The Anti-Atherosclerosis Effect of Anakinra, a Recombinant Human Interleukin-1 Receptor Antagonist, in Apolipoprotein E Knockout Mice
Abstract
:1. Introduction
2. Results
2.1. Focus on Atherosclerosis
2.1.1. Anakinra Reduces the Atherosclerotic Plaque Area in ApoE–/– Mice
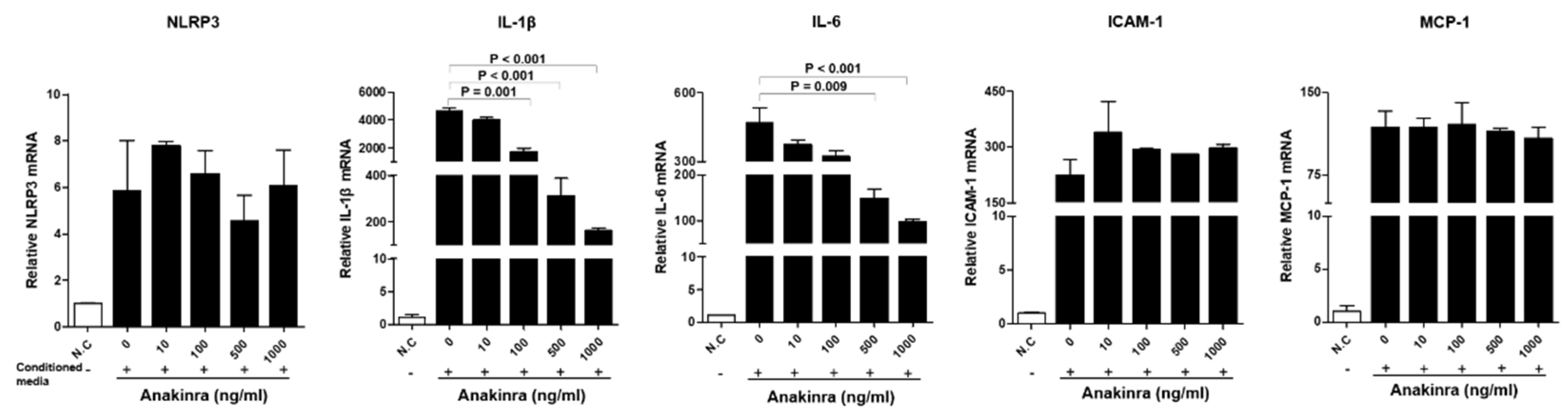
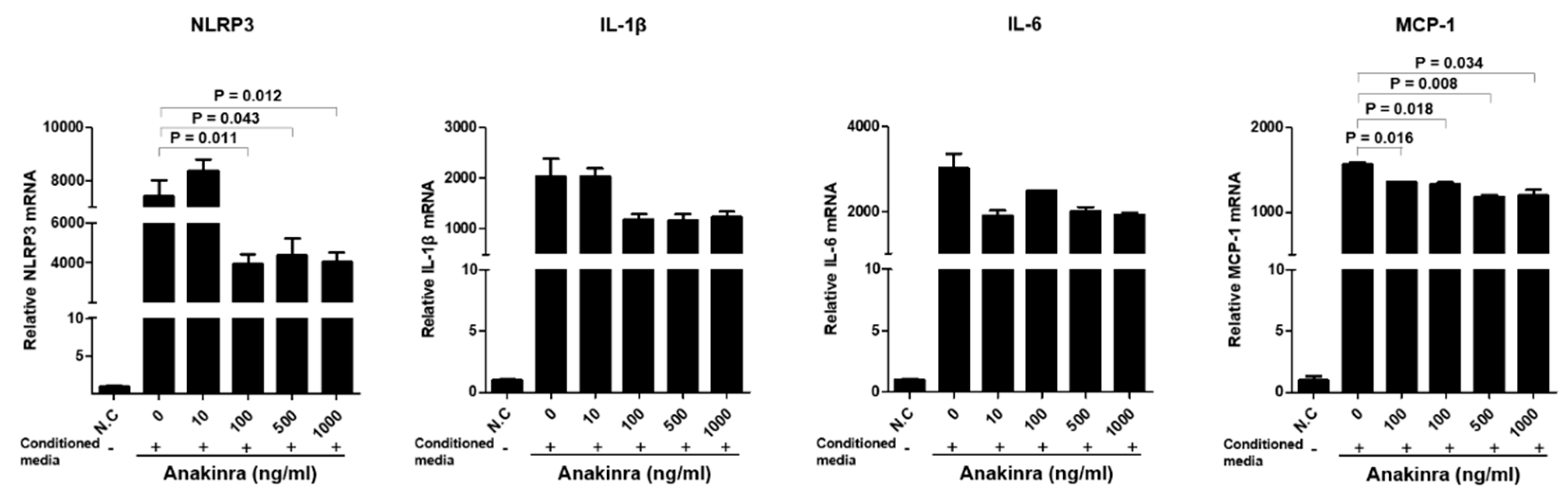
2.1.2. The Dose-Dependent Effect of Anakinra on the Activation of the NLRP3 Inflammasome and Upregulated Expression of Inflammatory Adhesion Molecules in Human Umbilical Vein Endothelial Cells (HUVECs)
2.1.3. The Dose-Dependent Effect of Anakinra on the Activated Nlrp3 Inflammasome and Upregulated Expression of Angiogenesis Molecules in Rat Aortic Smooth Muscle Cells (RAOSMCs)
2.1.4. The Effect of Anakinra on the p38 Mitogen-Activated Protein Kinase (MAPK)/Nuclear Factor-κB (NF-κB) Pathway in RAOSMCs and HUVECs
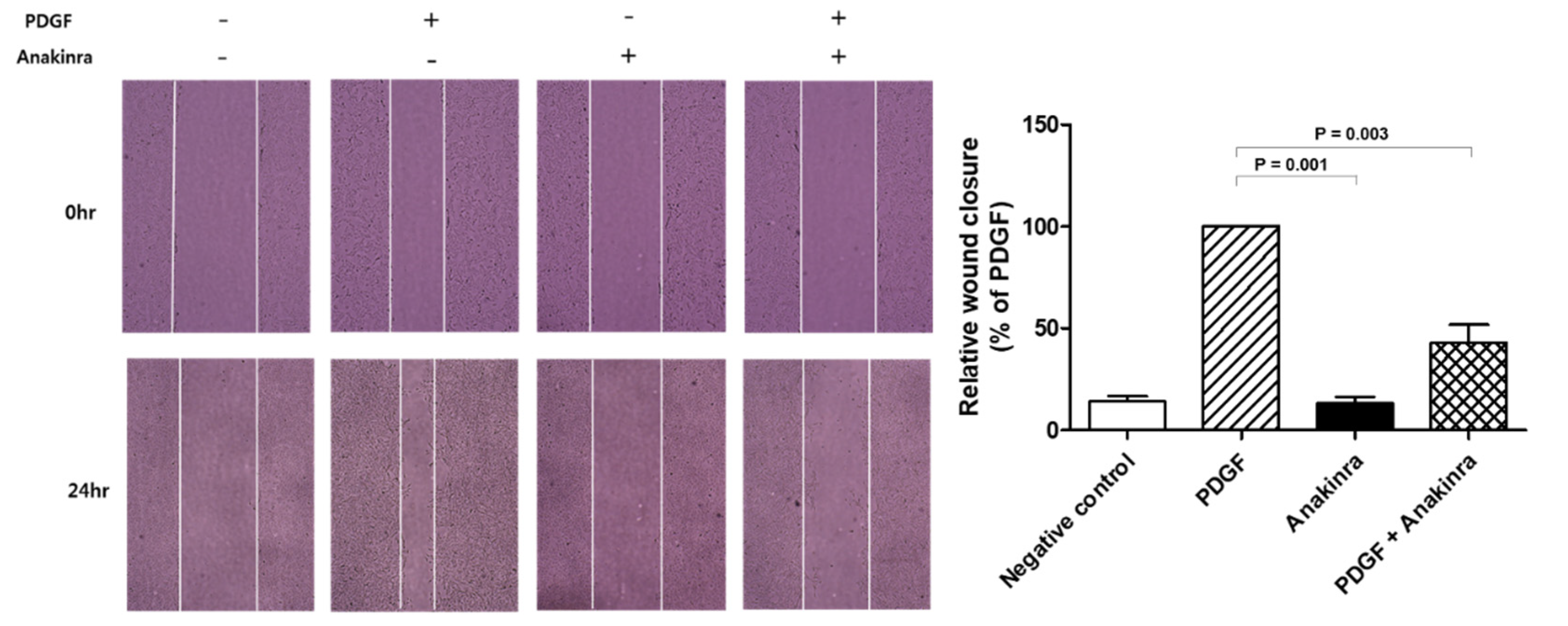
2.1.5. Anakinra Inhibits Migration of RAOSMCs
2.2. Focus on Fat
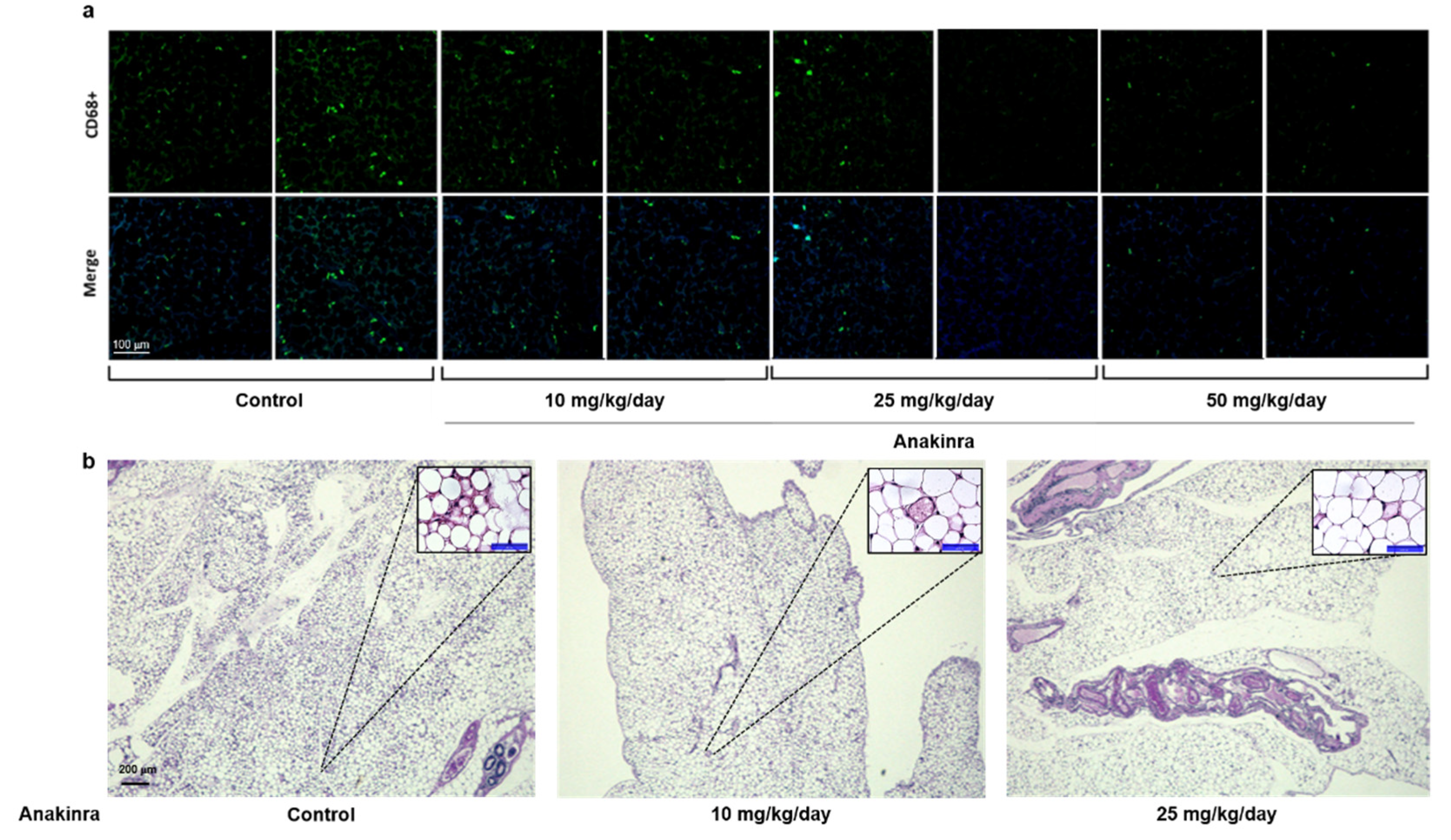
2.2.1. Immunofluorescent Staining of CD68 and Hematoxylin and Eosin (H&E) Staining in Visceral Adipose Tissue
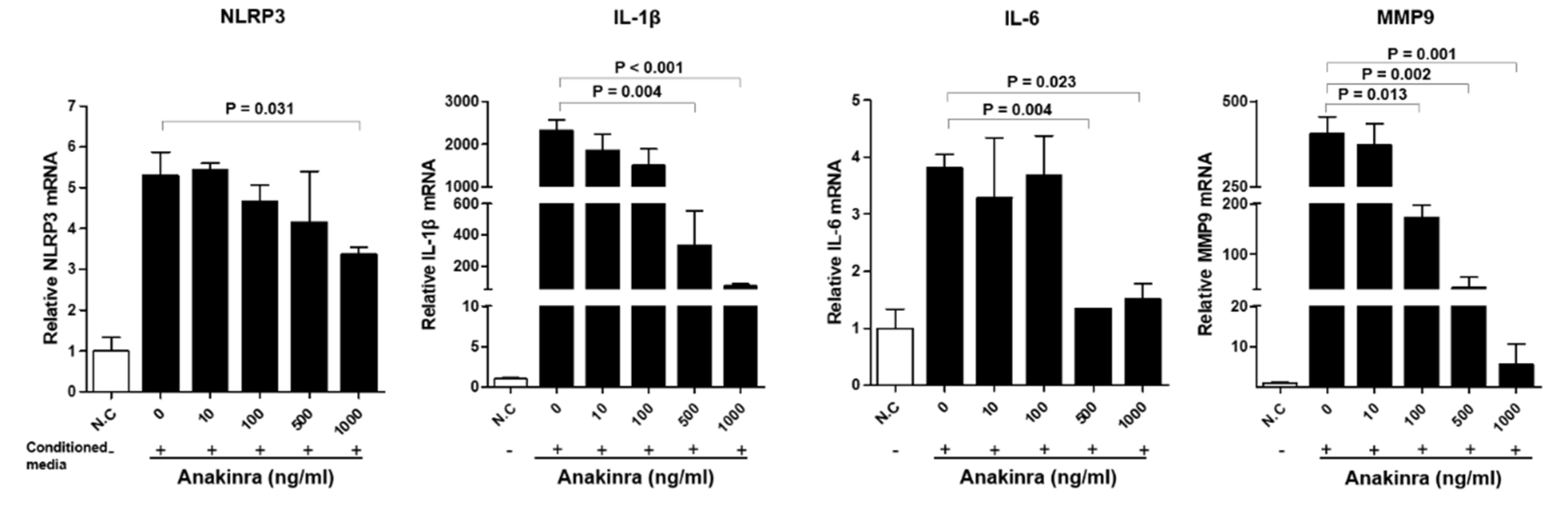
2.2.2. The Dose-Dependent Effect of Anakinra on the Activated NLRP3 Inflammasome and Upregulated Expression of Inflammatory Molecules in 3T3-L1 Adipocytes
2.2.3. Expression of Phosphorylated c-Jun N-Terminal Kinase (p-JNK), p-p38, and p-ERK in the Liver and Visceral Fat Tissue
3. Discussion
4. Materials and Methods
4.1. Animals, Diet, and Treatment Protocols
4.2. Immunofluorescent Staining of CD68 and H&E Staining in the Adipose Tissue
4.3. THP-1-Conditioned Media and Induction of NLRP3 Inflammasome Expression in HUVECs, RAOSMCs, and 3T3-L1 Cells
4.4. Reverse Transcription–Quantitative Polymerase Chain Reaction (RT–qPCR)
4.5. Western Blot Analysis
4.6. RAOSMC Migration Assays
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations and Acronyms
| ANOVA | Analysis of variance |
| ApoE–/– | Apolipoprotein E knockout |
| CRP | C-reactive protein |
| CVD | Cardiovascular disease |
| ERK | Extracellular-regulated kinase |
| H&E | Hematoxylin and eosin |
| HUVEC | Human umbilical vein endothelial cell |
| ICAM | Intracellular adhesion molecule |
| IL | Interleukin |
| LDL-C | Low-density lipoprotein cholesterol |
| LPS | Lipopolysaccharide |
| MAPK | Mitogen-activated protein kinase |
| MCP-1 | Monocyte chemoattractant protein-1 |
| MI | Myocardial infarction |
| MMP-9 | Matrix metalloproteinase-9 |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cell |
| NLRP3 | NACHT, LRR, and PYD domain-containing protein 3 |
| PDGF | Platelet-derived growth factor |
| P-JNK | Phosphorylated c-Jun N-terminal kinase |
| RAOSMC | Rat aortic smooth muscle cell |
| SMA | Smooth muscle actin |
| TG | Triglycerides |
| TNF-α | Tumor necrosis factor-α. |
References
- Benjamin, E.J.; Blaha, M.J.; Chiuve, S.E.; Cushman, M.; Das, S.R.; Deo, R.; de Ferranti, S.D.; Floyd, J.; Fornage, M.; Gillespie, C. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation 2017, 135, e146–e603. [Google Scholar] [CrossRef] [PubMed]
- Biondi-Zoccai, G.G.; Abbate, A.; Liuzzo, G.; Biasucci, L.M. Atherothrombosis, inflammation, and diabetes. J. Am. Coll. Cardiol. 2003, 41, 1071–1077. [Google Scholar] [CrossRef] [Green Version]
- Maffia, P.; Grassia, G.; Di Meglio, P.; Carnuccio, R.; Berrino, L.; Garside, P.; Ianaro, A.; Ialenti, A. Neutralization of interleukin-18 inhibits neointimal formation in a rat model of vascular injury. Circulation 2006, 114, 430–437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, G.; Mannam, A.P.; Wu, J.; Kirbis, S.; Shie, J.L.; Chen, C.; Laham, R.J.; Sellke, F.W.; Li, J. Hypoxia induces myocyte-dependent COX-2 regulation in endothelial cells: Role of VEGF. Am. J. Physiol. Heart Circ. Physiol. 2003, 285, H2420–H2429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Isoda, K.; Shiigai, M.; Ishigami, N.; Matsuki, T.; Horai, R.; Nishikawa, K.; Kusuhara, M.; Nishida, Y.; Iwakura, Y.; Ohsuzu, F. Deficiency of interleukin-1 receptor antagonist promotes neointimal formation after injury. Circulation 2003, 108, 516–518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Feuerstein, G.Z.; Gu, I.L.; Lysko, P.G.; Yue, T.L. Interleukin-1 beta induces expression of adhesion molecules in human vascular smooth muscle cells and enhances adhesion of leukocytes to smooth muscle cells. Atherosclerosis 1995, 115, 89–98. [Google Scholar] [CrossRef]
- Broz, P.; Dixit, V.M. Inflammasomes: Mechanism of assembly, regulation and signaling. Nat. Rev. Immunol. 2016, 16, 407–420. [Google Scholar] [CrossRef]
- Abbate, A.; Toldo, S.; Marchetti, C.; Kron, J.; Van Tassell, B.W.; Dinarello, C.A. Interleukin-1 and the Inflammasome as Therapeutic Targets in Cardiovascular Disease. Circ. Res. 2020, 126, 1260–1280. [Google Scholar] [CrossRef]
- Ridker, P.M.; Libby, P.; MacFadyen, J.G.; Thuren, T.; Ballantyne, C.; Fonseca, F.; Koenig, W.; Shimokawa, H.; Everett, B.M.; Glynn, R.J. Modulation of the interleukin-6 signalling pathway and incidence rates of atherosclerotic events and all-cause mortality: Analyses from the Canakinumab Anti-Inflammatory Thrombosis Outcomes Study (CANTOS). Eur. Heart J. 2018, 39, 3499–3507. [Google Scholar] [CrossRef] [Green Version]
- Van Tassell, B.W.; Raleigh, J.M.; Abbate, A. Targeting interleukin-1 in heart failure and inflammatory heart disease. Curr. Heart Fail. Rep. 2015, 12, 33–41. [Google Scholar] [CrossRef]
- Van Tassell, B.W.; Toldo, S.; Mezzaroma, E.; Abbate, A. Targeting interleukin-1 in heart disease. Circulation 2013, 128, 1910–1923. [Google Scholar] [CrossRef] [Green Version]
- Emmi, G.; Urban, M.L.; Imazio, M.; Gattorno, M.; Maestroni, S.; Lopalco, G.; Cantarini, L.; Prisco, D.; Brucato, A. Use of Interleukin-1 Blockers in Pericardial and Cardiovascular Diseases. Curr. Cardiol. Rep. 2018, 20, 61. [Google Scholar] [CrossRef]
- Szekely, Y.; Arbel, Y. A Review of Interleukin-1 in Heart Disease: Where Do We Stand Today? Cardiol. Ther. 2018, 7, 25–44. [Google Scholar] [CrossRef] [Green Version]
- Van Tassell, B.W.; Buckley, L.F.; Carbone, S.; Trankle, C.R.; Canada, J.M.; Dixon, D.L.; Abouzaki, N.; Oddi-Erdle, C.; Biondi-Zoccai, G.; Arena, R.; et al. Interleukin-1 blockade in heart failure with preserved ejection fraction: Rationale and design of the Diastolic Heart Failure Anakinra Response Trial 2 (D-HART2). Clin. Cardiol. 2017, 40, 626–632. [Google Scholar] [CrossRef]
- Garces, K. Anakinra: Interleukin-1 receptor antagonist therapy for rheumatoid arthritis. Issues Emerg. Health Technol. 2001, 1, 1–4. [Google Scholar]
- Van Tassell, B.W.; Abouzaki, N.A.; Oddi Erdle, C.; Carbone, S.; Trankle, C.R.; Melchior, R.D.; Turlington, J.S.; Thurber, C.J.; Christopher, S.; Dixon, D.L.; et al. Interleukin-1 Blockade in Acute Decompensated Heart Failure: A Randomized, Double-Blinded, Placebo-Controlled Pilot Study. J. Cardiovasc. Pharmacol. 2016, 67, 544–551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Tassell, B.W.; Arena, R.; Biondi-Zoccai, G.; Canada, J.M.; Oddi, C.; Abouzaki, N.A.; Jahangiri, A.; Falcao, R.A.; Kontos, M.C.; Shah, K.B.; et al. Effects of interleukin-1 blockade with anakinra on aerobic exercise capacity in patients with heart failure and preserved ejection fraction (from the D-HART pilot study). Am. J. Cardiol. 2014, 113, 321–327. [Google Scholar] [CrossRef] [Green Version]
- Ikonomidis, I.; Lekakis, J.P.; Nikolaou, M.; Paraskevaidis, I.; Andreadou, I.; Kaplanoglou, T.; Katsimbri, P.; Skarantavos, G.; Soucacos, P.N.; Kremastinos, D.T. Inhibition of interleukin-1 by anakinra improves vascular and left ventricular function in patients with rheumatoid arthritis. Circulation 2008, 117, 2662–2669. [Google Scholar] [CrossRef]
- Kullenberg, T.; Löfqvist, M.; Leinonen, M.; Goldbach-Mansky, R.; Olivecrona, H. Long-term safety profile of anakinra in patients with severe cryopyrin-associated periodic syndromes. Rheumatology 2016, 55, 1499–1506. [Google Scholar] [CrossRef] [Green Version]
- Dinarello, C.A.; Simon, A.; Van der Meer, J.W. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat. Rev. Drug Discov. 2012, 11, 633–652. [Google Scholar] [CrossRef] [Green Version]
- Vallejo, S.; Palacios, E.; Romacho, T.; Villalobos, L.; Peiró, C.; Sánchez-Ferrer, C.F. The interleukin-1 receptor antagonist anakinra improves endothelial dysfunction in streptozotocin-induced diabetic rats. Cardiovasc. Diabetol. 2014, 13, 158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shikama, Y.; Aki, N.; Hata, A.; Nishimura, M.; Oyadomari, S.; Funaki, M. Palmitate-stimulated monocytes induce adhesion molecule expression in endothelial cells via IL-1 signaling pathway. J. Cell Physiol. 2015, 230, 732–742. [Google Scholar] [CrossRef] [PubMed]
- Abbate, A.; Salloum, F.N.; Vecile, E.; Das, A.; Hoke, N.N.; Straino, S.; Biondi-Zoccai, G.G.; Houser, J.-E.; Qureshi, I.Z.; Ownby, E.D.; et al. Anakinra, a recombinant human interleukin-1 receptor antagonist, inhibits apoptosis in experimental acute myocardial infarction. Circulation 2008, 117, 2670–2683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhaskar, V.; Yin, J.; Mirza, A.M.; Phan, D.; Vanegas, S.; Issafras, H.; Michelson, K.; Hunter, J.J.; Kantak, S.S. Monoclonal antibodies targeting IL-1 beta reduce biomarkers of atherosclerosis in vitro and inhibit atherosclerotic plaque formation in Apolipoprotein E-deficient mice. Atherosclerosis 2011, 216, 313–320. [Google Scholar] [CrossRef]
- Wang, X.; Su, B.; Liu, W.; He, X.; Gao, Y.; Castellani, R.J.; Perry, G.; Smith, M.A.; Zhu, X. DLP1-dependent mitochondrial fragmentation mediates 1-methyl-4-phenylpyridinium toxicity in neurons: Implications for Parkinson’s disease. Aging Cell 2011, 10, 807–823. [Google Scholar] [CrossRef] [Green Version]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef]
- Vromman, A.; Ruvkun, V.; Shvartz, E.; Wojtkiewicz, G.; Masson, G.S.; Tesmenitsky, Y.; Folco, E.; Gram, H.; Nahrendorf, M.; Swirski, F.K.; et al. Stage-dependent differential effects of interleukin-1 isoforms on experimental atherosclerosis. Eur. Heart J. 2019, 40, 2482–2491. [Google Scholar] [CrossRef]
- Gomez, D.; Baylis, R.A.; Durgin, B.G.; Newman, A.A.C.; Alencar, G.F.; Mahan, S.; St Hilaire, C.; Müller, W.; Waisman, A.; Francis, S.E.; et al. Interleukin-1β has atheroprotective effects in advanced atherosclerotic lesions of mice. Nat. Med. 2018, 24, 1418–1429. [Google Scholar] [CrossRef]
- Plump, A.S.; Smith, J.D.; Hayek, T.; Aalto-Setälä, K.; Walsh, A.; Verstuyft, J.G.; Rubin, E.M.; Breslow, J.L. Severe hypercholesterolemia and atherosclerosis in apolipoprotein E-deficient mice created by homologous recombination in ES cells. Cell 1992, 71, 343–353. [Google Scholar] [CrossRef]
- Elhage, R.; Maret, A.; Pieraggi, M.T.; Thiers, J.C.; Arnal, J.F.; Bayard, F. Differential effects of interleukin-1 receptor antagonist and tumor necrosis factor binding protein on fatty-streak formation in apolipoprotein E-deficient mice. Circulation 1998, 97, 242–244. [Google Scholar] [CrossRef] [Green Version]
- Dragoljevic, D.; Lee, M.K.S.; Louis, C.; Shihata, W.; Kraakman, M.J.; Hansen, J.; Masters, S.L.; Hanaoka, B.Y.; Nagareddy, P.R.; Lancaster, G.I.; et al. Inhibition of interleukin-1β signaling promotes atherosclerotic lesion remodeling in mice with inflammatory arthritis. Clin. Transl. Immunol. 2020, 9, e1206. [Google Scholar] [CrossRef]
- van Asseldonk, E.J.; Stienstra, R.; Koenen, T.B.; Joosten, L.A.; Netea, M.G.; Tack, C.J. Treatment with Anakinra improves disposition index but not insulin sensitivity in nondiabetic subjects with the metabolic syndrome: A randomized, double-blind, placebo-controlled study. J. Clin. Endocrinol. Metab. 2011, 96, 2119–2126. [Google Scholar] [CrossRef] [Green Version]
- Ridker, P.M.; Howard, C.P.; Walter, V.; Everett, B.; Libby, P.; Hensen, J.; Thuren, T. Effects of interleukin-1β inhibition with canakinumab on hemoglobin A1c, lipids, C-reactive protein, interleukin-6, and fibrinogen: A phase IIb randomized, placebo-controlled trial. Circulation 2012, 126, 2739–2748. [Google Scholar] [CrossRef] [Green Version]
- Ghigliotti, G.; Barisione, C.; Garibaldi, S.; Fabbi, P.; Brunelli, C.; Spallarossa, P.; Altieri, P.; Rosa, G.; Spinella, G.; Palombo, D.; et al. Adipose tissue immune response: Novel triggers and consequences for chronic inflammatory conditions. Inflammation 2014, 37, 1337–1353. [Google Scholar] [CrossRef] [Green Version]
- Pietiläinen, K.H.; Róg, T.; Seppänen-Laakso, T.; Virtue, S.; Gopalacharyulu, P.; Tang, J.; Rodriguez-Cuenca, S.; Maciejewski, A.; Naukkarinen, J.; Ruskeepää, A.L.; et al. Association of lipidome remodeling in the adipocyte membrane with acquired obesity in humans. PLoS Biol. 2011, 9, e1000623. [Google Scholar] [CrossRef] [Green Version]
- Kolodgie, F.D.; Virmani, R.; Burke, A.P.; Farb, A.; Weber, D.K.; Kutys, R.; Finn, A.V.; Gold, H.K. Pathologic assessment of the vulnerable human coronary plaque. Heart 2004, 90, 1385–1391. [Google Scholar] [CrossRef] [Green Version]
- Virmani, R.; Kolodgie, F.D.; Burke, A.P.; Farb, A.; Schwartz, S.M. Lessons from sudden coronary death: A comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1262–1275. [Google Scholar] [CrossRef] [Green Version]
- Libby, P.; Buring, J.E.; Badimon, L.; Hansson, G.K.; Deanfield, J.; Bittencourt, M.S.; Tokgözoğlu, L.; Lewis, E.F. Atherosclerosis. Nat. Rev. Dis. Primers 2019, 5, 56. [Google Scholar] [CrossRef]
- Borén, J.; Williams, K.J. The central role of arterial retention of cholesterol-rich apolipoprotein-B-containing lipoproteins in the pathogenesis of atherosclerosis: A triumph of simplicity. Curr. Opin. Lipidol. 2016, 27, 473–483. [Google Scholar] [CrossRef]
- Martinet, W.; Coornaert, I.; Puylaert, P.; De Meyer, G.R.Y. Macrophage Death as a Pharmacological Target in Atherosclerosis. Front. Pharmacol. 2019, 10, 306. [Google Scholar] [CrossRef]
- Abbate, A.; Trankle, C.R.; Buckley, L.F.; Lipinski, M.J.; Appleton, D.; Kadariya, D.; Canada, J.M.; Carbone, S.; Roberts, C.S.; Abouzaki, N.; et al. Interleukin-1 Blockade Inhibits the Acute Inflammatory Response in Patients With ST-Segment-Elevation Myocardial Infarction. J. Am. Heart Assoc. 2020, 9, e014941. [Google Scholar] [CrossRef] [PubMed]
- Weber, A.; Wasiliew, P.; Kracht, M. Interleukin-1 (IL-1) pathway. Sci. Signal. 2010, 3, cm1. [Google Scholar] [CrossRef] [PubMed]
- Mai, W.; Liao, Y. Targeting IL-1β in the Treatment of Atherosclerosis. Front. Immunol. 2020, 11, 589654. [Google Scholar] [CrossRef] [PubMed]
- Kanda, H.; Tateya, S.; Tamori, Y.; Kotani, K.; Hiasa, K.; Kitazawa, R.; Kitazawa, S.; Miyachi, H.; Maeda, S.; Egashira, K.; et al. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J. Clin. Investig. 2006, 116, 1494–1505. [Google Scholar] [CrossRef]
- Syed, F.M.; Hahn, H.S.; Odley, A.; Guo, Y.; Vallejo, J.G.; Lynch, R.A.; Mann, D.L.; Bolli, R.; DornII, G.W., 2nd. Pro-poptotic effects of caspase-1/interleukin-converting enzyme dominate in myocardial ischemia. Circ. Res. 2005, 96, 1103–1109. [Google Scholar] [CrossRef] [Green Version]
- Isoda, K.; Sawada, S.; Ishigami, N.; Matsuki, T.; Miyazaki, K.; Kusuhara, M.; Iwakura, Y.; Ohsuzu, F. Lack of interleukin-1 receptor antagonist modulates plaque composition in apolipoprotein E-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 1068–1073. [Google Scholar] [CrossRef] [Green Version]
- Eissner, G.; Lindner, H.; Reisbach, G.; Klauke, I.; Holler, E. Differential modulation of IL-1-induced endothelial adhesion molecules and transendothelial migration of granulocytes by G-CSF. Br. J. Haematol. 1997, 97, 726–733. [Google Scholar] [CrossRef]
- Dhar-Mascareno, M.; Rozenberg, I.; Iqbal, J.; Hussain, M.M.; Beckles, D.; Mascareno, E. Hexim1 heterozygosity stabilizes atherosclerotic plaque and decreased steatosis in ApoE null mice fed atherogenic diet. Int. J. Biochem. Cell Biol. 2017, 83, 56–64. [Google Scholar] [CrossRef]
- Tian, Y.; Chen, T.; Wu, Y.; Yang, L.; Wang, L.; Fan, X.; Zhang, W.; Feng, J.; Yu, H.; Yang, Y.; et al. Pioglitazone stabilizes atherosclerotic plaque by regulating the Th17/Treg balance in AMPK-dependent mechanisms. Cardiovasc. Diabetol. 2017, 16, 140. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ku, E.J.; Kim, B.-R.; Lee, J.-I.; Lee, Y.K.; Oh, T.J.; Jang, H.C.; Choi, S.H. The Anti-Atherosclerosis Effect of Anakinra, a Recombinant Human Interleukin-1 Receptor Antagonist, in Apolipoprotein E Knockout Mice. Int. J. Mol. Sci. 2022, 23, 4906. https://doi.org/10.3390/ijms23094906
Ku EJ, Kim B-R, Lee J-I, Lee YK, Oh TJ, Jang HC, Choi SH. The Anti-Atherosclerosis Effect of Anakinra, a Recombinant Human Interleukin-1 Receptor Antagonist, in Apolipoprotein E Knockout Mice. International Journal of Molecular Sciences. 2022; 23(9):4906. https://doi.org/10.3390/ijms23094906
Chicago/Turabian StyleKu, Eu Jeong, Bo-Rahm Kim, Jee-In Lee, Yun Kyung Lee, Tae Jung Oh, Hak C. Jang, and Sung Hee Choi. 2022. "The Anti-Atherosclerosis Effect of Anakinra, a Recombinant Human Interleukin-1 Receptor Antagonist, in Apolipoprotein E Knockout Mice" International Journal of Molecular Sciences 23, no. 9: 4906. https://doi.org/10.3390/ijms23094906





