The Molecular Quality and Mitochondrial Activity of Porcine Cumulus–Oocyte Complexes Are Affected by Their Exposure to Three Endocrine-Active Compounds under 3D In Vitro Maturation Conditions
Abstract
:1. Introduction
2. Results
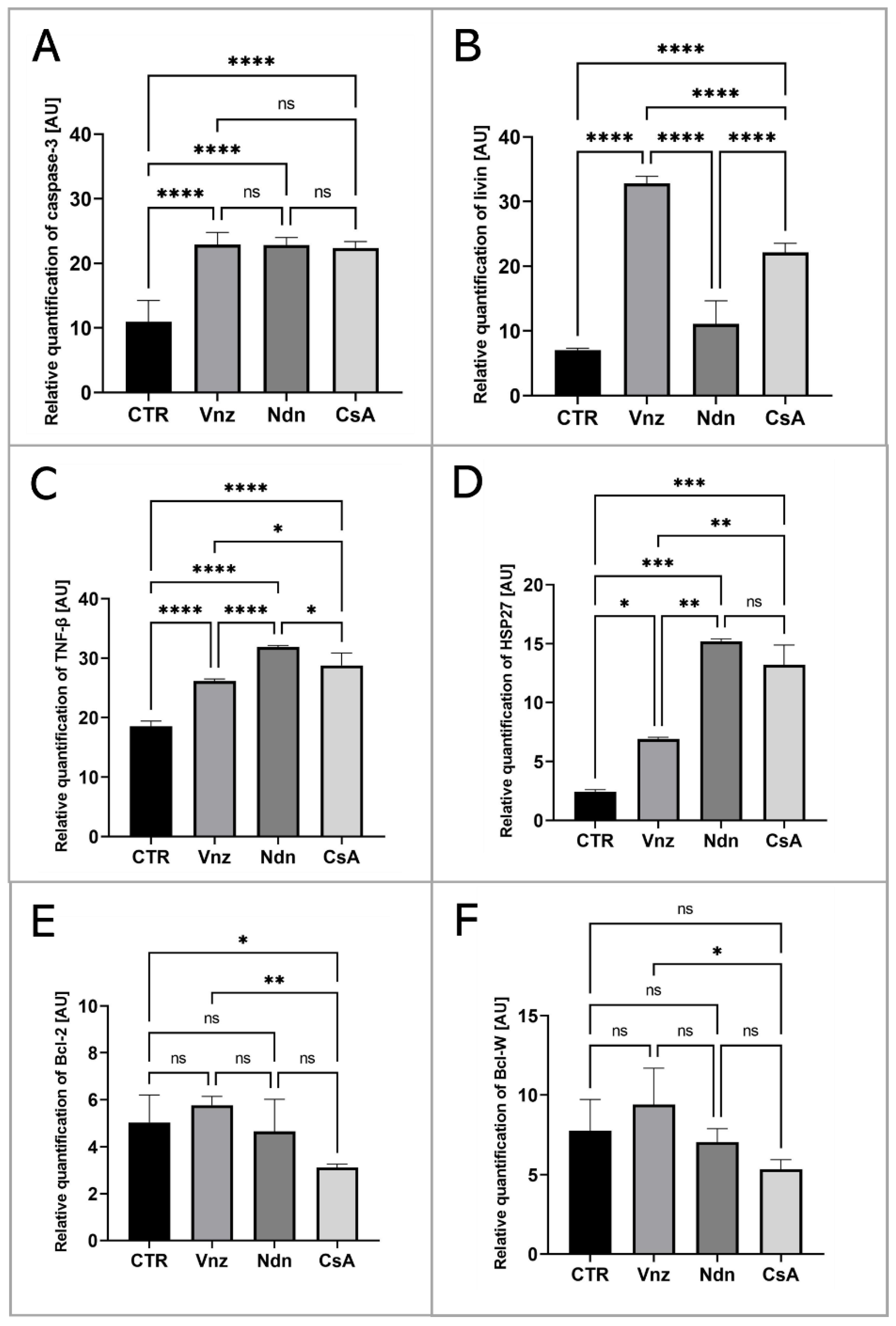
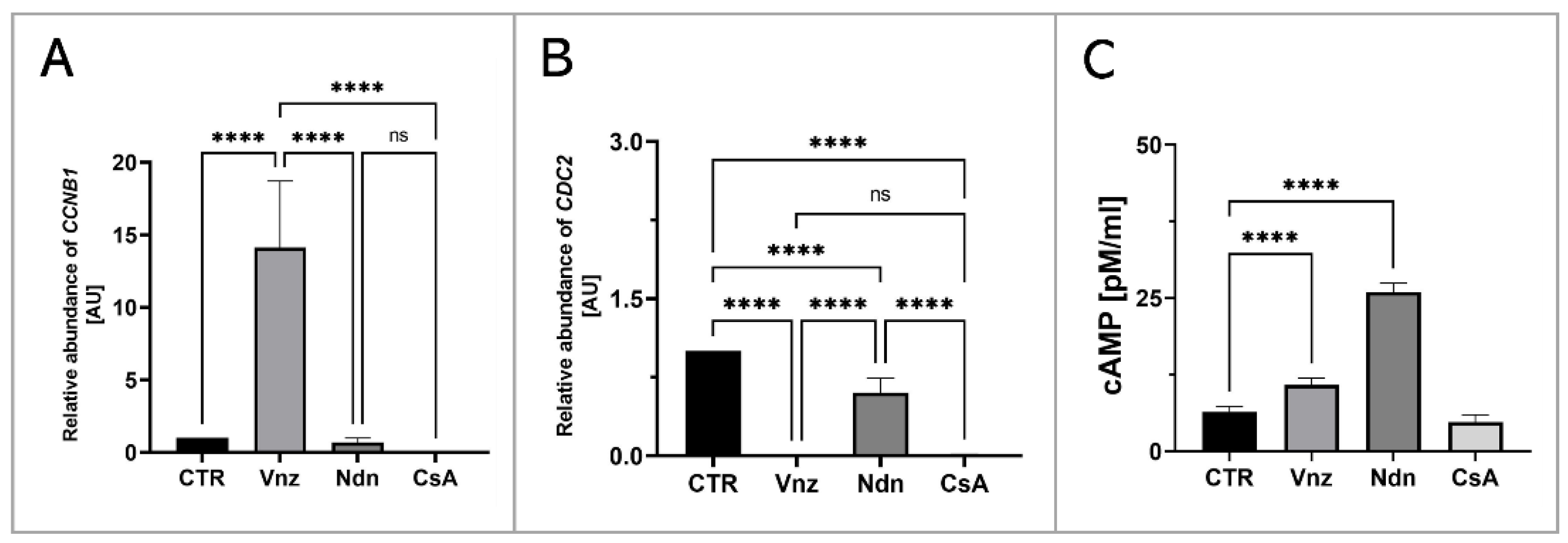
2.1. Vinclozolin and Nandrolone Accelerate Apoptosis of Cumulus Cells
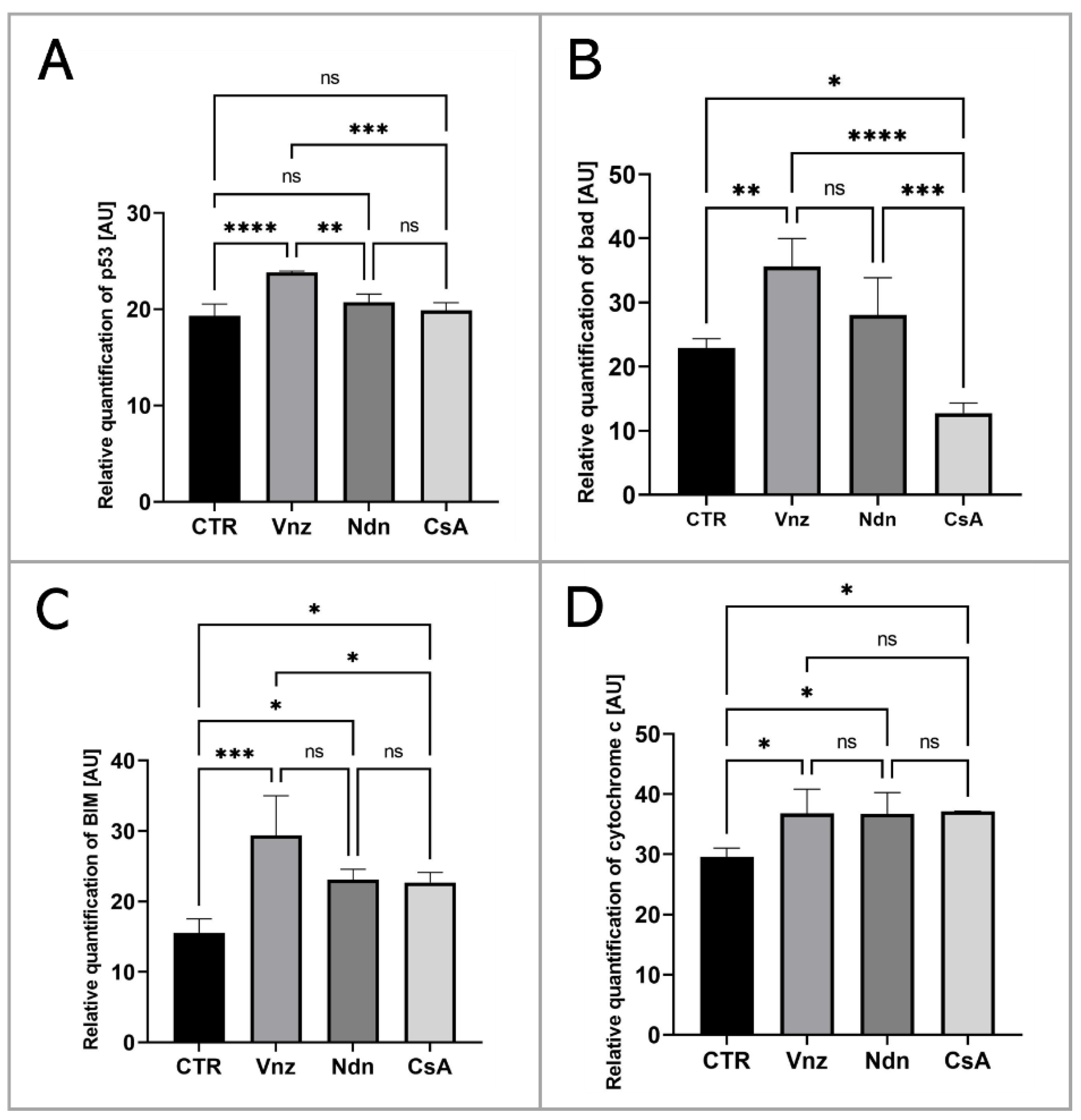
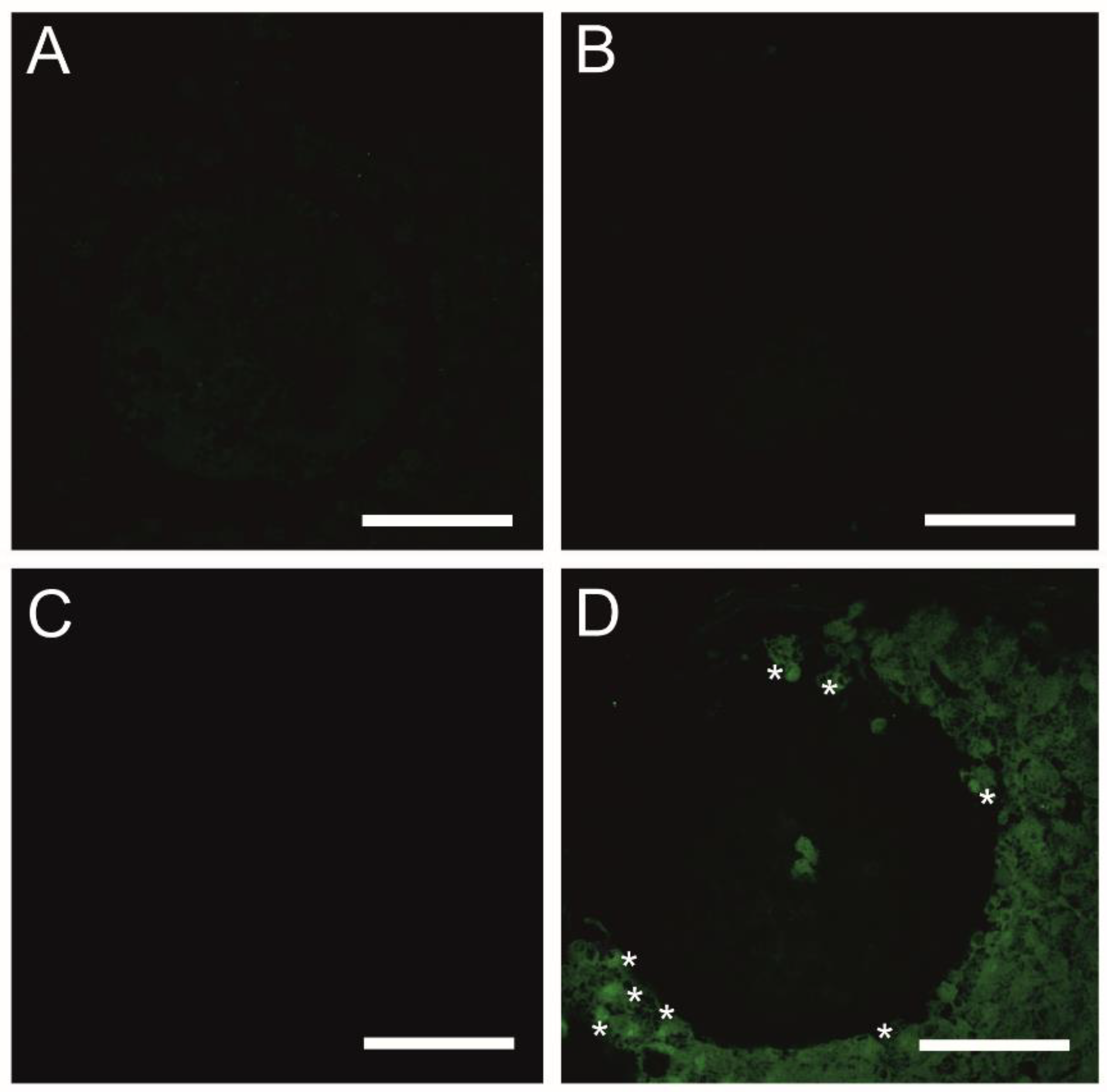
2.2. Analysis of the Apoptosis Mechanism in 3D-IVM-Generated Cumulus–Oocyte Complexes Treated with the Selected Endocrine Disruptors
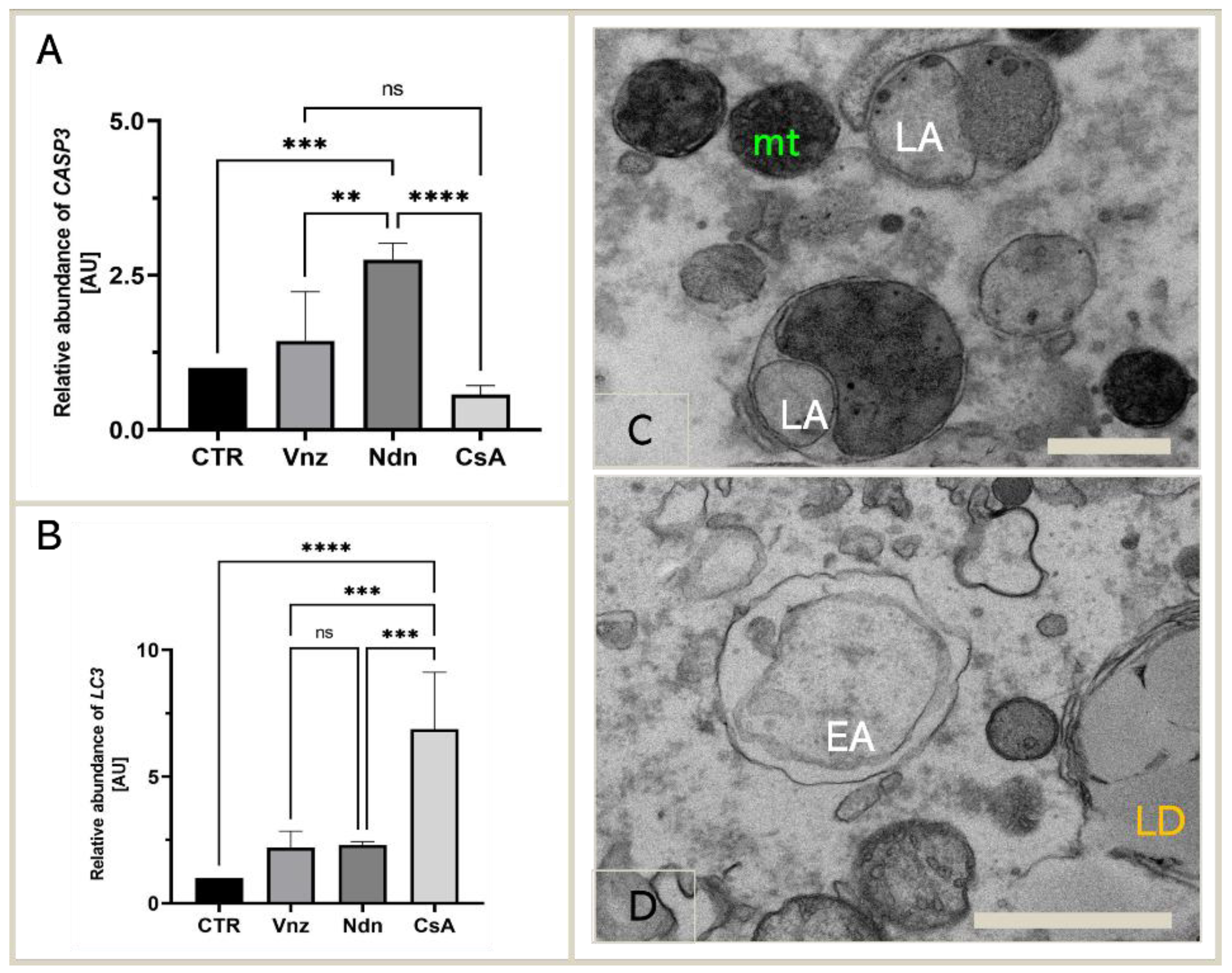
2.3. Gene Expression of Main Apoptosis and Autophagy Mediators
2.4. Cyclosporin A Induces Mitophagy in COCs Undergoing IVM under 3D Culture Conditions
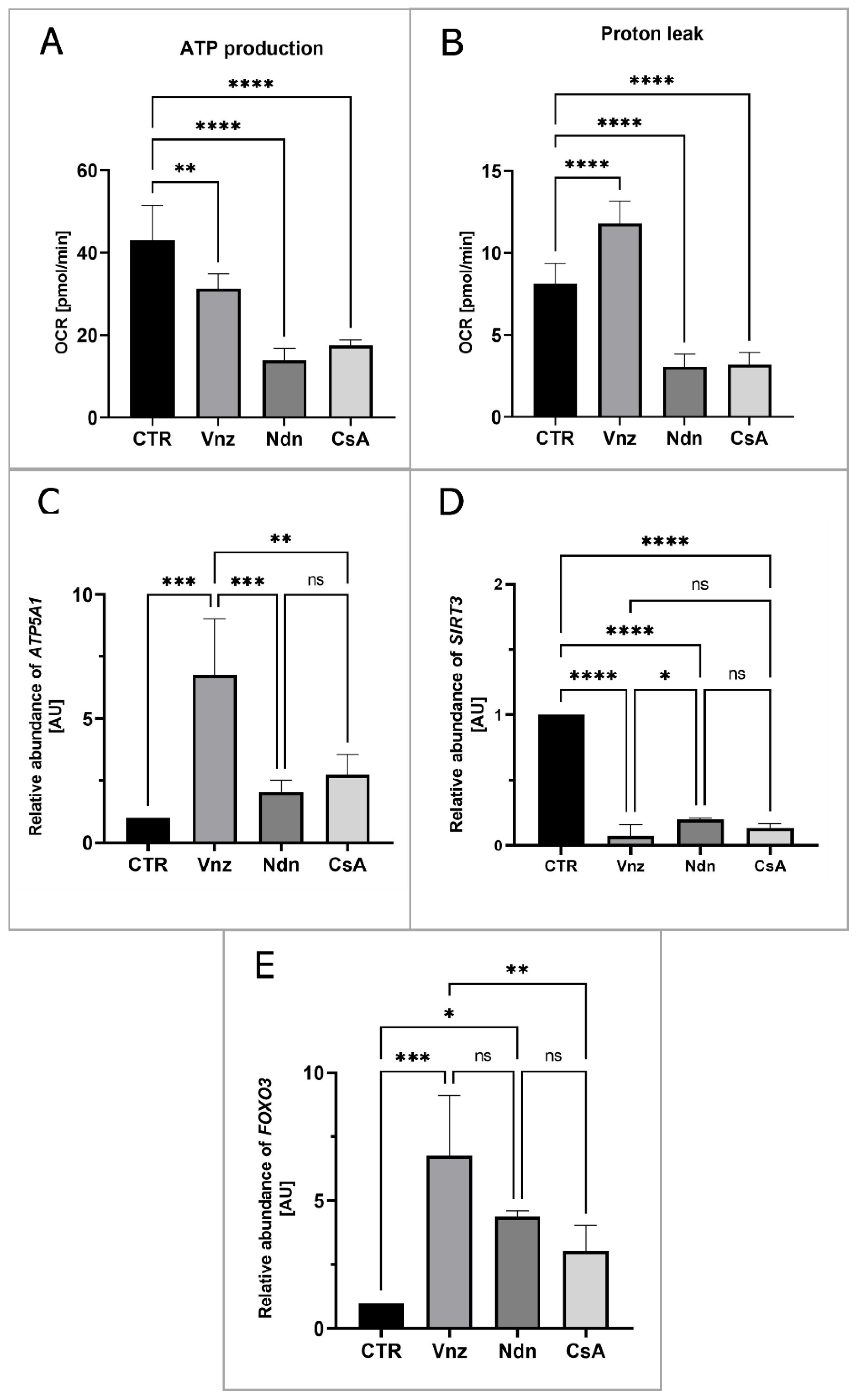
2.5. Analysis of Cellular Metabolism in 3D-IVM-Generated COCs Treated with the Selected Endocrine Disruptors
2.5.1. Analysis of the Distribution and Ultrastructure of Mitochondria in COCs Subjected to 3D-IVM in the Presence of Selected EACs
2.5.2. The Live Cell-Based Analysis of Mitochondrial Activity in 3D-IVM-Derived COCs Treated with the Selected Endocrine Disruptors
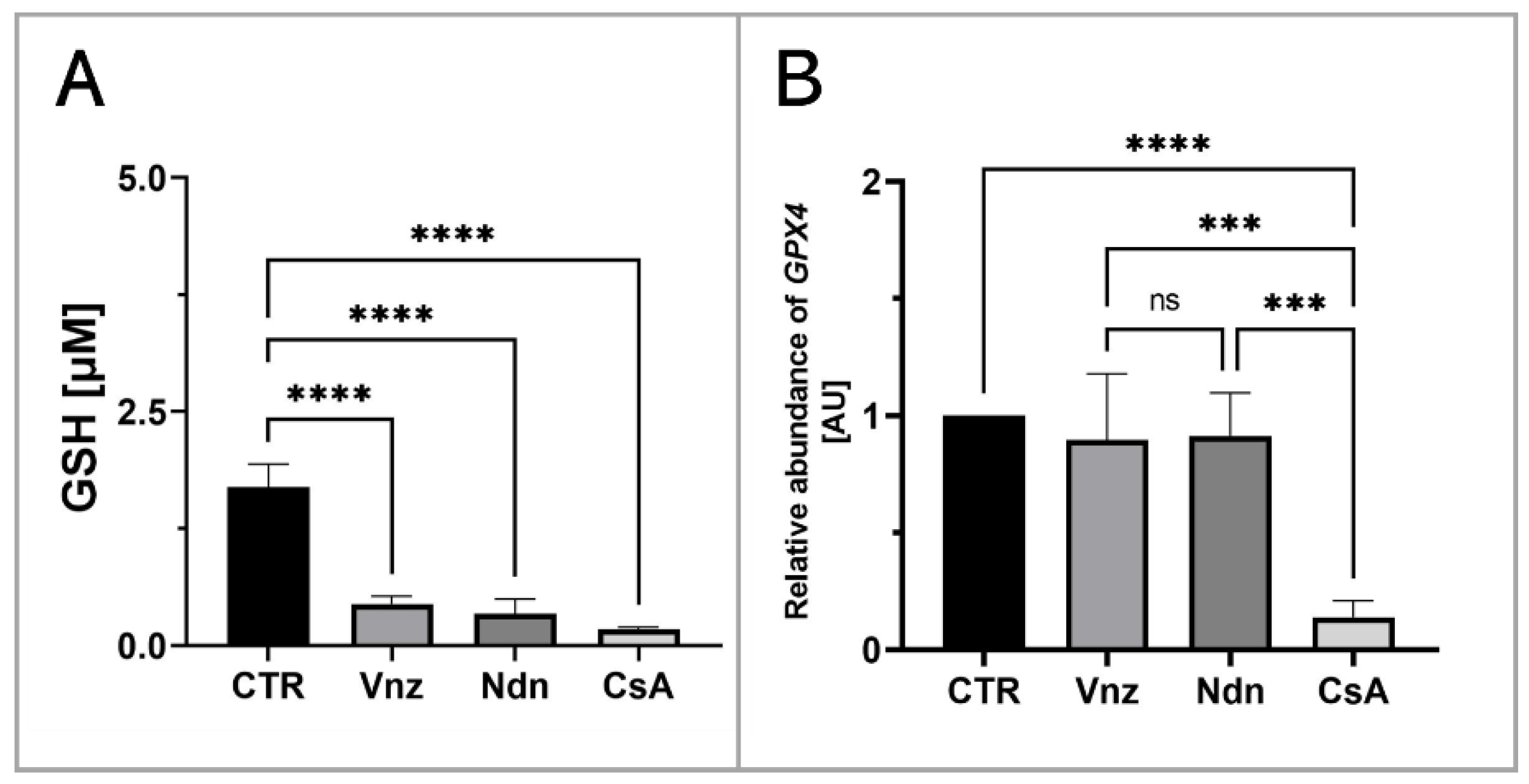
2.5.3. Quantitative Analysis of Intracytoplasmic Glutathione Concentration in 3D-IVM-Generated COCs Treated with the Selected Endocrine Disruptors
2.6. Analyzing the Extent of Meiosis/Maturation Progression in Oocytes Treated with the Selected Endocrine Disruptors during IVM Procedure under 3D Culture Conditions
3. Discussion
4. Materials and Methods
4.1. Collection and In Vitro Maturation of Porcine Cumulus–Oocyte Complexes under 3D Culture Conditions
4.2. Total RNA Isolation and cDNA Synthesis
4.3. Quantitative Reverse Transcriptase Real-Time Polymerase Chain Reaction (qRT-PCR)
4.4. The Use of Apoptosis Proteome Profiler Arrays for Detailed Evaluation of Pro- and Antiapoptotic Pathways in 3D-IVM-Derived COCs Treated with the Selected Endocrine Disruptors
4.5. TUNEL-Assisted Detection of Late-Apoptotic Cells in COCs Undergoing Exposure to the Selected EACs during 3D-IVM
4.6. Determination of cAMP Concentration in EAC-Treated COCs Subjected to 3D-IVM
4.7. Quantitatively Ascertaining the Intracytoplasmic Glutathione Concentration in 3D-IVM-Produced COCs Exposed to the Selected Endocrine Disruptors
4.8. Transmission Electron Microscope Analysis of Porcine COCs Undergoing 3D-IVM and Simultaneous EDC Treatment
4.9. The Live Cell-Based Assay of Mitochondrial Metabolic Activity Assisted by the Seahorse XFp Analyzer
Seahorse XF Measurement of ECAR and OCR Using Seahorse XF Cell Mito Stress Test
4.10. Assessment of Mitochondrial Distribution Pattern in 3D-IVM-Generated COCs Exposed to the Selected EDCs
4.11. Detection of Mitophagy Incidence in COCs Subjected to 3D-IVM and Simultaneously Treated with the Selected EACs
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ΔΨm | Mitochondrial membrane/transmembrane potential |
| 3D | Three-dimensional |
| 3D-IVM | Three-dimensional in vitro maturation |
| AAS | Anabolic androgenic steroids |
| ARTs | Assisted reproductive technologies |
| ATP | Adenosine-5′-triphosphate |
| ATP5A1 | ATP synthase F1 subunit α |
| bad | Bcl2-associated agonist of cell death |
| Bcl-2 | B-cell lymphoma 2 |
| BIM | Bcl2-interacting mediator of cell death |
| cAMP | Cyclic adenosine 3′,5′-monophosphate |
| CCNB1 | Cyclin B1 gene |
| CCs | Cumulus cells |
| CDC2 | Cell division cycle 2 |
| Cdc25 | Cell division cycle 25 |
| CDK1 | Cyclin-dependent kinase 1 |
| cg | Cortical granule |
| COCs | Cumulus–oocyte complexes |
| CsA | Cyclosporin A |
| DSCN | Donor somatic cell nuclei |
| DAPI | 4′,6-Diamidino-2-phenylindole |
| dUTP | 2′-Deoxyuridine-5′-triphosphate |
| EA | Early autophagosome |
| EACs ECAR | Endocrine-active compounds Extracellular acidification rate |
| EDCs | Endocrine-disrupting chemicals |
| FAB | Fibrin-alginate hydrogel bead |
| FasL | Fas ligand |
| FasR | Fas receptor |
| FBS | Fetal bovine serum |
| FITC | Fluorescein-5-isothiocyanate |
| FOXO3a | Forkhead box O3a |
| FSH | Follicle-stimulating hormone |
| GAPDH | Glyceraldehyde 3-phosphate dehydrogenase |
| GSH GV | Glutathione Germinal vesicle |
| GVBD | Germinal vesicle breakdown |
| hCG HepG2 | Human chorionic gonadotropin Human hepatocarcinoma-derived cell lines |
| HSP27 | Heat shock protein 27 |
| ICSI | Intracytoplasmic sperm injection |
| IVF | In vitro fertilization |
| IVM | In vitro maturation |
| IVP | In vitro embryo production |
| LA | Late autophagosome |
| LC3 | Microtubule-associated protein 1A/1B light chain 3β |
| LD | Lipid droplets |
| LH | Luteinizing hormone |
| MII | Metaphase II |
| MM | Maturation medium |
| MPF | Maturation/meiosis-promoting factor |
| mPTP | Mitochondrial permeability transition pore |
| MQ | Molecular quality |
| mRNA | Messenger RNA |
| mt | Mitochondria |
| MtOR | MitoTracker Orange |
| NADPH | Nicotinamide adenine dinucleotide phosphate (reduced form) |
| Ndn | Nandrolone |
| NSCLC | Non-small cell lung cancer |
| OCR PB | Oxygen consumption rate Polar body |
| pFF | Porcine follicular fluid |
| PMSG | Pregnant mare serum gonadotropin |
| RA | Relative abundance |
| qRT-PCR | Quantitative reverse transcriptase real-time polymerase chain reaction |
| ROS | Reactive oxygen species |
| RT | Reverse transcription |
| SCNT | Somatic cell nuclear transfer |
| SIRT3 | Sirtuin 3 |
| TNF-β | Tumor necrosis factor-β |
| TUNEL | Terminal deoxynucleotidyl transferase-mediated dUTP nick-end labelling |
| Vnz | Vinclozolin |
| ZP | Zona pellucida |
References
- Gore, A.C.; Chappell, V.A.; Fenton, S.E.; Flaws, J.E.; Nadal, A.; Prins, G.S.; Toppari, J.; Zoeller, R.T. EDC-2: The Endocrine Society’s Second Scientific Statement on Endocrine-Disrupting Chemicals. Endocr. Rev. 2015, 36, E1–E150. [Google Scholar] [CrossRef] [PubMed]
- Combarnous, Y.; Nguyen, T.M.D. Comparative Overview of the Mechanisms of Action of Hormones and Endocrine Disruptor Compounds. Toxics 2019, 7, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Ravenzwaay, B.; Kolle, S.N.; Ramirez, T.; Kamp, H.G. Vinclozolin: A case study on the identification of endocrine active substances in the past and a future perspective. Toxicol. Lett. 2013, 223, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Kavlock, R.; Cummings, A. Mode of action: Inhibition of androgen receptor function--vinclozolin-induced malformations in reproductive development. Crit. Rev. Toxicol. 2005, 35, 721–726. [Google Scholar] [CrossRef]
- Kiparissis, Y.; Metcalfe, T.L.; Balch, G.C.; Metcalfe, C.D. Effects of the antiandrogens, vinclozolin and cyproterone acetate on gonadal development in the Japanese medaka (Oryzias latipes). Aquat. Toxicol. 2003, 63, 391–403. [Google Scholar] [CrossRef]
- Dang, Z.; Kienzler, A. Changes in fish sex ratio as a basis for regulating endocrine disruptors. Environ. Int. 2019, 130, 104928. [Google Scholar] [CrossRef]
- Nilsson, E.E.; Anway, M.D.; Stanfield, J.; Skinner, M.K. Transgenerational epigenetic effects of the endocrine disruptor vinclozolin on pregnancies and female adult onset disease. Reproduction 2008, 135, 713–721. [Google Scholar] [CrossRef] [Green Version]
- Buckley, J.; Willingham, E.; Agras, K.; Baskin, L.S. Embryonic exposure to the fungicide vinclozolin causes virilization of females and alteration of progesterone receptor expression in vivo: An experimental study in mice. Environ. Health 2006, 5, 4. [Google Scholar] [CrossRef] [Green Version]
- Knet, M.; Tabarowski, Z.; Slomczynska, M.; Duda, M. The effects of the environmental antiandrogen vinclozolin on the induction of granulosa cell apoptosis during follicular atresia in pigs. Theriogenology 2014, 81, 1239–1247. [Google Scholar] [CrossRef]
- Knet, M.; Wartalski, K.; Hoja-Lukowicz, D.; Tabarowski, Z.; Slomczynska, M.; Duda, M. Analysis of porcine granulosa cell death signaling pathways induced by vinclozolin. Theriogenology 2015, 84, 927–939. [Google Scholar] [CrossRef]
- Wartalski, K.; Knet-Seweryn, M.; Hoja-Lukowicz, D.; Tabarowski, Z.; Duda, M. Androgen receptor-mediated non-genomic effects of vinclozolin on porcine ovarian follicles and isolated granulosa cells: Vinclozolin and non-genomic effects in porcine ovarian follicles. Acta Histochem. 2016, 118, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Gorczyca, G.; Wartalski, K.; Tabarowski, Z.; Duda, M. Effects of vinclozolin exposure on the expression and activity of SIRT1 and SIRT6 in the porcine ovary. J. Physiol. Pharmacol. 2019, 70, 153–165. [Google Scholar]
- Kicman, A.T. Pharmacology of anabolic steroids. Br. J. Pharmacol. 2008, 154, 502–521. [Google Scholar] [CrossRef]
- Llewellyn, W. Part III: Drug profiles. In Anabolics; Llewellyn, W., Ed.; Molecular Nutrition LLC: Jupiter, FL, USA, 2011; pp. 739–780. [Google Scholar]
- Basaria, S.; Wahlstrom, J.T.; Dobs, A.S. Clinical review 138: Anabolic-androgenic steroid therapy in the treatment of chronic diseases. J. Clin. Endocrinol. Metab. 2001, 86, 5108–5117. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.A. Use of androgens in patients with renal failure. Semin. Dial. 2000, 13, 36–39. [Google Scholar] [CrossRef]
- Karbalay-Doust, S.; Noorafshan, A. Stereological study of the effects of nandrolone decanoate on the rat prostate. Micron 2006, 37, 617–623. [Google Scholar] [CrossRef]
- Arlt, W. Androgen therapy in women. Eur. J. Endocrinol. 2006, 154, 1–11. [Google Scholar] [CrossRef]
- Evans, N.A. Current concepts in anabolic-androgenic steroids. Am. J. Sports Med. 2004, 32, 534–542. [Google Scholar] [CrossRef]
- Bahrke, M.S.; Yesalis, C.E. Abuse of anabolic androgenic steroids and related substances in sport and exercise. Curr. Opin. Pharmacol. 2004, 4, 614–620. [Google Scholar] [CrossRef]
- Sjöqvist, F.; Garle, M.; Rane, A. Use of doping agents, particularly anabolic steroids, in sports and society. Lancet 2008, 371, 1872–1882. [Google Scholar] [CrossRef]
- Kanayama, G.; Pope, H.G., Jr. History and epidemiology of anabolic androgens in athletes and non-athletes. Mol. Cell. Endocrinol. 2017, 464, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Fink, J.; Schoenfeld, B.J.; Hackney, A.C.; Matsumoto, M.; Maekawa, T.; Nakazato, K.; Horie, S. Anabolic-androgenic steroids: Procurement and administration practices od doping athletes. Phys. Sportsmed. 2019, 47, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Iriart, J.A.; Chaves, J.C.; Orleans, R.G. Body cult and use of anabolic steroids by bodybuilders (Article in Portuguese). Cad. Saude Publica 2009, 25, 773–782. [Google Scholar] [CrossRef] [PubMed]
- Hall, R.C.W.; Hall, R.C.W. Abuse of supraphysiological doses of anabolic steroids. South. Med. J. 2005, 98, 550–555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cannavò, S.; Curtò, L.; Trimarchi, F. Exercise-related female reproductive dysfunction. J. Endocrinol. Investig. 2001, 24, 823–832. [Google Scholar] [CrossRef]
- Tripathi, A.; Tekkalaki, B.; Saxena, S.; Himanshu, D. Iatrogenic dependence of anabolic-androgenic steroid in Indian non-athletic women. BMJ Case Rep. 2014, 2014, bcr2013202472. [Google Scholar] [CrossRef] [Green Version]
- Belardin, L.B.; Simão, V.A.; Leite, G.A.A.; Chuffa, L.G.A.; Camargo, I.C.C. Dose-dependent effects and reversibility of the injuries caused by nandrolone decanoate in uterine tissue and fertility of rats. Birth Defects Res. B Dev. Reprod. Toxicol. 2014, 101, 168–177. [Google Scholar] [CrossRef]
- Kam, P.C.A.; Yarrow, M. Anabolic steroid abuse: Physiological and anaesthetic considerations. Anaesthesia 2005, 60, 685–692. [Google Scholar] [CrossRef]
- Groot, M.J.; Biolatti, B. Histopathological effects of boldenone in cattle. J. Vet. Med. A Physiol. Pathol. Clin. Med. 2004, 51, 58–63. [Google Scholar] [CrossRef]
- Patanè, F.G.; Liberto, A.; Maria Maglitto, A.N.; Malandrino, P.; Esposito, M.; Amico, F.; Cocimano, G.; Li Rosi, G.; Condorelli, D.; Di Nunno, N.; et al. Nandrolone Decanoate: Use, Abuse and Side Effects. Medicina 2020, 56, 606. [Google Scholar] [CrossRef]
- Elks, J.; Ganellin, C.R. The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Handelsman, D.J. Androgen physiology, pharmacology and abuse. In Endocrinology-E-Book: Adult and Pediatric, 6th ed.; Jameson, J.L., De Groot, L.J., Eds.; Elsevier Health Sciences: Amsterdam, The Netherlands, 2010; pp. 2469–2498. [Google Scholar]
- Agriesti, F.; Tataranni, T.; Pacelli, C.; Scrima, R.; Laurenzana, I.; Ruggieri, V.; Cela, O.; Mazzoccoli, C.; Salerno, M.; Sessa, F.; et al. Nandrolone induces a stem cell-like phenotype in human hepatocarcinoma-derived cell line inhibiting mitochondrial respiratory activity. Sci. Rep. 2020, 10, 2287. [Google Scholar] [CrossRef] [Green Version]
- Gorczyca, G.; Wartalski, K.; Wiater, J.; Samiec, M.; Tabarowski, Z.; Duda, M. Anabolic steroids-driven regulation of porcine ovarian putative stem cells favors the onset of their neoplastic transformation. Int. J. Mol. Sci. 2021, 22, 11800. [Google Scholar] [CrossRef] [PubMed]
- Mobini Far, H.R.; Agren, G.; Lindqvist, A.S.; Marmendal, M.; Fahlke, C.; Thiblin, I. Administration of the anabolic androgenic steroid nandrolone decanoate to female rats causes alterations in the morphology of their uterus and a reduction in reproductive capacity. Eur. J. Obstet. Gynecol. Reprod. Biol. 2007, 131, 189–197. [Google Scholar] [CrossRef]
- Chuffa, L.G.A.; Souza, R.B.; Frei, F.; Mesquita, S.F.P.; Camargo, I.C.C. Nandrolone decanoate and physical effort: Histological and morphometrical assessment in adult rat uterus. Anat. Rec. 2011, 294, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Karbalay-Doust, S.; Noorafshan, A. Stereological estimation of ovarian oocyte volume, surface area and number: Application on mice treated with nandrolone decanoate. Folia Histochem. Cytobiol. 2012, 50, 275–279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simão, V.A.; Berloffa Belardin, L.; Araujo Leite, G.A.; de Almeida Chuffa, L.G.; Camargo, I.C.C. Effects of different doses of nandrolone decanoate on estrous cycle and ovarian tissue of rats after treatment and recovery periods. Int. J. Exp. Pathol. 2015, 96, 338–349. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Laslop, N.; Mankin, A.S. How Macrolide Antibiotics Work. Trends Biochem. Sci. 2018, 43, 668–684. [Google Scholar] [CrossRef]
- Maarten, N.; Kuypers, D.R.J.; Minnie, S. Calcineurin inhibitor nephrotoxicity. Clin. J. Am. Soc. Nephrol. 2009, 4, 481–508. [Google Scholar]
- Russell, G.; Graveley, R.; Seid, J.; al-Humidan, A.K.; Skjodt, H. Mechanisms of action of cyclosporine and effects on connective tissues. Semin. Arthritis Rheum. 1992, 21, 16–22. [Google Scholar] [CrossRef]
- Wiesner, R.H.; Goldstein, R.M.; Donovan, J.P.; Miller, C.M.; Lake, J.R.; Lucey, M.R. The impact of cyclosporine dose and level on acute rejection and patient and graft survival in liver transplant recipients. Liver Transpl. Surg. 1998, 4, 34–41. [Google Scholar] [CrossRef]
- Efimov, S.V.; Dubinin, M.V.; Kobchikova, P.P.; Zgadzay, Y.O.; Khodov, I.A.; Belosludtsev, K.N.; Klochkov, V.V. Comparison of cyclosporin variants B–E based on their structural properties and activity in mitochondrial membranes. Biochem. Biophys. Res. Commun. 2020, 526, 1054–1060. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; He, L.; Lemasters, J.J. Mitochondrial permeability transition: A common pathway to necrosis and apoptosis. Biochem. Biophys. Res. Commun. 2003, 304, 463–470. [Google Scholar] [CrossRef]
- Sharov, V.G.; Todor, A.; Khanal, S.; Imai, M.; Sabbah, H.N. Cyclosporine A attenuates mitochondrial permeability transition and improves mitochondrial respiratory function in cardiomyocytes isolated from dogs with heart failure. J. Mol. Cell. Cardiol. 2007, 42, 150–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zakerkish, F.; Soriano, M.J.; Nocella-Mestre, E.; Brännström, M.; Díaz-García, C. Differential effects of the immunosuppressive calcineurin inhibitors cyclosporine-A and tacrolimus on ovulation in a murine model. Hum. Reprod. Open 2021, 2021, hoab012. [Google Scholar] [CrossRef]
- Groth, K.; Brännström, M.; Mölne, J.; Wranning, C.A. Cyclosporine A exposure during pregnancy in mice: Effects on reproductive performance in mothers and offspring. Hum. Reprod. 2010, 25, 697–704. [Google Scholar] [CrossRef] [Green Version]
- Camargo, I.C.C.; Leite, G.A.A.; Pinto, T.; Ribeiro-Paes, J.T. Histopathologycal findings in the ovaries and uterus of albino female rats promoted by co-administration of synthetic steroids and nicotine. Exp. Toxicol. Pathol. 2014, 66, 195–202. [Google Scholar] [CrossRef]
- Mesbah, F.; Bordbar, H.; Khozani, T.T.; Dehghani, F.; Mirkhani, H. The non-preventive effects of human menopausal gonadotropins on ovarian tissues in nandrolone decanoate-treated female rats: A histochemical and ultra-structural study. Int. J. Reprod. Biomed. 2018, 16, 159–174. [Google Scholar] [CrossRef] [Green Version]
- Motlik, J.; Crozet, N.; Fulka, J. Meiotic competence in vitro pf pig oocytes isolated from early antral follicles. J. Reprod. Fertil. 1984, 72, 323–328. [Google Scholar] [CrossRef] [Green Version]
- Marchal, R.; Feugang, J.M.; Perreau, C.; Venturi, E.; Terqui, M.; Mermillod, P. Meiotic and developmental competence of prepubertal and adult swine oocyte. Theriogenology 2001, 56, 17–29. [Google Scholar] [CrossRef]
- Wang, H.; Cui, W.; Meng, C.; Zhang, J.; Li, Y.; Qian, Y.; Xing, G.; Zhao, D.; Cao, S. MC1568 Enhances Histone Acetylation During Oocyte Meiosis and Improves Development of Somatic Cell Nuclear Transfer Embryos in Pig. Cell. Reprogram. 2018, 20, 55–65. [Google Scholar] [CrossRef]
- Samiec, M.; Skrzyszowska, M. High developmental capability of porcine cloned embryos following trichostatin A-dependent epigenomic transformation during in vitro maturation of oocytes pre-exposed to R-roscovitine. Anim. Sci. Pap. Rep. 2012, 30, 383–393. [Google Scholar]
- Gupta, M.K.; Heo, Y.T.; Kim, D.K.; Lee, H.T.; Uhm, S.J. 5-Azacytidine improves the meiotic maturation and subsequent in vitro development of pig oocytes. Anim. Reprod. Sci. 2019, 208, 106118. [Google Scholar] [CrossRef] [PubMed]
- Watson, A.J. Oocyte cytoplasmic maturation: A key mediator of oocyte and embryo developmental competence. J. Anim. Sci. 2007, 85 (Suppl. 13), E1–E3. [Google Scholar] [CrossRef] [Green Version]
- Coticchio, G.; Dal Canto, M.; Mignini Renzini, M.; Guglielmo, M.C.; Brambillasca, F.; Turchi, D.; Novara, P.V.; Fadini, R. Oocyte maturation: Gamete-somatic cells interactions, meiotic resumption, cytoskeletal dynamics and cytoplasmic reorganization. Hum. Reprod. Update 2015, 21, 427–454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conti, M.; Franciosi, F. Acquisition of oocyte competence to develop as an embryo: Integrated nuclear and cytoplasmic events. Hum. Reprod. Update 2018, 24, 245–266. [Google Scholar] [CrossRef]
- Morselli, M.G.; Luvoni, G.C.; Comizzoli, P. The nuclear and developmental competence of cumulus–oocyte complexes is enhanced by three-dimensional coculture with conspecific denuded oocytes during in vitro maturation in the domestic cat model. Reprod. Domest. Anim. 2017, 52, 82–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira, A.F.; Soares, M.; Almeida Reis, S.; Ramalho-Santos, J.; Sousa, A.P.; Almeida-Santos, T. Does supplementation with mitochondria improve oocyte competence? A systematic review. Reproduction 2021, 161, 269–287. [Google Scholar] [CrossRef]
- Takeda, K. Functional consequences of mitochondrial mismatch in reconstituted embryos and offspring. J. Reprod. Dev. 2019, 65, 485–489. [Google Scholar] [CrossRef] [Green Version]
- Lee, W.J.; Lee, J.H.; Jeon, R.H.; Jang, S.J.; Lee, S.C.; Park, J.S.; Lee, S.L.; King, W.A.; Rho, G.J. Supplement of autologous ooplasm into porcine somatic cell nuclear transfer embryos does not alter embryo development. Reprod. Domest. Anim. 2017, 52, 437–445. [Google Scholar] [CrossRef]
- Srirattana, K.; St. John, J.C. Additional mitochondrial DNA influences the interactions between the nuclear and mitochondrial genomes in a bovine embryo model of nuclear transfer. Sci. Rep. 2018, 8, 7246. [Google Scholar] [CrossRef]
- Eppig, J.J.; O’Brien, M.J. Development in vitro of mouse oocytes from primordial follicles. Biol. Reprod. 1996, 54, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Hunter, M.G. Oocyte maturation and ovum quality in pigs. Rev. Reprod. 2000, 5, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Cran, D.G. Qualitative and quantitative structural changes during pig oocyte maturation. J. Reprod. Fertil. 1985, 74, 237–245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sasseville, M.; Gagnon, M.C.; Guillemette, C.; Sullivan, R.; Gilchrist, R.B.; Richard, F.J. Regulation of gap junctions in porcine cumulus-oocyte complexes: Contributions of granulosa cell contact, gonadotropins, and lipid rafts. Mol. Endocrinol. 2009, 23, 700–710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santiquet, N.W.; Develle, Y.; Laroche, A.; Robert, C.; Richard, F.J. Regulation of gap-junctional communication between cumulus cells during in vitro maturation in swine, a gap-FRAP study. Biol. Reprod. 2012, 87, 46. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.J.; Wu, S.N.; Shen, J.P.; Wang, D.H.; Kong, X.W.; Lu, A.; Li, Y.J.; Zhou, H.X.; Zhao, Y.F.; Liang, C.G. The beneficial effects of cumulus cells and oocyte-cumulus cell gap junctions depends on oocyte maturation and fertilization methods in mice. PeerJ 2016, 4, e1761. [Google Scholar] [CrossRef]
- Milakovic, I.; Jeseta, M.; Hanulakova, S.; Knitlova, D.; Hanzalova, K.; Hulinska, P.; Machal, L.; Kempisty, B.; Antosik, P.; Machatkova, M. Energy Status Characteristics of Porcine Oocytes During In Vitro Maturation is Influenced by Their Meiotic Competence. Reprod. Domest. Anim. 2015, 50, 812–819. [Google Scholar] [CrossRef]
- Tsai, T.S.; Tyagi, S.; St. John, J.C. The molecular characterisation of mitochondrial DNA deficient oocytes using a pig model. Hum. Reprod. 2018, 33, 942–953. [Google Scholar] [CrossRef]
- Zhao, J.; Li, Y. Adenosine triphosphate content in human unfertilized oocytes, undivided zygotes and embryos unsuitable for transfer or cryopreservation. J. Int. Med. Res. 2012, 40, 734–739. [Google Scholar] [CrossRef]
- Zuidema, D.; Sutovsky, P. The domestic pig as a model for the study of mitochondrial inheritance. Cell Tissue Res. 2020, 380, 263–271. [Google Scholar] [CrossRef]
- Dumollard, R.; Duchen, M.; Carroll, J. The role of mitochondrial function in the oocyte and embryo. Curr. Top. Dev. Biol. 2007, 77, 21–49. [Google Scholar] [PubMed]
- Cagnone, G.L.; Tsai, T.S.; Makanji, Y.; Matthews, P.; Gould, J.; Bonkowski, M.S.; Elgass, K.D.; Wong, A.S.; Wu, L.E.; McKenzie, M.; et al. Restoration of normal embryogenesis by mitochondrial supplementation in pig oocytes exhibiting mitochondrial DNA deficiency. Sci. Rep. 2016, 6, 23229. [Google Scholar] [CrossRef] [PubMed]
- El Shourbagy, S.H.; Spikings, E.C.; Freitas, M.; St John, J.C. Mitochondria directly influence fertilisation outcome in the pig. Reproduction 2006, 131, 233–245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsai, T.S.; St John, J.C. The effects of mitochondrial DNA supplementation at the time of fertilization on the gene expression profiles of porcine preimplantation embryos. Mol. Reprod. Dev. 2018, 85, 490–504. [Google Scholar] [CrossRef]
- Labarta, E.; de Los Santos, M.J.; Herraiz, S.; Escribá, M.J.; Marzal, A.; Buigues, A.; Pellicer, A. Autologous mitochondrial transfer as a complementary technique to intracytoplasmic sperm injection to improve embryo quality in patients undergoing in vitro fertilization-a randomized pilot study. Fertil. Steril. 2019, 111, 86–96. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Mesalam, A.; Lee, K.L.; Song, S.H.; Khan, I.; Chowdhury, M.M.R.; Lv, W.; Kong, I.K. Improves the in vitro developmental competence and reprogramming efficiency of cloned bovine embryos by additional complimentary cytoplasm. Cell. Reprogram. 2019, 21, 51–60. [Google Scholar] [CrossRef] [Green Version]
- Bebbere, D.; Ulbrich, S.E.; Giller, K.; Zakhartchenko, V.; Reichenbach, H.D.; Reichenbach, M.; Verma, P.J.; Wolf, E.; Ledda, S.; Hiendleder, S. Mitochondrial DNA Depletion in Granulosa Cell Derived Nuclear Transfer Tissues. Front. Cell Dev. Biol. 2021, 9, 664099. [Google Scholar] [CrossRef]
- Song, S.H.; Lee, K.L.; Xu, L.; Joo, M.D.; Hwang, J.Y.; Oh, S.H.; Kong, I.K. Production of cloned cats using additional complimentary cytoplasm. Anim. Reprod. Sci. 2019, 208, 106125. [Google Scholar] [CrossRef]
- Babayev, E.; Seli, E. Oocyte mitochondrial function and reproduction. Curr. Opin. Obstet. Gynecol. 2015, 27, 175–181. [Google Scholar] [CrossRef] [Green Version]
- Srirattana, K.; St John, J.C. Manipulating the mitochondrial genome to enhance cattle embryo development. G3 (Bethesda) 2017, 7, 2065–2080. [Google Scholar] [CrossRef]
- Srirattana, K.; St John, J.C. Transmission of dysfunctional mitochondrial DNA and its implications for mammalian reproduction. Adv. Anat. Embryol. Cell Biol. 2019, 231, 75–103. [Google Scholar] [PubMed]
- Tokumoto, T.; Tokumoto, M.; Nagahama, M.Y. Induction and inhibition of oocyte maturation by EDCs in zebrafish. Reprod. Biol. Endocrinol. 2005, 3, 69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Angelo, J.; Freeman, E. Effects of endocrine-disrupting chemical exposure on zebrafish ovarian follicles. Bios 2017, 88, 9–18. [Google Scholar] [CrossRef]
- Roth, Z.; Komsky-Elbaz, A.; Kalo, D. Effect of environmental contamination on female and male gametes—A lesson from bovines. Anim. Reprod. 2020, 17, e20200041. [Google Scholar] [CrossRef] [PubMed]
- Jeong, P.S.; Lee, S.; Park, S.H.; Kim, M.J.; Kang, H.G.; Nanjidsuren, T.; Son, H.C.; Song, B.S.; Koo, D.B.; Sim, B.W.; et al. Butylparaben Is Toxic to Porcine Oocyte Maturation and Subsequent Embryonic Development Following In Vitro Fertilization. Int. J. Mol. Sci. 2020, 21, 3692. [Google Scholar] [CrossRef] [PubMed]
- Sobek, A.; Tkadlec, E.; Klaskova, E.; Prochazka, M. Cytoplasmic Transfer Improves Human Egg Fertilization and Embryo Quality: An Evaluation of Sibling Oocytes in Women with Low Oocyte Quality. Reprod. Sci. 2021, 28, 1362–1369. [Google Scholar] [CrossRef]
- Do, M.; Jang, W.G.; Hwang, J.; Jang, H.; Kim, E.J.; Jeong, E.J.; Shim, H.; Hwang, S.; Oh, K.; Byun, S.; et al. Inheritance of mitochondrial DNA in serially recloned pigs by somatic cell nuclear transfer (SCNT). Biochem. Biophys. Res. Commun. 2012, 424, 765–770. [Google Scholar] [CrossRef]
- Song, S.H.; Oh, S.H.; Xu, L.; Lee, K.L.; Hwang, J.Y.; Joo, M.D.; Kong, I.K. Effect of additional cytoplasm of cloned embryo on in vitro developmental competence and reprogramming efficiency in mice. Cell. Reprogram. 2020, 22, 236–243. [Google Scholar] [CrossRef]
- Srirattana, K.; Kaneda, M.; Parnpai, R. Strategies to Improve the Efficiency of Somatic Cell Nuclear Transfer. Int. J. Mol. Sci. 2022, 23, 1969. [Google Scholar] [CrossRef]
- Martinović, D.; Blake, L.S.; Durhan, E.J.; Greene, K.J.; Kahl, M.D.; Jensen, K.J.; Makynen, E.A.; Villeneuve, D.L.; Ankley, G.T. Reproductive toxicity of vinclozolin in the fathead minnow: Confirming an anti-androgenic mode of action. Environ. Toxicol. Chem. 2008, 27, 478–488. [Google Scholar] [CrossRef]
- Makynen, E.A.; Kahl, M.D.; Jensen, K.M.; Tietge, J.E.; Wells, K.L.; Van Der Kraak, G.; Ankley, G.T. Effects of the mammalian antiandrogen vinclozolin on development and reproduction of the fathead minnow (Pimephales promelas). Aquat. Toxicol. 2000, 48, 461–475. [Google Scholar] [CrossRef]
- Anway, M.D.; Skinner, M.K. Epigenetic programming of the germ line: Effects of endocrine disruptors on the development of transgenerational disease. Reprod. Biomed. Online 2008, 16, 23–25. [Google Scholar] [CrossRef]
- Uzumcu, M.; Zachow, R. Developmental exposure to environmental endocrine disruptors: Consequences within the ovary and on female reproductive function. Reprod. Toxicol. 2007, 23, 337–352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merviel, P.; Cabry, R.; Chardon, K.; Haraux, E.; Scheffler, F.; Mansouri, N.B.; Devaux, A.; Hikmat Chahine, H.; Bach, V.; Copin, H. Impact of oocytes with CLCG on ICSI outcomes and their potential relation to pesticide exposure. J. Ovarian Res. 2017, 10, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Renault, V.M.; Thekkat, P.U.; Hoang, K.L.; White, J.L.; Brad, C.A.; Kenzelmann Broz, D.; Venturelli, O.S.; Johnson, T.M.; Oskoui, P.R.; Xuan, Z.; et al. The pro-longevity gene FoxO3 is a direct target of the p53 tumor suppressor. Oncogene 2011, 30, 3207–3221. [Google Scholar] [CrossRef] [Green Version]
- Sionov, R.V.; Vlahopoulos, S.A.; Granot, Z. Regulation of Bim in Health and Disease. Oncotarget 2015, 6, 23058–23134. [Google Scholar] [CrossRef] [Green Version]
- Obexer, P.; Geiger, K.; Ambros, P.F.; Meister, B.; Ausserlechner, M.J. FKHRL1-mediated expression of Noxa and Bim induces apoptosis via the mitochondria in neuroblastoma cells. Cell Death Differ. 2007, 14, 534–547. [Google Scholar] [CrossRef] [Green Version]
- Hagenbuchner, J.; Kuznetsov, A.; Hermann, M.; Hausott, B.; Obexer, P.; Ausserlechner, M.J. FOXO3-induced reactive oxygen species are regulated by BCL2L11 (Bim) and SESN3. J. Cell Sci. 2012, 125, 1191–1203. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.S.; Joo, B.S.; Na, Y.J.; Yoon, M.S.; Choi, O.H.; Kim, W.W. Clinical assisted reproduction: Cumulus cells apoptosis as an indicator to predict the quality of oocytes and the outcome of IVF–ET. J. Assist. Reprod. Genet. 2001, 18, 490–498. [Google Scholar] [CrossRef]
- Kasof, G.M.; Gomes, B.C. Livin, a novel inhibitor of apoptosis protein family member. J. Biol. Chem. 2001, 276, 3238–3246. [Google Scholar] [CrossRef] [Green Version]
- Sugihara, E.; Hashimoto, N.; Osuka, S.; Shimizu, T.; Ueno, S.; Okazaki, S.; Yaguchi, T.; Kawakami, Y.; Kosaki, K.; Sato, T.A. The Inhibitor of Apoptosis Protein Livin Confers Resistance to Fas-Mediated Immune Cytotoxicity in Refractory Lymphoma. Cancer Res. 2020, 80, 4439–4450. [Google Scholar] [CrossRef] [PubMed]
- Aki, T.; Uemura, K. Cell Death and Survival Pathways Involving ATM Protein Kinase. Genes 2021, 12, 1581. [Google Scholar] [CrossRef] [PubMed]
- Rueda, C.B.; Llorente-Folch, I.; Traba, J.; Amigo, I.; Gonzalez-Sanchez, P.; Contreras, L.; Juaristi, I.; Martinez-Valero, P.; Pardo, B.; Del Arco, A.; et al. Glutamate excitotoxicity and Ca2+-regulation of respiration: Role of the Ca2+ activated mitochondrial transporters (CaMCs). Biochim. Biophys. Acta 2016, 1857, 1158–1166. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Fang, P.; Li, Y.; Kuo, Y.M.; Andrews, A.J.; Nanayakkara, G.; Johnson, C.; Fu, H.; Shan, H.; Du, F. Mitochondrial reactive oxygen species mediate lysophosphatidylcholine-induced endothelial cell activation. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 1090–1100. [Google Scholar] [CrossRef] [Green Version]
- Mazat, J.P.; Ransac, S.; Heiske, M.; Devin, A.; Rigoulet, M. Mitochondrial energetic metabolism-some general principles. IUBMB Life 2013, 65, 171–179. [Google Scholar] [CrossRef]
- Baines, C.P. Role of the mitochondrion in programmed necrosis. Front. Physiol. 2010, 1, 156. [Google Scholar] [CrossRef] [Green Version]
- Cabon, L.; Galán-Malo, P.; Bouharrour, A.; Delavallée, L.; Brunelle-Navas, M.N.; Lorenzo, H.K.; Gross, A.; Susin, S.A. BID regulates AIF-mediated caspase-independent necroptosis by promoting BAX activation. Cell Death Differ. 2012, 19, 245–256. [Google Scholar] [CrossRef]
- Chaube, S.K.; Prasad, P.V.; Thakur, S.C.; Shrivastav, T.G. Hydrogen peroxide modulates meiotic cell cycle and induces morphological features characteristic of apoptosis in rat oocytes cultured in vitro. Apoptosis 2005, 10, 863–874. [Google Scholar] [CrossRef]
- Yu, Y.; Dumollard, R.; Rossbach, A.; Lai, F.A.; Swann, K. Redistribution of mitochondria leads to bursts of ATP production during spontaneous mouse oocyte maturation. J. Cell. Physiol. 2010, 224, 672–680. [Google Scholar] [CrossRef] [Green Version]
- Marangos, P.; Carroll, J. The dynamics of cyclin B1 distribution during meiosis I in mouse oocytes. Reproduction 2004, 128, 153–162. [Google Scholar] [CrossRef] [Green Version]
- Conti, M.; Hsieh, M.; Zamah, A.M.; Oh, J.S. Novel signaling mechanisms in the ovary during oocyte maturation and ovulation. Mol. Cell. Endocrinol. 2012, 356, 65–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holt, J.E.; Lane, S.I.R.; Jones, K.T. The control of meiotic maturation in mammalian oocytes. Curr. Top. Dev. Biol. 2013, 102, 207–226. [Google Scholar] [PubMed]
- Prates, E.G.; Marques, C.C.; Baptista, M.C.; Vasques, M.I.; Carolino, N.; Horta, A.E.M.; Charneca, R.; Nunes, J.T.; Pereira, R.M. Fat area and lipid droplet morphology of porcine oocytes during in vitro maturation with trans-10, cis-12 conjugated linoleic acid and forskolin. Animal 2013, 7, 602–609. [Google Scholar] [CrossRef] [PubMed]
- Abazarikia, A.; Ariu, F.; Rasekhi, M.; Zhandi, M.; Ledda, S. Distribution and size of lipid droplets in oocytes recovered from young lamb and adult ovine ovaries. Reprod. Fertil. Dev. 2020, 32, 1022–1026. [Google Scholar] [CrossRef]
- He, B.; Yin, C.; Gong, Y.; Liu, J.; Guo, H.; Zhao, R. Melatonin-induced increase of lipid droplets accumulation and in vitro maturation in porcine oocytes is mediated by mitochondrial quiescence. J. Cell. Physiol. 2018, 233, 302–312. [Google Scholar] [CrossRef]
- Bordbar, H.; Mesbah, F.; Talaei, T.; Dehghani, F.; Mirkhani, H. Modulatory effect of gonadotropins on rats’ ovaries after nandrolone decanoate administration: A stereological study. Iran. J. Med. Sci. 2014, 39, 44–50. [Google Scholar]
- Carteri, R.B.; Kopczynski, A.; Menegassi, L.N.; Rodolphi, M.S.; Strogulski, N.R.; Portela, L.V. Anabolic-androgen steroids effects on bioenergetics responsiveness of synaptic and extrasynaptic mitochondria. Toxicol. Lett. 2019, 307, 72–80. [Google Scholar] [CrossRef]
- Nagano, M.; Katagiri, S.; Takahashi, Y. ATP content and maturational/developmental ability of bovine oocytes with various cytoplasmic morphologies. Zygote 2006, 14, 299–304. [Google Scholar] [CrossRef] [Green Version]
- Duran, H.E.; Simsek-Duran, F.; Oehninger, S.C.; Jones, H.W., Jr.; Castora, F.J. The association of reproductive senescence with mitochondrial quantity, function, and DNA integrity in human oocytes at different stages of maturation. Fertil. Steril. 2011, 96, 384–388. [Google Scholar] [CrossRef]
- Kumar, M.; Pathak, D.; Kriplani, A.; Ammini, A.C.; Talwar, P.; Dada, R. Nucleotide variations in mitochondrial DNA and supra-physiological ROS levels in cytogenetically normal cases of premature ovarian insufficiency. Arch. Gynecol. Obstet. 2010, 282, 695–705. [Google Scholar] [CrossRef]
- Lanneau, D.; Brunet, M.; Frisan, E.; Solary, E.; Fontenay, M.; Garrido, C. Heat shock proteins: Essential proteins for apoptosis regulation. J. Cell. Mol. Med. 2008, 12, 743–761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bodzek, P.; Damasiewicz-Bodzek, A.; Janosz, I.; Witek, L.; Olejek, A. Heat shock protein 27 (HSP27) in patients with ovarian cancer. Ginekol. Pol. 2021, 92, 837–843. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, M.T.; Dejardin, E.; dos Santos, N.R. Context-dependent roles for lymphotoxin-β receptor signaling in cancer development. Biochim. Biophys. Acta 2016, 1865, 204–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dzafic, E.; Stimpfel, M.; Virant-Klun, I. Plasticity of granulosa cells: On the crossroad of stemness and transdifferentiation potential. J. Assist. Reprod. Genet. 2013, 30, 1255–1261. [Google Scholar] [CrossRef] [Green Version]
- Dompe, C.; Kulus, M.; Stefańska, K.; Kranc, W.; Chermuła, B.; Bryl, R.; Pieńkowski, W.; Nawrocki, M.J.; Petitte, J.N.; Stelmach, B.; et al. Human granulosa cells—stemness properties, molecular cross-talk and follicular angiogenesis. Cells 2021, 10, 1396. [Google Scholar] [CrossRef]
- Skinner, M.K.; Schmidt, M.; Savenkova, M.I.; Sadler-Riggleman, I.; Nilsson, E.E. Regulation of granulosa and theca cell transcriptomes during ovarian antral follicle development. Mol. Reprod. Dev. 2008, 75, 1457–1472. [Google Scholar] [CrossRef] [Green Version]
- Lavranos, T.C.; Rodgers, H.F.; Bertoncello, I.; Rodgers, R.J. Anchorage-independent culture of bovine granulosa cells: The effects of basic fibroblast growth factor and dibutyryl cAMP on cell division and differentiation. Exp. Cell Res. 1994, 211, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Kossowska-Tomaszczuk, K.; De Geyter, C.; De Geyter, M.; Martin, I.; Holzgreve, W.; Scherberich, A.; Zhang, H. The multipotency of luteinizing granulosa cells collected from mature ovarian follicles. Stem Cells 2009, 27, 210–219. [Google Scholar] [CrossRef]
- Riva, F.; Omes, C.; Bassani, R.; Nappi, R.E.; Mazzini, G.; Cornaglia, A.I.; Casasco, A. In-vitro culture system for mesenchymal progenitor cells derived from waste human ovarian follicular fluid. Reprod. Biomed. Online 2014, 29, 457–469. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Chu, K.; Wang, Y.; Li, J.; Fu, J.; Zeng, Y.A.; Li, W. Procr-expressing granulosa cells are highly proliferative and are important for follicle development. iScience 2021, 24, 102065. [Google Scholar] [CrossRef]
- Oki, Y.; Ono, H.; Motohashi, T.; Sugiura, N.; Nobusue, H.; Kano, K. Dedifferentiated follicular granulosa cells derived from pig ovary can transdifferentiate into osteoblasts. Biochem. J. 2012, 447, 239–248. [Google Scholar] [CrossRef] [Green Version]
- Merkwitz, C.; Ricken, A.M.; Lösche, A.; Sakurai, M.; Spanel-Borowski, K. Progenitor cells harvested from bovine follicles become endothelial cells. Differentiation 2010, 79, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Elsherif, S.; Bourne, M.; Soule, E.; Lall, C.; Bhosale, P. Multimodality imaging and genomics of granulosa cell tumors. Abdom. Radiol. 2020, 45, 812–827. [Google Scholar] [CrossRef] [PubMed]
- Pectasides, D.; Pectasides, E.; Psyrri, A. Granulosa cell tumor of the ovary. Cancer Treat. Rev. 2008, 34, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Sekkate, S.; Kairouani, M.; Serji, B.; Tazi, A.; Mrabti, H.; Boutayeb, S.; Errihani, H. Ovarian granulosa cell tumors: A retrospective study of 27 cases and a review of the literature. World J. Surg. Oncol. 2013, 11, 142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiesner, R.; Rabkin, J.; Klintmalm, G.; McDiarmid, S.; Langnas, A.; Punch, J.; McMaster, P.; Kalayoglu, M.; Levy, G.; Freeman, R.; et al. A randomized double-blind comparative study of mycophenolate mofetil and azathioprine in combination with cyclosporine and corticosteroids in primary liver transplant recipients. Liver Transpl. 2001, 7, 442–450. [Google Scholar] [CrossRef]
- Ahlbach, C.L.; Lexa, K.W.; Bockus, A.T.; Chen, V.; Crews, P.; Jacobson, M.P.; Lokey, R.S. Beyond cyclosporine A: Conformation-dependent passive membrane permeabilities of cyclic peptide natural products. Future Med. Chem. 2015, 7, 2121–2130. [Google Scholar] [CrossRef] [Green Version]
- Jimenez, R.; Galan, A.I.; Gonzalez de Buitrago, J.M.; Palomero, J.; Munoz, M.E. Glutathione Metabolism In Cyclosporine A-Treated Rats: Dose-And Time-Related Changes In Liver And Kidney. Clin. Exp. Pharmacol. Physiol. 2000, 27, 991–996. [Google Scholar] [CrossRef]
- Pallet, N.; Bouvier, N.; Legendre, C.; Gilleron, J.; Codogno, P.; Beaune, P.; Thervet, E.; Anglicheau, D. Autophagy protects renal tubular cells against cyclosporine toxicity. Autophagy 2008, 4, 783–791. [Google Scholar] [CrossRef] [Green Version]
- Pallet, N.; Bouvier, N.; Bandjallabah, A.; Rabant, M.; Flinois, J.P.; Hertig, A.; Legendre, C.; Beaune, P.; Thervet, E.; Anglicheau, D. Cyclosporine-induced endoplasmic reticulum stress triggers tubular phenotypic changes and death. Am. J. Transplant. 2008, 8, 2283–2296. [Google Scholar] [CrossRef]
- Pallet, N.; Anglicheau, D. Autophagy: A protective mechanism against nephrotoxicant-induced renal injury. Kidney Int. 2009, 75, 118–119. [Google Scholar] [CrossRef] [Green Version]
- Ogata, M.; Hino, S.; Saito, A.; Morikawa, K.; Kondo, S.; Kanemoto, S.; Murakami, T.; Taniguchi, M.; Tanii, I.; Yoshinaga, K. Autophagy is activated for cell survival after endoplasmic reticulum stress. Mol. Cell. Biol. 2006, 26, 9220–9231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciechomska, I.A.; Gabrusiewicz, K.; Szczepankiewicz, A.; Kaminska, B. Endoplasmic reticulum stress triggers autophagy in malignant glioma cells undergoing cyclosporine A-induced cell death. Oncogene 2013, 32, 1518–1529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, Z.B.; Lan, G.C.; Wu, Y.G.; Han, D.; Feng, W.G.; Wang, J.Z.; Tan, J.H. Interactive effects of granulosa cell apoptosis, follicle size, cumulus–oocyte complex morphology, and cumulus expansion on the developmental competence of goat oocytes: A study using the well-in-drop culture system. Reproduction 2006, 132, 749–758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nunnari, J.; Suomalainen, A. Mitochondria: In sickness and in health. Cell 2012, 148, 1145–1159. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Hekimi, S. Mitochondrial dysfunction and longevity in animals: Untangling the knot. Science 2015, 350, 1204–1207. [Google Scholar] [CrossRef]
- Jiang, K.; He, B.; Lai, L.; Chen, Q.; Liu, Y.; Guo, Q.; Wang, Q. Cyclosporine A inhibits breast cancer cell growth by downregulating the expression of pyruvate kinase subtype M2. Int. J. Mol. Med. 2012, 30, 302–308. [Google Scholar] [CrossRef]
- Jeon, S.H.; Piao, Y.J.; Choi, K.J.; Hong, F.; Baek, H.W.; Kang, I.; Ha, J.; Kim, S.S.; Chang, S.G. Prednisolone suppresses cyclosporin A-induced apoptosis but not cell cycle arrest in MDCK cells. Arch. Biochem. Biophys. 2005, 435, 382–392. [Google Scholar] [CrossRef]
- Roy, M.K.; Takenaka, M.; Kobori, M.; Nakahara, K.; Isobe, S.; Tsushida, T. Apoptosis, necrosis and cell proliferation-inhibition by cyclosporine A in U937 cells (a human monocytic cell line). Pharmacol. Res. 2006, 53, 293–302. [Google Scholar] [CrossRef]
- Zorova, L.D.; Popkov, V.A.; Plotnikov, E.Y.; Silachev, D.N.; Pevzner, I.B.; Jankauskas, S.S.; Babenko, V.A.; Zorov, S.D.; Balakireva, A.V.; Juhaszova, M.; et al. Mitochondrial membrane potential. Anal. Biochem. 2018, 552, 50–59. [Google Scholar] [CrossRef]
- Zeng, H.T.; Ren, Z.; Yeung, W.S.; Shu, Y.M.; Xu, Y.W.; Zhuang, G.L.; Liang, X.Y. Low mitochondrial DNA and ATP contents contribute to the absence of birefringent spindle imaged with PolScope in in vitro matured human oocytes. Hum. Reprod. 2007, 22, 1681–1686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gore-Langton, R.E. Cyclosporine differentially affects estrogen and progestin synthesis by rat granulosa cells in vitro. Mol. Cell. Endocrinol. 1988, 57, 187–198. [Google Scholar] [CrossRef]
- Ross, H.J.; Cho, J.; Osann, K.; Wong, S.F.; Ramsinghani, N.; Williams, J.; Downey-Hurtado, N.; Slater, L.M. Phase I/II trial of low dose cyclosporin A with EP for advanced non-small cell lung cancer. Lung Cancer 1997, 18, 189–198. [Google Scholar] [CrossRef]
- Qin, X.; Chen, Z. Metabolic dependence of cyclosporine A on cell proliferation of human non-small cell lung cancer A549 cells and its implication in post-transplant malignancy. Oncol. Rep. 2019, 41, 2997–3004. [Google Scholar] [CrossRef] [PubMed]
- Cevik, O.; Turut, F.A.; Acidereli, H.; Yildirim, S. Cyclosporine-A induces apoptosis in human prostate cancer cells PC3 and DU145 via downregulation of COX-2 and upregulation of TGFβ. Turk. Biyokim. Derg. 2019, 44, 47–54. [Google Scholar] [CrossRef]
- Pedersen, H.S.; Løvendahl, P.; Nikolaisen, N.K.; Holm, P.; Hyttel, P.; Nyengaard, J.R.; Chen, F.; Callesen, H. Mitochondrial dynamics in pre- and post-pubertal pig oocytes before and after in vitro maturation. Reprod. Fertil. Dev. 2014, 26, 189–190. [Google Scholar] [CrossRef]
- Gorczyca, G.; Wartalski, K.; Tabarowski, Z.; Duda, M. Proteolytically Degraded Alginate Hydrogels and Hydrophobic Microbioreactors for Porcine Oocyte Encapsulation. J. Vis. Exp. 2019, 161, e61325. [Google Scholar] [CrossRef]
- Pawlak, P.; Warzych, E.; Chabowska, A.; Lechniak, D. Differences in cytoplasmic maturation between the BCB+ and control porcine oocytes do not justify application of the BCB test for a standard IVM protocol. J. Reprod. Dev. 2014, 60, 28–36. [Google Scholar] [CrossRef] [Green Version]
- Jia, B.Y.; Xiang, D.C.; Shao, Q.Y.; Zhang, B.; Liu, S.N.; Hong, Q.H.; Quan, G.B.; Wu, G.Q. Inhibitory effects of astaxanthin on postovulatory porcine oocyte aging in vitro. Sci. Rep. 2020, 10, 20217. [Google Scholar] [CrossRef]
- Matsuda, F.; Inoue, N.; Maeda, A.; Cheng, Y.; Sai, T.; Gonda, H.; Goto, Y.; Sakamaki, K.; Manabe, N. Expression and function of apoptosis initiator FOXO3 in granulosa cells during follicular atresia in pig ovaries. J. Reprod. Dev. 2011, 57, 151–158. [Google Scholar] [CrossRef] [Green Version]
- Jin, D.; Tan, H.J.; Lei, T.; Gan, L.; Chen, X.D.; Long, Q.Q.; Feng, B.; Yang, Z.Q. Molecular cloning and characterization of porcine sirtuin genes. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2009, 153, 348–358. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.K.; Zhao, M.H.; Kwon, J.W.; Li, Y.H.; Lin, Z.L.; Jin, Y.X.; Kim, N.H.; Cui, X.S. The association of mitochondrial potential and copy number with pig oocyte maturation and developmental potential. J. Reprod. Dev. 2014, 60, 128–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
- Duda, M.; Durlej, M.; Knet, M.; Knapczyk-Stwora, K.; Tabarowski, Z.; Slomczynska, M. Does 2-hydroxyflutamide inhibit apoptosis in porcine granulosa cells?—An in vitro study. J. Reprod. Dev. 2012, 58, 438–444. [Google Scholar] [CrossRef] [Green Version]
- Romek, M.; Gajda, B.; Rolka, M.; Smorąg, Z. Mitochondrial activity and morphology in developing porcine oocytes and pre-implantation non-cultured and cultured embryos. Reprod. Domest. Anim. 2011, 46, 471–480. [Google Scholar] [CrossRef]
- De Moura, M.B.; Van Houten, B. Bioenergetic analysis of intact mammalian cells using the Seahorse XF24 Extracellular Flux analyzer and a luciferase ATP assay. In Molecular Toxicology Protocols; Keohavong, P., Grant, S., Eds.; Humana Press: Totowa, NJ, USA, 2014; pp. 589–602. [Google Scholar]
- Muller, B.; Lewis, N.; Adeniyi, T.; Leese, H.J.; Brison, D.R.; Sturmey, R.G. Application of extracellular flux analysis for determining mitochondrial function in mammalian oocytes and early embryos. Sci. Rep. 2019, 9, 16778. [Google Scholar] [CrossRef] [Green Version]
- Romek, M.; Kucia, M.; Gajda, B.; Krzysztofowicz, E.; Smorag, Z. Effect of high hydrostatic pressure on mitochondrial activity, reactive oxygen species level and developmental competence of cultured pig embryos. Theriogenology 2019, 68, 99–108. [Google Scholar] [CrossRef]
- Adegoke, E.O.; Xue, W.; Machebe, N.S.; Adeniran, S.O.; Hao, W.; Chen, W.; Han, Z.; Guixue, Z.; Peng, Z. Sodium Selenite inhibits mitophagy, downregulation and mislocalization of blood-testis barrier proteins of bovine Sertoli cell exposed to microcystin-leucine arginine (MC-LR) via TLR4/NF-κB and mitochondrial signaling pathways blockage. Ecotoxicol. Environ. Saf. 2018, 166, 165–175. [Google Scholar] [CrossRef]


| Gene | F/R | Primer Sequence (5′→3′) | Tm (°C) | Reference |
|---|---|---|---|---|
| GAPDH | F | CCCACGAGCACACCTCAGAA | 55.9 | [161] |
| R | TGCAGCCTGTACTCCCGCT | 55.4 | [161] | |
| GPX4 | F | ATTCTCAGCCAAGGACATCG | 51.8 | [162] |
| R | CCTCATTGAGAGGCCACATT | 51.8 | [162] | |
| FOXO3 | F | GGGGAGTTTGGTCAATCAGA | 51.8 | [163] |
| R | TGCATAGACTGGCTGACAGG | 53.8 | [163] | |
| SIRT3 | F | CAGCGGCATTCCAGACTTCA | 53.8 | [164] |
| R | GTCCCAACCATCAAACTTTCCA | 53.0 | [164] | |
| CASP3 | F | GAGGCAGACTTCTTGTATGC | 51.8 | [162] |
| R | CATGGACACAATACATGGAA | 47.7 | [162] | |
| LC3 | F | CCGAACCTTCGAACAGAGAG | 53.8 | [162] |
| R | AGGCTTGGTTAGCATTGAGC | 51.8 | [162] | |
| CDC2 | F | TGGGCACTCCCAATAATGAA | 49.7 | [165] |
| R | TCCAAGCCATTTTCATCCAA | 47.7 | [165] | |
| CCNB1 | F | GCTCCAGTGCTCTGCTTCTC | 55.9 | [165] |
| R | ACAAACTTTATTAAAAGTAAATAAGTG | 47.6 | [165] | |
| ATP5A1 | F | AGTTGCTGAAGCAAGGACAGTAT | 53.5 | [161] |
| R | GTGTTGGCTGATAACGTGAGAC | 54.8 | [161] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gorczyca, G.; Wartalski, K.; Romek, M.; Samiec, M.; Duda, M. The Molecular Quality and Mitochondrial Activity of Porcine Cumulus–Oocyte Complexes Are Affected by Their Exposure to Three Endocrine-Active Compounds under 3D In Vitro Maturation Conditions. Int. J. Mol. Sci. 2022, 23, 4572. https://doi.org/10.3390/ijms23094572
Gorczyca G, Wartalski K, Romek M, Samiec M, Duda M. The Molecular Quality and Mitochondrial Activity of Porcine Cumulus–Oocyte Complexes Are Affected by Their Exposure to Three Endocrine-Active Compounds under 3D In Vitro Maturation Conditions. International Journal of Molecular Sciences. 2022; 23(9):4572. https://doi.org/10.3390/ijms23094572
Chicago/Turabian StyleGorczyca, Gabriela, Kamil Wartalski, Marek Romek, Marcin Samiec, and Małgorzata Duda. 2022. "The Molecular Quality and Mitochondrial Activity of Porcine Cumulus–Oocyte Complexes Are Affected by Their Exposure to Three Endocrine-Active Compounds under 3D In Vitro Maturation Conditions" International Journal of Molecular Sciences 23, no. 9: 4572. https://doi.org/10.3390/ijms23094572
APA StyleGorczyca, G., Wartalski, K., Romek, M., Samiec, M., & Duda, M. (2022). The Molecular Quality and Mitochondrial Activity of Porcine Cumulus–Oocyte Complexes Are Affected by Their Exposure to Three Endocrine-Active Compounds under 3D In Vitro Maturation Conditions. International Journal of Molecular Sciences, 23(9), 4572. https://doi.org/10.3390/ijms23094572






