Liver Steatosis: A Marker of Metabolic Risk in Children
Abstract
:1. Introduction
2. Methods
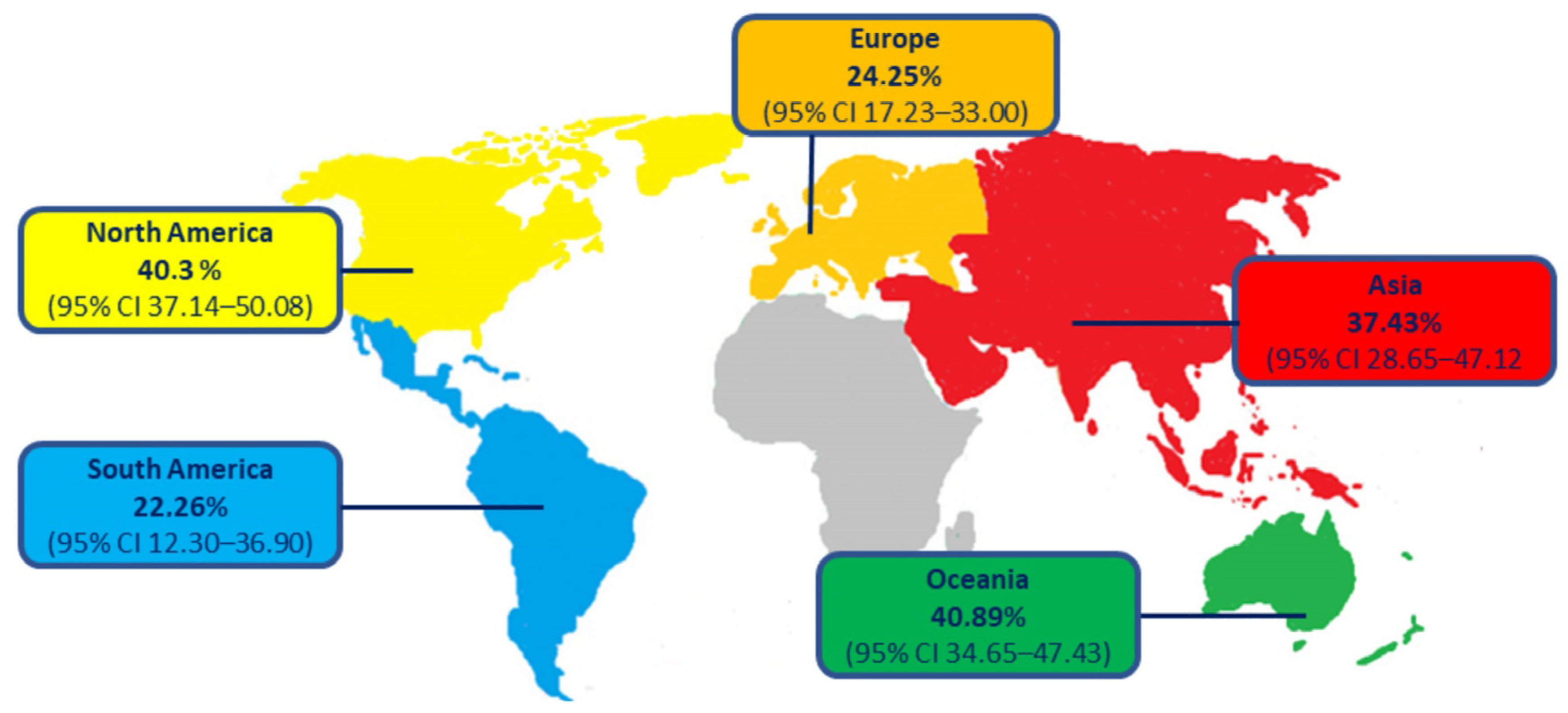
3. Epidemiology
4. Genetic Risk Factors
5. NAFLD and Metabolic Dysregulation
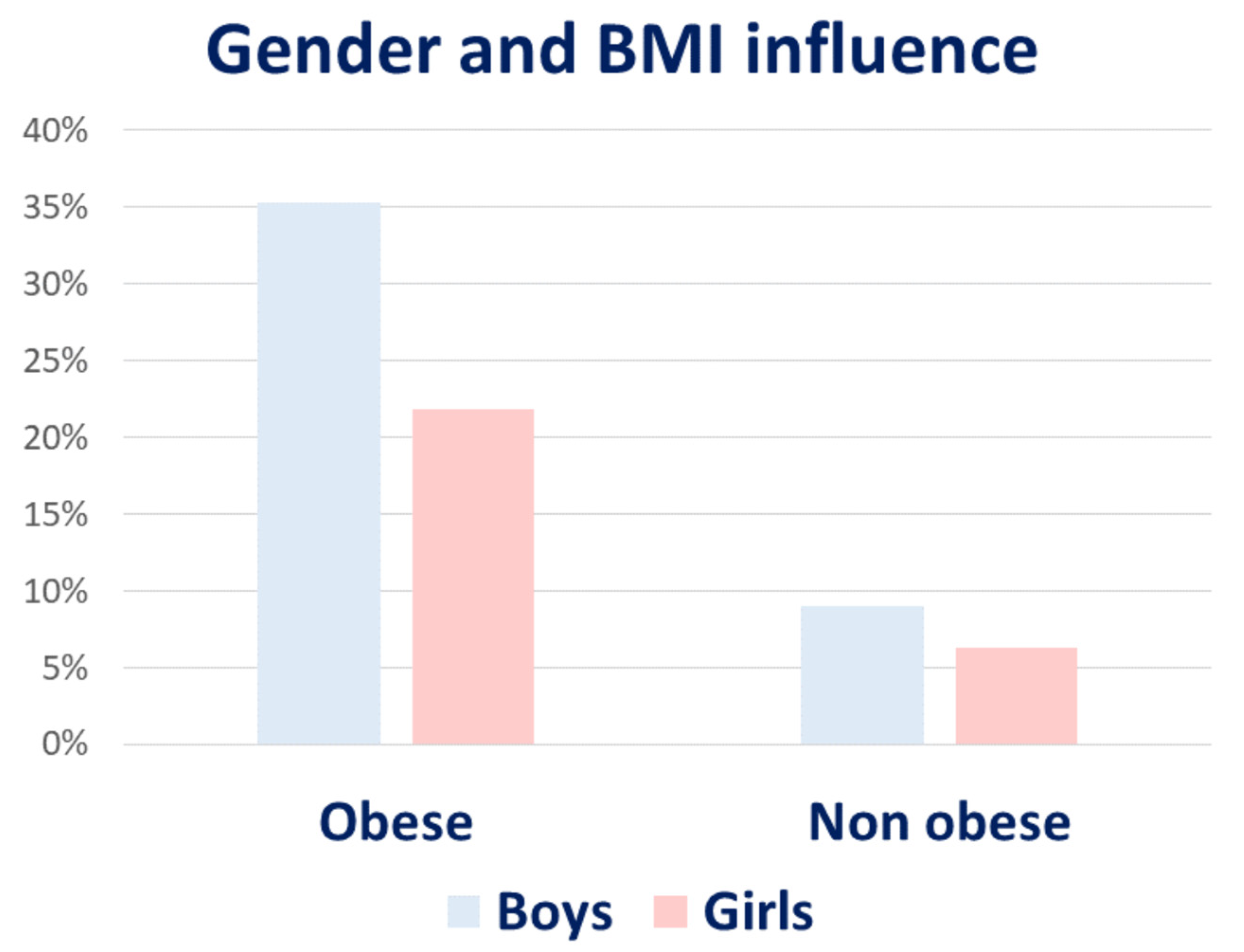
5.1. Obesity and Diet
5.2. Insulin Resistance and T2DM
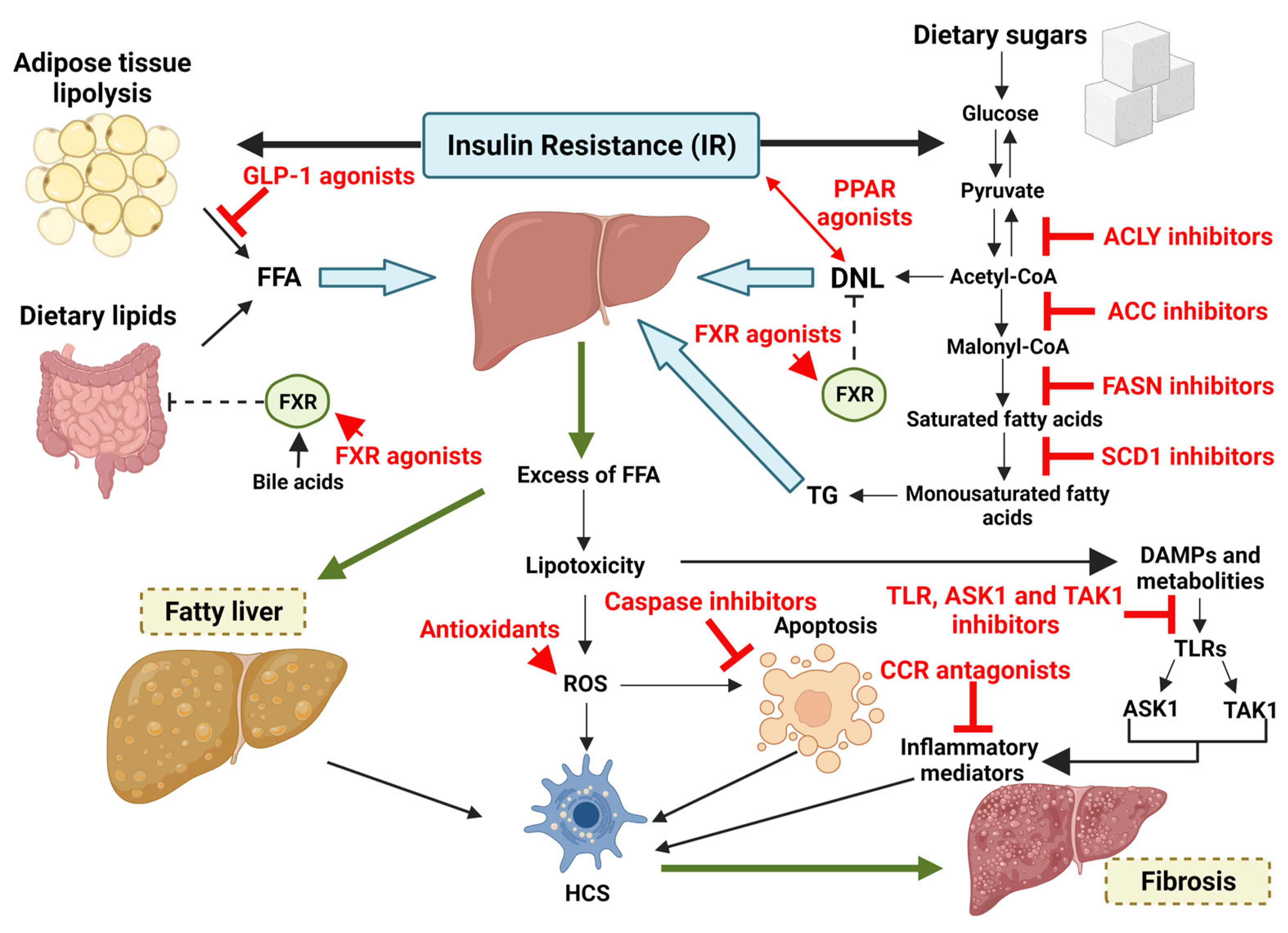
5.3. Alterations in Lipid Metabolism
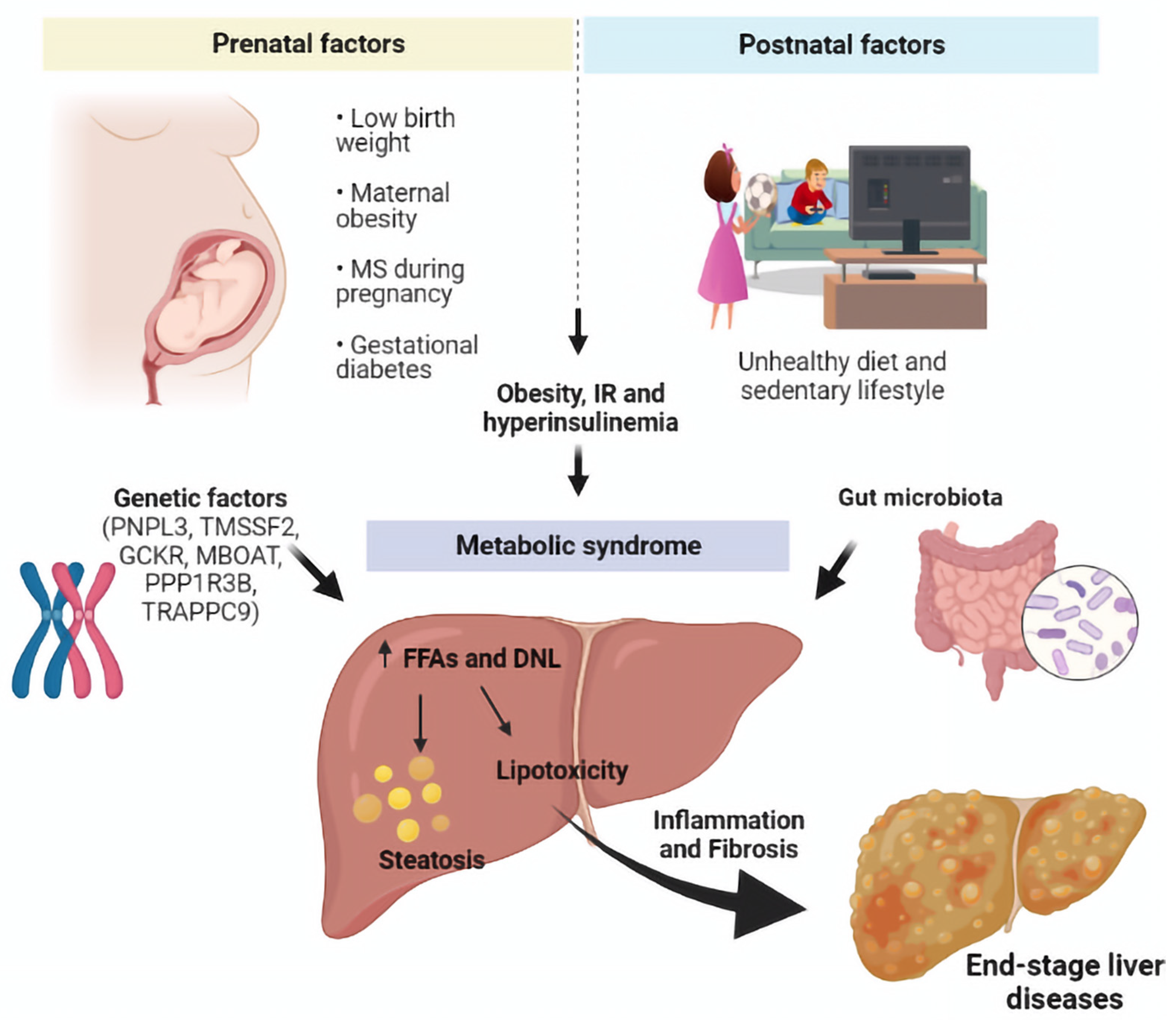
5.4. Prenatal Factors
6. The Role of Microbiota
7. Pathogenesis
8. Diagnosis and Outcomes
9. Treatment
10. Pharmacological Treatment
11. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Schwimmer, J.B.; Deutsch, R.; Kahen, T.; Lavine, J.E.; Stanley, C.; Behling, C. Prevalence of Fatty Liver in Children and Adolescents. Pediatrics 2006, 118, 1388–1393. [Google Scholar] [CrossRef] [PubMed]
- Wieckowska, A.; Feldstein, A.E. Diagnosis of Nonalcoholic Fatty Liver Disease: Invasive versus Noninvasive. Semin. Liver Dis. 2008, 28, 386–395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nobili, V.; Alisi, A.; Newton, K.P.; Schwimmer, J.B. Comparison of the Phenotype and Approach to Pediatric vs Adult Patients with Nonalcoholic Fatty Liver Disease. Gastroenterology 2016, 150, 1798–1810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brumbaugh, D.E.; Friedman, J.E. Developmental Origins of Nonalcoholic Fatty Liver Disease. Pediatr. Res. 2014, 75, 140–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jou, J.; Choi, S.S.; Diehl, A.M. Mechanisms of Disease Progression in Nonalcoholic Fatty Liver Disease. Semin. Liver Dis. 2008, 28, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Vajro, P.; Lenta, S.; Socha, P.; Dhawan, A.; McKiernan, P.; Baumann, U.; Durmaz, O.; Lacaille, F.; McLin, V.; Nobili, V. Diagnosis of Nonalcoholic Fatty Liver Disease in Children and Adolescents: Position Paper of the ESPGHAN Hepatology Committee. J. Pediatr. Gastroenterol. Nutr. 2012, 54, 700–713. [Google Scholar] [CrossRef]
- Geh, D.; Manas, D.M.; Reeves, H.L. Hepatocellular Carcinoma in Non-Alcoholic Fatty Liver Disease-a Review of an Emerging Challenge Facing Clinicians. Hepatobiliary Surg. Nutr. 2021, 10, 59–75. [Google Scholar] [CrossRef]
- Flisiak-Jackiewicz, M.; Bobrus-Chociej, A.; Wasilewska, N.; Lebensztejn, D.M. From Nonalcoholic Fatty Liver Disease (NAFLD) to Metabolic Dysfunction-Associated Fatty Liver Disease (MAFLD)—New Terminology in Pediatric Patients as a Step in Good Scientific Direction? J. Clin. Med. 2021, 10, 924. [Google Scholar] [CrossRef]
- Eslam, M.; Sanyal, A.J.; George, J.; Sanyal, A.; Neuschwander-Tetri, B.; Tiribelli, C.; Kleiner, D.E.; Brunt, E.; Bugianesi, E.; Yki-Järvinen, H.; et al. MAFLD: A Consensus-Driven Proposed Nomenclature for Metabolic Associated Fatty Liver Disease. Gastroenterology 2020, 158, 1999–2014.e1. [Google Scholar] [CrossRef]
- Eslam, M.; Alkhouri, N.; Vajro, P.; Baumann, U.; Weiss, R.; Socha, P.; Marcus, C.; Lee, W.S.; Kelly, D.; Porta, G.; et al. Defining Paediatric Metabolic (Dysfunction)-Associated Fatty Liver Disease: An International Expert Consensus Statement. Lancet Gastroenterol. Hepatol. 2021, 6, 864–873. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Rinella, M.E.; Sanyal, A.J.; Harrison, S.A.; Brunt, E.M.; Goodman, Z.; Cohen, D.E.; Loomba, R. From NAFLD to MAFLD: Implications of a Premature Change in Terminology. Hepatology 2021, 73, 1194–1198. [Google Scholar] [CrossRef]
- Huang, Q.; Zou, X.; Wen, X.; Zhou, X.; Ji, L. NAFLD or MAFLD: Which Has Closer Association With All-Cause and Cause-Specific Mortality?—Results From NHANES III. Front. Med. 2021, 8, 693507. [Google Scholar] [CrossRef]
- Lin, S.; Huang, J.; Wang, M.; Kumar, R.; Liu, Y.; Liu, S.; Wu, Y.; Wang, X.; Zhu, Y. Comparison of MAFLD and NAFLD Diagnostic Criteria in Real World. Liver Int. 2020, 40, 2082–2089. [Google Scholar] [CrossRef]
- Anderson, E.L.; Howe, L.D.; Jones, H.E.; Higgins, J.P.T.; Lawlor, D.A.; Fraser, A. The Prevalence of Non-Alcoholic Fatty Liver Disease in Children and Adolescents: A Systematic Review and Meta-Analysis. PLoS ONE 2015, 10, e0140908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stanfield, D.; Lucey, M.R. The Heightened Risk of Fatty Liver Disorders in the Time of COVID-19. Mayo Clin. Proc. 2020, 95, 2580–2581. [Google Scholar] [CrossRef] [PubMed]
- CDC. Childhood Obesity Facts. Overweight & Obesity. Available online: https://www.cdc.gov/obesity/data/childhood.html (accessed on 22 February 2022).
- Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 23 January 2022).
- Welsh, J.A.; Karpen, S.; Vos, M.B. Increasing Prevalence of Nonalcoholic Fatty Liver Disease Among United States Adolescents, 1988–1994 to 2007–2010. J. Pediatr. 2013, 162, 496. [Google Scholar] [CrossRef] [Green Version]
- Le Garf, S.; Nègre, V.; Anty, R.; Gual, P. Metabolic Fatty Liver Disease in Children: A Growing Public Health Problem. Biomedicines 2021, 9, 1915. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Mu, C.; Li, K.; Luo, H.; Liu, Y.; Li, Z. Estimating Global Prevalence of Metabolic Dysfunction-Associated Fatty Liver Disease in Overweight or Obese Children and Adolescents: Systematic Review and Meta-Analysis. Int. J. Public Health 2021, 66, 1604371. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Wang, M.; Zheng, S.; Xia, M.; Yang, H.; Zhang, D.; Yin, C.; Cheng, N.; Bai, Y. Comparing the Diagnostic Criteria of MAFLD and NAFLD in the Chinese Population: A Population-Based Prospective Cohort Study. J. Clin. Transl. Hepatol. 2022, 10, 6–16. [Google Scholar] [CrossRef]
- Rundle, A.G.; Park, Y.; Herbstman, J.B.; Kinsey, E.W.; Wang, Y.C. COVID-19-Related School Closings and Risk of Weight Gain Among Children. Obesity 2020, 28, 1008–1009. [Google Scholar] [CrossRef] [Green Version]
- Di Renzo, L.; Gualtieri, P.; Pivari, F.; Soldati, L.; Attinà, A.; Cinelli, G.; Cinelli, G.; Leggeri, C.; Caparello, G.; Barrea, L.; et al. Eating Habits and Lifestyle Changes during COVID-19 Lockdown: An Italian Survey. J. Transl. Med. 2020, 18, 229. [Google Scholar] [CrossRef] [PubMed]
- Fore, H.H.; Dongyu, Q.; Beasley, D.M.; Ghebreyesus, T.A. Child Malnutrition and COVID-19: The Time to Act Is Now. Lancet 2020, 396, 517–518. [Google Scholar] [CrossRef]
- Kim, D.; Kim, W.R.; Kim, H.J.; Therneau, T.M. Association between Noninvasive Fibrosis Markers and Mortality among Adults with Nonalcoholic Fatty Liver Disease in the United States. Hepatology 2013, 57, 1357–1365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nobili, V.; Svegliati-Baroni, G.; Alisi, A.; Miele, L.; Valenti, L.; Vajro, P. A 360-Degree Overview of Paediatric NAFLD: Recent Insights. J. Hepatol. 2013, 58, 1218–1229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Wu, M.; Liu, Z.; Yuan, H.; Wu, X.; Shi, T.; Chen, X.; Zhang, T. Increasing Prevalence of NAFLD/NASH among Children, Adolescents and Young Adults from 1990 to 2017: A Population-Based Observational Study. BMJ Open 2021, 11, e042843. [Google Scholar] [CrossRef]
- Guerrero, R.; Vega, G.L.; Grundy, S.M.; Browning, J.D. Ethnic Differences in Hepatic Steatosis: An Insulin Resistance Paradox? Hepatology 2009, 49, 791–801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Y.C.; Wu, C.C.; Ni, Y.H. New Perspectives on Genetic Prediction for Pediatric Metabolic Associated Fatty Liver Disease. Front. Pediatr. 2020, 8, 849. [Google Scholar] [CrossRef]
- Romeo, S.; Kozlitina, J.; Xing, C.; Pertsemlidis, A.; Cox, D.; Pennacchio, L.A.; Boerwinkle, E.; Cohen, J.C.; Hobbs, H.H. Genetic Variation in PNPLA3 Confers Susceptibility to Nonalcoholic Fatty Liver Disease. Nat. Genet. 2008, 40, 1461. [Google Scholar] [CrossRef] [Green Version]
- Draijer, L.; Benninga, M.; Koot, B. Pediatric NAFLD: An Overview and Recent Developments in Diagnostics and Treatment. Expert Rev. Gastroenterol. Hepatol. 2019, 13, 447–461. [Google Scholar] [CrossRef]
- Eslam, M.; Valenti, L.; Romeo, S. Genetics and Epigenetics of NAFLD and NASH: Clinical Impact. J. Hepatol. 2018, 68, 268–279. [Google Scholar] [CrossRef]
- Estes, C.; Razavi, H.; Loomba, R.; Younossi, Z.; Sanyal, A.J. Modeling the Epidemic of Nonalcoholic Fatty Liver Disease Demonstrates an Exponential Increase in Burden of Disease. Hepatology 2018, 67, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Loomba, R.; Schork, N.; Chen, C.H.; Bettencourt, R.; Bhatt, A.; Ang, B.; Nguyen, P.; Hernandez, C.; Richards, L.; Salotti, J.; et al. Heritability of Hepatic Fibrosis and Steatosis Based on a Prospective Twin Study. Gastroenterology 2015, 149, 1784–1793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwimmer, J.B.; Celedon, M.A.; Lavine, J.E.; Salem, R.; Campbell, N.; Schork, N.J.; Shiehmorteza, M.; Yokoo, T.; Chavez, A.; Middleton, M.S.; et al. Heritability of Nonalcoholic Fatty Liver Disease. Gastroenterology 2009, 136, 1585–1592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makkonen, J.; Pietiläinen, K.H.; Rissanen, A.; Kaprio, J.; Yki-Järvinen, H. Genetic Factors Contribute to Variation in Serum Alanine Aminotransferase Activity Independent of Obesity and Alcohol: A Study in Monozygotic and Dizygotic Twins q. J. Hepatol. 2009, 50, 1035–1042. [Google Scholar] [CrossRef]
- Kozlitina, J.; Smagris, E.; Stender, S.; Nordestgaard, B.G.; Zhou, H.H.; Tybjærg-Hansen, A.; Vogt, T.F.; Hobbs, H.H.; Cohen, J.C. Exome-Wide Association Study Identifies a TM6SF2 Variant That Confers Susceptibility to Nonalcoholic Fatty Liver Disease. Nat. Genet. 2014, 46, 352. [Google Scholar] [CrossRef] [Green Version]
- Mancina, R.M.; Dongiovanni, P.; Petta, S.; Pingitore, P.; Meroni, M.; Rametta, R.; Borén, J.; Montalcini, T.; Pujia, A.; Wiklund, O.; et al. The MBOAT7-TMC4 Variant Rs641738 Increases Risk of Nonalcoholic Fatty Liver Disease in Individuals of European Descent. Gastroenterology 2016, 150, 1219–1230.e6. [Google Scholar] [CrossRef] [Green Version]
- Nobili, V.; Alisi, A.; Valenti, L.; Miele, L.; Feldstein, A.E.; Alkhouri, N. NAFLD in Children: New Genes, New Diagnostic Modalities and New Drugs. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 517–530. [Google Scholar] [CrossRef]
- Dongiovanni, P.; Petta, S.; Maglio, C.; Fracanzani, A.L.; Pipitone, R.; Mozzi, E.; Motta, B.M.; Kaminska, D.; Rametta, R.; Grimaudo, S.; et al. Transmembrane 6 Superfamily Member 2 Gene Variant Disentangles Nonalcoholic Steatohepatitis from Cardiovascular Disease. Hepatology 2015, 61, 506–514. [Google Scholar] [CrossRef]
- Liu, Y.L.; Reeves, H.L.; Burt, A.D.; Tiniakos, D.; McPherson, S.; Leathart, J.B.S.; Allison, M.E.D.; Alexander, G.J.; Piguet, A.C.; Anty, R.; et al. TM6SF2 Rs58542926 Influences Hepatic Fibrosis Progression in Patients with Non-Alcoholic Fatty Liver Disease. Nat. Commun. 2014, 5, 4309. [Google Scholar] [CrossRef] [Green Version]
- Wattacheril, J.; Lavine, J.E.; Chalasani, N.P.; Guo, X.; Kwon, S.; Schwimmer, J.; Molleston, J.P.; Loomba, R.; Brunt, E.M.; Chen, Y.D.I.; et al. Genome-Wide Associations Related to Hepatic Histology in Nonalcoholic Fatty Liver Disease in Hispanic Boys. J. Pediatr. 2017, 190, 100–107.e2. [Google Scholar] [CrossRef]
- Cefalù, A.B.; Pirruccello, J.P.; Noto, D.; Gabriel, S.; Valenti, V.; Gupta, N.; Spina, R.; Tarugi, P.; Kathiresan, S.; Averna, M.R. A Novel APOB Mutation Identified by Exome Sequencing Cosegregates with Steatosis, Liver Cancer and Hypocholesterolemia. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 2021–2025. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donati, B.; Pietrelli, A.; Pingitore, P.; Dongiovanni, P.; Caddeo, A.; Walker, L.; Baselli, G.; Pelusi, S.; Rosso, C.; Vanni, E.; et al. Telomerase Reverse Transcriptase Germline Mutations and Hepatocellular Carcinoma in Patients with Nonalcoholic Fatty Liver Disease. Cancer Med. 2017, 6, 1930–1940. [Google Scholar] [CrossRef] [PubMed]
- Calado, R.T.; Regal, J.A.; Kleiner, D.E.; Schrump, D.S.; Peterson, N.R.; Pons, V.; Chanock, S.J.; Lansdorp, P.M.; Young, N.S. A Spectrum of Severe Familial Liver Disorders Associate with Telomerase Mutations. PLoS ONE 2009, 4, e7926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suomela, E.; Oikonen, M.; Pitkänen, N.; Ahola-Olli, A.; Virtanen, J.; Parkkola, R.; Jokinen, E.; Laitinen, T.; Hutri-Kähönen, N.; Kähönen, M.; et al. Childhood Predictors of Adult Fatty Liver. The Cardiovascular Risk in Young Finns Study. J. Hepatol. 2016, 65, 784–790. [Google Scholar] [CrossRef] [Green Version]
- Zusi, C.; Mantovani, A.; Olivieri, F.; Morandi, A.; Corradi, M.; Miraglia Del Giudice, E.; Dauriz, M.; Valenti, L.; Byrne, C.D.; Targher, G.; et al. Contribution of a Genetic Risk Score to Clinical Prediction of Hepatic Steatosis in Obese Children and Adolescents. Dig. Liver Dis. 2019, 51, 1586–1592. [Google Scholar] [CrossRef]
- Sun, C.; Fan, J.G.; Qiao, L. Potential Epigenetic Mechanism in Non-Alcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2015, 16, 5161–5179. [Google Scholar] [CrossRef]
- Bayoumi, A.; Grønbæk, H.; George, J.; Eslam, M. The Epigenetic Drug Discovery Landscape for Metabolic-Associated Fatty Liver Disease. Trends Genet. TIG 2020, 36, 429–441. [Google Scholar] [CrossRef]
- Willeit, P.; Skroblin, P.; Kiechl, S.; Fernández-Hernando, C.; Mayr, M. Liver MicroRNAs: Potential Mediators and Biomarkers for Metabolic and Cardiovascular Disease? Eur. Heart J. 2016, 37, 3260–3266. [Google Scholar] [CrossRef] [Green Version]
- Weiland, M.; Gao, X.H.; Zhou, L.; Mi, Q.S. Small RNAs Have a Large Impact: Circulating MicroRNAs as Biomarkers for Human Diseases. RNA Biol. 2012, 9, 850–859. [Google Scholar] [CrossRef] [Green Version]
- Hegazy, M.A.; Abd ALgwad, I.; Abuel Fadl, S.; Sayed Hassan, M.; Ahmed Rashed, L.; Hussein, M.A. Serum Micro-RNA-122 Level as a Simple Noninvasive Marker of MAFLD Severity. Diabetes Metab. Syndr. Obes. Targets Ther. 2021, 14, 2247–2254. [Google Scholar] [CrossRef]
- Chai, C.; Rivkin, M.; Berkovits, L.; Simerzin, A.; Zorde-Khvalevsky, E.; Rosenberg, N.; Klein, S.; Yaish, D.; Durst, R.; Shpitzen, S.; et al. Metabolic Circuit Involving Free Fatty Acids, MicroRNA 122, and Triglyceride Synthesis in Liver and Muscle Tissues. Gastroenterology 2017, 153, 1404–1415. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.J.; Cheung, R.; Ahmed, A. Nonalcoholic Steatohepatitis Is the Most Rapidly Growing Indication for Liver Transplantation in Patients with Hepatocellular Carcinoma in the U.S. Hepatology 2014, 59, 2188–2195. [Google Scholar] [CrossRef] [PubMed]
- Brandt, S.; Roos, J.; Inzaghi, E.; Kotnik, P.; Kovac, J.; Battelino, T.; Cianfarani, S.; Nobili, V.; Colajacomo, M.; Kratzer, W.; et al. Circulating Levels of MiR-122 and Nonalcoholic Fatty Liver Disease in Pre-Pubertal Obese Children. Pediatr. Obes. 2018, 13, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Xu, M.; Cao, Z.; Li, W.; Chen, L.; Xie, X.; Wang, W.; Liu, J. Ultrasound-Assisted MiR-122-Loaded Polymeric Nanodroplets for Hepatocellular Carcinoma Gene Therapy. Mol. Pharm. 2020, 17, 541–553. [Google Scholar] [CrossRef] [PubMed]
- Becker, P.P.; Rau, M.; Schmitt, J.; Malsch, C.; Hammer, C.; Bantel, H.; Möllhaupt, B.; Geier, A. Performance of Serum MicroRNAs-122,-192 and-21 as Biomarkers in Patients with Non-Alcoholic Steatohepatitis. PLoS ONE 2015, 10, e0142661. [Google Scholar] [CrossRef] [PubMed]
- Pirola, C.J.; Gianotti, T.F.; Castaño, G.O.; Mallardi, P.; Martino, J.S.; Ledesma, M.M.G.L.; Flichman, D.; Mirshahi, F.; Sanyal, A.J.; Sookoian, S. Circulating MicroRNA Signature in Non-Alcoholic Fatty Liver Disease: From Serum Non-Coding RNAs to Liver Histology and Disease Pathogenesis. Gut 2015, 64, 800–812. [Google Scholar] [CrossRef] [Green Version]
- Tan, Y.; Ge, G.; Pan, T.; Wen, D.; Gan, J. A Pilot Study of Serum MicroRNAs Panel as Potential Biomarkers for Diagnosis of Nonalcoholic Fatty Liver Disease. PLoS ONE 2014, 9, e105192. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.L.; Pan, Q.; Cao, H.X.; Xin, F.Z.; Zhao, Z.H.; Yang, R.X.; Zeng, J.; Zhou, H.; Fan, J.G. Lipotoxic Hepatocyte-Derived Exosomal MicroRNA 192-5p Activates Macrophages Through Rictor/Akt/Forkhead Box Transcription Factor O1 Signaling in Nonalcoholic Fatty Liver Disease. Hepatology 2020, 72, 454–469. [Google Scholar] [CrossRef]
- Long, J.K.; Dai, W.; Zheng, Y.W.; Zhao, S.P. MiR-122 Promotes Hepatic Lipogenesis via Inhibiting the LKB1/AMPK Pathway by Targeting Sirt1 in Non-Alcoholic Fatty Liver Disease. Mol. Med. Camb. Mass 2019, 25, 26. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.L.; Cao, H.X.; Wang, B.C.; Xin, F.Z.; Zhang, R.N.; Zhou, D.; Yang, R.X.; Zhao, Z.H.; Pan, Q.; Fan, J.G. MiR-192-5p Regulates Lipid Synthesis in Non-Alcoholic Fatty Liver Disease through SCD-1. World J. Gastroenterol. 2017, 23, 8140. [Google Scholar] [CrossRef]
- Iguchi, T.; Niino, N.; Tamai, S.; Sakurai, K.; Mori, K. Comprehensive Analysis of Circulating MicroRNA Specific to the Liver, Heart, and Skeletal Muscle of Cynomolgus Monkeys. Int. J. Toxicol. 2017, 36, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Socha, P.; Wierzbicka, A.; Neuhoff-Murawska, J.; Włodarek, D.; Podleśny, J.; Socha, J.; Choroba, N.; Wątroby, S.; Cecha, J.; Metabolicznego, Z. Nonalcoholic fatty liver disease as a feature of the metabolic syndrome. Rocz. Panstw. Zakl. Hig. 2007, 58, 129–137. [Google Scholar] [PubMed]
- Mikolasevic, I.; Milic, S.; Wensveen, T.T.; Grgic, I.; Jakopcic, I.; Stimac, D.; Wensveen, F.; Orlic, L. Nonalcoholic Fatty Liver Disease—A Multisystem Disease? World J. Gastroenterol. 2016, 22, 9488. [Google Scholar] [CrossRef] [PubMed]
- Selvakumar, P.K.C.; Kabbany, M.N.; Nobili, V.; Alkhouri, N. Nonalcoholic Fatty Liver Disease in Children: Hepatic and Extrahepatic Complications. Pediatr. Clin. N. Am. 2017, 64, 659–675. [Google Scholar] [CrossRef] [PubMed]
- Lonardo, A.; Ballestri, S.; Marchesini, G.; Angulo, P.; Loria, P. Nonalcoholic Fatty Liver Disease: A Precursor of the Metabolic Syndrome. Dig. Liver Dis. 2015, 47, 181–190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parsons, T.J.; Power, C.; Logan, S.; Summerbell, C.D. Childhood Predictors of Adult Obesity: A Systematic Review. Int. J. Obes. 1999, 23 (Suppl. S8), S1–S107. [Google Scholar]
- Guo, S.S.; Roche, A.F.; Chumlea, W.C.; Gardner, J.D.; Siervogel, R.M. The Predictive Value of Childhood Body Mass Index Values for Overweight at Age 35 y. Am. J. Clin. Nutr. 1994, 59, 810–819. [Google Scholar] [CrossRef] [Green Version]
- Zimmermann, E.; Gamborg, M.; Holst, C.; Baker, J.L.; Sørensen, T.I.A.; Berentzen, T.L. Body Mass Index in School-Aged Children and the Risk of Routinely Diagnosed Non-Alcoholic Fatty Liver Disease in Adulthood: A Prospective Study Based on the Copenhagen School Health Records Register. BMJ Open 2015, 5, e006998. [Google Scholar] [CrossRef] [Green Version]
- Leung, J.C.F.; Loong, T.C.W.; Wei, J.L.; Wong, G.L.H.; Chan, A.W.H.; Choi, P.C.L.; Shu, S.S.T.; Chim, A.M.L.; Chan, H.L.Y.; Wong, V.W.S. Histological Severity and Clinical Outcomes of Nonalcoholic Fatty Liver Disease in Nonobese Patients. Hepatology 2017, 65, 54–64. [Google Scholar] [CrossRef]
- Shulman, G.I. Ectopic Fat in Insulin Resistance, Dyslipidemia, and Cardiometabolic Disease. N. Engl. J. Med. 2014, 371, 2237–2238. [Google Scholar] [CrossRef] [PubMed]
- Mokha, J.S.; Srinivasan, S.R.; DasMahapatra, P.; Fernandez, C.; Chen, W.; Xu, J.; Berenson, G.S. Utility of Waist-to-Height Ratio in Assessing the Status of Central Obesity and Related Cardiometabolic Risk Profile among Normal Weight and Overweight/Obese Children: The Bogalusa Heart Study. BMC Pediatr. 2010, 10, 73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawano, Y.; Cohen, D.E. Mechanisms of Hepatic Triglyceride Accumulation in Non-Alcoholic Fatty Liver Disease. J. Gastroenterol. 2013, 48, 434–441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rius, B.; López-Vicario, C.; González-Périz, A.; Morán-Salvador, E.; García-Alonso, V.; Clària, J.; Titos, E. Resolution of Inflammation in Obesity-Induced Liver Disease. Front. Immunol. 2012, 3, 257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mendez-Sanchez, N.; Cruz-Ramon, V.C.; Ramirez-Perez, O.L.; Hwang, J.P.; Barranco-Fragoso, B.; Cordova-Gallardo, J. New Aspects of Lipotoxicity in Nonalcoholic Steatohepatitis. Int. J. Mol. Sci. 2018, 19, 2034. [Google Scholar] [CrossRef] [Green Version]
- Nobili, V.; Liccardo, D.; Bedogni, G.; Salvatori, G.; Gnani, D.; Bersani, I.; Alisi, A.; Valenti, L.; Raponi, M. Influence of Dietary Pattern, Physical Activity, and I148M PNPLA3 on Steatosis Severity in at-Risk Adolescents. Genes Nutr. 2014, 9, 392. [Google Scholar] [CrossRef] [Green Version]
- Dongiovanni, P.; Lanti, C.; Riso, P.; Valenti, L. Nutritional Therapy for Nonalcoholic Fatty Liver Disease. J. Nutr. Biochem. 2016, 29, 1–11. [Google Scholar] [CrossRef]
- Vandevijvere, S.; De Ridder, K.; Fiolet, T.; Bel, S.; Tafforeau, J. Consumption of Ultra-Processed Food Products and Diet Quality among Children, Adolescents and Adults in Belgium. Eur. J. Nutr. 2019, 58, 3267–3278. [Google Scholar] [CrossRef] [PubMed]
- Oddy, W.H.; Herbison, C.E.; Jacoby, P.; Ambrosini, G.L.; O’Sullivan, T.A.; Ayonrinde, O.T.; Olynyk, J.K.; Black, L.J.; Beilin, L.J.; Mori, T.A.; et al. The Western Dietary Pattern Is Prospectively Associated with Nonalcoholic Fatty Liver Disease in Adolescence. Am. J. Gastroenterol. 2013, 108, 778–785. [Google Scholar] [CrossRef] [Green Version]
- Després, J.P.; Lemieux, I. Abdominal Obesity and Metabolic Syndrome. Nature 2006, 444, 881–887. [Google Scholar] [CrossRef] [PubMed]
- Vacca, M.; Allison, M.; Griffin, J.L.; Vidal-Puig, A. Fatty Acid and Glucose Sensors in Hepatic Lipid Metabolism: Implications in NAFLD. Semin. Liver Dis. 2015, 35, 250–261. [Google Scholar] [CrossRef] [PubMed]
- Santoro, N.; Savoye, M.; Kim, G.; Marotto, K.; Shaw, M.M.; Pierpont, B.; Caprio, S. Hepatic Fat Accumulation Is Modulated by the Interaction between the Rs738409 Variant in the PNPLA3 Gene and the Dietary Omega6/Omega3 PUFA Intake. PLoS ONE 2012, 7, e37827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daniel, P.V.; Mondal, P. Causative and Sanative Dynamicity of ChREBP in Hepato-Metabolic Disorders. Eur. J. Cell Biol. 2020, 99, 151128. [Google Scholar] [CrossRef] [PubMed]
- Vos, M.B.; Lavine, J.E. Dietary Fructose in Nonalcoholic Fatty Liver Disease. Hepatology 2013, 57, 2525–2531. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, J.M.; Noworolski, S.M.; Erkin-Cakmak, A.; Korn, N.J.; Wen, M.J.; Tai, V.W.; Jones, G.M.; Palii, S.P.; Velasco-Alin, M.; Pan, K.; et al. Effects of Dietary Fructose Restriction on Liver Fat, De Novo Lipogenesis, and Insulin Kinetics in Children with Obesity. Gastroenterology 2017, 153, 743–752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Softic, S.; Gupta, M.K.; Wang, G.X.; Fujisaka, S.; O’Neill, B.T.; Rao, T.N.; Willoughby, J.; Harbison, C.; Fitzgerald, K.; Ilkayeva, O.; et al. Divergent Effects of Glucose and Fructose on Hepatic Lipogenesis and Insulin Signaling. J. Clin. Investig. 2017, 127, 4059–4074. [Google Scholar] [CrossRef] [Green Version]
- Mosca, A.; Nobili, V.; De Vito, R.; Crudele, A.; Scorletti, E.; Villani, A.; Alisi, A.; Byrne, C.D. Serum Uric Acid Concentrations and Fructose Consumption Are Independently Associated with NASH in Children and Adolescents. J. Hepatol. 2017, 66, 1031–1036. [Google Scholar] [CrossRef] [Green Version]
- Lustig, R.H.; Mulligan, K.; Noworolski, S.M.; Tai, V.W.; Wen, M.J.; Erkin-Cakmak, A.; Gugliucci, A.; Schwarz, J.M. Isocaloric Fructose Restriction and Metabolic Improvement in Children with Obesity and Metabolic Syndrome. Obesity 2016, 24, 453–460. [Google Scholar] [CrossRef]
- Schwimmer, J.B.; Ugalde-Nicalo, P.; Welsh, J.A.; Angeles, J.E.; Cordero, M.; Harlow, K.E.; Alazraki, A.; Durelle, J.; Knight-Scott, J.; Newton, K.P.; et al. Effect of a Low Free Sugar Diet vs Usual Diet on Nonalcoholic Fatty Liver Disease in Adolescent Boys: A Randomized Clinical Trial. JAMA 2019, 321, 256–265. [Google Scholar] [CrossRef] [Green Version]
- Angelico, F.; Del Ben, M.; Conti, R.; Francioso, S.; Feole, K.; Maccioni, D.; Antonini, T.M.; Alessandri, C. Non-Alcoholic Fatty Liver Syndrome: A Hepatic Consequence of Common Metabolic Diseases. J. Gastroenterol. Hepatol. 2003, 18, 588–594. [Google Scholar] [CrossRef]
- Angelico, F.; Del Ben, M.; Conti, R.; Francioso, S.; Feole, K.; Fiorello, S.; Cavallo, M.G.; Zalunardo, B.; Lirussi, F.; Alessandri, C.; et al. Insulin Resistance, the Metabolic Syndrome, and Nonalcoholic Fatty Liver Disease. J. Clin. Endocrinol. Metab. 2005, 90, 1578–1582. [Google Scholar] [CrossRef]
- Nobili, V.; Marcellini, M.; Devito, R.; Ciampalini, P.; Piemonte, F.; Comparcola, D.; Sartorelli, M.R.; Angulo, P. NAFLD in Children: A Prospective Clinical-Pathological Study and Effect of Lifestyle Advice. Hepatology 2006, 44, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Burgert, T.S.; Taksali, S.E.; Dziura, J.; Goodman, T.R.; Yeckel, C.W.; Papademetris, X.; Constable, R.T.; Weiss, R.; Tamborlane, W.V.; Savoye, M.; et al. Alanine Aminotransferase Levels and Fatty Liver in Childhood Obesity: Associations with Insulin Resistance, Adiponectin, and Visceral Fat. J. Clin. Endocrinol. Metab. 2006, 91, 4287–4294. [Google Scholar] [CrossRef] [PubMed]
- Patton, H.M.; Yates, K.; Unalp-Arida, A.; Behling, C.A.; Huang, T.T.K.; Rosenthal, P.; Sanyal, A.J.; Schwimmer, J.B.; Lavine, J.E. Association Between Metabolic Syndrome and Liver Histology Among Children with Nonalcoholic Fatty Liver Disease. Am. J. Gastroenterol. 2010, 105, 2093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilcox, G. Insulin and Insulin Resistance. Clin. Biochem. Rev. 2005, 26, 19. [Google Scholar] [PubMed]
- Hari Kumar, K.V.S. The Good, the Bad, and the Ugly Facets of Insulin Resistance. Med. J. Armed Forces India 2020, 76, 4–7. [Google Scholar] [CrossRef] [PubMed]
- Caprio, S.; Perry, R.; Kursawe, R. Adolescent Obesity and Insulin Resistance: Roles of Ectopic Fat Accumulation and Adipose Inflammation. Gastroenterology 2017, 152, 1638–1646. [Google Scholar] [CrossRef]
- Newton, K.P.; Hou, J.; Crimmins, N.A.; Lavine, J.E.; Barlow, S.E.; Xanthakos, S.A.; Africa, J.; Behling, C.; Donithan, M.; Clark, J.M.; et al. Prevalence of Prediabetes and Type 2 Diabetes in Children With Nonalcoholic Fatty Liver Disease. JAMA Pediatr. 2016, 170, e161971. [Google Scholar] [CrossRef]
- Gastaldelli, A.; Cusi, K. From NASH to Diabetes and from Diabetes to NASH: Mechanisms and Treatment Options. JHEP Rep. 2019, 1, 312–328. [Google Scholar] [CrossRef] [Green Version]
- Armstrong, M.J.; Adams, L.A.; Canbay, A.; Syn, W.-K. Extrahepatic Complications of Nonalcoholic Fatty Liver Disease. Hepatology 2014, 59, 1174–1197. [Google Scholar] [CrossRef]
- D’Adamo, E.; Cali, A.M.G.; Weiss, R.; Santoro, N.; Pierpont, B.; Northrup, V.; Caprio, S. Central Role of Fatty Liver in the Pathogenesis of Insulin Resistance in Obese Adolescents. Diabetes Care 2010, 33, 1817–1822. [Google Scholar] [CrossRef] [Green Version]
- Morandi, A.; Di Sessa, A.; Zusi, C.; Umano, G.R.; El Mazloum, D.; Fornari, E.; Miraglia Del Giudice, E.; Targher, G.; Maffeis, C. Nonalcoholic Fatty Liver Disease and Estimated Insulin Resistance in Obese Youth: A Mendelian Randomization Analysis. J. Clin. Endocrinol. Metab. 2020, 105, e4046–e4054. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S.; Jackson, A.U.; Li, Y.K.; Stringham, H.M.; Kuusisto, J.; Kangas, A.J.; Soininen, P.; Ala-Korpela, M.; Burant, C.F.; Salomaa, V.; et al. Novel Association of TM6SF2 Rs58542926 Genotype with Increased Serum Tyrosine Levels and Decreased ApoB-100 Particles in Finns. J. Lipid Res. 2017, 58, 1471–1481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahajan, A.; Wessel, J.; Willems, S.M.; Zhao, W.; Robertson, N.R.; Chu, A.Y.; Gan, W.; Kitajima, H.; Taliun, D.; Rayner, N.W.; et al. Refining the Accuracy of Validated Target Identification through Coding Variant Fine-Mapping in Type 2 Diabetes. Nat. Genet. 2018, 50, 559–571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dongiovanni, P.; Stender, S.; Pietrelli, A.; Mancina, R.M.; Cespiati, A.; Petta, S.; Pelusi, S.; Pingitore, P.; Badiali, S.; Maggioni, M.; et al. Causal Relationship of Hepatic Fat with Liver Damage and Insulin Resistance in Nonalcoholic Fatty Liver. J. Intern. Med. 2018, 283, 356–370. [Google Scholar] [CrossRef] [PubMed]
- De Silva, N.M.G.; Borges, M.C.; Hingorani, A.D.; Engmann, J.; Shah, T.; Zhang, X.; Luan, J.; Langenberg, C.; Wong, A.; Kuh, D.; et al. Liver Function and Risk of Type 2 Diabetes: Bidirectional Mendelian Randomization Study. Diabetes 2019, 68, 1681–1691. [Google Scholar] [CrossRef] [Green Version]
- Weiss, R.; Dufour, S.; Taksali, S.E.; Tamborlane, W.V.; Petersen, K.F.; Bonadonna, R.C.; Boselli, L.; Barbetta, G.; Allen, K.; Rife, F.; et al. Prediabetes in Obese Youth: A Syndrome of Impaired Glucose Tolerance, Severe Insulin Resistance, and Altered Myocellular and Abdominal Fat Partitioning. Lancet 2003, 362, 951–957. [Google Scholar] [CrossRef] [Green Version]
- Kim, G.; Giannini, C.; Pierpont, B.; Feldstein, A.E.; Santoro, N.; Kursawe, R.; Shaw, M.; Duran, E.; Goldberg, R.; Dziura, J.; et al. Longitudinal Effects of MRI-Measured Hepatic Steatosis on Biomarkers of Glucose Homeostasis and Hepatic Apoptosis in Obese Youth. Diabetes Care 2013, 36, 130–136. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, S.H.; Kohli, R.; Gores, G.J. Mechanisms of Lipotoxicity in NAFLD and Clinical Implications. J. Pediatr. Gastroenterol. Nutr. 2011, 53, 131–140. [Google Scholar] [CrossRef] [Green Version]
- Pereira, S.; Park, E.; Mori, Y.; Andrew Haber, C.; Han, P.; Uchida, T.; Stavar, L.; Oprescu, A.I.; Koulajian, K.; Ivovic, A.; et al. FFA-Induced Hepatic Insulin Resistance in Vivo Is Mediated by PKCδ, NADPH Oxidase, and Oxidative Stress. Am. J. Physiol. Endocrinol. Metab. 2014, 307, E34–E46. [Google Scholar] [CrossRef] [Green Version]
- Amor, A.J.; Perea, V. Dyslipidemia in Nonalcoholic Fatty Liver Disease. Curr. Opin. Endocrinol. Diabetes Obes. 2019, 26, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Zhou, Y.; Zhang, K.; Qi, Y.; An, S.; Wang, S.; Zhao, X.; Tang, Y.D. Association between Nonalcoholic Fatty Liver Disease and Subclinical Atherosclerosis: A Cross-Sectional Study on Population over 40 Years Old. BMC Cardiovasc. Disord. 2018, 18, 147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahaling, D.U.; Basavaraj, M.M.; Bika, A.J. Comparison of Lipid Profile in Different Grades of Non-Alcoholic Fatty Liver Disease Diagnosed on Ultrasound Asian Pacific Journal of Tropical Biomedicine. Doc. Head. Asian Pac. J. Trop. Biomed 2013, 3, 907–912. [Google Scholar] [CrossRef] [Green Version]
- Peng, K.; Mo, Z.; Tian, G. Serum Lipid Abnormalities and Nonalcoholic Fatty Liver Disease in Adult Males. Am. J. Med. Sci. 2017, 353, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Nobili, V.; Alkhouri, N.; Bartuli, A.; Manco, M.; Lopez, R.; Alisi, A.; Feldstein, A.E. Severity of Liver Injury and Atherogenic Lipid Profile in Children with Nonalcoholic Fatty Liver Disease. Pediatr. Res. 2010, 67, 665–670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deeb, A.; Attia, S.; Mahmoud, S.; Elhaj, G.; Elfatih, A. Dyslipidemia and Fatty Liver Disease in Overweight and Obese Children. J. Obes. 2018, 2018, 8626818. [Google Scholar] [CrossRef] [Green Version]
- Products—Data Briefs—Number 228. December 2015. Available online: https://www.cdc.gov/nchs/products/databriefs/db228.htm (accessed on 28 February 2022).
- Modi, N.; Murgasova, D.; Ruager-Martin, R.; Thomas, E.L.; Hyde, M.J.; Gale, C.; Santhakumaran, S.; Doré, C.J.; Alavi, A.; Bell, J.D. The Influence of Maternal Body Mass Index on Infant Adiposity and Hepatic Lipid Content. Pediatr. Res. 2011, 70, 287–291. [Google Scholar] [CrossRef] [Green Version]
- Brumbaugh, D.E.; Tearse, P.; Cree-Green, M.; Fenton, L.Z.; Brown, M.; Scherzinger, A.; Reynolds, R.; Alston, M.; Hoffman, C.; Pan, Z.; et al. Intrahepatic Fat Is Increased in the Neonatal Offspring of Obese Women with Gestational Diabetes. J. Pediatr. 2013, 162, 930–936. [Google Scholar] [CrossRef] [Green Version]
- Pascale, A.; Marchesi, N.; Marelli, C.; Coppola, A.; Luzi, L.; Govoni, S.; Giustina, A.; Gazzaruso, C. Microbiota and Metabolic Diseases. Endocrine 2018, 61, 357–371. [Google Scholar] [CrossRef]
- Fianchi, F.; Liguori, A.; Gasbarrini, A.; Grieco, A.; Miele, L. Nonalcoholic Fatty Liver Disease (Nafld) as Model of Gut–Liver Axis Interaction: From Pathophysiology to Potential Target of Treatment for Personalized Therapy. Int. J. Mol. Sci. 2021, 22, 6485. [Google Scholar] [CrossRef]
- Arab, J.P.; Martin-Mateos, R.M.; Shah, V.H. Gut-Liver Axis, Cirrhosis and Portal Hypertension: The Chicken and the Egg. Hepatol. Int. 2018, 12, 24–33. [Google Scholar] [CrossRef]
- Miura, K.; Ohnishi, H. Role of Gut Microbiota and Toll-like Receptors in Nonalcoholic Fatty Liver Disease. World J. Gastroenterol. 2014, 20, 7381–7391. [Google Scholar] [CrossRef] [PubMed]
- Zulian, A.; Cancello, R.; Cesana, E.; Rizzi, E.; Consolandi, C.; Severgnini, M.; Panizzo, V.; Di Blasio, A.M.; Micheletto, G.; Invitti, C. Adipose Tissue Microbiota in Humans: An Open Issue. Int. J. Obes. 2016, 40, 1643–1648. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Johnston, L.J.; Wu, C.; Ma, X. Gut Microbiota and Its Metabolites: Bridge of Dietary Nutrients and Obesity-Related Diseases. Crit. Rev. Food Sci. Nutr. 2021, 2021, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.D. The Gut Microbiome and Its Role in Obesity. Nutr. Today 2016, 51, 167. [Google Scholar] [CrossRef] [Green Version]
- Zwartjes, M.S.Z.; Gerdes, V.E.A.; Nieuwdorp, M. The Role of Gut Microbiota and Its Produced Metabolites in Obesity, Dyslipidemia, Adipocyte Dysfunction, and Its Interventions. Metabolites 2021, 11, 531. [Google Scholar] [CrossRef] [PubMed]
- Kalliomäki, M.; Collado, M.C.; Salminen, S.; Isolauri, E. Early Differences in Fecal Microbiota Composition in Children May Predict Overweight. Am. J. Clin. Nutr. 2008, 87, 534–538. [Google Scholar] [CrossRef]
- Bertrando, S.; Vajro, P. NAFLD at the Interface of the Mother-Infant Dyad. Curr. Pharm. Des. 2020, 26, 1119–1125. [Google Scholar] [CrossRef]
- Mouzaki, M.; Comelli, E.M.; Arendt, B.M.; Bonengel, J.; Fung, S.K.; Fischer, S.E.; Mcgilvray, I.D.; Allard, J.P. Intestinal Microbiota in Patients with Nonalcoholic Fatty Liver Disease. Hepatology 2013, 58, 120–127. [Google Scholar] [CrossRef] [Green Version]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Microbial Ecology: Human Gut Microbes Associated with Obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef]
- Boursier, J.; Mueller, O.; Barret, M.; Machado, M.; Fizanne, L.; Araujo-Perez, F.; Guy, C.D.; Seed, P.C.; Rawls, J.F.; David, L.A.; et al. The Severity of Nonalcoholic Fatty Liver Disease Is Associated with Gut Dysbiosis and Shift in the Metabolic Function of the Gut Microbiota. Hepatology 2016, 63, 764–775. [Google Scholar] [CrossRef] [Green Version]
- Chen, F.; Esmaili, S.; Rogers, G.B.; Bugianesi, E.; Petta, S.; Marchesini, G.; Bayoumi, A.; Metwally, M.; Azardaryany, M.K.; Coulter, S.; et al. Lean NAFLD: A Distinct Entity Shaped by Differential Metabolic Adaptation. Hepatology 2020, 71, 1213–1227. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Stepanova, M.; Negro, F.; Hallaji, S.; Younossi, Y.; Lam, B.; Srishord, M. Nonalcoholic Fatty Liver Disease in Lean Individuals in the United States. Medicine 2012, 91, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Conjeevaram Selvakumar, P.K.; Kabbany, M.N.; Lopez, R.; Rayas, M.S.; Lynch, J.L.; Alkhouri, N. Prevalence of Suspected Nonalcoholic Fatty Liver Disease in Lean Adolescents in the United States. J. Pediatr. Gastroenterol. Nutr. 2018, 67, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Qu, W.; Ma, T.; Cai, J.; Zhang, X.; Zhang, P.; She, Z.; Wan, F.; Li, H. Liver Fibrosis and MAFLD: From Molecular Aspects to Novel Pharmacological Strategies. Front. Med. 2021, 8, 761538. [Google Scholar] [CrossRef]
- Day, C.P.; James, O.F.W. Steatohepatitis: A Tale of Two “Hits”? Gastroenterology 1998, 114, 842–845. [Google Scholar] [CrossRef]
- Berardis, S.; Sokal, E. Pediatric Non-Alcoholic Fatty Liver Disease: An Increasing Public Health Issue. Eur. J. Pediatr. 2014, 173, 131–139. [Google Scholar] [CrossRef] [Green Version]
- Ratziu, V.; Bellentani, S.; Cortez-Pinto, H.; Day, C.; Marchesini, G. A Position Statement on NAFLD/NASH Based on the EASL 2009 Special Conference. J. Hepatol. 2010, 53, 372–384. [Google Scholar] [CrossRef] [Green Version]
- Fang, Y.-L.; Chen, H.; Wang, C.-L.; Liang, L. Pathogenesis of Non-Alcoholic Fatty Liver Disease in Children and Adolescence: From “Two Hit Theory” to “Multiple Hit Model”. World J. Gastroenterol. 2018, 24, 2974. [Google Scholar] [CrossRef]
- Tilg, H.; Moschen, A.R. Evolution of Inflammation in Nonalcoholic Fatty Liver Disease: The Multiple Parallel Hits Hypothesis. Hepatology 2010, 52, 1836–1846. [Google Scholar] [CrossRef]
- Yang, A.; Mottillo, E.P. Adipocyte Lipolysis: From Molecular Mechanisms of Regulation to Disease and Therapeutics. Biochem. J. 2020, 477, 985–1008. [Google Scholar] [CrossRef]
- Ferré, P.; Foufelle, F. Hepatic Steatosis: A Role for de Novo Lipogenesis and the Transcription Factor SREBP-1c. Diabetes Obes. Metab. 2010, 12 (Suppl. S2), 83–92. [Google Scholar] [CrossRef] [PubMed]
- Denechaud, P.-D.; Dentin, R.; Girard, J.; Postic, C. Role of ChREBP in Hepatic Steatosis and Insulin Resistance. FEBS Lett. 2008, 582, 68–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alkhouri, N.; Lawitz, E.; Noureddin, M.; DeFronzo, R.; Shulman, G.I. GS-0976 (Firsocostat): An Investigational Liver-Directed Acetyl-CoA Carboxylase (ACC) Inhibitor for the Treatment of Non-Alcoholic Steatohepatitis (NASH). Expert Opin. Investig. Drugs 2020, 29, 135–141. [Google Scholar] [CrossRef] [PubMed]
- McGarry, J.D.; Brown, N.F. The Mitochondrial Carnitine Palmitoyltransferase System. From Concept to Molecular Analysis. Eur. J. Biochem. 1997, 244, 1–14. [Google Scholar] [CrossRef]
- Ruderman, N.B.; Saha, A.K.; Kraegen, E.W. Minireview: Malonyl CoA, AMP-Activated Protein Kinase, and Adiposity. Endocrinology 2003, 144, 5166–5171. [Google Scholar] [CrossRef] [Green Version]
- Buhl, E.S.; Jessen, N.; Pold, R.; Ledet, T.; Flyvbjerg, A.; Pedersen, S.B.; Pedersen, O.; Schmitz, O.; Lund, S. Long-Term AICAR Administration Reduces Metabolic Disturbances and Lowers Blood Pressure in Rats Displaying Features of the Insulin Resistance Syndrome. Diabetes 2002, 51, 2199–2206. [Google Scholar] [CrossRef] [Green Version]
- Li, T.; Fang, T.; Xu, L.; Liu, X.; Li, X.; Xue, M.; Yu, X.; Sun, B.; Chen, L. Empagliflozin Alleviates Hepatic Steatosis by Activating the AMPK-TET2-Autophagy Pathway in Vivo and in Vitro. Front. Pharmacol. 2020, 11, 622153. [Google Scholar] [CrossRef]
- Friedman, S.L.; Neuschwander-Tetri, B.A.; Rinella, M.; Sanyal, A.J. Mechanisms of NAFLD Development and Therapeutic Strategies. Nat. Med. 2018, 24, 908–922. [Google Scholar] [CrossRef]
- MacHado, M.V.; Diehl, A.M. Pathogenesis of Nonalcoholic Steatohepatitis. Gastroenterology 2016, 150, 1769–1777. [Google Scholar] [CrossRef] [Green Version]
- Akazawa, Y.; Nakao, K. Lipotoxicity Pathways Intersect in Hepatocytes: Endoplasmic Reticulum Stress, c-Jun N-Terminal Kinase-1, and Death Receptors. Hepatol. Res. 2016, 46, 977–984. [Google Scholar] [CrossRef]
- Shojaie, L.; Iorga, A.; Dara, L. Cell Death in Liver Diseases: A Review. Int. J. Mol. Sci. 2020, 21, 9682. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, D.M.S.; Castro, R.E.; Machado, M.V.; Evangelista, T.; Silvestre, A.; Costa, A.; Coutinho, J.; Carepa, F.; Cortez-Pinto, H.; Rodrigues, C.M.P. Apoptosis and Insulin Resistance in Liver and Peripheral Tissues of Morbidly Obese Patients Is Associated with Different Stages of Non-Alcoholic Fatty Liver Disease. Diabetologia 2011, 54, 1788–1798. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.K.; Perito, E.R. Nonalcoholic Liver Disease in Children and Adolescents. Clin. Liver Dis. 2018, 22, 723–733. [Google Scholar] [CrossRef] [PubMed]
- Roh, Y.-S.; Seki, E. Chemokines and Chemokine Receptors in the Development of NAFLD. Adv. Exp. Med. Biol. 2018, 1061, 45–53. [Google Scholar] [CrossRef]
- Bai, L.; Li, H. Innate Immune Regulatory Networks in Hepatic Lipid Metabolism. J. Mol. Med. Berl. Ger. 2019, 97, 593–604. [Google Scholar] [CrossRef]
- Loomba, R.; Friedman, S.L.; Shulman, G.I. Mechanisms and Disease Consequences of Nonalcoholic Fatty Liver Disease. Cell 2021, 184, 2537–2564. [Google Scholar] [CrossRef]
- Delli Bovi, A.P.; Marciano, F.; Mandato, C.; Siano, M.A.; Savoia, M.; Vajro, P. Oxidative Stress in Non-Alcoholic Fatty Liver Disease. An Updated Mini Review. Front. Med. 2021, 8, 595371. [Google Scholar] [CrossRef]
- Chen, Z.; Tian, R.; She, Z.; Cai, J.; Li, H. Role of Oxidative Stress in the Pathogenesis of Nonalcoholic Fatty Liver Disease. Free Radic. Biol. Med. 2020, 152, 116–141. [Google Scholar] [CrossRef]
- Roh, Y.S.; Seki, E. Toll-like Receptors in Alcoholic Liver Disease, Non-Alcoholic Steatohepatitis and Carcinogenesis. J. Gastroenterol. Hepatol. 2013, 28 (Suppl. S1), 38–42. [Google Scholar] [CrossRef] [Green Version]
- Parola, M.; Pinzani, M. Liver Fibrosis: Pathophysiology, Pathogenetic Targets and Clinical Issues. Mol. Aspects Med. 2019, 65, 37–55. [Google Scholar] [CrossRef]
- Breitkopf, K.; Godoy, P.; Ciuclan, L.; Singer, M.V.; Dooley, S. TGF-Beta/Smad Signaling in the Injured Liver. Z. Gastroenterol. 2006, 44, 57–66. [Google Scholar] [CrossRef]
- Tsuchida, T.; Friedman, S.L. Mechanisms of Hepatic Stellate Cell Activation. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 397–411. [Google Scholar] [CrossRef] [PubMed]
- Kazankov, K.; Jørgensen, S.M.D.; Thomsen, K.L.; Møller, H.J.; Vilstrup, H.; George, J.; Schuppan, D.; Grønbæk, H. The Role of Macrophages in Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 145–159. [Google Scholar] [CrossRef] [PubMed]
- Schwabe, R.F.; Tabas, I.; Pajvani, U.B. Mechanisms of Fibrosis Development in Nonalcoholic Steatohepatitis. Gastroenterology 2020, 158, 1913–1928. [Google Scholar] [CrossRef] [PubMed]
- Juanola, O.; Martínez-López, S.; Francés, R.; Gómez-Hurtado, I. Non-Alcoholic Fatty Liver Disease: Metabolic, Genetic, Epigenetic and Environmental Risk Factors. Int. J. Environ. Res. Public Health 2021, 18, 5227. [Google Scholar] [CrossRef]
- Kovalic, A.J.; Satapathy, S.K.; Chalasani, N. Targeting Incretin Hormones and the ASK-1 Pathway as Therapeutic Options in the Treatment of Non-Alcoholic Steatohepatitis. Hepatol. Int. 2018, 12, 97–106. [Google Scholar] [CrossRef]
- Wang, P.-X.; Ji, Y.-X.; Zhang, X.-J.; Zhao, L.-P.; Yan, Z.-Z.; Zhang, P.; Shen, L.-J.; Yang, X.; Fang, J.; Tian, S.; et al. Targeting CASP8 and FADD-like Apoptosis Regulator Ameliorates Nonalcoholic Steatohepatitis in Mice and Nonhuman Primates. Nat. Med. 2017, 23, 439–449. [Google Scholar] [CrossRef]
- Tanase, D.M.; Gosav, E.M.; Costea, C.F.; Ciocoiu, M.; Lacatusu, C.M.; Maranduca, M.A.; Ouatu, A.; Floria, M. The Intricate Relationship between Type 2 Diabetes Mellitus (T2DM), Insulin Resistance (IR), and Nonalcoholic Fatty Liver Disease (NAFLD). J. Diabetes Res. 2020, 2020, 3920196. [Google Scholar] [CrossRef]
- Vos, M.B.; Abrams, S.H.; Barlow, S.E.; Caprio, S.; Daniels, S.R.; Kohli, R.; Mouzaki, M.; Sathya, P.; Schwimmer, J.B.; Sundaram, S.S.; et al. NASPGHAN Clinical Practice Guideline for the Diagnosis and Treatment of Nonalcoholic Fatty Liver Disease in Children: Recommendations from the Expert Committee on NAFLD (ECON) and the North American Society of Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN). J. Pediatr. Gastroenterol. Nutr. 2017, 64, 319–334. [Google Scholar] [CrossRef] [Green Version]
- Zimmet, P.; Alberti, G.K.M.M.; Kaufman, F.; Tajima, N.; Silink, M.; Arslanian, S.; Wong, G.; Bennett, P.; Shaw, J.; Caprio, S. The Metabolic Syndrome in Children and Adolescents—An IDF Consensus Report. Pediatr. Diabetes 2007, 8, 299–306. [Google Scholar] [CrossRef]
- Yamamura, S.; Eslam, M.; Kawaguchi, T.; Tsutsumi, T.; Nakano, D.; Yoshinaga, S.; Takahashi, H.; Anzai, K.; George, J.; Torimura, T. MAFLD Identifies Patients with Significant Hepatic Fibrosis Better than NAFLD. Liver Int. 2020, 40, 3018–3030. [Google Scholar] [CrossRef]
- Kim, D.; Konyn, P.; Sandhu, K.K.; Dennis, B.B.; Cheung, A.C.; Ahmed, A. Metabolic Dysfunction-Associated Fatty Liver Disease Is Associated with Increased All-Cause Mortality in the United States. J. Hepatol. 2021, 75, 1284–1291. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, Y.; Byrne, C.D.; Musso, G. A Single-Letter Change in an Acronym: Signals, Reasons, Promises, Challenges, and Steps Ahead for Moving from NAFLD to MAFLD. Expert Rev. Gastroenterol. Hepatol. 2021, 15, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Pal, P.; Palui, R.; Ray, S. Heterogeneity of Non-Alcoholic Fatty Liver Disease: Implications for Clinical Practice and Research Activity. World J. Hepatol. 2021, 13, 1584–1610. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.P.; Anirvan, P.; Reddy, K.R.; Conjeevaram, H.S.; Marchesini, G.; Rinella, M.E.; Madan, K.; Petroni, M.L.; Al-Mahtab, M.; Caldwell, S.H.; et al. Non-Alcoholic Fatty Liver Disease: Not Time for an Obituary Just Yet! J. Hepatol. 2021, 74, 972–974. [Google Scholar] [CrossRef] [PubMed]
- Mato, J.M.; Alonso, C.; Noureddin, M.; Lu, S.C. Biomarkers and Subtypes of Deranged Lipid Metabolism in Non-Alcoholic Fatty Liver Disease. World J. Gastroenterol. 2019, 25, 3009–3020. [Google Scholar] [CrossRef]
- Wu, T.; Ye, J.; Shao, C.; Li, F.; Lin, Y.; Ma, Q.; Wang, W.; Feng, S.; Zhong, B. Varied Relationship of Lipid and Lipoprotein Profiles to Liver Fat Content in Phenotypes of Metabolic Associated Fatty Liver Disease. Front. Endocrinol. 2021, 12, 1458. [Google Scholar] [CrossRef]
- Vandromme, M.; Jun, T.; Perumalswami, P.; Dudley, J.T.; Branch, A.; Li, L. Automated Phenotyping of Patients with Non-Alcoholic Fatty Liver Disease Reveals Clinically Relevant Disease Subtypes. Pac. Symp. Biocomput. 2020, 25, 91–102. [Google Scholar]
- Hoang, S.A.; Oseini, A.; Feaver, R.E.; Cole, B.K.; Asgharpour, A.; Vincent, R.; Siddiqui, M.; Lawson, M.J.; Day, N.C.; Taylor, J.M.; et al. Gene Expression Predicts Histological Severity and Reveals Distinct Molecular Profiles of Nonalcoholic Fatty Liver Disease. Sci. Rep. 2019, 9, 12541. [Google Scholar] [CrossRef] [Green Version]
- Mandala, A.; Janssen, R.C.; Palle, S.; Short, K.R.; Friedman, J.E. Pediatric Non-Alcoholic Fatty Liver Disease: Nutritional Origins and Potential Molecular Mechanisms. Nutrients 2020, 12, 3166. [Google Scholar] [CrossRef]
- Janczyk, W.; Lebensztejn, D.; Wierzbicka-Rucińska, A.; Mazur, A.; Neuhoff-Murawska, J.; Matusik, P.; Socha, P. Omega-3 Fatty Acids Therapy in Children with Nonalcoholic Fatty Liver Disease: A Randomized Controlled Trial. J. Pediatr. 2015, 166, 1358–1363.e3. [Google Scholar] [CrossRef] [PubMed]
- Boyraz, M.; Pirgon, Ö.; Dündar, B.; Çekmez, F.; Hatipoğlu, N. Long-Term Treatment with n-3 Polyunsaturated Fatty Acids as a Monotherapy in Children with Nonalcoholic Fatty Liver Disease. J. Clin. Res. Pediatr. Endocrinol. 2015, 7, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Nobili, V.; Bedogni, G.; Alisi, A.; Pietrobattista, A.; Risé, P.; Galli, C.; Agostoni, C. Docosahexaenoic Acid Supplementation Decreases Liver Fat Content in Children with Non-Alcoholic Fatty Liver Disease: Double-Blind Randomised Controlled Clinical Trial. Arch. Dis. Child. 2011, 96, 350–353. [Google Scholar] [CrossRef] [PubMed]
- Bauer, T.M.; Schwacha, H.; Steinbrückner, B.; Brinkmann, F.E.; Ditzen, A.K.; Aponte, J.J.; Pelz, K.; Berger, D.; Kist, M.; Blum, H.E. Small Intestinal Bacterial Overgrowth in Human Cirrhosis Is Associated with Systemic Endotoxemia. Am. J. Gastroenterol. 2002, 97, 2364–2370. [Google Scholar] [CrossRef] [PubMed]
- Bauer, T.M.; Steinbruckner, B.; Brinkmann, F.E.; Ditzen, A.K.; Schwacha, H.; Aponte, J.J.; Pelz, K.; Kist, M.; Blum, H.E. Small Intestinal Bacterial Overgrowth in Patients with Cirrhosis: Prevalence and Relation with Spontaneous Bacterial Peritonitis. Am. J. Gastroenterol. 2001, 96, 2962–2967. [Google Scholar] [CrossRef] [PubMed]
- Harte, A.L.; Da Silva, N.F.; Creely, S.J.; McGee, K.C.; Billyard, T.; Youssef-Elabd, E.M.; Tripathi, G.; Ashour, E.; Abdalla, M.S.; Sharada, H.M.; et al. Elevated Endotoxin Levels in Non-Alcoholic Fatty Liver Disease. J. Inflamm. Lond. Engl. 2010, 7, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cani, P.D.; Amar, J.; Iglesias, M.A.; Poggi, M.; Knauf, C.; Bastelica, D.; Neyrinck, A.M.; Fava, F.; Tuohy, K.M.; Chabo, C.; et al. Metabolic Endotoxemia Initiates Obesity and Insulin Resistance. Diabetes 2007, 56, 1761–1772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Den Besten, G.; Bleeker, A.; Gerding, A.; Van Eunen, K.; Havinga, R.; Van Dijk, T.H.; Oosterveer, M.H.; Jonker, J.W.; Groen, A.K.; Reijngoud, D.J.; et al. Short-Chain Fatty Acids Protect Against High-Fat Diet-Induced Obesity via a PPARγ-Dependent Switch from Lipogenesis to Fat Oxidation. Diabetes 2015, 64, 2398–2408. [Google Scholar] [CrossRef] [Green Version]
- Abbate, J.M.; Macrì, F.; Capparucci, F.; Iaria, C.; Briguglio, G.; Cicero, L.; Salvo, A.; Arfuso, F.; Ieni, A.; Piccione, G.; et al. Administration of Protein Hydrolysates from Anchovy (Engraulis Encrasicolus) Waste for Twelve Weeks Decreases Metabolic Dysfunction-associated Fatty Liver Disease Severity in ApoE−/−Mice. Animals 2020, 10, 2303. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Reyes, M.J.; Mishra, A.; Mehta, R.; Henry, L. Systematic Review with Meta-Analysis: Non-Alcoholic Steatohepatitis—A Case for Personalised Treatment Based on Pathogenic Targets. Aliment. Pharmacol. Ther. 2014, 39, 3–14. [Google Scholar] [CrossRef]
- Ciardullo, S.; Perseghin, G. Prevalence of NAFLD, MAFLD and Associated Advanced Fibrosis in the Contemporary United States Population. Liver Int. 2021, 41, 1290–1293. [Google Scholar] [CrossRef] [PubMed]
- Wong, V.W.-S.; Wong, G.L.-H.; Woo, J.; Abrigo, J.M.; Chan, C.K.-M.; Shu, S.S.-T.; Leung, J.K.-Y.; Chim, A.M.-L.; Kong, A.P.-S.; Lui, G.C.-Y.; et al. Impact of the New Definition of Metabolic Associated Fatty Liver Disease on the Epidemiology of the Disease. Clin. Gastroenterol. Hepatol. 2021, 19, 2161–2171.e5. [Google Scholar] [CrossRef] [PubMed]
- Müller, T.D.; Finan, B.; Bloom, S.R.; D’Alessio, D.; Drucker, D.J.; Flatt, P.R.; Fritsche, A.; Gribble, F.; Grill, H.J.; Habener, J.F.; et al. Glucagon-like Peptide 1 (GLP-1). Mol. Metab. 2019, 30, 72–130. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, M.J.; Gaunt, P.; Aithal, G.P.; Barton, D.; Hull, D.; Parker, R.; Hazlehurst, J.M.; Guo, K.; LEAN Trial Team; Abouda, G.; et al. Liraglutide Safety and Efficacy in Patients with Non-Alcoholic Steatohepatitis (LEAN): A Multicentre, Double-Blind, Randomised, Placebo-Controlled Phase 2 Study. Lancet 2016, 387, 679–690. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Yan, H.; Xia, M.; Zhao, L.; Lv, M.; Zhao, N.; Rao, S.; Yao, X.; Wu, W.; Pan, B.; et al. Efficacy of Exenatide and Insulin Glargine on Nonalcoholic Fatty Liver Disease in Patients with Type 2 Diabetes. Diabetes Metab. Res. Rev. 2020, 36, e3292. [Google Scholar] [CrossRef]
- Newsome, P.N.; Buchholtz, K.; Cusi, K.; Linder, M.; Okanoue, T.; Ratziu, V.; Sanyal, A.J.; Sejling, A.-S.; Harrison, S.A.; NN9931-4296 Investigators. A Placebo-Controlled Trial of Subcutaneous Semaglutide in Nonalcoholic Steatohepatitis. N. Engl. J. Med. 2021, 384, 1113–1124. [Google Scholar] [CrossRef]
- Boland, M.L.; Laker, R.C.; Mather, K.; Nawrocki, A.; Oldham, S.; Boland, B.B.; Lewis, H.; Conway, J.; Naylor, J.; Guionaud, S.; et al. Resolution of NASH and Hepatic Fibrosis by the GLP-1R/GcgR Dual-Agonist Cotadutide via Modulating Mitochondrial Function and Lipogenesis. Nat. Metab. 2020, 2, 413–431. [Google Scholar] [CrossRef]
- Hartman, M.L.; Sanyal, A.J.; Loomba, R.; Wilson, J.M.; Nikooienejad, A.; Bray, R.; Karanikas, C.A.; Duffin, K.L.; Robins, D.A.; Haupt, A. Effects of Novel Dual GIP and GLP-1 Receptor Agonist Tirzepatide on Biomarkers of Nonalcoholic Steatohepatitis in Patients with Type 2 Diabetes. Diabetes Care 2020, 43, 1352–1355. [Google Scholar] [CrossRef]
- Clifford, B.L.; Sedgeman, L.R.; Williams, K.J.; Morand, P.; Cheng, A.; Jarrett, K.E.; Chan, A.P.; Brearley-Sholto, M.C.; Wahlström, A.; Ashby, J.W.; et al. FXR Activation Protects against NAFLD via Bile-Acid-Dependent Reductions in Lipid Absorption. Cell Metab. 2021, 33, 1671–1684.e4. [Google Scholar] [CrossRef]
- Schumacher, J.D.; Kong, B.; Wu, J.; Rizzolo, D.; Armstrong, L.E.; Chow, M.D.; Goedken, M.; Lee, Y.-H.; Guo, G.L. Direct and Indirect Effects of Fibroblast Growth Factor (FGF) 15 and FGF19 on Liver Fibrosis Development. Hepatology 2020, 71, 670–685. [Google Scholar] [CrossRef]
- Chen, Z.; Yu, Y.; Cai, J.; Li, H. Emerging Molecular Targets for Treatment of Nonalcoholic Fatty Liver Disease. Trends Endocrinol. Metab. TEM 2019, 30, 903–914. [Google Scholar] [CrossRef] [PubMed]
- Polyzos, S.A.; Kountouras, J.; Mantzoros, C.S. Obeticholic Acid for the Treatment of Nonalcoholic Steatohepatitis: Expectations and Concerns. Metab. Clin. Exp. 2020, 104, 154144. [Google Scholar] [CrossRef] [PubMed]
- Neuschwander-Tetri, B.A.; Loomba, R.; Sanyal, A.J.; Lavine, J.E.; Van Natta, M.L.; Abdelmalek, M.F.; Chalasani, N.; Dasarathy, S.; Diehl, A.M.; Hameed, B.; et al. Farnesoid X Nuclear Receptor Ligand Obeticholic Acid for Non-Cirrhotic, Non-Alcoholic Steatohepatitis (FLINT): A Multicentre, Randomised, Placebo-Controlled Trial. Lancet 2015, 385, 956–965. [Google Scholar] [CrossRef] [Green Version]
- Younossi, Z.M.; Ratziu, V.; Loomba, R.; Rinella, M.; Anstee, Q.M.; Goodman, Z.; Bedossa, P.; Geier, A.; Beckebaum, S.; Newsome, P.N.; et al. Obeticholic Acid for the Treatment of Non-Alcoholic Steatohepatitis: Interim Analysis from a Multicentre, Randomised, Placebo-Controlled Phase 3 Trial. Lancet 2019, 394, 2184–2196. [Google Scholar] [CrossRef] [Green Version]
- Pockros, P.J.; Fuchs, M.; Freilich, B.; Schiff, E.; Kohli, A.; Lawitz, E.J.; Hellstern, P.A.; Owens-Grillo, J.; Van Biene, C.; Shringarpure, R.; et al. CONTROL: A Randomized Phase 2 Study of Obeticholic Acid and Atorvastatin on Lipoproteins in Nonalcoholic Steatohepatitis Patients. Liver Int. 2019, 39, 2082–2093. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.; Harrison, S.A.; Elkhashab, M.; Trotter, J.F.; Herring, R.; Rojter, S.E.; Kayali, Z.; Wong, V.W.-S.; Greenbloom, S.; Jayakumar, S.; et al. Cilofexor, a Nonsteroidal FXR Agonist, in Patients with Noncirrhotic NASH: A Phase 2 Randomized Controlled Trial. Hepatology 2020, 72, 58–71. [Google Scholar] [CrossRef] [PubMed]
- An, P.; Wei, G.; Huang, P.; Li, W.; Qi, X.; Lin, Y.; Vaid, K.A.; Wang, J.; Zhang, S.; Li, Y.; et al. A Novel Non-Bile Acid FXR Agonist EDP-305 Potently Suppresses Liver Injury and Fibrosis without Worsening of Ductular Reaction. Liver Int. 2020, 40, 1655–1669. [Google Scholar] [CrossRef]
- Fiorucci, S.; Biagioli, M.; Sepe, V.; Zampella, A.; Distrutti, E. Bile Acid Modulators for the Treatment of Nonalcoholic Steatohepatitis (NASH). Expert Opin. Investig. Drugs 2020, 29, 623–632. [Google Scholar] [CrossRef]
- Erstad, D.J.; Farrar, C.T.; Ghoshal, S.; Masia, R.; Ferreira, D.S.; Chen, Y.-C.I.; Choi, J.-K.; Wei, L.; Waghorn, P.A.; Rotile, N.J.; et al. Molecular Magnetic Resonance Imaging Accurately Measures the Antifibrotic Effect of EDP-305, a Novel Farnesoid X Receptor Agonist. Hepatol. Commun. 2018, 2, 821–835. [Google Scholar] [CrossRef]
- Stiede, K.; Miao, W.; Blanchette, H.S.; Beysen, C.; Harriman, G.; Harwood, H.J.; Kelley, H.; Kapeller, R.; Schmalbach, T.; Westlin, W.F. Acetyl-Coenzyme A Carboxylase Inhibition Reduces de Novo Lipogenesis in Overweight Male Subjects: A Randomized, Double-Blind, Crossover Study. Hepatology 2017, 66, 324–334. [Google Scholar] [CrossRef] [Green Version]
- Loomba, R.; Kayali, Z.; Noureddin, M.; Ruane, P.; Lawitz, E.J.; Bennett, M.; Wang, L.; Harting, E.; Tarrant, J.M.; McColgan, B.J.; et al. GS-0976 Reduces Hepatic Steatosis and Fibrosis Markers in Patients with Nonalcoholic Fatty Liver Disease. Gastroenterology 2018, 155, 1463–1473.e6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baardman, J.; Verberk, S.G.S.; van der Velden, S.; Gijbels, M.J.J.; van Roomen, C.P.P.A.; Sluimer, J.C.; Broos, J.Y.; Griffith, G.R.; Prange, K.H.M.; van Weeghel, M.; et al. Macrophage ATP Citrate Lyase Deficiency Stabilizes Atherosclerotic Plaques. Nat. Commun. 2020, 11, 6296. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Guo, Y.-Y.; Li, B.-Y.; Peng, W.-Q.; Chang, X.-X.; Gao, X.; Tang, Q.-Q. Enhanced Acetylation of ATP-Citrate Lyase Promotes the Progression of Nonalcoholic Fatty Liver Disease. J. Biol. Chem. 2019, 294, 11805–11816. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Sun, D.; Xiang, H.; Wang, S.; Huang, Y.; Li, L.; Cheng, X.; Liu, H.; Hu, F.; Cheng, Y.; et al. Hepatocyte SH3RF2 Deficiency Is a Key Aggravator for NAFLD. Hepatology 2021, 74, 1319–1338. [Google Scholar] [CrossRef] [PubMed]
- Covarrubias, A.J.; Aksoylar, H.I.; Yu, J.; Snyder, N.W.; Worth, A.J.; Iyer, S.S.; Wang, J.; Ben-Sahra, I.; Byles, V.; Polynne-Stapornkul, T.; et al. Akt-MTORC1 Signaling Regulates Acly to Integrate Metabolic Input to Control of Macrophage Activation. eLife 2016, 5, e11612. [Google Scholar] [CrossRef]
- Infantino, V.; Iacobazzi, V.; Palmieri, F.; Menga, A. ATP-Citrate Lyase Is Essential for Macrophage Inflammatory Response. Biochem. Biophys. Res. Commun. 2013, 440, 105–111. [Google Scholar] [CrossRef]
- Feng, X.; Zhang, L.; Xu, S.; Shen, A.-Z. ATP-Citrate Lyase (ACLY) in Lipid Metabolism and Atherosclerosis: An Updated Review. Prog. Lipid Res. 2020, 77, 101006. [Google Scholar] [CrossRef]
- Heeren, J.; Scheja, L. Metabolic-Associated Fatty Liver Disease and Lipoprotein Metabolism. Mol. Metab. 2021, 50, 101238. [Google Scholar] [CrossRef]
- Hu, Y.; He, W.; Huang, Y.; Xiang, H.; Guo, J.; Che, Y.; Cheng, X.; Hu, F.; Hu, M.; Ma, T.; et al. Fatty Acid Synthase-Suppressor Screening Identifies Sorting Nexin 8 as a Therapeutic Target for NAFLD. Hepatology 2021, 74, 2508–2525. [Google Scholar] [CrossRef]
- Loomba, R.; Mohseni, R.; Lucas, K.J.; Gutierrez, J.A.; Perry, R.G.; Trotter, J.F.; Rahimi, R.S.; Harrison, S.A.; Ajmera, V.; Wayne, J.D.; et al. TVB-2640 (FASN Inhibitor) for the Treatment of Nonalcoholic Steatohepatitis: FASCINATE-1, a Randomized, Placebo-Controlled Phase 2a Trial. Gastroenterology 2021, 161, 1475–1486. [Google Scholar] [CrossRef]
- Sagimet Biosciences Inc. A Phase 2B, Multi-Center, Double-Blind, Randomized, Placebo-Controlled Study of the Safety and Efficacy of TVB-2640 in Subjects with Nonalcoholic Steatohepatitis (FASCINATE-2); Sagimet Biosciences Inc.: San Mateo, CA, USA, 2022. [Google Scholar]
- Iruarrizaga-Lejarreta, M.; Varela-Rey, M.; Fernández-Ramos, D.; Martínez-Arranz, I.; Delgado, T.C.; Simon, J.; Juan, V.G.; delaCruz-Villar, L.; Azkargorta, M.; Lavin, J.L.; et al. Role of Aramchol in Steatohepatitis and Fibrosis in Mice. Hepatol. Commun. 2017, 1, 911–927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lai, K.K.Y.; Kweon, S.-M.; Chi, F.; Hwang, E.; Kabe, Y.; Higashiyama, R.; Qin, L.; Yan, R.; Wu, R.P.; Lai, K.; et al. Stearoyl-CoA Desaturase Promotes Liver Fibrosis and Tumor Development in Mice via a Wnt Positive-Signaling Loop by Stabilization of Low-Density Lipoprotein-Receptor-Related Proteins 5 and 6. Gastroenterology 2017, 152, 1477–1491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, X.; Sun, R.; Chen, L.; Chu, S.; Doll, M.A.; Li, X.; Feng, W.; Siskind, L.; McClain, C.J.; Deng, Z. Neutral Ceramidase Mediates Nonalcoholic Steatohepatitis by Regulating Monounsaturated Fatty Acids and Gut IgA+ B Cells. Hepatology 2021, 73, 901–919. [Google Scholar] [CrossRef] [PubMed]
- Rau, M.; Geier, A. An Update on Drug Development for the Treatment of Nonalcoholic Fatty Liver Disease—From Ongoing Clinical Trials to Future Therapy. Expert Rev. Clin. Pharmacol. 2021, 14, 333–340. [Google Scholar] [CrossRef]
- Han, X.; Wu, Y.; Yang, Q.; Cao, G. Peroxisome Proliferator-Activated Receptors in the Pathogenesis and Therapies of Liver Fibrosis. Pharmacol. Ther. 2021, 222, 107791. [Google Scholar] [CrossRef]
- Tong, J.; Han, C.-J.; Zhang, J.-Z.; He, W.-Z.; Zhao, G.-J.; Cheng, X.; Zhang, L.; Deng, K.-Q.; Liu, Y.; Fan, H.-F.; et al. Hepatic Interferon Regulatory Factor 6 Alleviates Liver Steatosis and Metabolic Disorder by Transcriptionally Suppressing Peroxisome Proliferator-Activated Receptor γ in Mice. Hepatology 2019, 69, 2471–2488. [Google Scholar] [CrossRef]
- Sandoval-Rodriguez, A.; Monroy-Ramirez, H.C.; Meza-Rios, A.; Garcia-Bañuelos, J.; Vera-Cruz, J.; Gutiérrez-Cuevas, J.; Silva-Gomez, J.; Staels, B.; Dominguez-Rosales, J.; Galicia-Moreno, M.; et al. Pirfenidone Is an Agonistic Ligand for PPARα and Improves NASH by Activation of SIRT1/LKB1/PAMPK. Hepatol. Commun. 2020, 4, 434–449. [Google Scholar] [CrossRef] [Green Version]
- Ratziu, V.; Harrison, S.A.; Francque, S.; Bedossa, P.; Lehert, P.; Serfaty, L.; Romero-Gomez, M.; Boursier, J.; Abdelmalek, M.; Caldwell, S.; et al. Elafibranor, an Agonist of the Peroxisome Proliferator-Activated Receptor-α and -δ, Induces Resolution of Nonalcoholic Steatohepatitis Without Fibrosis Worsening. Gastroenterology 2016, 150, 1147–1159.e5. [Google Scholar] [CrossRef] [Green Version]
- Gawrieh, S.; Noureddin, M.; Loo, N.; Mohseni, R.; Awasty, V.; Cusi, K.; Kowdley, K.V.; Lai, M.; Schiff, E.; Parmar, D.; et al. Saroglitazar, a PPAR-α/γ Agonist, for Treatment of NAFLD: A Randomized Controlled Double-Blind Phase 2 Trial. Hepatology 2021, 74, 1809–1824. [Google Scholar] [CrossRef]
- Lavine, J.E.; Schwimmer, J.B.; Van Natta, M.L.; Molleston, J.P.; Murray, K.F.; Rosenthal, P.; Abrams, S.H.; Scheimann, A.O.; Sanyal, A.J.; Chalasani, N.; et al. Effect of Vitamin E or Metformin for Treatment of Nonalcoholic Fatty Liver Disease in Children and Adolescents: The TONIC Randomized Controlled Trial. JAMA 2011, 305, 1659–1668. [Google Scholar] [CrossRef] [Green Version]
- Sanyal, A.J.; Chalasani, N.; Kowdley, K.V.; McCullough, A.; Diehl, A.M.; Bass, N.M.; Neuschwander-Tetri, B.A.; Lavine, J.E.; Tonascia, J.; Unalp, A.; et al. Pioglitazone, Vitamin E, or Placebo for Nonalcoholic Steatohepatitis. N. Engl. J. Med. 2010, 362, 1675–1685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eliades, M.; Spyrou, E.; Agrawal, N.; Lazo, M.; Brancati, F.L.; Potter, J.J.; Koteish, A.A.; Clark, J.M.; Guallar, E.; Hernaez, R. Meta-Analysis: Vitamin D and Non-Alcoholic Fatty Liver Disease. Aliment. Pharmacol. Ther. 2013, 38, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Abramovitch, S.; Sharvit, E.; Weisman, Y.; Bentov, A.; Brazowski, E.; Cohen, G.; Volovelsky, O.; Reif, S. Vitamin D Inhibits Development of Liver Fibrosis in an Animal Model but Cannot Ameliorate Established Cirrhosis. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 308, G112–G120. [Google Scholar] [CrossRef] [PubMed]
- Corte, C.D.; Carpino, G.; De Vito, R.; De Stefanis, C.; Alisi, A.; Cianfarani, S.; Overi, D.; Mosca, A.; Stronati, L.; Cucchiara, S.; et al. Docosahexanoic Acid Plus Vitamin D Treatment Improves Features of NAFLD in Children with Serum Vitamin D Deficiency: Results from a Single Centre Trial. PLoS ONE 2016, 11, e0168216. [Google Scholar] [CrossRef] [Green Version]
- Akazawa, Y.; Nakao, K. To Die or Not to Die: Death Signaling in Nonalcoholic Fatty Liver Disease. J. Gastroenterol. 2018, 53, 893–906. [Google Scholar] [CrossRef] [Green Version]
- Kanda, T.; Matsuoka, S.; Yamazaki, M.; Shibata, T.; Nirei, K.; Takahashi, H.; Kaneko, T.; Fujisawa, M.; Higuchi, T.; Nakamura, H.; et al. Apoptosis and Non-Alcoholic Fatty Liver Diseases. World J. Gastroenterol. 2018, 24, 2661–2672. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Dong, G.; Xiong, H.; Diao, H. A Narrative Review of the Role of Necroptosis in Liver Disease: A Double-Edged Sword. Ann. Transl. Med. 2021, 9, 422. [Google Scholar] [CrossRef] [PubMed]
- Barreyro, F.J.; Holod, S.; Finocchietto, P.V.; Camino, A.M.; Aquino, J.B.; Avagnina, A.; Carreras, M.C.; Poderoso, J.J.; Gores, G.J. The Pan-Caspase Inhibitor Emricasan (IDN-6556) Decreases Liver Injury and Fibrosis in a Murine Model of Non-Alcoholic Steatohepatitis. Liver Int. 2015, 35, 953–966. [Google Scholar] [CrossRef] [Green Version]
- Harrison, S.A.; Goodman, Z.; Jabbar, A.; Vemulapalli, R.; Younes, Z.H.; Freilich, B.; Sheikh, M.Y.; Schattenberg, J.M.; Kayali, Z.; Zivony, A.; et al. A Randomized, Placebo-Controlled Trial of Emricasan in Patients with NASH and F1-F3 Fibrosis. J. Hepatol. 2020, 72, 816–827. [Google Scholar] [CrossRef]
- Jenne, C.N.; Kubes, P. Immune Surveillance by the Liver. Nat. Immunol. 2013, 14, 996–1006. [Google Scholar] [CrossRef]
- Cai, J.; Zhang, X.-J.; Li, H. The Role of Innate Immune Cells in Nonalcoholic Steatohepatitis. Hepatology 2019, 70, 1026–1037. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.A.; Wong, V.W.-S.; Okanoue, T.; Bzowej, N.; Vuppalanchi, R.; Younes, Z.; Kohli, A.; Sarin, S.; Caldwell, S.H.; Alkhouri, N.; et al. Selonsertib for Patients with Bridging Fibrosis or Compensated Cirrhosis Due to NASH: Results from Randomized Phase III STELLAR Trials. J. Hepatol. 2020, 73, 26–39. [Google Scholar] [CrossRef]
- Loomba, R.; Lawitz, E.; Mantry, P.S.; Jayakumar, S.; Caldwell, S.H.; Arnold, H.; Diehl, A.M.; Djedjos, C.S.; Han, L.; Myers, R.P.; et al. The ASK1 Inhibitor Selonsertib in Patients with Nonalcoholic Steatohepatitis: A Randomized, Phase 2 Trial. Hepatology 2018, 67, 549–559. [Google Scholar] [CrossRef]
- Zhang, L.; Tian, R.; Yao, X.; Zhang, X.-J.; Zhang, P.; Huang, Y.; She, Z.-G.; Li, H.; Ji, Y.-X.; Cai, J. Milk Fat Globule-Epidermal Growth Factor-Factor 8 Improves Hepatic Steatosis and Inflammation. Hepatology 2021, 73, 586–605. [Google Scholar] [CrossRef]
- An, S.Y.; Jang, Y.J.; Lim, H.-J.; Han, J.; Lee, J.; Lee, G.; Park, J.Y.; Park, S.-Y.; Kim, J.H.; Do, B.-R.; et al. Milk Fat Globule-EGF Factor 8, Secreted by Mesenchymal Stem Cells, Protects Against Liver Fibrosis in Mice. Gastroenterology 2017, 152, 1174–1186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, P.; Wang, P.-X.; Zhao, L.-P.; Zhang, X.; Ji, Y.-X.; Zhang, X.-J.; Fang, C.; Lu, Y.-X.; Yang, X.; Gao, M.-M.; et al. The Deubiquitinating Enzyme TNFAIP3 Mediates Inactivation of Hepatic ASK1 and Ameliorates Nonalcoholic Steatohepatitis. Nat. Med. 2018, 24, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wen, H.; Fu, J.; Cai, L.; Li, P.-L.; Zhao, C.-L.; Dong, Z.-F.; Ma, J.-P.; Wang, X.; Tian, H.; et al. Hepatocyte TNF Receptor-Associated Factor 6 Aggravates Hepatic Inflammation and Fibrosis by Promoting Lysine 6-Linked Polyubiquitination of Apoptosis Signal-Regulating Kinase 1. Hepatology 2020, 71, 93–111. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, K.; Shirakabe, K.; Shibuya, H.; Irie, K.; Oishi, I.; Ueno, N.; Taniguchi, T.; Nishida, E.; Matsumoto, K. Identification of a Member of the MAPKKK Family as a Potential Mediator of TGF-Beta Signal Transduction. Science 1995, 270, 2008–2011. [Google Scholar] [CrossRef]
- Roh, Y.S.; Song, J.; Seki, E. TAK1 Regulates Hepatic Cell Survival and Carcinogenesis. J. Gastroenterol. 2014, 49, 185–194. [Google Scholar] [CrossRef] [Green Version]
- Inokuchi, S.; Aoyama, T.; Miura, K.; Osterreicher, C.H.; Kodama, Y.; Miyai, K.; Akira, S.; Brenner, D.A.; Seki, E. Disruption of TAK1 in Hepatocytes Causes Hepatic Injury, Inflammation, Fibrosis, and Carcinogenesis. Proc. Natl. Acad. Sci. USA 2010, 107, 844–849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, D.; Zhang, P.; Zhou, J.; Liao, R.; Che, Y.; Gao, M.-M.; Sun, J.; Cai, J.; Cheng, X.; Huang, Y.; et al. TNFAIP3 Interacting Protein 3 Overexpression Suppresses Nonalcoholic Steatohepatitis by Blocking TAK1 Activation. Cell Metab. 2020, 31, 726–740.e8. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, F.; Gao, L.; Xu, L.; Tong, R.; Lin, N.; Su, Y.; Yan, Y.; Gao, Y.; He, J.; et al. Ubiquitin-Specific Protease 4 Is an Endogenous Negative Regulator of Metabolic Dysfunctions in Nonalcoholic Fatty Liver Disease in Mice. Hepatology 2018, 68, 897–917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- An, S.; Zhao, L.-P.; Shen, L.-J.; Wang, S.; Zhang, K.; Qi, Y.; Zheng, J.; Zhang, X.-J.; Zhu, X.-Y.; Bao, R.; et al. USP18 Protects against Hepatic Steatosis and Insulin Resistance through Its Deubiquitinating Activity. Hepatology 2017, 66, 1866–1884. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Jeong, W.-I.; Tian, Z. Liver: An Organ with Predominant Innate Immunity. Hepatology 2008, 47, 729–736. [Google Scholar] [CrossRef]
- Hsu, M.-C.; Liu, S.-H.; Wang, C.-W.; Hu, N.-Y.; Wu, E.S.C.; Shih, Y.-C.; Chiu, P.J.S. JKB-122 Is Effective, Alone or in Combination with Prednisolone in Con A-Induced Hepatitis. Eur. J. Pharmacol. 2017, 812, 113–120. [Google Scholar] [CrossRef]
- TaiwanJ Pharmaceuticals Co., Ltd. A Phase 2, Randomized, Multiple-Dose, Double-Blind, Placebo-Controlled Study of JKB-122 in Patients with Non-Alcoholic Steatohepatitis (NASH) and Fibrosis; TaiwanJ Pharmaceuticals Co., Ltd.: La Grange, GA, USA, 2020. [Google Scholar]
- Abdel-Razik, A.; Mousa, N.; Shabana, W.; Refaey, M.; Elzehery, R.; Elhelaly, R.; Zalata, K.; Abdelsalam, M.; Eldeeb, A.A.; Awad, M.; et al. Rifaximin in Nonalcoholic Fatty Liver Disease: Hit Multiple Targets with a Single Shot. Eur. J. Gastroenterol. Hepatol. 2018, 30, 1237–1246. [Google Scholar] [CrossRef] [PubMed]
- Yan, N.; Wang, L.; Li, Y.; Wang, T.; Yang, L.; Yan, R.; Wang, H.; Jia, S. Metformin Intervention Ameliorates AS in ApoE−/−Mice through Restoring Gut Dysbiosis and Anti-Inflammation. PLoS ONE 2021, 16, e0254321. [Google Scholar] [CrossRef]
| NAFLD (Nonalcoholic Fatty Liver Disease) | MAFLD (Metabolic Fatty Liver Disease) |
|---|---|
| Histological, imaging (ultrasound), or blood biomarker (e.g., ALT) evidence of steatosis | Histological, imaging (ultrasound), or blood biomarker (e.g., ALT) evidence of steatosis |
| Exclusion of other causes of hepatic steatosis besides NAFLD (e.g., HBV, HCV, drugs, hemochromatosis, autoimmunity, Wilson’s disease, alpha 1 anti-trypsin deficiency, rapid weight loss) | Excess adiposity Presence of prediabetes or Type 2 Diabetes |
Metabolic dysregulation defined by 2 or more altered results on standardized biometric parameters, with a different cut off for each ethnic group *, including:
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neri, C.R.; Scapaticci, S.; Chiarelli, F.; Giannini, C. Liver Steatosis: A Marker of Metabolic Risk in Children. Int. J. Mol. Sci. 2022, 23, 4822. https://doi.org/10.3390/ijms23094822
Neri CR, Scapaticci S, Chiarelli F, Giannini C. Liver Steatosis: A Marker of Metabolic Risk in Children. International Journal of Molecular Sciences. 2022; 23(9):4822. https://doi.org/10.3390/ijms23094822
Chicago/Turabian StyleNeri, Costanza Renata, Serena Scapaticci, Francesco Chiarelli, and Cosimo Giannini. 2022. "Liver Steatosis: A Marker of Metabolic Risk in Children" International Journal of Molecular Sciences 23, no. 9: 4822. https://doi.org/10.3390/ijms23094822
APA StyleNeri, C. R., Scapaticci, S., Chiarelli, F., & Giannini, C. (2022). Liver Steatosis: A Marker of Metabolic Risk in Children. International Journal of Molecular Sciences, 23(9), 4822. https://doi.org/10.3390/ijms23094822







