Extracellular Vesicles as an Index for Endothelial Injury and Cardiac Dysfunction in a Rodent Model of GDM
Abstract
1. Introduction
2. Results
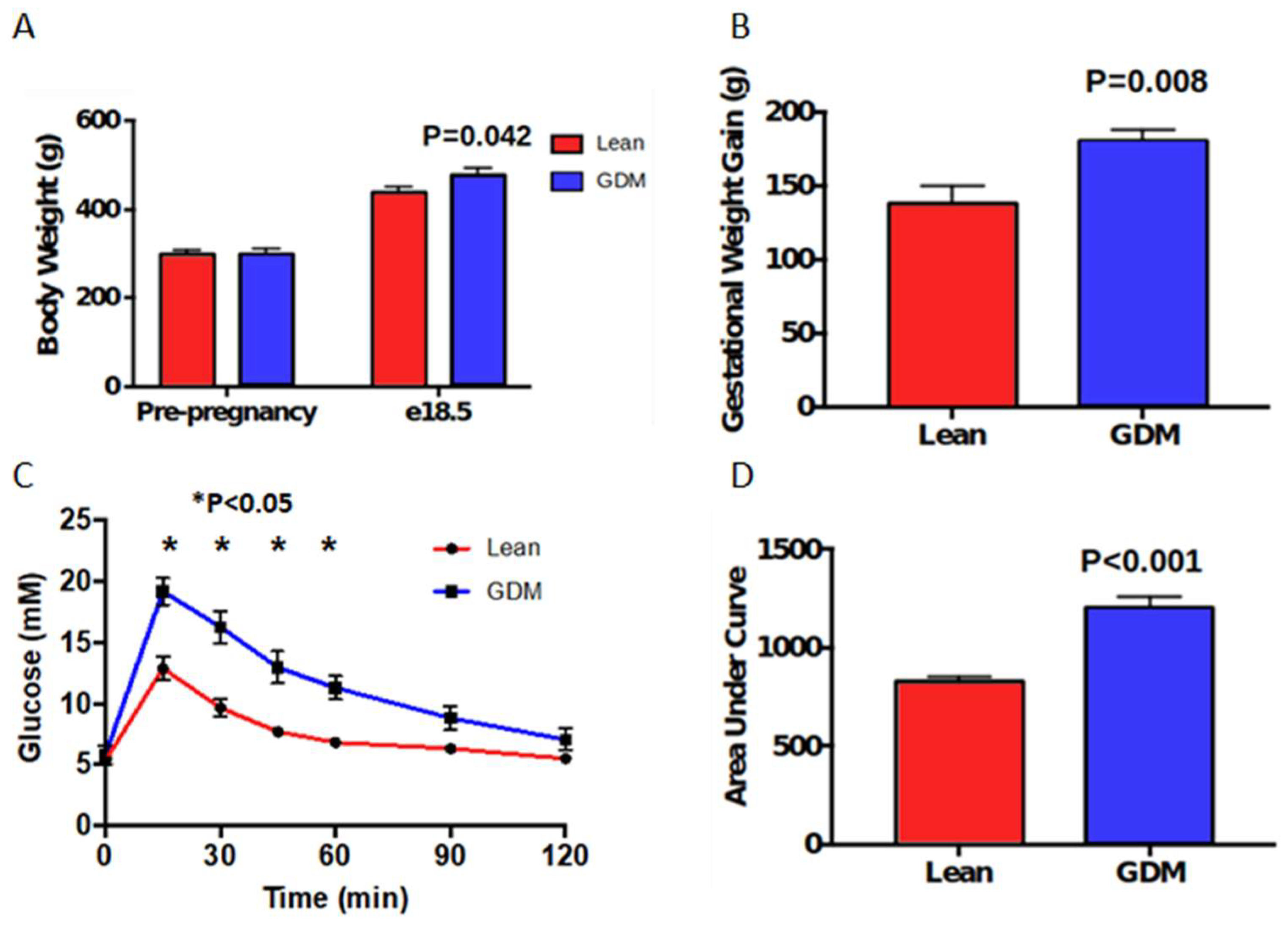
2.1. Maternal Characteristics
2.2. Measures of Vascular Injury
2.3. Aortic NAD+, and Mitochondrial DNA Shedding
2.4. Maternal Cardiac Structure and Function
3. Discussion
4. Materials and Methods
4.1. Maternal GDM Model
4.2. Metabolic Assessment in Pregnancy
4.3. Isolation of Circulating Extracellular Vesicles
4.4. Nanoparticle Tracking Analysis
4.5. Quantification of Endothelial EVs by Flow Cytometry
4.6. Assessment of mtDNA in Circulating Extracellular Vesicles
4.7. vWF Measurement
4.8. NAD Measurement
4.9. Echocardiography Assessment in Pregnancy
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Melchiorre, K.; Sharma, R.; Thilaganathan, B. Cardiac Structure and Function in Normal Pregnancy. Curr. Opin. Obstet. Gynecol. 2012, 24, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Moyce, B.L.; Dolinsky, V.W. Maternal β-Cell Adaptations in Pregnancy and Placental Signalling: Implications for Gestational Diabetes. Int. J. Mol. Sci. 2018, 19, 3467. [Google Scholar] [CrossRef] [PubMed]
- International Association of Diabetes and Pregnancy Study Groups Consensus Panel. International Association of Diabetes and Pregnancy Study Groups Recommendations on the Diagnosis and Classification of Hyperglycemia in Pregnancy. Diabetes Care 2010, 33, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Billionnet, C.; Mitanchez, D.; Weill, A.; Nizard, J.; Alla, F.; Hartemann, A.; Jacqueminet, S. Gestational Diabetes and Adverse Perinatal Outcomes from 716,152 Births in France in 2012. Diabetologia 2017, 60, 636–644. [Google Scholar] [CrossRef]
- Wendland, E.M.; Torloni, M.R.; Falavigna, M.; Trujillo, J.; Dode, M.A.; Campos, M.A.; Duncan, B.B.; Schmidt, M.I. Gestational Diabetes and Pregnancy Outcomes—A Systematic Review of the World Health Organization (WHO) and the International Association of Diabetes in Pregnancy Study Groups (IADPSG) Diagnostic Criteria. BMC Pregnancy Childbirth 2012, 12, 23. [Google Scholar] [CrossRef]
- Bellamy, L.; Casas, J.-P.; Hingorani, A.D.; Williams, D. Type 2 Diabetes Mellitus after Gestational Diabetes: A Systematic Review and Meta-Analysis. Lancet 2009, 373, 1773–1779. [Google Scholar] [CrossRef]
- Feig, D.S.; Berger, H.; Donovan, L.; Godbout, A.; Kader, T.; Keely, E.; Sanghera, R. Diabetes and Pregnancy. Can. J. Diabetes 2018, 42, S255–S282. [Google Scholar] [CrossRef]
- Shah, B.R.; Feig, D.S.; Herer, E.; Hladunewich, M.A.; Kiss, A.; Kohly, R.P.; Lipscombe, L.L.; Yip, P.M.; Cherney, D.Z. Increased Risk for Microvascular Complications among Women with Gestational Diabetes in the Third Trimester. Diabetes Res. Clin. Pract. 2021, 180, 109068. [Google Scholar] [CrossRef]
- Triggle, C.R.; Ding, H.; Marei, I.; Anderson, T.J.; Hollenberg, M.D. Why the Endothelium? The Endothelium as a Target to Reduce Diabetes-Associated Vascular Disease. Can. J. Physiol. Pharmacol. 2020, 98, 415–430. [Google Scholar] [CrossRef]
- Li, X.; Sun, X.; Carmeliet, P. Hallmarks of Endothelial Cell Metabolism in Health and Disease. Cell Metab. 2019, 30, 414–433. [Google Scholar] [CrossRef]
- Pangare, M.; Makino, A. Mitochondrial Function in Vascular Endothelial Cell in Diabetes. J. Smooth Muscle Res. Nihon Heikatsukin Gakkai Kikanshi 2012, 48, 1–26. [Google Scholar] [CrossRef]
- Shenouda, S.M.; Widlansky, M.E.; Chen, K.; Xu, G.; Holbrook, M.; Tabit, C.E.; Hamburg, N.M.; Frame, A.A.; Caiano, T.L.; Kluge, M.A.; et al. Altered Mitochondrial Dynamics Contributes to Endothelial Dysfunction in Diabetes Mellitus. Circulation 2011, 124, 444–453. [Google Scholar] [CrossRef]
- Kluge, M.A.; Fetterman, J.L.; Vita, J.A. Mitochondria and Endothelial Function. Circ. Res. 2013, 112, 1171–1188. [Google Scholar] [CrossRef]
- Meng, T.; Qin, W.; Liu, B. SIRT1 Antagonizes Oxidative Stress in Diabetic Vascular Complication. Front. Endocrinol. 2020, 11, 891. [Google Scholar] [CrossRef]
- Liu, G.; Cao, M.; Xu, Y.; Li, Y. SIRT3 Protects Endothelial Cells from High Glucose-Induced Cytotoxicity. Int. J. Clin. Exp. Pathol. 2015, 8, 353. [Google Scholar]
- Mattagajasingh, I.; Kim, C.-S.; Naqvi, A.; Yamamori, T.; Hoffman, T.A.; Jung, S.-B.; DeRicco, J.; Kasuno, K.; Irani, K. SIRT1 Promotes Endothelium-Dependent Vascular Relaxation by Activating Endothelial Nitric Oxide Synthase. Proc. Natl. Acad. Sci. USA 2007, 104, 14855–14860. [Google Scholar] [CrossRef]
- McElwain, C.J.; Tuboly, E.; McCarthy, F.P.; McCarthy, C.M. Mechanisms of Endothelial Dysfunction in Pre-Eclampsia and Gestational Diabetes Mellitus: Windows Into Future Cardiometabolic Health? Front. Endocrinol. 2020, 11, 655. [Google Scholar] [CrossRef]
- Erdbrugger, U.; Le, T.H. Extracellular Vesicles in Renal Diseases: More than Novel Biomarkers? J. Am. Soc. Nephrol. 2016, 27, 12–26. [Google Scholar] [CrossRef]
- Burger, D.; Schock, S.; Thompson, C.S.; Montezano, A.C.; Hakim, A.M.; Touyz, R.M. Microparticles: Biomarkers and Beyond. Clin. Sci. 2013, 124, 423–441. [Google Scholar] [CrossRef]
- Medeiros, T.; Myette, R.L.; Almeida, J.R.; Silva, A.A.; Burger, D. Extracellular Vesicles: Cell-Derived Biomarkers of Glomerular and Tubular Injury. Cell. Physiol. Biochem. 2020, 54, 88–109. [Google Scholar] [CrossRef]
- McVey, M.J.; Kuebler, W.M. Extracellular Vesicles: Biomarkers and Regulators of Vascular Function during Extracorporeal Circulation. Oncotarget 2018, 9, 37229–37251. [Google Scholar] [CrossRef][Green Version]
- Huang-Doran, I.; Zhang, C.-Y.; Vidal-Puig, A. Extracellular Vesicles: Novel Mediators of Cell Communication In Metabolic Disease. Trends Endocrinol. Metab. 2017, 28, 3–18. [Google Scholar] [CrossRef]
- Chen, Y.; Feng, B.; Li, X.; Ni, Y.; Luo, Y. Plasma Endothelial Microparticles and Their Correlation with the Presence of Hypertension and Arterial Stiffness in Patients with Type 2 Diabetes. J. Clin. Hypertens. Greenwich Conn 2012, 14, 455–460. [Google Scholar] [CrossRef]
- Burger, D.; Montezano, A.C.; Nishigaki, N.; He, Y.; Carter, A.; Touyz, R.M. Endothelial Microparticle Formation by Angiotensin II Is Mediated via Ang II Receptor Type I/NADPH Oxidase/ Rho Kinase Pathways Targeted to Lipid Rafts. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 1898–1907. [Google Scholar] [CrossRef]
- Amabile, N.; Cheng, S.; Renard, J.M.; Larson, M.G.; Ghorbani, A.; McCabe, E.; Griffin, G.; Guerin, C.; Ho, J.E.; Shaw, S.Y.; et al. Association of Circulating Endothelial Microparticles with Cardiometabolic Risk Factors in the Framingham Heart Study. Eur. Heart J. 2014, 35, 2972–2979. [Google Scholar] [CrossRef]
- Sabatier, F.; Darmon, P.; Hugel, B.; Combes, V.; Sanmarco, M.; Velut, J.-G.; Arnoux, D.; Charpiot, P.; Freyssinet, J.-M.; Oliver, C.; et al. Type 1 And Type 2 Diabetic Patients Display Different Patterns of Cellular Microparticles. Diabetes 2002, 51, 2840–2845. [Google Scholar] [CrossRef]
- Sinning, J.-M.; Losch, J.; Walenta, K.; Böhm, M.; Nickenig, G.; Werner, N. Circulating CD31+/Annexin V+ Microparticles Correlate with Cardiovascular Outcomes. Eur. Heart J. 2011, 32, 2034–2041. [Google Scholar] [CrossRef]
- Amabile, N.; Guérin, A.P.; Tedgui, A.; Boulanger, C.M.; London, G.M. Predictive Value of Circulating Endothelial Microparticles for Cardiovascular Mortality in End-Stage Renal Failure: A Pilot Study. Nephrol. Dial. Transplant. 2012, 27, 1873–1880. [Google Scholar] [CrossRef]
- Abolbaghaei, A.; Langlois, M.-A.; Murphy, H.R.; Feig, D.S.; Burger, D.; CONCEPTT Collaborative Group. Circulating Extracellular Vesicles during Pregnancy in Women with Type 1 Diabetes: A Secondary Analysis of the CONCEPTT Trial. Biomark. Res. 2021, 9, 67. [Google Scholar] [CrossRef]
- Bathla, T.; Abolbaghaei, A.; Reyes, A.B.; Burger, D. Extracellular Vesicles in Gestational Diabetes Mellitus: A Scoping Review. Diab. Vasc. Dis. Res. 2022, 19, 14791641221093900. [Google Scholar] [CrossRef]
- Nair, S.; Ormazabal, V.; Lappas, M.; McIntyre, H.D.; Salomon, C. Extracellular Vesicles and Their Potential Role Inducing Changes in Maternal Insulin Sensitivity during Gestational Diabetes Mellitus. Am. J. Reprod. Immunol. 2021, 85, e13361. [Google Scholar] [CrossRef] [PubMed]
- Palma, C.; McIntyre, H.D.; Salomon, C. Extracellular Vesicles—New Players in Cell-to-Cell Communication in Gestational Diabetes Mellitus. Biomedicines 2022, 10, 462. [Google Scholar] [CrossRef] [PubMed]
- James-Allan, L.B.; Devaskar, S.U. Extracellular Vesicles and Their Role in Gestational Diabetes Mellitus. Placenta 2021, 113, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Zheng, L.; Zou, X.; Wang, J.; Zhong, J.; Zhong, T. Extracellular Vesicles in Type 2 Diabetes Mellitus: Key Roles in Pathogenesis, Complications, and Therapy. J. Extracell. Vesicles 2019, 8, 1625677. [Google Scholar] [CrossRef] [PubMed]
- Salomon, C.; Scholz-Romero, K.; Sarker, S.; Sweeney, E.; Kobayashi, M.; Correa, P.; Longo, S.; Duncombe, G.; Mitchell, M.D.; Rice, G.E.; et al. Gestational Diabetes Mellitus Is Associated With Changes in the Concentration and Bioactivity of Placenta-Derived Exosomes in Maternal Circulation Across Gestation. Diabetes 2016, 65, 598–609. [Google Scholar] [CrossRef] [PubMed]
- James-Allan, L.B.; Rosario, F.J.; Barner, K.; Lai, A.; Guanzon, D.; McIntyre, H.D.; Lappas, M.; Powell, T.L.; Salomon, C.; Jansson, T. Regulation of Glucose Homeostasis by Small Extracellular Vesicles in Normal Pregnancy and in Gestational Diabetes. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2020, 34, 5724–5739. [Google Scholar] [CrossRef] [PubMed]
- Nair, S.; Jayabalan, N.; Guanzon, D.; Palma, C.; Scholz-Romero, K.; Elfeky, O.; Zuñiga, F.; Ormazabal, V.; Diaz, E.; Rice, G.E.; et al. Human Placental Exosomes in Gestational Diabetes Mellitus Carry a Specific Set of MiRNAs Associated with Skeletal Muscle Insulin Sensitivity. Clin. Sci. 2018, 132, 2451–2467. [Google Scholar] [CrossRef] [PubMed]
- Kandzija, N.; Zhang, W.; Motta-Mejia, C.; Mhlomi, V.; McGowan-Downey, J.; James, T.; Cerdeira, A.S.; Tannetta, D.; Sargent, I.; Redman, C.W.; et al. Placental Extracellular Vesicles Express Active Dipeptidyl Peptidase IV; Levels Are Increased in Gestational Diabetes Mellitus. J. Extracell. Vesicles 2019, 8, 1617000. [Google Scholar] [CrossRef]
- Motta-Mejia, C.; Kandzija, N.; Zhang, W.; Mhlomi, V.; Cerdeira, A.S.; Burdujan, A.; Tannetta, D.; Dragovic, R.; Sargent, I.L.; Redman, C.W.; et al. Placental Vesicles Carry Active Endothelial Nitric Oxide Synthase and Their Activity Is Reduced in Preeclampsia. Hypertens. Dallas Tex 1979 2017, 70, 372–381. [Google Scholar] [CrossRef]
- Franzago, M.; Lanuti, P.; Fraticelli, F.; Marchioni, M.; Buca, D.; Di Nicola, M.; Liberati, M.; Miscia, S.; Stuppia, L.; Vitacolonna, E. Biological Insight into the Extracellular Vesicles in Women with and without Gestational Diabetes. J. Endocrinol. Investig. 2021, 44, 49–61. [Google Scholar] [CrossRef]
- Gillet, V.; Ouellet, A.; Stepanov, Y.; Rodosthenous, R.S.; Croft, E.K.; Brennan, K.; Abdelouahab, N.; Baccarelli, A.; Takser, L. MiRNA Profiles in Extracellular Vesicles From Serum Early in Pregnancies Complicated by Gestational Diabetes Mellitus. J. Clin. Endocrinol. Metab. 2019, 104, 5157–5169. [Google Scholar] [CrossRef]
- Jayabalan, N.; Lai, A.; Nair, S.; Guanzon, D.; Scholz-Romero, K.; Palma, C.; McIntyre, H.D.; Lappas, M.; Salomon, C. Quantitative Proteomics by SWATH-MS Suggest an Association Between Circulating Exosomes and Maternal Metabolic Changes in Gestational Diabetes Mellitus. Proteomics 2019, 19, e1800164. [Google Scholar] [CrossRef]
- Pereira, T.J.; Fonseca, M.A.; Campbell, K.E.; Moyce, B.L.; Cole, L.K.; Hatch, G.M.; Doucette, C.A.; Klein, J.; Aliani, M.; Dolinsky, V.W. Maternal Obesity Characterized by Gestational Diabetes Increases the Susceptibility of Rat Offspring to Hepatic Steatosis via a Disrupted Liver Metabolome. J. Physiol. 2015, 593, 3181–3197. [Google Scholar] [CrossRef]
- Brawerman, G.M.; Kereliuk, S.M.; Brar, N.; Cole, L.K.; Seshadri, N.; Pereira, T.J.; Xiang, B.; Hunt, K.L.; Fonseca, M.A.; Hatch, G.M.; et al. Maternal Resveratrol Administration Protects against Gestational Diabetes-Induced Glucose Intolerance and Islet Dysfunction in the Rat Offspring. J. Physiol. 2019, 597, 4175–4192. [Google Scholar] [CrossRef]
- McLaughlin, K.; Audette, M.C.; Parker, J.D.; Kingdom, J.C. Mechanisms and Clinical Significance of Endothelial Dysfunction in High-Risk Pregnancies. Can. J. Cardiol. 2018, 34, 371–380. [Google Scholar] [CrossRef]
- Grandi, S.M.; Filion, K.B.; Yoon, S.; Ayele, H.T.; Doyle, C.M.; Hutcheon, J.A.; Smith, G.N.; Gore, G.C.; Ray, J.G.; Nerenberg, K.; et al. Cardiovascular Disease-Related Morbidity and Mortality in Women With a History of Pregnancy Complications. Circulation 2019, 139, 1069–1079. [Google Scholar] [CrossRef]
- Stanley, J.L.; Cheung, C.C.; Rueda-Clausen, C.F.; Sankaralingam, S.; Baker, P.N.; Davidge, S.T. Effect of Gestational Diabetes on Maternal Artery Function. Reprod. Sci. 2011, 18, 342–352. [Google Scholar] [CrossRef]
- Meera, S.J.; Ando, T.; Pu, D.; Manjappa, S.; Taub, C.C. Dynamic Left Ventricular Changes in Patients with Gestational Diabetes: A Speckle Tracking Echocardiography Study. J. Clin. Ultrasound 2017, 45, 20–27. [Google Scholar] [CrossRef]
- Buddeberg, B.S.; Sharma, R.; O’Driscoll, J.M.; Kaelin Agten, A.; Khalil, A.; Thilaganathan, B. Impact of Gestational Diabetes Mellitus on Maternal Cardiac Adaptation to Pregnancy. Ultrasound Obstet. Gynecol. 2020, 56, 240–246. [Google Scholar] [CrossRef]
- Amabile, N.; Guérin, A.P.; Leroyer, A.; Mallat, Z.; Nguyen, C.; Boddaert, J.; London, G.M.; Tedgui, A.; Boulanger, C.M. Circulating Endothelial Microparticles Are Associated with Vascular Dysfunction in Patients with End-Stage Renal Failure. J. Am. Soc. Nephrol. 2005, 16, 3381–3388. [Google Scholar] [CrossRef]
- Spiel, A.O.; Gilbert, J.C.; Jilma, B. Von Willebrand Factor in Cardiovascular Disease. Circulation 2008, 117, 1449–1459. [Google Scholar] [CrossRef]
- Xiao, Y.; Chen, W.; Chen, R.; Luo, A.; Chen, D.; Liang, Q.; Liu, T.; Chen, X.; Tan, W. Exosomal MicroRNA Expression Profiling Analysis of the Effects of Lycium Barbarum Polysaccharide on Gestational Diabetes Mellitus Mice. Evid.-Based Complement. Altern. Med. ECAM 2020, 2020, 2953502. [Google Scholar] [CrossRef]
- Herrera-Van Oostdam, A.S.; Toro-Ortíz, J.C.; López, J.A.; Noyola, D.E.; García-López, D.A.; Durán-Figueroa, N.V.; Martínez-Martínez, E.; Portales-Pérez, D.P.; Salgado-Bustamante, M.; López-Hernández, Y. Placental Exosomes Isolated from Urine of Patients with Gestational Diabetes Exhibit a Differential Profile Expression of MicroRNAs across Gestation. Int. J. Mol. Med. 2020, 46, 546–560. [Google Scholar] [CrossRef]
- Ramachandrarao, S.P.; Hamlin, A.A.; Awdishu, L.; Overcash, R.; Zhou, M.; Proudfoot, J.; Ishaya, M.; Aghania, E.; Madrigal, A.; Kokoy-Mondragon, C.; et al. Proteomic Analyses of Urine Exosomes Reveal New Biomarkers of Diabetes in Pregnancy. Madridge J. Diabetes 2016, 1, 11–22. [Google Scholar] [CrossRef]
- Monteiro, L.J.; Varas-Godoy, M.; Monckeberg, M.; Realini, O.; Hernández, M.; Rice, G.; Romero, R.; Saavedra, J.F.; Illanes, S.E.; Chaparro, A. Oral Extracellular Vesicles in Early Pregnancy Can Identify Patients at Risk of Developing Gestational Diabetes Mellitus. PLoS ONE 2019, 14, e0218616. [Google Scholar] [CrossRef]
- Arias, M.; Monteiro, L.J.; Acuña-Gallardo, S.; Varas-Godoy, M.; Rice, G.E.; Monckeberg, M.; Díaz, P.; Illanes, S.E. Extracellular vesicle concentration in maternal plasma as an early marker of gestational diabetes. Rev. Med. Chil. 2019, 147, 1503–1509. [Google Scholar] [CrossRef]
- Zhang, Y.; Feng, J.; Wang, Q.; Zhao, S.; Yang, S.; Tian, L.; Meng, P.; Li, J.; Li, H. Hyperglycaemia Stress-Induced Renal Injury Is Caused by Extensive Mitochondrial Fragmentation, Attenuated MKP1 Signalling, and Activated JNK-CaMKII-Fis1 Biological Axis. Cell. Physiol. Biochem. 2018, 51, 1778–1798. [Google Scholar] [CrossRef]
- Premer, C.; Kanelidis, A.J.; Hare, J.M.; Schulman, I.H. Rethinking Endothelial Dysfunction as a Crucial Target in Fighting Heart Failure. Mayo Clin. Proc. Innov. Qual. Outcomes 2019, 3, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Gamrat, A.; Surdacki, M.A.; Chyrchel, B.; Surdacki, A. Endothelial Dysfunction: A Contributor to Adverse Cardiovascular Remodeling and Heart Failure Development in Type 2 Diabetes beyond Accelerated Atherogenesis. J. Clin. Med. 2020, 9, 2090. [Google Scholar] [CrossRef] [PubMed]
- Knapp, M.; Tu, X.; Wu, R. Vascular Endothelial Dysfunction, a Major Mediator in Diabetic Cardiomyopathy. Acta Pharmacol. Sin. 2019, 40, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Severino, P.; D’Amato, A.; Prosperi, S.; Fanisio, F.; Birtolo, L.I.; Costi, B.; Netti, L.; Chimenti, C.; Lavalle, C.; Maestrini, V.; et al. Myocardial Tissue Characterization in Heart Failure with Preserved Ejection Fraction: From Histopathology and Cardiac Magnetic Resonance Findings to Therapeutic Targets. Int. J. Mol. Sci. 2021, 22, 7650. [Google Scholar] [CrossRef] [PubMed]
- Zuchi, C.; Tritto, I.; Carluccio, E.; Mattei, C.; Cattadori, G.; Ambrosio, G. Role of Endothelial Dysfunction in Heart Failure. Heart Fail. Rev. 2020, 25, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Biemmi, V.; Milano, G.; Ciullo, A.; Cervio, E.; Burrello, J.; Dei Cas, M.; Paroni, R.; Tallone, T.; Moccetti, T.; Pedrazzini, G.; et al. Inflammatory Extracellular Vesicles Prompt Heart Dysfunction via TRL4-Dependent NF-ΚB Activation. Theranostics 2020, 10, 2773–2790. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Huang, G.X.; Bonkowski, M.S.; Longchamp, A.; Li, C.; Schultz, M.B.; Kim, L.-J.; Osborne, B.; Joshi, S.; Lu, Y.; et al. Impairment of an Endothelial NAD+-H2S Signaling Network Is a Reversible Cause of Vascular Aging. Cell 2018, 173, 74–89.e20. [Google Scholar] [CrossRef]
- Csiszar, A.; Tarantini, S.; Yabluchanskiy, A.; Balasubramanian, P.; Kiss, T.; Farkas, E.; Baur, J.A.; Ungvari, Z. Role of Endothelial NAD+ Deficiency in Age-Related Vascular Dysfunction. Am. J. Physiol.-Heart Circ. Physiol. 2019, 316, H1253–H1266. [Google Scholar] [CrossRef]
- Tarantini, S.; Yabluchanskiy, A.; Ballabh, P.; Farkas, E.; Baur, J.; Sinclair, D.; Csiszar, A.; Ungvari, Z. NMN Rescues Endothelial Function and Neurovascular Coupling, Improving Cognitive Function in Aged Mice. Innov. Aging 2020, 4, 121. [Google Scholar] [CrossRef]
- Gui, J.; Potthast, A.; Rohrbach, A.; Borns, K.; Das, A.M.; von Versen-Höynck, F. Gestational Diabetes Induces Alterations of Sirtuins in Fetal Endothelial Cells. Pediatr. Res. 2016, 79, 788–798. [Google Scholar] [CrossRef]
- Vekic, J.; Zeljkovic, A.; Al Rasadi, K.; Cesur, M.; Silva-Nunes, J.; Stoian, A.P.; Rizzo, M. A New Look at Novel Cardiovascular Risk Biomarkers: The Role of Atherogenic Lipoproteins and Innovative Antidiabetic Therapies. Metabolites 2022, 12, 108. [Google Scholar] [CrossRef]
- He, Y.; Wu, N. Research Progress on Gestational Diabetes Mellitus and Endothelial Dysfunction Markers. Diabetes Metab. Syndr. Obes. Targets Ther. 2021, 14, 983–990. [Google Scholar] [CrossRef]
- de Rijk, E.P.C.T.; van Esch, E.; Flik, G. Pregnancy Dating in the Rat: Placental Morphology and Maternal Blood Parameters. Toxicol. Pathol. 2002, 30, 271–282. [Google Scholar] [CrossRef]
- Ruzicka, M.; Xiao, F.; Abujrad, H.; Al-Rewashdy, Y.; Tang, V.A.; Langlois, M.-A.; Sorisky, A.; Ooi, T.C.; Burger, D. Effect of Hemodialysis on Extracellular Vesicles and Circulating Submicron Particles. BMC Nephrol. 2019, 20, 294. [Google Scholar] [CrossRef]
- Munkonda, M.N.; Akbari, S.; Landry, C.; Sun, S.; Xiao, F.; Turner, M.; Holterman, C.E.; Nasrallah, R.; Hébert, R.L.; Kennedy, C.R.J.; et al. Podocyte-Derived Microparticles Promote Proximal Tubule Fibrotic Signaling via P38 MAPK and CD36. J. Extracell. Vesicles 2018, 7, 1432206. [Google Scholar] [CrossRef]
- Lytvyn, Y.; Xiao, F.; Kennedy, C.R.J.; Perkins, B.A.; Reich, H.N.; Scholey, J.W.; Cherney, D.Z.; Burger, D. Assessment of Urinary Microparticles in Normotensive Patients with Type 1 Diabetes. Diabetologia 2017, 60, 581–584. [Google Scholar] [CrossRef]
- Akbari, S.; Abou-Arkoub, R.; Sun, S.; Hiremath, S.; Reunov, A.; McCormick, B.B.; Ruzicka, M.; Burger, D. Microparticle Formation in Peritoneal Dialysis. Can. J. Kidney Health Dis. 2017, 4, 2054358117699829. [Google Scholar] [CrossRef] [PubMed]
- Soltesz, B.; Nagy, B. Chapter Seven—Quantification of MtDNA in Extracellular Vesicles. In Methods in Enzymology; Spada, S., Galluzzi, L., Eds.; Extracellular Vesicles; Academic Press: Cambridge, MA, USA, 2020; Volume 645, pp. 119–140. [Google Scholar]



| Pre-Pregnancy | E-18.5 | |||||
|---|---|---|---|---|---|---|
| Lean | GDM | p Value | Lean | GDM | p-Value | |
| LV mass (mg) | 662.1 8.0 | 681.1 11.1 | 0.70 | 709.9 9.7 | 811.3 18.7 | 0.11 |
| LV mass/g Body Weight | 2.21 0.01 | 2.29 0.03 | 0.37 | 1.63 0.02 | 1.68 0.02 | 0.52 |
| LVPWd (mm) | 1.64 0.02 | 1.65 0.04 | 0.93 | 1.73 0.02 | 1.96 0.02 | 0.01 * |
| LVAWd (mm) | 1.52 0.02 | 1.50 0.03 | 0.81 | 1.59 0.02 | 1.84 0.02 | 0.01 * |
| IVSd (mm) | 1.42 0.03 | 1.49 0.02 | 0.52 | 1.54 0.03 | 1.55 0.02 | 0.95 |
| LVIDd (mm) | 7.7 0.07 | 7.6 0.04 | 0.81 | 7.6 0.09 | 7.2 0.11 | 0.35 |
| LV Vol (uL) | 315.8 6.3 | 309.0 3.5 | 0.76 | 315.3 9.1 | 280.5 8.9 | 0.37 |
| EF (%) | 74.7 0.9 | 75.0 1.1 | 0.94 | 78.8 0.5 | 76.8 1.0 | 0.57 |
| FS (%) | 45.6 0.8 | 46.4 1.2 | 0.87 | 49.2 0.5 | 47.9 1.0 | 0.69 |
| CO (mL/min) | 86.8 1.9 | 85.6 1.8 | 0.88 | 86.7 2.1 | 80.2 2.5 | 0.51 |
| SV (uL) | 244.2 5.2 | 238.8 2.8 | 0.76 | 240.5 6.4 | 227.8 6.3 | 0.64 |
| IVRT (ms) | 13.4 0.3 | 14.7 0.3 | 0.31 | 14.6 0.8 | 18.2 1.0 | <0.001 * |
| E (mm/s) | 1021.1 20.8 | 978.6 24.5 | 0.68 | 1251.1 20.8 | 1104.5 11.3 | 0.033 * |
| A (mm/s) | 910.0 18.1 | 821 24.0 | 0.34 | 1020.7 23.4 | 800.9 27.9 | 0.037 * |
| E/A | 1.21 0.02 | 1.13 0.02 | 0.29 | 1.48 0.04 | 1.27 0.03 | 0.16 |
| E’/A’ | 0.78 0.01 | 0.83 0.03 | 0.65 | 1.19 0.04 | 1.00 0.01 | 0.09 |
| E/E’ | 25.4 0.7 | 28.9 0.7 | 0.26 | 25.4 0.5 | 30.3 0.8 | 0.05 |
| MPI | 0.35 0.01 | 0.32 0.01 | 0.26 | 0.39 0.01 | 0.45 0.01 | 0.04 * |
| Heart rate (bpm) | 355.5 2.8 | 357.2 5.2 | 0.92 | 363.0 3.6 | 353.1 5.1 | 0.60 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kereliuk, S.M.; Xiao, F.; Burger, D.; Dolinsky, V.W. Extracellular Vesicles as an Index for Endothelial Injury and Cardiac Dysfunction in a Rodent Model of GDM. Int. J. Mol. Sci. 2022, 23, 4970. https://doi.org/10.3390/ijms23094970
Kereliuk SM, Xiao F, Burger D, Dolinsky VW. Extracellular Vesicles as an Index for Endothelial Injury and Cardiac Dysfunction in a Rodent Model of GDM. International Journal of Molecular Sciences. 2022; 23(9):4970. https://doi.org/10.3390/ijms23094970
Chicago/Turabian StyleKereliuk, Stephanie M., Fengxia Xiao, Dylan Burger, and Vernon W. Dolinsky. 2022. "Extracellular Vesicles as an Index for Endothelial Injury and Cardiac Dysfunction in a Rodent Model of GDM" International Journal of Molecular Sciences 23, no. 9: 4970. https://doi.org/10.3390/ijms23094970
APA StyleKereliuk, S. M., Xiao, F., Burger, D., & Dolinsky, V. W. (2022). Extracellular Vesicles as an Index for Endothelial Injury and Cardiac Dysfunction in a Rodent Model of GDM. International Journal of Molecular Sciences, 23(9), 4970. https://doi.org/10.3390/ijms23094970







