Alleviation of Tris(2-chloroethyl) Phosphate Toxicity on the Marine Rotifer Brachionus plicatilis by Polystyrene Microplastics: Features and Molecular Evidence
Abstract
:1. Introduction
2. Results
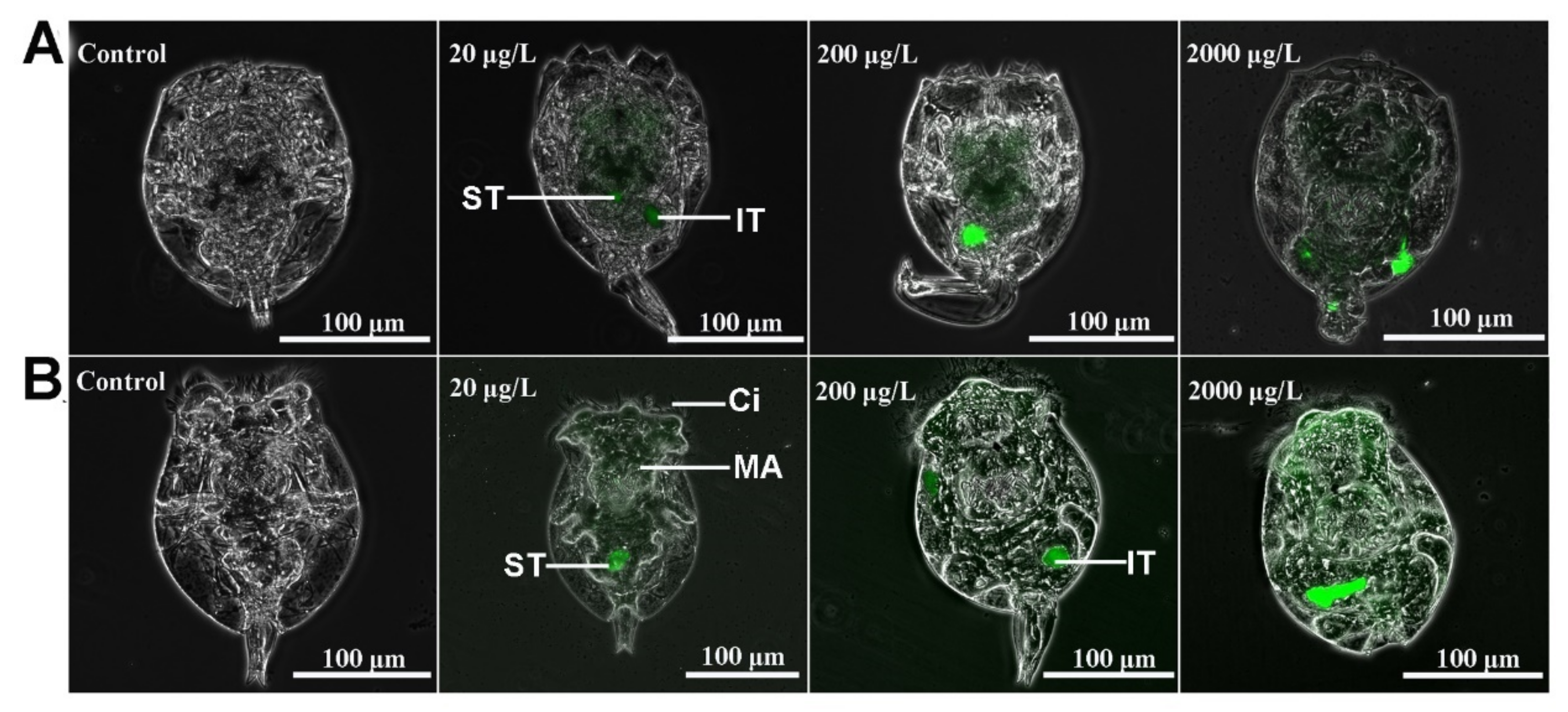
2.1. Ingestion of MPs in Rotifers
2.2. Stomach Microstructural Observations
2.3. Changes in the Life History Traits of Rotifers in Response to Two Sizes of MPs
2.4. The Combined Effect of 1-μm MPs and TCEP on the Population Growth and Oxidative Status of Rotifers
2.5. Transcriptomics Response
2.6. Changes in Multi-Xenobiotic Resistance System Activities
3. Discussion
4. Materials and Methods
4.1. Cultivation of B. plicatilis
4.2. Chemicals
4.3. Impact of Different Sized MPs on Key Life History Parameters of B. plicatilis
4.3.1. The Ingestion Test for MPs of Two Sizes
4.3.2. Microstructural Analysis of Rotifer Stomach
4.3.3. Life-Cycle Test
4.4. Coexposure Test of 1 μm MPs and TCEP
4.4.1. Population Growth
4.4.2. Oxidative Stress
4.4.3. Transcriptomic Analysis
RNA Extraction and RNA-seq
Analysis of Differentially Expressed Unigenes (DEGs)
Validation of Quantitative Real-Time PCR (qRT-PCR)
4.4.4. Activities of MXR Key Components
P-gp and MRP Activity
GST Activity
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cole, M.; Lindeque, P.; Halsband, C.; Galloway, T.S. Microplastics as contaminants in the marine environment: A review. Mar. Pollut. Bull. 2011, 62, 2588–2597. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Pu, S.; Liu, S.; Bai, Y.; Mandal, S.; Xing, B. Microplastics in aquatic environments: Toxicity to trigger ecological consequences. Environ. Pollut. 2020, 261, 114089. [Google Scholar] [CrossRef] [PubMed]
- Masry, M.; Rossignol, S.; Gardette, J.L.; Therias, S.; Bussiere, P.O.; Wong-Wah-Chung, P. Characteristics, fate, and impact of marine plastic debris exposed to sunlight: A review. Mar. Pollut. Bull. 2021, 171, 112701. [Google Scholar] [CrossRef] [PubMed]
- Rochman, C.M.; Browne, M.A.; Halpern, B.S.; Hentschel, B.T.; Hoh, E.; Karapanagioti, H.K.; Rios-Mendoza, L.M.; Takada, H.; Teh, S.; Thompson, R.C. Policy: Classify plastic waste as hazardous. Nature 2013, 494, 169–171. [Google Scholar] [CrossRef]
- Andrady, A.L. Microplastics in the marine environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef]
- Arthur, C.; Baker, J.; Bamford, H.; Barnea, N.; Mcelwee, K. Summary of the International Research Workshop on the Occurrence, Effects, and Fate of Microplastic Marine Debris. Natl. Ocean. Atmos. Adm. Tech. Memo. NOS-ORR30. 2009. Available online: https://repository.library.noaa.gov/view/noaa/2509 (accessed on 24 April 2022).
- Jeong, C.B.; Kang, H.M.; Lee, Y.H.; Kim, M.S.; Lee, J.S.; Seo, J.S.; Wang, M.; Lee, J.S. Nanoplastic ingestion enhances toxicity of Persistent Organic Pollutants (POPs) in the monogonont rotifer Brachionus koreanus via Multixenobiotic Resistance (MXR) Disruption. Environ. Sci. Technol. 2018, 52, 11411–11418. [Google Scholar] [CrossRef]
- Wang, J.; Wang, M.; Ru, S.; Liu, X. High levels of microplastic pollution in the sediments and benthic organisms of the South Yellow Sea, China. Sci. Total Environ. 2019, 651, 1661–1669. [Google Scholar] [CrossRef]
- Kanhai, D.K.; Gardfeldt, K.; Lyashevska, O.; Hassellov, M.; Thompson, R.C.; O’Connor, I. Microplastics in sub-surface waters of the Arctic Central Basin. Mar. Pollut. Bull. 2018, 130, 8–18. [Google Scholar] [CrossRef]
- Waller, C.L.; Griffiths, H.J.; Waluda, C.M.; Thorpe, S.E.; Loaiza, I.; Moreno, B.; Pacherres, C.O.; Hughes, K.A. Microplastics in the Antarctic marine system: An emerging area of research. Sci. Total Environ. 2017, 598, 220–227. [Google Scholar] [CrossRef] [Green Version]
- Setala, O.; Fleming-Lehtinen, V.; Lehtiniemi, M. Ingestion and transfer of microplastics in the planktonic food web. Environ. Pollut. 2014, 185, 77–83. [Google Scholar] [CrossRef]
- Bour, A.; Avio, C.G.; Gorbi, S.; Regoli, F.; Hylland, K. Presence of microplastics in benthic and epibenthic organisms: Influence of habitat, feeding mode and trophic level. Environ. Pollut. 2018, 243, 1217–1225. [Google Scholar] [CrossRef] [PubMed]
- Avio, C.G.; Gorbi, S.; Regoli, F. Experimental development of a new protocol for extraction and characterization of microplastics in fish tissues: First observations in commercial species from Adriatic Sea. Mar. Environ. Res. 2015, 111, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Trestrail, C.; Walpitagama, M.; Miranda, A.; Nugegoda, D.; Shimeta, J. Microplastics alter digestive enzyme activities in the marine bivalve, Mytilus galloprovincialis. Sci. Total Environ. 2021, 779, 146418. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, J.; Geng, X.; Jiang, Y. Does microplastic ingestion dramatically decrease the biomass of protozoa grazers? A case study on the marine ciliate Uronema marinum. Chemosphere 2021, 267, 129308. [Google Scholar] [CrossRef] [PubMed]
- Gambardella, C.; Morgana, S.; Bramini, M.; Rotini, A.; Manfra, L.; Migliore, L.; Piazza, V.; Garaventa, F.; Faimali, M. Ecotoxicological effects of polystyrene microbeads in a battery of marine organisms belonging to different trophic levels. Mar. Environ. Res. 2018, 141, 313–321. [Google Scholar] [CrossRef]
- Sun, Y.; Xu, W.; Gu, Q.; Chen, Y.; Zhou, Q.; Zhang, L.; Gu, L.; Huang, Y.; Lyu, K.; Yang, Z. Small-sized microplastics negatively affect rotifers: Changes in the key life-history traits and rotifer-Phaeocystis population dynamics. Environ. Sci. Technol. 2019, 53, 9241–9251. [Google Scholar] [CrossRef]
- Lusher, A.L.; McHugh, M.; Thompson, R.C. Occurrence of microplastics in the gastrointestinal tract of pelagic anddemersal fish from the English Channel. Mar. Pollut. Bull. 2013, 67, 94–99. [Google Scholar] [CrossRef]
- Wan, Z.; Wang, C.; Zhou, J.; Shen, M.; Wang, X.; Fu, Z.; Jin, Y. Effects of polystyrene microplastics on the composition of the microbiome and metabolism in larval zebrafish. Chemosphere 2019, 217, 646–658. [Google Scholar] [CrossRef]
- Beiras, R.; Bellas, J.; Cachot, J.; Cormier, B.; Cousin, X.; Engwall, M.; Gambardella, C.; Garaventa, F.; Keiter, S.; Le Bihanic, F. Ingestion and contact with polyethylene microplastics does not cause acute toxicity on marine zooplankton. J. Hazard. Mater. 2018, 360, 452–460. [Google Scholar] [CrossRef] [Green Version]
- Canniff, P.M.; Hoang, T.C. Microplastic ingestion by Daphnia magna and its enhancement on algal growth. Sci. Total Environ. 2018, 633, 500–507. [Google Scholar] [CrossRef] [Green Version]
- Barnes, D.K.; Galgani, F.; Thompson, R.C.; Barlaz, M. Accumulation and fragmentation of plastic debris in global environments. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 1985–1998. [Google Scholar] [CrossRef] [Green Version]
- Jeong, C.B.; Kang, H.M.; Byeon, E.; Kim, M.S.; Ha, S.Y.; Kim, M.; Jung, J.H.; Lee, J.S. Phenotypic and transcriptomic responses of the rotifer Brachionus koreanus by single and combined exposures to nano-sized microplastics and water-accommodated fractions of crude oil. J. Hazard. Mater. 2021, 416, 125703. [Google Scholar] [CrossRef]
- Wang, J.; Tan, Z.; Peng, J.; Qiu, Q.; Li, M. The behaviors of microplastics in the marine environment. Mar. Environ. Res. 2016, 113, 7–17. [Google Scholar] [CrossRef]
- Franzellitti, S.; Capolupo, M.; Wathsala, R.; Valbonesi, P.; Fabbri, E. The Multixenobiotic resistance system as a possible protective response triggered by microplastic ingestion in Mediterranean mussels (Mytilus galloprovincialis): Larvae and adult stages. Comp. Biochem. Physiol. C Toxicol. Pharm. 2019, 219, 50–58. [Google Scholar] [CrossRef]
- Xu, K.; Zhang, Y.; Huang, Y.; Wang, J. Toxicological effects of microplastics and phenanthrene to zebrafish (Danio rerio). Sci. Total Environ. 2021, 757, 143730. [Google Scholar] [CrossRef]
- Zhang, J.; Meng, H.; Kong, X.; Cheng, X.; Ma, T.; He, H.; Du, W.; Yang, S.; Li, S.; Zhang, L. Combined effects of polyethylene and organic contaminant on zebrafish (Danio rerio): Accumulation of 9-Nitroanthracene, biomarkers and intestinal microbiota. Environ. Pollut. 2021, 277, 116767. [Google Scholar] [CrossRef]
- Bihanic, F.L.; Clérandeau, C.; Cormier, B.; Crebassa, J.C.; Cachot, J. Organic contaminants sorbed to microplastics affect marine medaka fish early life stages development. Mar. Pollut. Bull. 2020, 154, 111059. [Google Scholar] [CrossRef]
- Beiras, R.; Tato, T. Microplastics do not increase toxicity of a hydrophobic organic chemical to marine plankton. Mar. Pollut. Bull. 2019, 138, 58–62. [Google Scholar] [CrossRef]
- Guven, O.; Bach, L.; Munk, P.; Dinh, K.V.; Mariani, P.; Nielsen, T.G. Microplastic does not magnify the acute effect of PAH pyrene on predatory performance of a tropical fish (Lates calcarifer). Aquat. Toxicol. 2018, 198, 287–293. [Google Scholar] [CrossRef]
- Zhang, Y.T.; Chen, M.; He, S.; Fang, C.; Chen, M.; Li, D.; Wu, D.; Chernick, M.; Hinton, D.E.; Bo, J.; et al. Microplastics decrease the toxicity of triphenyl phosphate (TPhP) in the marine medaka (Oryzias melastigma) larvae. Sci. Total Environ. 2021, 763, 143040. [Google Scholar] [CrossRef]
- Yoon, D.S.; Lee, Y.; Park, J.C.; Lee, M.C.; Lee, J.S. Alleviation of tributyltin-induced toxicity by diet and microplastics in the marine rotifer Brachionus koreanus. J. Hazard. Mater. 2021, 402, 123739. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.L.; Li, D.Q.; Zhuo, M.N.; Liao, Y.S.; Xie, Z.Y.; Guo, T.L.; Li, J.J.; Zhang, S.Y.; Liang, Z.Q. Organophosphorus flame retardants and plasticizers: Sources, occurrence, toxicity and human exposure. Environ. Pollut. 2015, 196, 29–46. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Su, G.; Tang, S.; Liu, W.; Ma, Z.; Zheng, X.; Liu, H.; Yu, H. The combination of in silico and in vivo approaches for the investigation of disrupting effects of tris (2-chloroethyl) phosphate (TCEP) toward core receptors of zebrafish. Chemosphere 2017, 168, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; He, Y.; Lin, L.; Zeng, F.; Luan, T. Application of fully automatic hollow fiber liquid phase microextraction to assess the distribution of organophosphate esters in the Pearl River Estuaries. Sci. Total Environ. 2014, 470–471, 263–269. [Google Scholar] [CrossRef]
- Lian, W.; Yi, X.; Huang, K.; Tang, T.; Wang, R.; Tao, X.; Zheng, Z.; Dang, Z.; Yin, H.; Lu, G. Degradation of tris(2-chloroethyl) phosphate (TCEP) in aqueous solution by using pyrite activating persulfate to produce radicals. Ecotoxicol. Environ. Saf. 2019, 174, 667–674. [Google Scholar] [CrossRef]
- Marklund, A.; Andersson, B.; Haglund, P. Screening of organophosphorus compounds and their distribution in various indoor environments. Chemosphere 2003, 53, 1137–1146. [Google Scholar] [CrossRef]
- Kawagoshi, Y.; Fukunaga, I.; Itoh, H. Distribution of organophosphoric acid triesters between water and sediment at a sea-based solid waste disposal site. J. Mater. Cycles Waste Manag. 1999, 1, 53–61. [Google Scholar] [CrossRef]
- Hou, R.; Xu, Y.; Wang, Z. Review of OPFRs in animals and humans: Absorption, bioaccumulation, metabolism, and internal exposure research. Chemosphere 2016, 153, 78–90. [Google Scholar] [CrossRef]
- Li, W.; Yuan, S.; Sun, Q.; Liu, C. Toxicity of tris(2-chloroethyl) phosphate in Daphnia magna after lifetime exposure: Changes in growth, reproduction, survival and gene transcription. Ecotoxicol Environ. Saf. 2020, 200, 110769. [Google Scholar] [CrossRef]
- Hu, F.; Zhao, Y.; Yuan, Y.; Yin, L.; Dong, F.; Zhang, W.; Chen, X. Effects of environmentally relevant concentrations of tris (2-chloroethyl) phosphate (TCEP) on early life stages of zebrafish (Danio rerio). Environ. Toxicol. Pharm. 2021, 83, 103600. [Google Scholar] [CrossRef]
- Hao, H.; Yuan, S.; Cheng, S.; Sun, Q.; Giesy, J.P.; Liu, C. Effects of tris (2-chloroethyl) phosphate (TCEP) on growth, reproduction and gene transcription in the protozoan Tetrahymena thermophila. Aquat. Toxicol. 2020, 222, 105477. [Google Scholar] [CrossRef] [PubMed]
- Dahms, H.U.; Hagiwara, A.; Lee, J.S. Ecotoxicology, ecophysiology, and mechanistic studies with rotifers. Aquat. Toxicol. 2011, 101, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Cooper, C.A.; Tait, T.; Gray, H.; Cimprich, G.; Santore, R.C.; McGeer, J.C.; Wood, C.M.; Smith, D.S. Influence of salinity and dissolved organic carbon on acute Cu toxicity to the rotifer Brachionus plicatilis. Environ. Sci. Technol. 2014, 48, 1213–1221. [Google Scholar] [CrossRef] [PubMed]
- Venâncio, C.; Ciubotariu, A.; Lopes, I.; Martins, M.A.; Oliveira, M. Is the toxicity of nanosized polymethylmethacrylate particles dependent on the exposure route and food items? J. Hazard. Mater. 2021, 413, 125443. [Google Scholar] [CrossRef]
- Byeon, E.; Yoon, C.; Lee, J.S.; Lee, Y.H.; Jeong, C.B.; Lee, J.S.; Kang, H.M. Interspecific biotransformation and detoxification of arsenic compounds in marine rotifer and copepod. J. Hazard. Mater. 2020, 391, 122196. [Google Scholar] [CrossRef]
- Zhao, K.; Wei, Y.; Dong, J.; Zhao, P.; Wang, Y.; Pan, X.; Wang, J. Separation and characterization of microplastic and nanoplastic particles in marine environment. Environ. Pollut. 2021, 297, 118773. [Google Scholar] [CrossRef]
- Hotos, G.N. Selectivity of the rotifer Brachionus plicatilis fed mixtures of algal species with various cell volumes and cell densities. Aquac. Res. 2002, 33, 949–957. [Google Scholar] [CrossRef]
- Zhang, C.; Jeong, C.B.; Lee, J.S.; Wang, D.; Wang, M. Transgenerational proteome pasticity in resilience of a marine Copepod in response to environmentally relevant concentrations of microplastics. Environ. Sci. Technol. 2019, 53, 8426–8436. [Google Scholar] [CrossRef]
- Aljaibachi, R.; Laird, W.B.; Stevens, F.; Callaghan, A. Impacts of polystyrene microplastics on Daphnia magna: A laboratory and a mesocosm study. Sci. Total Environ. 2019, 705, 135800. [Google Scholar] [CrossRef]
- Park, J.C.; Kim, D.H.; Lee, M.C.; Han, J.; Kim, H.J.; Hagiwara, A.; Hwang, U.K.; Park, H.G.; Lee, J.S. Genome-wide identification of the entire 90 glutathione S-transferase (GST) subfamily genes in four rotifer Brachionus species and transcriptional modulation in response to endocrine disrupting chemicals. Comp. Biochem. Physiol. Part D Genom. Proteom. 2018, 28, 183–195. [Google Scholar] [CrossRef]
- Bard, S.M. Multixenobiotic resistance as a cellular defense mechanism in aquatic organisms. Aquat. Toxicol. 2000, 48, 357–389. [Google Scholar] [CrossRef]
- Won, E.J.; Kim, R.O.; Kang, H.M.; Kim, H.S.; Hwang, D.S.; Han, J.; Lee, Y.H.; Hwang, U.K.; Zhou, B.; Lee, S.J. Adverse effects, expression of the Bk-CYP3045C1 gene, and activation of the ERK signaling pathway in the water accommodated fraction-exposed rotifer. Environ. Sci. Technol. 2016, 50, 6025–6035. [Google Scholar] [CrossRef]
- Dolui, D.; Saha, I.; Adak, M.K. 2, 4-D removal efficiency of Salvinia natans L. and its tolerance to oxidative stresses through glutathione metabolism under induction of light and darkness. Ecotoxicol Environ. Saf 2021, 208, 111708. [Google Scholar] [CrossRef] [PubMed]
- Niu, B.; Liao, K.; Zhou, Y.; Wen, T.; Quan, G.; Pan, X.; Wu, C. Application of glutathione depletion in cancer therapy: Enhanced ROS-based therapy, ferroptosis, and chemotherapy. Biomaterials 2021, 277, 121110. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.D.; Flanagan, J.U.; Jowsey, I.R. Glutathione transferases. Arab. Book 2005, 8, 131. [Google Scholar] [CrossRef]
- Jeong, C.B.; Won, E.J.; Kang, H.M.; Lee, M.C.; Hwang, D.S.; Hwang, U.K.; Zhou, B.; Souissi, S.; Lee, S.J.; Lee, J.S. Microplastic Size-Dependent Toxicity, Oxidative Stress Induction, and p-JNK and p-p38 Activation in the Monogonont Rotifer (Brachionus koreanus). Environ. Sci. Technol. 2016, 50, 8849–8857. [Google Scholar] [CrossRef]
- Hartl, F.U.; Hayer-Hartl, M. Molecular chaperones in the cytosol: From nascent chain to folded protein. Science 2002, 295, 1852–1858. [Google Scholar] [CrossRef] [Green Version]
- Ryan, M.T.; Pfanner, N. Hsp70 proteins in protein translocation. Adv. Protein Chem. 2001, 59, 223–242. [Google Scholar] [CrossRef]
- Park, S.; Jang, J.; Kim, H.; Dong, I.P.; Kim, K.; Yoon, H.J. Thermal conductance in single molecules and self-assembled monolayers: Physicochemical insights, progress, and challenges. J. Mater. Chem. 2020, 8, 19704–19728. [Google Scholar] [CrossRef]
- Lu, K.; Qiao, R.; An, H.; Zhang, Y. Influence of microplastics on the accumulation and chronic toxic effects of cadmium in zebrafish (Danio rerio). Chemosphere 2018, 202, 514–520. [Google Scholar] [CrossRef]
- Xia, B.; Zhang, J.; Zhao, X.; Feng, J.; Teng, Y.; Chen, B.; Sun, X.; Zhu, L.; Sun, X.; Qu, K. Polystyrene microplastics increase uptake, elimination and cytotoxicity of decabromodiphenyl ether (BDE-209) in the marine scallop Chlamys farreri. Environ. Pollut. 2020, 258, 113657. [Google Scholar] [CrossRef] [PubMed]
- Hansen, P.J.; Bjornsen, P.K.; Hansen, B.W. Zooplankton grazing grazing and growth: Scaling within the 2-2, 00- μm body size range. Limnol. Oceanogr. 1997, 42, 687–704. [Google Scholar] [CrossRef]
- Preston, B.L.; Snell, T.W. Direct and indirect effects of sublethal toxicant exposure on population dynamics of freshwater rotifers: A modeling approach. Aquat. Toxicol. 2001, 52, 87–99. [Google Scholar] [CrossRef]
- Chen, S.; Tan, Z.; Qi, Y.; Ouyang, C. Sorption of tri-n-butyl phosphate and tris(2-chloroethyl) phosphate on polyethylene and polyvinyl chloride microplastics in seawater. Mar. Pollut. Bull. 2019, 149, 110490. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Han, F.; Huang, K.; Zhang, J.; Qin, J.G.; Chen, L.; Li, E. Impact of imidacloprid exposure on the biochemical responses, transcriptome, gut microbiota and growth performance of the Pacific white shrimp Litopenaeus vannamei. J. Hazard. Mater. 2022, 424, 127513. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Kang, H.M.; Kim, M.S.; Lee, J.S.; Jeong, C.B.; Lee, J.S. The protective role of multixenobiotic resistance (MXR)-mediated ATP-binding cassette (ABC) transporters in biocides-exposed rotifer Brachionus koreanus. Aquat. Toxicol. 2018, 195, 129–136. [Google Scholar] [CrossRef]
- Rhee, J.S.; Jeong, C.B.; Kim, B.M.; Lee, J.S. P-glycoprotein (P-gp) in the monogonont rotifer, Brachionus koreanus: Molecular characterization and expression in response to pharmaceuticals. Aquat. Toxicol. 2012, 114–115, 104–118. [Google Scholar] [CrossRef]
- Jeong, C.B.; Kang, H.M.; Lee, M.C.; Kim, D.H.; Han, J.; Hwang, D.S.; Souissi, S.; Lee, S.J.; Shin, K.H.; Park, H.G.; et al. Adverse effects of microplastics and oxidative stress-induced MAPK/Nrf2 pathway-mediated defense mechanisms in the marine copepod Paracyclopina nana. Sci. Rep. 2017, 7, 41323. [Google Scholar] [CrossRef] [Green Version]
- Byeon, E.; Choi, B.S.; Park, J.C.; Kim, M.S.; Kim, D.H.; Lee, J.S.; Lee, Y.H.; Jeong, C.B.; Hwang, U.K.; Hagiwara, A.; et al. The genome of the freshwater monogonont rotifer Brachionus angularis: Identification of phase I, II, and III detoxification genes and their roles in molecular ecotoxicology. Comp. Biochem. Physiol. Part D Genom. Proteom. 2021, 38, 100821. [Google Scholar] [CrossRef]
- Jeong, C.B.; Kim, B.M.; Lee, J.S.; Rhee, J.S. Genome-wide identification of whole ATP-binding cassette (ABC) transporters in the intertidal copepod Tigriopus japonicus. BMC Genom. 2014, 15, 651. [Google Scholar] [CrossRef] [Green Version]
- Bosnjak, I.; Uhlinger, K.R.; Heim, W.; Smital, T.; Hamdoun, A. Multidrug Efflux Transporters Limit Accumulation of Inorganic, but Not Organic, Mercury in Sea Urchin Embryos. Environ. Sci. Technol. 2009, 43, 8374–8380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ASTM E1440-91; Standard Guide for Acute Toxicity Tests with the Rotifer Brachionus. American Society for Testing and Material: Philadelphia, PA, USA, 1991.
- Yang, Y.; Jian, X.; Tang, X.; Ma, W.; Sun, Z.; Zhang, X.; Fang, K.; Zhang, X. Feeding behavior toxicity in the marine rotifer Brachionus plicatilis caused by 2,2′,4,4′-tetrabromodiphenyl ether (BDE-47): Characteristics and mechanisms. Chemosphere 2021, 271, 129512. [Google Scholar] [CrossRef] [PubMed]
- Charles, J.; Krebs, C.J. Ecology: The Experimental Analysis of Distribution and Abundance; Harper & Row: New York, NY, USA, 1978. [Google Scholar]
- Lee, M.C.; Fonseca, E.; Park, J.C.; Yoon, D.S.; Choi, H.; Kim, M.; Han, J.; Cho, H.S.; Shin, K.H.; Santos, M.L.; et al. Tributyltin affects retinoid X receptor-mediated lipid metabolism in the marine rotifer Brachionus koreanus. Environ. Sci. Technol. 2019, 53, 7830–7839. [Google Scholar] [CrossRef]
- Lee, Y.H.; Kang, H.M.; Kim, M.S.; Lee, J.S.; Wang, M.; Hagiwara, A.; Jeong, C.B.; Lee, J.S. Multigenerational mitigating effects of ocean acidification on in vivo endpoints, antioxidant defense, DNA damage rsponse, and epigenetic modification in an asexual monogonont rotifer. Environ. Sci. Technol. 2020, 54, 7858–7869. [Google Scholar] [CrossRef] [PubMed]
- Andresen, J.A.; Grundmann, A.; Bester, K. Organophosphorus flame retardants and plasticisers in surface waters. Sci. Total Environ. 2004, 332, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Tang, X.; Yang, Y.; Sun, Z.; Ma, W.; Tong, X.; Wang, C.; Zhang, X. Responses of the reproduction, population growth and metabolome of the marine rotifer Brachionus plicatilis to tributyl phosphate (TnBP). Environ. Pollut. 2021, 273, 116462. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Araki, M.; Goto, S.; Hattori, M.; Hirakawa, M.; Itoh, M.; Katayama, T.; Kawashima, S.; Okuda, S.; Tokimatsu, T.; et al. KEGG for linking genomes to life and the environment. Nucleic Acids Res. 2008, 36, D480–D484. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, W.; Sun, Z.; Zhang, X.; Tang, X.; Zhang, X. Alleviation of Tris(2-chloroethyl) Phosphate Toxicity on the Marine Rotifer Brachionus plicatilis by Polystyrene Microplastics: Features and Molecular Evidence. Int. J. Mol. Sci. 2022, 23, 4934. https://doi.org/10.3390/ijms23094934
Ma W, Sun Z, Zhang X, Tang X, Zhang X. Alleviation of Tris(2-chloroethyl) Phosphate Toxicity on the Marine Rotifer Brachionus plicatilis by Polystyrene Microplastics: Features and Molecular Evidence. International Journal of Molecular Sciences. 2022; 23(9):4934. https://doi.org/10.3390/ijms23094934
Chicago/Turabian StyleMa, Wenqian, Zijie Sun, Xin Zhang, Xuexi Tang, and Xinxin Zhang. 2022. "Alleviation of Tris(2-chloroethyl) Phosphate Toxicity on the Marine Rotifer Brachionus plicatilis by Polystyrene Microplastics: Features and Molecular Evidence" International Journal of Molecular Sciences 23, no. 9: 4934. https://doi.org/10.3390/ijms23094934
APA StyleMa, W., Sun, Z., Zhang, X., Tang, X., & Zhang, X. (2022). Alleviation of Tris(2-chloroethyl) Phosphate Toxicity on the Marine Rotifer Brachionus plicatilis by Polystyrene Microplastics: Features and Molecular Evidence. International Journal of Molecular Sciences, 23(9), 4934. https://doi.org/10.3390/ijms23094934






