- Maekawa, M.; Kamimura, K.; Nagano, T. Peritubular myoid cells in the testis: Their structure and function. Arch. Histol. Cytol. 1996, 59, 1–13. [Google Scholar] [CrossRef]
- Nakagawa, T.; Nabeshima, Y.-I.; Yoshida, S. Functional Identification of the Actual and Potential Stem Cell Compartments in Mouse Spermatogenesis. Dev. Cell 2007, 12, 195–206. [Google Scholar] [CrossRef]
- Bhang, D.H.; Kim, B.J.; Kim, B.G.; Schadler, K.; Baek, K.H.; Kim, Y.H.; Hsiao, W.; Ding, B.S.; Rafii, S.; Weiss, M.J.; et al. Testicular endothelial cells are a critical population in the germline stem cell niche. Nat. Commun. 2018, 9, 4379. [Google Scholar] [CrossRef]
- Chen, L.-Y.; Willis, W.D.; Eddy, E.M. Targeting the Gdnf Gene in peritubular myoid cells disrupts undifferentiated spermatogonial cell development. Proc. Natl. Acad. Sci. USA 2016, 113, 1829–1834. [Google Scholar] [CrossRef] [PubMed]
- Spinnler, K.; Köhn, F.M.; Schwarzer, U.; Mayerhofer, A. Glial cell line-derived neurotrophic factor is constitutively produced by human testicular peritubular cells and may contribute to the spermatogonial stem cell niche in man. Hum. Reprod. 2010, 25, 2181–2187. [Google Scholar] [CrossRef] [PubMed]
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Catalano, M.; O’Driscoll, L. Inhibiting extracellular vesicles formation and release: A review of EV inhibitors. J. Extracell. Vesicles 2019, 9, 1703244. [Google Scholar] [CrossRef] [PubMed]
- Zaborowski, M.P.; Balaj, L.; Breakefield, X.O.; Lai, C.P. Extracellular Vesicles: Composition, Biological Relevance, and Methods of Study. Bioscience 2015, 65, 783–797. [Google Scholar] [CrossRef]
- Hessvik, N.P.; Llorente, A. Current knowledge on exosome biogenesis and release. Cell. Mol. Life Sci. 2018, 75, 193–208. [Google Scholar] [CrossRef]
- Théry, C. Exosomes: Secreted vesicles and intercellular communications. F1000 Biol. Rep. 2011, 3, 15. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Liu, H.; Tang, W.H. Exosomes: Biogenesis, biologic function and clinical potential. Cell Biosci. 2019, 9, 19. [Google Scholar] [CrossRef]
- Vodička, P.; Smetana, K.; Dvořánková, B.; Emerick, T.; Xu, Y.Z.; Ourednik, J.; Ourednik, V.; Motlík, J. The miniature pig as an animal model in biomedical research. Ann. N. Y. Acad. Sci. 2005, 1049, 161–171. [Google Scholar] [CrossRef]
- González, R.; Dobrinski, I. Beyond the mouse monopoly: Studying the male germ line in domestic animal models. ILAR J. 2015, 56, 83–98. [Google Scholar] [CrossRef]
- Dawson, H.D.; Smith, A.D.; Chen, C.; Urban, J.F. An in-depth comparison of the porcine, murine and human inflammasomes; lessons from the porcine genome and transcriptome. Vet. Microbiol. 2017, 202, 2–15. [Google Scholar] [CrossRef] [PubMed]
- Sakib, S.; Yu, Y.; Voigt, A.; Ungrin, M.; Dobrinski, I. Generation of Porcine Testicular Organoids with Testis Specific Architecture using Microwell Culture. J. Vis. Exp. 2019, 2019, e60387. [Google Scholar] [CrossRef] [PubMed]
- Tung, P.S.; Fritz, I.B. Characterization of Rat Testicular Peritubular Myoid Cells in Culture: α-Smooth Muscle Isoactin is a Specific Differentiation Marker. Biol. Reprod. 1990, 42, 351–365. [Google Scholar] [CrossRef] [PubMed]
- Maretta, M.; Marettová, E. Immunohistochemical demonstration of myoid cells in the testis and its excurrent ducts in the domestic fowl. Br. Poult. Sci. 2004, 45, 585–589. [Google Scholar] [CrossRef]
- Kyrönlahti, A.; Euler, R.; Bielinska, M.; Schoeller, E.L.; Moley, K.H.; Toppari, J.; Heikinheimo, M.; Wilson, D.B. GATA4 regulates Sertoli cell function and fertility in adult male mice. Mol. Cell. Endocrinol. 2011, 333, 85. [Google Scholar] [CrossRef]
- Ketola, I.; Pentikäinen, V.; Vaskivuo, T.; Ilvesmäki, V.; Herva, R.; Dunkel, L.; Tapanainen, J.S.; Toppari, J.; Heikinheimo, M. Expression of Transcription Factor GATA-4 during Human Testicular Development and Disease. J. Clin. Endocrinol. Metab. 2000, 85, 3925–3931. [Google Scholar] [CrossRef]
- Kim, Y.-H.; Oh, M.-G.; Bhang, D.H.; Kim, B.-J.; Jung, S.-E.; Kim, S.-M.; Dohr, G.; Kim, S.-U.; Ryeom, S.; Ryu, B.-Y. Testicular endothelial cells promote self-renewal of spermatogonial stem cells in rats. Biol. Reprod. 2019, 101, 360–367. [Google Scholar] [CrossRef]
- Luo, J.; Megee, S.; Rathi, R.; Dobrinski, I. Protein gene product 9.5 is a spermatogonia-specific marker in the pig testis: Application to enrichment and culture of porcine spermatogonia. Mol. Reprod. Dev. 2006, 73, 1531–1540. [Google Scholar] [CrossRef]
- He, Z.; Kokkinaki, M.; Jiang, J.; Dobrinski, I.; Dym, M. Isolation, Characterization, and Culture of Human Spermatogonia1. Biol. Reprod. 2010, 82, 363–372. [Google Scholar] [CrossRef]
- Alpaugh, W.F.; Voigt, A.L.; Dardari, R.; Su, L.; Al Khatib, I.; Shin, W.; Goldsmith, T.M.; Coyle, K.M.; Tang, L.A.; Shutt, T.E.; et al. Loss of Ubiquitin Carboxy-Terminal Hydrolase L1 Impairs Long-Term Differentiation Competence and Metabolic Regulation in Murine Spermatogonial Stem Cells. Cells 2021, 10, 2265. [Google Scholar] [CrossRef]
- Ryu, B.-Y.; Nagano, M.; Brinster, C.J.; Avarbock, M.R.; Brinster, R.L. Maintenance of Mouse Male Germ Line Stem Cells In Vitro. Biol. Reprod. 2004, 68, 2207–2214. [Google Scholar] [CrossRef]
- Kokabu, S.; Gamer, L.; Cox, K.; Lowery, J.; Tsuji, K.; Raz, R.; Economides, A.; Katagiri, T.; Rosen, V. BMP3 Suppresses Osteoblast Differentiation of Bone Marrow Stromal Cells via Interaction with Acvr2b. Mol. Endocrinol. 2012, 26, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, M.C. Gdnf Signaling Pathways within the Mammalian Spermatogonial Stem Cell Niche. Mol. Cell. Endocrinol. 2008, 288, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Lindahl, M.; Hyvönen, M.E.; Parvinen, M.; De Rooij, D.G.; Hess, M.W.; Raatikainen-Ahokas, A.; Sainio, K.; Rauvala, H.; Lakso, M.; et al. Regulation of cell fate decision of undifferentiated spermatogonia by GDNF. Science 2000, 287, 1489–1493. [Google Scholar] [CrossRef] [PubMed]
- Costa, G.M.J.; Avelar, G.F.; Rezende-Neto, J.V.; Campos-Junior, P.H.A.; Lacerda, S.M.S.N.; Andrade, B.S.C.; Thomé, R.G.; Hofmann, M.C.; Franca, L.R. Spermatogonial Stem Cell Markers and Niche in Equids. PLoS ONE 2012, 7, e44091. [Google Scholar] [CrossRef]
- Oatley, J.M.; Oatley, M.J.; Avarbock, M.R.; Tobias, J.W.; Brinster, R.L. Colony stimulating factor 1 is an extrinsic stimulator of mouse spermatogonial stem cell self-renewal. Development 2009, 136, 1191–1199. [Google Scholar] [CrossRef]
- Hamra, F.K.; Chapman, K.M.; Nguyen, D.M.; Williams-Stephens, A.A.; Hammer, R.E.; Garbers, D.L. Self renewal, expansion, and transfection of rat spermatogonial stem cells in culture. Proc. Natl. Acad. Sci. USA 2005, 102, 17430–17435. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Zhang, X.; Sun, J.; Hao, J. BMP4/Smad signaling pathway induces the differentiation of mouse spermatogonial stem cells via upregulation of Sohlh2. Anat. Rec. 2014, 297, 749–757. [Google Scholar] [CrossRef]
- Yang, Y.; Feng, Y.; Feng, X.; Liao, S.; Wang, X.; Gan, H.; Wang, L.; Lin, X.; Han, C. BMP4 Cooperates with Retinoic Acid to Induce the Expression of Differentiation Markers in Cultured Mouse Spermatogonia. Stem Cells Int. 2016, 2016, 9536192. [Google Scholar] [CrossRef]
- Garcia, T.X.; Hofmann, M.C. Regulation of germ line stem cell homeostasis. Anim. Reprod. 2015, 12, 35–45. [Google Scholar]
- Jing, S.; Wen, D.; Yu, Y.; Holst, P.L.; Luo, Y.; Fang, M.; Tamir, R.; Antonio, L.; Hu, Z.; Cupples, R.; et al. GDNF-induced activation of the Ret protein tyrosine kinase is mediated by GDNFR-α, a novel receptor for GDNF. Cell 1996, 85, 1113–1124. [Google Scholar] [CrossRef]
- Kawase, E.; Wong, M.D.; Ding, B.C.; Xie, T. Gbb/Bmp signaling is essential for maintaining germline stem cells and for repressing bam transcription in the Drosophila testis. Development 2004, 131, 1365–1375. [Google Scholar] [CrossRef] [PubMed]
- Skryabin, G.O.; Komelkov, A.V.; Savelyeva, E.E.; Tchevkina, E.M. Lipid Rafts in Exosome Biogenesis. Biochemistry 2020, 85, 177–191. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.S.; Yin, Y.; Lee, T.; Lai, R.C.; Yeo, R.W.Y.; Zhang, B.; Choo, A.; Lim, S.K. Therapeutic MSC exosomes are derived from lipid raft microdomains in the plasma membrane. J. Extracell. Vesicles 2013, 2, 22614. [Google Scholar] [CrossRef] [PubMed]
- Evans IV, W.E.; Coyer, R.L.; Sandusky, M.F.; Van Fleet, M.J.; Moore, J.G.; Nyquist, S.E. Characterization of membrane rafts isolated from rat sertoli cell cultures: Caveolin and flotillin-1 content. J. Androl. 2003, 24, 812–821. [Google Scholar] [CrossRef]
- Wei, D.; Zhan, W.; Gao, Y.; Huang, L.; Gong, R.; Wang, W.; Zhang, R.; Wu, Y.; Gao, S.; Kang, T. RAB31 marks and controls an ESCRT-independent exosome pathway. Cell Res. 2020, 31, 157–177. [Google Scholar] [CrossRef]
- Essandoh, K.; Yang, L.; Wang, X.; Huang, W.; Qin, D.; Hao, J.; Wang, Y.; Zingarelli, B.; Peng, T.; Fan, G.C. Blockade of exosome generation with GW4869 dampens the sepsis-induced inflammation and cardiac dysfunction. Biochim. Biophys. Acta-Mol. Basis Dis. 2015, 1852, 2362–2371. [Google Scholar] [CrossRef]
- Wang, X.; Huang, W.; Liu, G.; Cai, W.; Millard, R.W.; Wang, Y.; Chang, J.; Peng, T.; Fan, G.C. Cardiomyocytes mediate anti-angiogenesis in type 2 diabetic rats through the exosomal transfer of miR-320 into endothelial cells. J. Mol. Cell. Cardiol. 2014, 74, 139–150. [Google Scholar] [CrossRef]
- Kosaka, N.; Iguchi, H.; Yoshioka, Y.; Takeshita, F.; Matsuki, Y.; Ochiya, T. Secretory mechanisms and intercellular transfer of microRNAs in living cells. J. Biol. Chem. 2010, 285, 17442–17452. [Google Scholar] [CrossRef]
- Pramod, R.K.; Mitra, A. In vitro culture and characterization of spermatogonial stem cells on Sertoli cell feeder layer in goat (Capra hircus). J. Assist. Reprod. Genet. 2014, 31, 993–1001. [Google Scholar] [CrossRef]
- Chen, L.-Y.; Brown, P.R.; Willis, W.B.; Eddy, E.M. Peritubular myoid cells participate in male mouse spermatogonial stem cell maintenance. Endocrinology 2014, 155, 4964–4974. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Sukeno, M.; Nabeshima, Y.I. A vasculature-associated niche for undifferentiated spermatogonia in the mouse testis. Science 2007, 317, 1722–1726. [Google Scholar] [CrossRef] [PubMed]
- Chiarini-Garcia, H.; Hornick, J.R.; Griswold, M.D.; Russell, L.D. Distribution of type A spermatogonia in the mouse is not random. Biol. Reprod. 2001, 65, 1179–1185. [Google Scholar] [CrossRef] [PubMed]
- Chan, F.; Oatley, M.J.; Kaucher, A.V.; Yang, Q.-E.; Bieberich, C.J.; Shashikant, C.S.; Oatley, J.M. Functional and molecular features of the Id4+ germline stem cell population in mouse testes. Genes Dev. 2014, 28, 1351–1362. [Google Scholar] [CrossRef]
- Sakib, S.; Voigt, A.; de Lima e Martins Lara, N.; Su, L.; Ungrin, M.; Rancourt, D.; Dobrinski, I. The Proliferation of Pre-Pubertal Porcine Spermatogonia in Stirred Suspension Bioreactors Is Partially Mediated by the Wnt/β-Catenin Pathway. Int. J. Mol. Sci. 2021, 22, 13549. [Google Scholar] [CrossRef]
- Voigt, A.L.; Kondro, D.A.; Powell, D.; Valli-Pulaski, H.; Ungrin, M.; Stukenborg, J.B.; Klein, C.; Lewis, I.A.; Orwig, K.E.; Dobrinski, I. Unique metabolic phenotype and its transition during maturation of juvenile male germ cells. FASEB J. 2021, 35, e21513. [Google Scholar] [CrossRef]
- Matsui, Y.; Zsebo, K.; Hogan, B.L.M. Derivation of pluripotential embryonic stem cells from murine primordial germ cells in culture. Cell 1992, 70, 841–847. [Google Scholar] [CrossRef]
- Resnick, J.L.; Bixler, L.S.; Cheng, L.; Donovan, P.J. Long-term proliferation of mouse primordial germ cells in culture. Nature 1992, 359, 550–551. [Google Scholar] [CrossRef]
- Spradling, A.; Drummond-Barbosa, D.; Kai, T. Stem cells find their niche. Nature 2001, 414, 98–104. [Google Scholar] [CrossRef]
- Discher, D.E.; Mooney, D.J.; Zandstra, P.W. Growth factors, matrices, and forces combine and control stem cells. Science 2009, 324, 1673–1677. [Google Scholar] [CrossRef]
- Guilak, F.; Cohen, D.M.; Estes, B.T.; Gimble, J.M.; Liedtke, W.; Chen, C.S. Control of stem cell fate by physical interactions with the extracellular matrix. Cell Stem Cell 2009, 5, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H.C.; Thampatty, B.P. Mechanobiology of adult and stem cells. Int. Rev. Cell Mol. Biol. 2008, 271, 301–346. [Google Scholar] [CrossRef] [PubMed]
- Geiger, B.; Spatz, J.P.; Bershadsky, A.D. Environmental sensing through focal adhesions. Nat. Rev. Mol. Cell Biol. 2009, 10, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, M.A.; DeSimone, D.W. Cell adhesion receptors in mechanotransduction. Curr. Opin. Cell Biol. 2008, 20, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Park, J.E.; Park, M.H.; Kim, M.S.; Park, Y.R.; Yun, J.I.; Cheong, H.T.; Kim, M.; Choi, J.H.; Lee, E.; Lee, S.T. Porcine spermatogonial stem cells self-renew effectively in a three dimensional culture microenvironment. Cell Biol. Int. 2017, 41, 1316–1324. [Google Scholar] [CrossRef]
- Zhao, X.; Wan, W.; Li, B.; Zhang, X.; Zhang, M.; Wu, Z.; Yang, H. Isolation and in vitro expansion of porcine spermatogonial stem cells. Reprod. Domest. Anim. 2022, 57, 210–220. [Google Scholar] [CrossRef]
- Yang, Y.; Lin, Q.; Zhou, C.; Li, Q.; Li, Z.; Cao, Z.; Liang, J.; Li, H.; Mei, J.; Zhang, Q.; et al. A Testis-Derived Hydrogel as an Efficient Feeder-Free Culture Platform to Promote Mouse Spermatogonial Stem Cell Proliferation and Differentiation. Front. Cell Dev. Biol. 2020, 8, 250. [Google Scholar] [CrossRef]
- Faught, E.; Henrickson, L.; Vijayan, M.M. Plasma exosomes are enriched in Hsp70 and modulated by stress and cortisol in rainbow trout. J. Endocrinol. 2017, 232, 237–246. [Google Scholar] [CrossRef]
- De Jong, O.G.; Verhaar, M.C.; Chen, Y.; Vader, P.; Gremmels, H.; Posthuma, G.; Schiffelers, R.M.; Gucek, M.; van Balkom, B.W.M. Cellular stress conditions are reflected in the protein and RNA content of endothelial cell-derived exosomes. J. Extracell. Vesicles 2012, 1, 18396. [Google Scholar] [CrossRef]
- Zou, W.; Lai, M.; Zhang, Y.; Zheng, L.; Xing, Z.; Li, T.; Zou, Z.; Song, Q.; Zhao, X.; Xia, L.; et al. Exosome Release Is Regulated by mTORC1. Adv. Sci. 2019, 6, 1801313. [Google Scholar] [CrossRef]
- Baskaran, S.; Panner Selvam, M.K.; Agarwal, A. Exosomes of male reproduction. Adv. Clin. Chem. 2020, 95, 149–163. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Li, H.; Liang, J.; Mei, J.; Cao, Z.; Zhang, L.; Luo, J.; Tang, Y.; Huang, R.; Xia, H.; et al. Sertoli cell-derived exosomal MicroRNA-486-5p regulates differentiation of spermatogonial stem cell through PTEN in mice. J. Cell. Mol. Med. 2021, 25, 3950–3962. [Google Scholar] [CrossRef] [PubMed]
- Menck, K.; Sönmezer, C.; Worst, T.S.; Schulz, M.; Dihazi, G.H.; Streit, F.; Erdmann, G.; Kling, S.; Boutros, M.; Binder, C.; et al. Neutral sphingomyelinases control extracellular vesicles budding from the plasma membrane. J. Extracell. Vesicles 2017, 6, 1378056. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Bondareva, A.; González, R.; Rodriguez-Sosa, J.R.; Carlson, D.F.; Webster, D.; Fahrenkrug, S.; Dobrinski, I. TALEN-mediated gene targeting in porcine spermatogonia. Mol. Reprod. Dev. 2018, 85, 250–261. [Google Scholar] [CrossRef]
- Lou, J.; Megee, S.; Dobrinski, I. Asymmetric distribution of UCH-L1 in spermatogonia is associated with maintenance and differentiation of spermatogonial stem cells. J. Cell. Physiol. 2009, 220, 460–468. [Google Scholar] [CrossRef]
- Théry, C.; Amigorena, S.; Raposo, G.; Clayton, A. Isolation and Characterization of Exosomes from Cell Culture Supernatants and Biological Fluids. Curr. Protoc. Cell Biol. 2006, 30, 3.22.1–3.22.29. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thiageswaran, S.; Steele, H.; Voigt, A.L.; Dobrinski, I. A Role for Exchange of Extracellular Vesicles in Porcine Spermatogonial Co-Culture. Int. J. Mol. Sci. 2022, 23, 4535. https://doi.org/10.3390/ijms23094535
Thiageswaran S, Steele H, Voigt AL, Dobrinski I. A Role for Exchange of Extracellular Vesicles in Porcine Spermatogonial Co-Culture. International Journal of Molecular Sciences. 2022; 23(9):4535. https://doi.org/10.3390/ijms23094535
Chicago/Turabian StyleThiageswaran, Shiama, Heather Steele, Anna Laura Voigt, and Ina Dobrinski. 2022. "A Role for Exchange of Extracellular Vesicles in Porcine Spermatogonial Co-Culture" International Journal of Molecular Sciences 23, no. 9: 4535. https://doi.org/10.3390/ijms23094535
APA StyleThiageswaran, S., Steele, H., Voigt, A. L., & Dobrinski, I. (2022). A Role for Exchange of Extracellular Vesicles in Porcine Spermatogonial Co-Culture. International Journal of Molecular Sciences, 23(9), 4535. https://doi.org/10.3390/ijms23094535





