Preservation of Organs to Be Transplanted: An Essential Step in the Transplant Process
Abstract
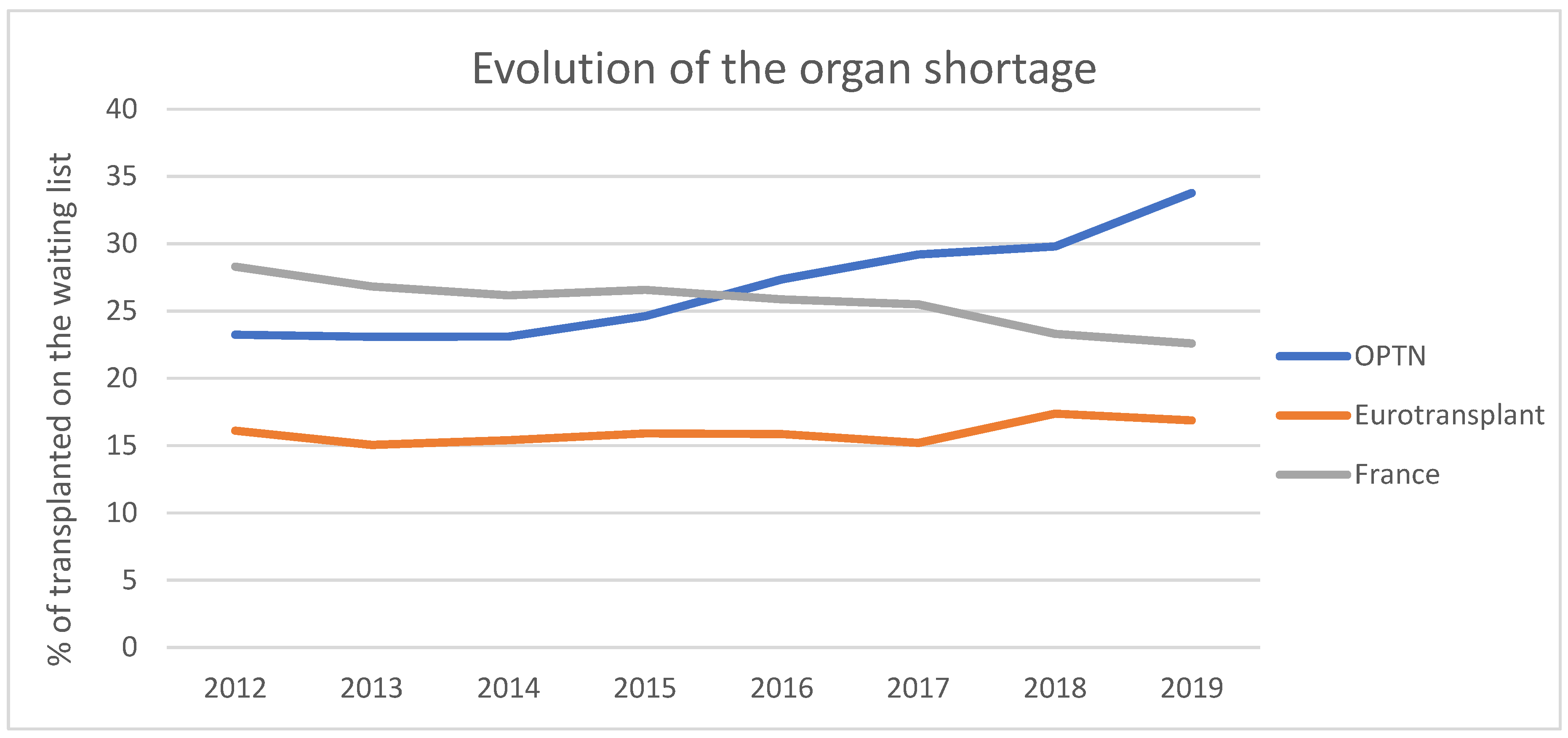
:1. Introduction
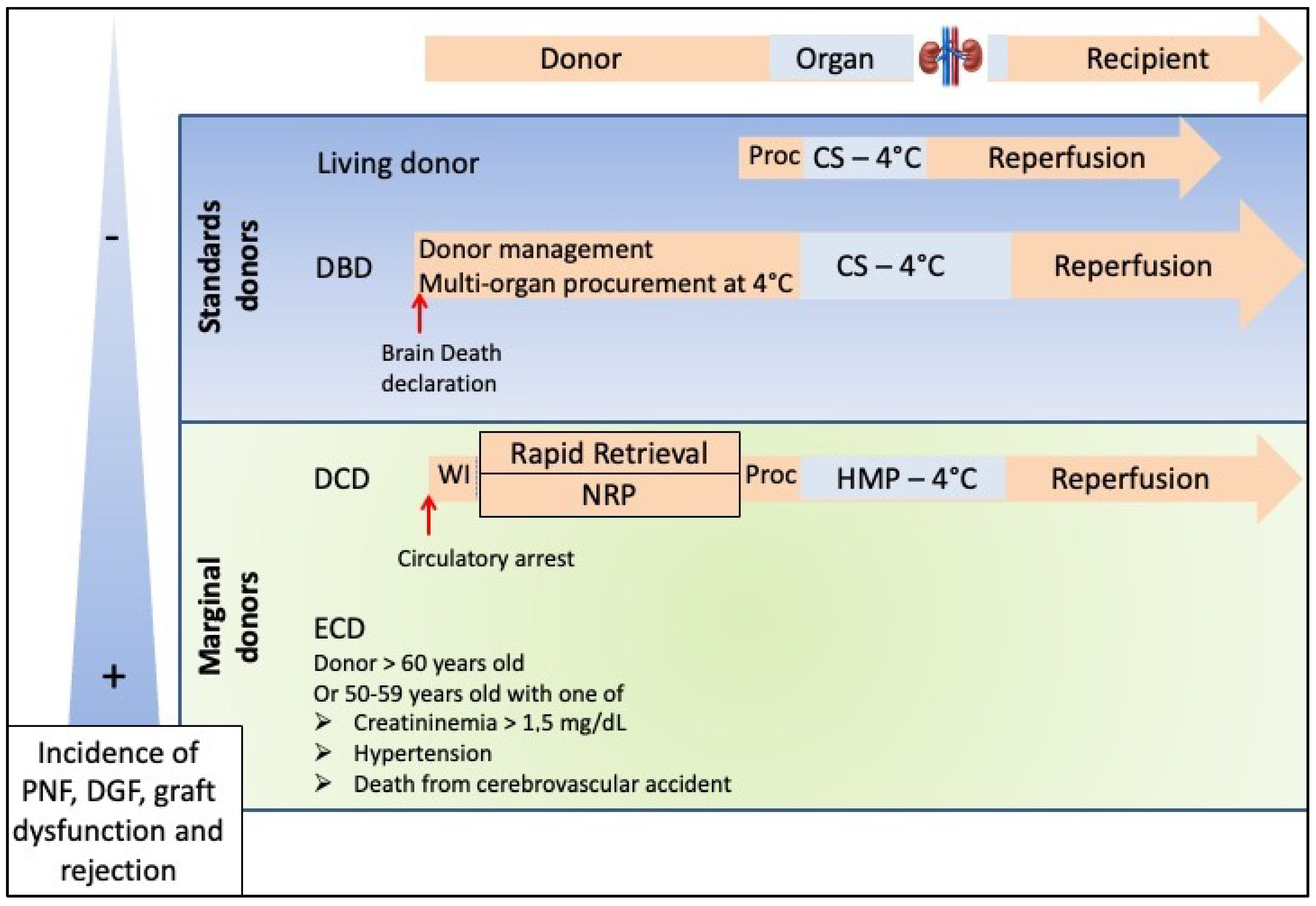
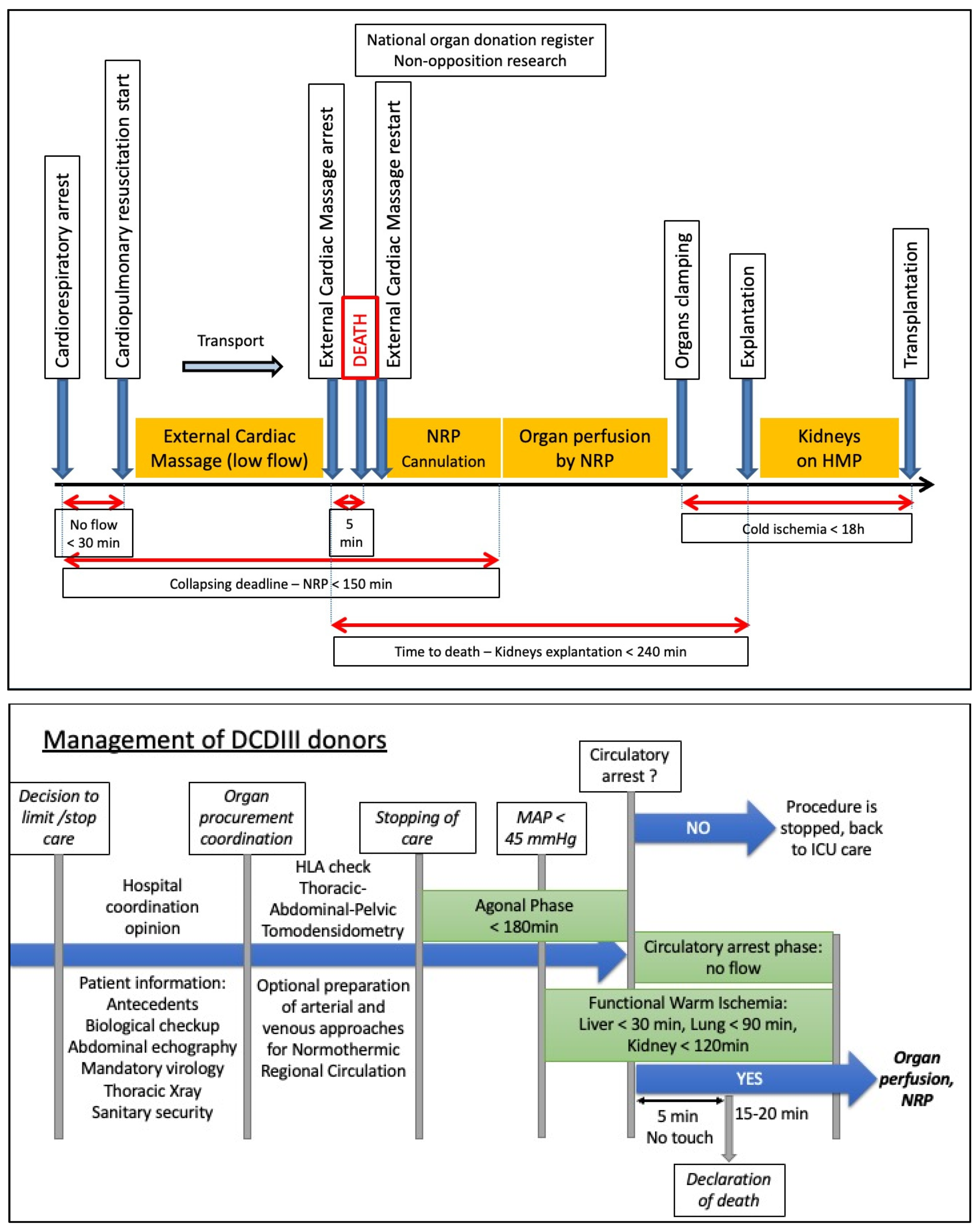
2. The Different Types of Donors
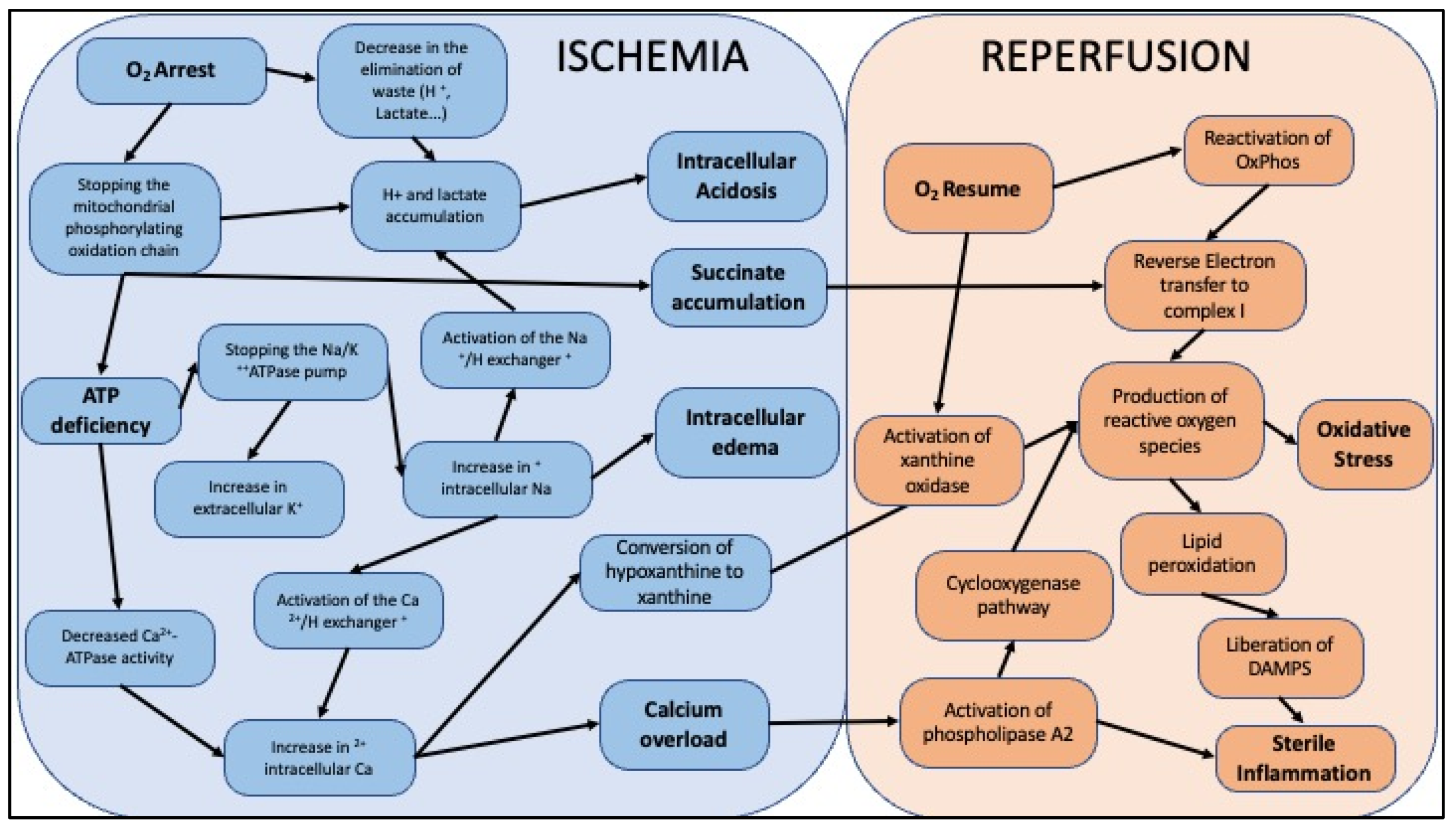
3. Ischemia-Reperfusion
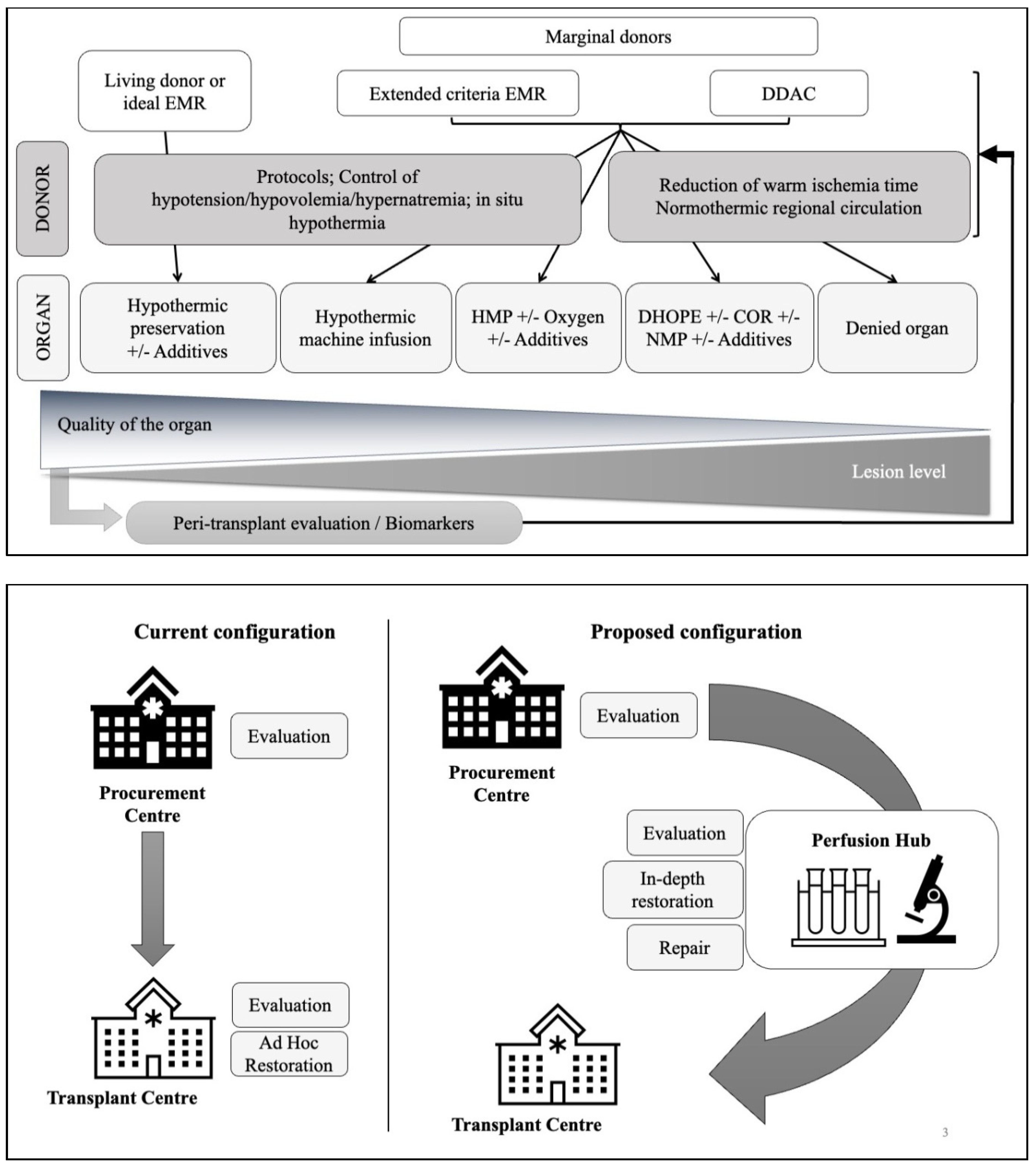
4. Means of Organ Preservation
4.1. Temperature
4.2. Oxygen
4.3. Pharmacological Agents
5. Perspectives and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- ABM. Rapport Médicale et Scientifique de l’agence de La Biomédecine. 2020. Available online: https://rams.agence-biomedecine.fr/ (accessed on 25 February 2022).
- Smith, M.; Dominguez-Gil, B.; Greer, D.M.; Manara, A.R.; Souter, M.J. Organ donation after circulatory death: Current status and future potential. Intensive Care Med. 2019, 45, 310–321. [Google Scholar] [CrossRef] [PubMed]
- Port, F.K.; Bragg-Gresham, J.L.; Metzger, R.A.; Dykstra, D.M.; Gillespie, B.W.; Young, E.W.; Delmonico, F.L.; Wynn, J.J.; Merion, R.M.; Wolfe, R.A.; et al. Donor characteristics associated with reduced graft survival: An approach to expanding the pool of kidney donors. Transplantation 2002, 74, 1281–1286. [Google Scholar] [CrossRef] [PubMed]
- Durand, F.; Antoine, C.; Soubrane, O. Liver Transplantation in France. Liver Transplant. 2019, 25, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Bodzin, A.S.; Baker, T.B. Liver Transplantation Today: Where We Are Now and Where We Are Going. Liver Transplant. 2018, 24, 1470–1475. [Google Scholar] [CrossRef] [Green Version]
- Vanholder, R.; Domínguez-Gil, B.; Busic, M.; Cortez-Pinto, H.; Craig, J.C.; Jager, K.J.; Mahillo, B.; Stel, V.S.; Valentin, M.O.; Zoccali, C.; et al. Organ donation and transplantation: A multi-stakeholder call to action. Nat. Rev. Nephrol. 2021, 17, 554–568. [Google Scholar] [CrossRef]
- Resch, T.; Cardini, B.; Oberhuber, R.; Weissenbacher, A.; Dumfarth, J.; Krapf, C.; Boesmueller, C.; Oefner, D.; Grimm, M.; Schneeberger, S. Transplanting Marginal Organs in the Era of Modern Machine Perfusion and Advanced Organ Monitoring. Front. Immunol. 2020, 11, 631. [Google Scholar] [CrossRef]
- Hidalgo, J.M.S.; Rodríguez-Ortiz, L.; Arjona-Sánchez, Á.; Ayllón-Terán, M.; Gómez-Luque, I.; Ciria-Bru, R.; Luque-Molina, A.; López-Cillero, P.; Rufián-Peña, S.; Briceño-Delgado, J. “Super-rapid” Technique in Donation After Circulatory Death Liver Donors: Advantages and Disadvantages. Transplant. Proc. 2019, 51, 25–27. [Google Scholar] [CrossRef]
- Jochmans, I.; Hessheimer, A.J.; Neyrinck, A.P.; Paredes, D.; Bellini, M.I.; Dark, J.H.; Kimenai, H.J.A.N.; Pengel, L.H.M.; Watson, C.J.E. ESOT Workstream 04 of the TLJ (Transplant Learning Journey) project Consensus Statement on Normothermic Regional Perfusion in Donation after Circulatory Death: Report from the European Society for Organ Transplantation’s Transplant Learning Journey. Transpl. Int. 2021, 34, 2019–2030. [Google Scholar] [CrossRef]
- De Beule, J.; Jochmans, I. Kidney Perfusion as an Organ Quality Assessment Tool—Are We Counting Our Chickens before They Have Hatched? J. Clin. Med. 2020, 9, 879. [Google Scholar] [CrossRef] [Green Version]
- Kerforne, T.; Allain, G.; Giraud, S.; Bon, D.; Ameteau, V.; Couturier, P.; Hebrard, W.; Danion, J.; Goujon, J.-M.; Thuillier, R.; et al. Defining the optimal duration for normothermic regional perfusion in the kidney donor: A porcine preclinical study. Am. J. Transplant. 2019, 19, 737–751. [Google Scholar] [CrossRef]
- Franzin, R.; Stasi, A.; Fiorentino, M.; Simone, S.; Oberbauer, R.; Castellano, G.; Gesualdo, L. Renal Delivery of Pharmacologic Agents during Machine Perfusion to Prevent Ischaemia-Reperfusion Injury: From Murine Model to Clinical Trials. Front. Immunol. 2021, 12, 673562. [Google Scholar] [CrossRef] [PubMed]
- Arnoux, V.; Dorez, D.; Muller, M.; Gignoux, A.; Valignat, C.; Faucher, A.; Dechamboux, E.; Gour, A.-S.; Jakkel, D.; Saint Marcel, L.; et al. Non-heart-beating renal donors: Organization in a non-university hospital. Prog. Urol. 2014, 24, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Carcy, R.; Cougnon, M.; Poet, M.; Durandy, M.; Sicard, A.; Counillon, L.; Blondeau, N.; Hauet, T.; Tauc, M.; Pisani, D.F. Targeting oxidative stress, a crucial challenge in renal transplantation outcome. Free Radic. Biol. Med. 2021, 169, 258–270. [Google Scholar] [CrossRef]
- Kerforne, T.; Favreau, F.; Thuillier, R.; Hauet, T.; Pinsard, M. Toward a customized preservation for each kidney graft? Nephrol. Ther. 2016, 12, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Fernández, A.R.; Sánchez-Tarjuelo, R.; Cravedi, P.; Ochando, J.; López-Hoyos, M. Review: Ischemia Reperfusion Injury—A Translational Perspective in Organ Transplantation. Int. J. Mol. Sci. 2020, 21, 8549. [Google Scholar] [CrossRef] [PubMed]
- Dziodzio, T.; Biebl, M.; Pratschke, J. Impact of brain death on ischemia/reperfusion injury in liver transplantation. Curr. Opin. Organ Transplant. 2014, 19, 108–114. [Google Scholar] [CrossRef]
- Bon, D.; Chatauret, N.; Giraud, S.; Thuillier, R.; Favreau, F.; Hauet, T. New strategies to optimize kidney recovery and preservation in transplantation. Nat. Rev. Nephrol. 2012, 8, 339–347. [Google Scholar] [CrossRef]
- Eltzschig, H.K.; Bratton, D.L.; Colgan, S.P. Targeting hypoxia signalling for the treatment of ischaemic and inflammatory diseases. Nat. Rev. Drug Discov. 2014, 13, 852–869. [Google Scholar] [CrossRef] [Green Version]
- Kerforne, T.; Favreau, F.; Khalifeh, T.; Maiga, S.; Allain, G.; Thierry, A.; Dierick, M.; Baulier, E.; Steichen, C.; Hauet, T. Hypercholesterolemia-induced increase in plasma oxidized LDL abrogated pro angiogenic response in kidney grafts. J. Transl. Med. 2019, 17, 26. [Google Scholar] [CrossRef]
- Maïga, S.; Allain, G.; Hauet, T.; Roumy, J.; Baulier, E.; Scepi, M.; Dierick, M.; Van Hoorebeke, L.; Hannaert, P.; Guy, F.; et al. Renal auto-transplantation promotes cortical microvascular network remodeling in a preclinical porcine model. PLoS ONE 2017, 12, e0181067. [Google Scholar] [CrossRef] [Green Version]
- Chatauret, N.; Favreau, F.; Giraud, S.; Thierry, A.; Rossard, L.; Le Pape, S.; Lerman, L.O.; Hauet, T. Diet-induced increase in plasma oxidized LDL promotes early fibrosis in a renal porcine auto-transplantation model. J. Transl. Med. 2014, 12, 76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbier, L.; Ferhat, M.; Salamé, E.; Robin, A.; Herbelin, A.; Gombert, J.-M.; Silvain, C.; Barbarin, A. Interleukin-1 Family Cytokines: Keystones in Liver Inflammatory Diseases. Front. Immunol. 2019, 10, 2014. [Google Scholar] [CrossRef] [PubMed]
- Issa, N.; Stephany, B.; Fatica, R.; Nurko, S.; Krishnamurthi, V.; Goldfarb, D.A.; Braun, W.E.; Dennis, V.W.; Heeger, P.S.; Poggio, E.D. Donor Factors Influencing Graft Outcomes in Live Donor Kidney Transplantation. Transplantation 2007, 83, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Hessheimer, A.J.; Billault, C.; Barrou, B.; Fondevila, C. Hypothermic or normothermic abdominal regional perfusion in high-risk donors with extended warm ischemia times: Impact on outcomes? Transpl. Int. 2014, 28, 700–707. [Google Scholar] [CrossRef]
- Hosgood, S.A.; Brown, R.J.; Nicholson, M.L. Advances in kidney preservation techniques and their application in clinical practice. Transplantation 2021, 105, e202. [Google Scholar] [CrossRef]
- Chazelas, P.; Steichen, C.; Favreau, F.; Trouillas, P.; Hannaert, P.; Thuillier, R.; Giraud, S.; Hauet, T.; Guillard, J. Oxidative Stress Evaluation in Ischemia Reperfusion Models: Characteristics, Limits and Perspectives. Int. J. Mol. Sci. 2021, 22, 2366. [Google Scholar] [CrossRef]
- Steichen, C.; Giraud, S.; Bon, D.; Barrou, B.; Badet, L.; Salamé, E.; Kerforne, T.; Allain, G.; Roumy, J.; Jayle, C.; et al. Barriers and Advances in Kidney Preservation. BioMed Res. Int. 2018, 2018, 9206257. [Google Scholar] [CrossRef] [Green Version]
- Southard, M.J.H.; Belzer, M.F.O. ORGAN PRESERVATION. Annu. Rev. Med. 1995, 46, 235–247. [Google Scholar] [CrossRef]
- Thuillier, R.; Giraud, S.; Favreau, F.; Goujon, J.-M.; Desurmont, T.; Eugene, M.; Barrou, B.; Hauet, T. Improving Long-Term Outcome in Allograft Transplantation: Role of Ionic Composition and Polyethylene Glycol. Transplantation 2011, 91, 605–614. [Google Scholar] [CrossRef]
- Giraud, S.; Codas, R.; Hauet, T.; Eugene, M.; Badet, L. Polyethylene glycols and organ protection against I/R injury. Prog. Urol. 2014, 24 (Suppl. 1), S37–S43. [Google Scholar] [CrossRef]
- Chen, Y.; Shi, J.; Xia, T.C.; Xu, R.; He, X.; Xia, Y. Preservation Solutions for Kidney Transplantation: History, Advances and Mechanisms. Cell Transplant. 2019, 28, 1472–1489. [Google Scholar] [CrossRef] [PubMed]
- Petrenko, A.; Carnevale, M.; Somov, A.; Osorio, J.; Rodríguez, J.; Guibert, E.; Fuller, B.; Froghi, F. Organ Preservation into the 2020s: The Era of Dynamic Intervention. Transfus. Med. Hemother. 2019, 46, 151–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chatauret, N.; Coudroy, R.; Delpech, P.O.; Vandebrouck, C.; Hosni, S.; Scepi, M.; Hauet, T. Mechanistic Analysis of Nonoxygenated Hypothermic Machine Perfusion’s Protection on Warm Ischemic Kidney Uncovers Greater eNOS Phosphorylation and Vasodilation. Am. J. Transplant. 2014, 14, 2500–2514. [Google Scholar] [CrossRef] [PubMed]
- Brat, A.; de Vries, K.M.; van Heurn, E.W.; Huurman, V.A.; de Jongh, W.; Leuvenink, H.G.; van Zuilen, A.D.; Haase-Kromwijk, B.J.; de Jonge, J.; Berger, S.P.; et al. Hypothermic Machine Perfusion as a National Standard Preservation Method for Deceased Donor Kidneys. Transplantation, 2021; ahead of print. [Google Scholar] [CrossRef]
- Singh, N.; Logan, A.; Schenk, A.; Bumgardner, G.; Brock, G.; El-Hinnawi, A.; Rajab, A.; Washburn, K. Machine perfusion of kidney allografts affects early but not late graft function. Am. J. Surg. 2021, 223, 804–811. [Google Scholar] [CrossRef] [PubMed]
- Val, A.R.; Guarrera, J.; Porte, R.J.; Selzner, M.; Spiro, M.; Raptis, D.A.; Friend, P.J. Does machine perfusion improve immediate and short-term outcomes by enhancing graft function and recipient recovery after liver transplantation?—A systematic review of the literature, meta-analysis and expert panel recommendations. Clin. Transplant. 2022, e14638. [Google Scholar] [CrossRef]
- Qin, G.; Jernryd, V.; Sjöberg, T.; Steen, S.; Nilsson, J. Machine Perfusion for Human Heart Preservation: A Systematic Review. Transpl. Int. 2022, 35, 10258. [Google Scholar] [CrossRef]
- Dirito, J.R.; Hosgood, S.A.; Tietjen, G.T.; Nicholson, M.L. The future of marginal kidney repair in the context of normothermic machine perfusion. Am. J. Transplant. 2018, 18, 2400–2408. [Google Scholar] [CrossRef]
- Elliott, T.R.; Nicholson, M.L.; Hosgood, S.A. Normothermic kidney perfusion: An overview of protocols and strategies. Am. J. Transplant. 2021, 21, 1382–1390. [Google Scholar] [CrossRef]
- Hamar, M.; Urbanellis, P.; Kaths, M.J.; Kollmann, D.; Linares, I.; Ganesh, S.; Wiebe, A.; Cen, J.Y.; Yip, P.M.; John, R.; et al. Normothermic Ex Vivo Kidney Perfusion Reduces Warm Ischemic Injury of Porcine Kidney Grafts Retrieved after Circulatory Death. Transplantation 2018, 102, 1262–1270. [Google Scholar] [CrossRef]
- Pool, M.B.F.; Hamelink, T.L.; van Goor, H.; Heuvel, M.C.V.D.; Leuvenink, H.G.D.; Moers, C. Prolonged ex-vivo normothermic kidney perfusion: The impact of perfusate composition. PLoS ONE 2021, 16, e0251595. [Google Scholar] [CrossRef]
- Hosgood, S.A.; Nicholson, M.L. A Short Period of Normothermic Machine Perfusion May Not Be Able to Predict Primary Nonfunction in Uncontrolled Circulatory Death Kidneys. Transplantation 2021, 105, e11–e12. [Google Scholar] [CrossRef] [PubMed]
- Hosgood, S.; Saeb-Parsy, K.; Wilson, C.; Callaghan, C.; Collett, D.; Nicholson, M.L. Protocol of a randomised controlled, open-label trial of ex vivo normothermic perfusion versus static cold storage in donation after circulatory death renal transplantation. BMJ Open 2017, 7, e012237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rijkse, E.; Bouari, S.; Kimenai, H.J.A.N.; de Jonge, J.; de Bruin, R.W.F.; Slagter, J.S.; Hoogen, M.W.F.V.D.; Ijzermans, J.N.M.; Hoogduijn, M.J.; Minnee, R.C. Additional Normothermic Machine Perfusion versus Hypothermic Machine Perfusion in Suboptimal Donor Kidney Transplantation: Protocol of a Randomized, Controlled, Open-Label Trial. Int. J. Surg. Protoc. 2021, 25, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Muller, X.; Mohkam, K.; Mueller, M.; Schlegel, A.; Dondero, F.; Sepulveda, A.; Savier, E.; Scatton, O.; Bucur, P.; Salame, E.; et al. Hypothermic Oxygenated Perfusion Versus Normothermic Regional Perfusion in Liver Transplantation From Controlled Donation after Circulatory Death: First International Comparative Study. Ann. Surg. 2020, 272, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Hessheimer, A.J.; Riquelme, F.; Fundora-Suárez, Y.; Pérez, R.G.; Fondevila, C. Normothermic perfusion and outcomes after liver transplantation. Transplant. Rev. 2019, 33, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Nasralla, D.; Coussios, C.C.; Mergental, H.; Akhtar, M.Z.; Butler, A.J.; Ceresa, C.D.L.; Chiocchia, V.; Dutton, S.J.; García-Valdecasas, J.C.; Heaton, N.; et al. A randomized trial of normothermic preservation in liver transplantation. Nature 2018, 557, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Cypel, M.; Yeung, J.; Liu, M.; Anraku, M.; Chen, F.; Karolak, W.; Sato, M.; Laratta, J.; Azad, S.; Madonik, M.; et al. Normothermic Ex Vivo Lung Perfusion in Clinical Lung Transplantation. N. Engl. J. Med. 2011, 364, 1431–1440. [Google Scholar] [CrossRef] [Green Version]
- Loor, G.; Warnecke, G.; Villavicencio, M.A.; Smith, M.A.; Kukreja, J.; Ardehali, A.; Hartwig, M.; Daneshmand, M.A.; Hertz, M.I.; Huddleston, S.; et al. Portable normothermic ex-vivo lung perfusion, ventilation, and functional assessment with the Organ Care System on donor lung use for transplantation from extended-criteria donors (EXPAND): A single-arm, pivotal trial. Lancet Respir. Med. 2019, 7, 975–984. [Google Scholar] [CrossRef]
- Niederberger, P.; Farine, E.; Raillard, M.; Dornbierer, M.; Freed, D.H.; Large, S.R.; Chew, H.C.; MacDonald, P.S.; Messer, S.J.; White, C.W.; et al. Heart Transplantation with Donation after Circulatory Death. Circ. Heart Fail. 2019, 12, e005517. [Google Scholar] [CrossRef]
- Minor, T.; von Horn, C. Reduction of Renal Preservation/Reperfusion Injury by Controlled Hyperthermia During Ex Vivo Machine Perfusion. Clin. Transl. Sci. 2020, 14, 544–549. [Google Scholar] [CrossRef]
- Fabry, G.; Doorschodt, B.M.; Grzanna, T.; Boor, P.; Elliott, A.; Stollenwerk, A.; Tolba, R.H.; Rossaint, R.; Bleilevens, C. Cold Preflush of Porcine Kidney Grafts Prior to Normothermic Machine Perfusion Aggravates Ischemia Reperfusion Injury. Sci. Rep. 2019, 9, 13897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaths, J.M.; Echeverri, J.; Chun, Y.M.; Cen, J.Y.; Goldaracena, N.; Linares, I.; Dingwell, L.; Yip, P.; John, R.; Bagli, D.; et al. Continuous Normothermic Ex Vivo Kidney Perfusion Improves Graft Function in Donation after Circulatory Death Pig Kidney Transplantation. Transplantation 2017, 101, 754–763. [Google Scholar] [CrossRef] [PubMed]
- Weissenbacher, A.; Faro, M.L.L.; Boubriak, O.; Soares, M.F.; Roberts, I.S.; Hunter, J.P.; Voyce, D.; Mikov, N.; Cook, A.; Ploeg, R.J.; et al. Twenty-four–hour normothermic perfusion of discarded human kidneys with urine recirculation. Am. J. Transplant. 2019, 19, 178–192. [Google Scholar] [CrossRef] [PubMed]
- Minor, T.; Von Horn, C.; Gallinat, A.; Kaths, M.; Kribben, A.; Treckmann, J.; Paul, A. First-in-man controlled rewarming and normothermic perfusion with cell-free solution of a kidney prior to transplantation. Am. J. Transplant. 2020, 20, 1192–1195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jochmans, I.; Nicholson, M.L.; Hosgood, S.A. Kidney perfusion: Some like it Hot Others Prefer to Keep it Cool. Curr. Opin. Organ Transplant. 2017, 22, 260–266. [Google Scholar] [CrossRef]
- Hamelink, T.L.; Ogurlu, B.; De Beule, J.; Lantinga, V.A.; Pool, M.B.; Venema, L.H.; Leuvenink, H.G.; Jochmans, I.; Moers, C. Renal Normothermic Machine Perfusion: The Road Toward Clinical Implementation of a Promising Pretransplant Organ Assessment Tool. Transplantation 2021, 106, 268–279. [Google Scholar] [CrossRef]
- Xu, J.; Buchwald, J.; Martins, P.N. Review of Current Machine Perfusion Therapeutics for Organ Preservation. Transplantation 2020, 104, 1792–1803. [Google Scholar] [CrossRef]
- Minor, T.; von Horn, C.; Paul, A. Role of temperature in reconditioning and evaluation of cold preserved kidney and liver grafts. Curr. Opin. Organ Transplant. 2017, 22, 267–273. [Google Scholar] [CrossRef] [Green Version]
- Kasil, A.; Giraud, S.; Couturier, P.; Amiri, A.; Danion, J.; Donatini, G.; Matillon, X.; Hauet, T.; Badet, L. Individual and Combined Impact of Oxygen and Oxygen Transporter Supplementation during Kidney Machine Preservation in a Porcine Preclinical Kidney Transplantation Model. Int. J. Mol. Sci. 2019, 20, 1992. [Google Scholar] [CrossRef] [Green Version]
- Jochmans, I.; Brat, A.; Davies, L.; Hofker, H.S.; van de Leemkolk, F.E.M.; Leuvenink, H.G.D.; Knight, S.R.; Pirenne, J.; Ploeg, R.J.; COMPARE Trial Collaboration and Consortium for Organ Preservation in Europe (COPE). Oxygenated versus standard cold perfusion preservation in kidney transplantation (COMPARE): A randomised, double-blind, paired, phase 3 trial. Lancet 2020, 396, 1653–1662. [Google Scholar] [CrossRef]
- Peters, S.M.; Rauen, U.; Tijsen, M.J.; Bindels, R.J.; van Os, C.H.; de Groot, H.; Wetzels, J.F. Cold preservation of isolated rabbit proximal tubules induces radical-mediated cell Injury. Transplantation 1998, 65, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.; Smith, T.B.; Neil, D.A.; Thakker, A.; Tsuchiya, Y.; Higgs, E.B.; Hodges, N.J.; Ready, A.R.; Nath, J.; Ludwig, C. The Effects of Oxygenation on Ex Vivo Kidneys Undergoing Hypothermic Machine Perfusion. Transplantation 2019, 103, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Hoyer, D.P.; Gallinat, A.; Swoboda, S.; Wohlschlaeger, J.; Rauen, U.; Paul, A.; Minor, T. Influence of Oxygen Concentration during Hypothermic Machine Perfusion on Porcine Kidneys from Donation after Circulatory Death. Transplantation 2014, 98, 944–950. [Google Scholar] [CrossRef] [PubMed]
- Darius, T.; Gianello, P.; Vergauwen, M.; Mourad, N.; Buemi, A.; De Meyer, M.; Mourad, M. The effect on early renal function of various dynamic preservation strategies in a preclinical pig ischemia-reperfusion autotransplant model. Am. J. Transplant. 2018, 19, 752–762. [Google Scholar] [CrossRef] [PubMed]
- Thuillier, R.; Allain, G.; Celhay, O.; Hebrard, W.; Barrou, B.; Badet, L.; Leuvenink, H.; Hauet, T. Benefits of active oxygenation during hypothermic machine perfusion of kidneys in a preclinical model of deceased after cardiac death donors. J. Surg. Res. 2013, 184, 1174–1181. [Google Scholar] [CrossRef]
- Meister, F.A.; Czigany, Z.; Rietzler, K.; Miller, H.; Reichelt, S.; Liu, W.-J.; Boecker, J.; Moeller, M.J.; Tolba, R.H.; Hamesch, K.; et al. Decrease of renal resistance during hypothermic oxygenated machine perfusion is associated with early allograft function in extended criteria donation kidney transplantation. Sci. Rep. 2020, 10, 17726. [Google Scholar] [CrossRef]
- Husen, P.; Boffa, C.; Jochmans, I.; Krikke, C.; Davies, L.; Mazilescu, L.; Brat, A.; Knight, S.; Wettstein, D.; Cseprekal, O.; et al. Oxygenated End-Hypothermic Machine Perfusion in Expanded Criteria Donor Kidney Transplant; A Randomized Clinical Trial. JAMA Surg. 2021, 156, 517–525. [Google Scholar] [CrossRef]
- Mazilescu, L.I.; Urbanellis, P.; Kaths, M.J.; Ganesh, S.; Goto, T.; Noguchi, Y.; John, R.; Konvalinka, A.; Mucsi, I.; Ghanekar, A.; et al. Prolonged Normothermic Ex Vivo Kidney Perfusion Is Superior to Cold Nonoxygenated and Oxygenated Machine Perfusion for the Preservation of DCD Porcine Kidney Grafts. Transplant. Direct 2021, 7, e751. [Google Scholar] [CrossRef]
- Thuillier, R.; Delpy, E.; Matillon, X.; Kaminski, J.; Kasil, A.; Soussi, D.; Danion, J.; Sauvageon, Y.; Rod, X.; Donatini, G.; et al. Preventing acute kidney injury during transplantation: The application of novel oxygen carriers. Expert Opin. Investig. Drugs 2019, 28, 643–657. [Google Scholar] [CrossRef]
- Bhattacharjee, R.N.; Patel, S.V.; Sun, Q.; Jiang, L.; Richard-Mohamed, M.; Ruthirakanthan, A.; Aquil, S.; Al-Ogaili, R.; Juriasingani, S.; Sener, A.; et al. Renal Protection Against Ischemia Reperfusion Injury: Hemoglobin-based Oxygen Carrier-201 Versus Blood as an Oxygen Carrier in Ex Vivo Subnormothermic Machine Perfusion. Transplantation 2020, 104, 482–489. [Google Scholar] [CrossRef]
- Alix, P.; Val-Laillet, D.; Turlin, B.; Ben Mosbah, I.; Burel, A.; Bobillier, E.; Bendavid, C.; Delpy, E.; Zal, F.; Corlu, A.; et al. Adding the oxygen carrier M101 to a cold-storage solution could be an alternative to HOPE for liver graft preservation. JHEP Rep. 2020, 2, 100119. [Google Scholar] [CrossRef] [PubMed]
- Sakai, H.; Tomiyama, K.-I.; Sou, K.; Takeoka, S.; Tsuchida, E. Poly(ethylene glycol)-Conjugation and Deoxygenation Enable Long-Term Preservation of Hemoglobin-Vesicles as Oxygen Carriers in a Liquid State. Bioconj. Chem. 2000, 11, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Mot, A.C.; Roman, A.; Lupan, I.; Kurtz, D.M.; Silaghi-Dumitrescu, R. Towards the Development of Hemerythrin-Based Blood Substitutes. J. Protein Chem. 2010, 29, 387–393. [Google Scholar] [CrossRef]
- Mallet, V.; Dutheil, D.; Polard, V.; Rousselot, M.; Leize, E.; Hauet, T.; Goujon, J.M.; Zal, F. Dose-Ranging Study of the Performance of the Natural Oxygen Transporter HEMO2Life in Organ Preservation. Artif. Organs 2014, 38, 691–701. [Google Scholar] [CrossRef]
- Le Meur, Y.; Badet, L.; Essig, M.; Thierry, A.; Büchler, M.; Drouin, S.; Deruelle, C.; Morelon, E.; Pesteil, F.; Delpech, P.; et al. First-in-human use of a marine oxygen carrier (M101) for organ preservation: A safety and proof-of-principle study. Am. J. Transplant. 2020, 20, 1729–1738. [Google Scholar] [CrossRef] [PubMed]
- van Leeuwen, O.; De Vries, Y.; Fujiyoshi, M.; Nijsten, M.W.N.; Ubbink, R.; Pelgrim, G.J.; Werner, M.J.M.; Reyntjens, K.M.E.M.; Berg, A.P.V.D.; De Boer, M.T.; et al. Transplantation of High-risk Donor Livers After Ex Situ Resuscitation and Assessment Using Combined Hypo- and Normothermic Machine Perfusion: A Prospective Clinical Trial. Ann. Surg. 2019, 270, 906–914. [Google Scholar] [CrossRef]
- De Vries, Y.; Matton, A.P.M.; Nijsten, M.W.N.; Werner, M.J.M.; van den Berg, A.P.; De Boer, M.T.; Buis, C.I.; Fujiyoshi, M.; De Kleine, R.H.J.; van Leeuwen, O.; et al. Pretransplant sequential hypo- and normothermic machine perfusion of suboptimal livers donated after circulatory death using a hemoglobin-based oxygen carrier perfusion solution. Am. J. Transplant. 2019, 19, 1202–1211. [Google Scholar] [CrossRef] [Green Version]
- He, X.; Guo, Z.; Zhao, Q.; Ju, W.; Wang, D.; Wu, L.; Yang, L.; Ji, F.; Tang, Y.; Zhang, Z.; et al. The first case of ischemia-free organ transplantation in humans: A proof of concept. Am. J. Transplant. 2018, 18, 737–744. [Google Scholar] [CrossRef] [Green Version]
- He, X.; Chen, G.; Zhu, Z.; Zhang, Z.; Yuan, X.; Han, M.; Zhao, Q.; Zheng, Y.; Tang, Y.; Huang, S.; et al. The First Case of Ischemia-Free Kidney Transplantation in Humans. Front. Med. 2019, 6, 276. [Google Scholar] [CrossRef] [Green Version]
- Mesnard, B.; Ogbemudia, A.E.; Karam, G.; Dengu, F.; Hackim, G.; Rigaud, J.; Blancho, G.; Drouin, S.; Timsit, M.O.; Branchereau, J. What is the evidence for oxygenation during kidney preservation for transplantation in 2021? A scoping review. World J. Urol. 2021. [Google Scholar] [CrossRef]
- De Vries, R.J.; Tessier, S.N.; Banik, P.D.; Nagpal, S.; Cronin, S.E.J.; Ozer, S.; Hafiz, E.O.A.; Van Gulik, T.M.; Yarmush, M.L.; Markmann, J.F.; et al. Supercooling extends preservation time of human livers. Nat. Biotechnol. 2019, 37, 1131–1136. [Google Scholar] [CrossRef] [PubMed]
- De Vries, R.J.; Tessier, S.N.; Banik, P.D.; Nagpal, S.; Cronin, S.E.J.; Ozer, S.; Hafiz, E.O.A.; Van Gulik, T.M.; Yarmush, M.L.; Markmann, J.F.; et al. Subzero non-frozen preservation of human livers in the supercooled state. Nat. Protoc. 2020, 15, 2024–2040. [Google Scholar] [CrossRef] [PubMed]
- Chandak, P.; Phillips, B.; Uwechue, R.; Thompson, E.; Bates, L.; Ibrahim, I.; Sewpaul, A.; Figueiredo, R.; Olsburgh, J.; Hosgood, S.; et al. Dissemination of a novel organ perfusion technique: Ex vivo normothermic perfusion of deceased donor kidneys. Artif. Organs 2019, 43, E308–E319. [Google Scholar] [CrossRef] [PubMed]
- Bruinsma, B.G.; Uygun, K. Subzero organ preservation: The Dawn of a New Ice Age? Curr. Opin. Organ Transplant. 2017, 22, 281–286. [Google Scholar] [CrossRef]
- Buchwald, J.; Xu, J.; Bozorgzadeh, A.; Martins, P.N. Therapeutics administered during ex vivo liver machine perfusion: An overview. World J. Transplant. 2020, 10, 1–14. [Google Scholar] [CrossRef]
- Melis, N.; Rubera, I.; Cougnon, M.; Giraud, S.; Mograbi, B.; Belaid, A.; Pisani, D.; Huber, S.M.; Lacas-Gervais, S.; Fragaki, K.; et al. Targeting eIF5A Hypusination Prevents Anoxic Cell Death through Mitochondrial Silencing and Improves Kidney Transplant Outcome. J. Am. Soc. Nephrol. 2017, 28, 811–822. [Google Scholar] [CrossRef]
- Giraud, S.; Kerforne, T.; Zely, J.; Ameteau, V.; Couturier, P.; Tauc, M.; Hauet, T. The inhibition of eIF5A hypusination by GC7, a preconditioning protocol to prevent brain death-induced renal injuries in a preclinical porcine kidney transplantation model. Am. J. Transplant. 2020, 20, 3326–3340. [Google Scholar] [CrossRef]
- Barrera-Chimal, J.; Jaisser, F. Pathophysiologic mechanisms in diabetic kidney disease: A focus on current and future therapeutic targets. Diabetes Obes. Metab. 2020, 22 (Suppl. 1), 16–31. [Google Scholar] [CrossRef]
- Barrera-Chimal, J.; André-Grégoire, G.; Cat, A.N.D.; Lechner, S.M.; Cau, J.; Prince, S.; Kolkhof, P.; Loirand, G.; Sauzeau, V.; Hauet, T.; et al. Benefit of Mineralocorticoid Receptor Antagonism in AKI: Role of Vascular Smooth Muscle Rac1. J. Am. Soc. Nephrol. 2017, 28, 1216–1226. [Google Scholar] [CrossRef]
- Rao, P.S.; Schaubel, D.E.; Guidinger, M.K.; Andreoni, K.A.; Wolfe, R.A.; Merion, R.M.; Port, F.K.; Sung, R.S. A Comprehensive Risk Quantification Score for Deceased Donor Kidneys: The Kidney Donor Risk Index. Transplantation 2009, 88, 231–236. [Google Scholar] [CrossRef]
- Querard, A.-H.; Foucher, Y.; Combescure, C.; Dantan, E.; Larmet, D.; Lorent, M.; Pouteau, L.-M.; Giral, M.; Gillaizeau, F. Comparison of survival outcomes between Expanded Criteria Donor and Standard Criteria Donor kidney transplant recipients: A systematic review and meta-analysis. Transpl. Int. 2016, 29, 403–415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loupy, A.; Aubert, O.; Orandi, B.J.; Naesens, M.; Bouatou, Y.; Raynaud, M.; Divard, G.; Jackson, A.M.; Viglietti, D.; Giral, M.; et al. Prediction system for risk of allograft loss in patients receiving kidney transplants: International derivation and validation study. BMJ 2019, 366, l4923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benmoussa, K.; Garaude, J.; Acín-Pérez, R. How Mitochondrial Metabolism Contributes to Macrophage Phenotype and Functions. J. Mol. Biol. 2018, 430, 3906–3921. [Google Scholar] [CrossRef] [PubMed]
- Bekkering, S.; Domínguez-Andrés, J.; Joosten, L.A.; Riksen, N.P.; Netea, M.G. Trained Immunity: Reprogramming Innate Immunity in Health and Disease. Annu. Rev. Immunol. 2021, 39, 667–693. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Andrés, J.; Fanucchi, S.; Joosten, L.A.; Mhlanga, M.M.; Netea, M.G. Advances in understanding molecular regulation of innate immune memory. Curr. Opin. Cell Biol. 2020, 63, 68–75. [Google Scholar] [CrossRef]
| Categories | Short Description | Description |
|---|---|---|
| Standard-Criteria Donor (SCD) | Donor under 60 years of age and do not meet any of the criteria of Expanded Criteria Donors (ECD) | |
| Expanded Criteria Donor (ECD) | Donor either > 60 years or aged 50 to 59 years with at least 2 of the following three criteria: (i) cerebrovascular accident as cause of death; (ii) serum creatinine level > 1.5 mg/dL (137 mmol/L); (iii) preexisting history of systemic hypertension | |
| Uncontrolled DCD I | Found dead I a. Out of hospital I b. In the hospital | Unexpected circulatory arrest with no resuscitation. Can donate tissue (cannot donate organs). |
| Uncontrolled DCD II | Cardiac arrest in front of a witness II a. Out-of-hospital II b. In-hospital | Unexpected circulatory arrest with failed resuscitation |
| Controlled DCD III | Withdrawal of life-sustaining therapy | Planned withdrawal of life-sustaining therapy. Limiting and stopping treatment in the intensive care unit (ICU). Primary donor type (only type in some countries). |
| Controlled DCD IV | Circulatory arrest while brain dead | Circulatory arrest in a brain-dead candidate for donation. |
| Controlled DCD V | Medical assisted circulatory arrest | Expected circulatory arrest as a result of euthanasia (depend of countries legislation). |
| Preservation in Static Hypothermic Condition | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Organ Preservation Solutions Adaptable to Static or Sometimes Dynamic Preservation | |||||||||
| Solutions | K+ (mM) | Na+ (mM) | Buffer | pH | Impermeants | Adenosine (mM) | Anti-Oxidant | Colloid (g/L) | Organs |
| Blood | 4.25 | 139 | HCO3− | 7.4 | + | 0 | + | Albumin (50 g/L) | All |
| HTK (Custodiol®) | 10 | 15 | Histidine | 7.2 | + | 5 | - | - | Kidney, liver, pancreas, heart, |
| UW (Viaspan®) (Bridge to life®) (SPS-1®) (Bel-Gen®) | 100 | 28.5 | (K)H2PO4 HEPES | 7.4 | + | 5 | Glutathione | HES (50 g/L) | Kidney, liver, pancreas |
| Celsior® | 15 | 100 | HEPES | 7.3 | + | 0 | Glutathione | - | Kidney, liver, pancreas, heart, lung |
| IGL-1® | 30 | 125 | (K)H2PO4 | 7.3 | + | 5 | Glutathione Allopurinol | PEG 35 kDa (1 g/L) | Kidney, liver, pancreas |
| SCOT 15® | 5 | 118 | HCO3− | 7.4 | + | 0 | - | PEG 20 kDa (15 g/L) | Kidney, liver |
| PERFADEX® Plus | 6 | 138 | - | 5.5 | + | - | - | Dextran 40 (5%) | Lungs |
| Preservation in dynamic condition | |||||||||
| Infusion machines | |||||||||
| Machine | Pulsatile perfusion | Temperature | Oxygenation | ||||||
| ORS—LifePort® | Hypothermia | No | Kidney | ||||||
| IGL—WAVES® | + | Hypothermia | Yes | Kidney pancreas | |||||
| Kidney Assist (XVIVO) | + | Hypothermia to normothermia | Yes | Kidney | |||||
| Liver Assist (XVIVO) | + | Hypothermia to normothermia | Yes | Liver | |||||
| Lung Assist (XVIVO) | +/− | Hypothermia to normothermia | Yes | Lung | |||||
| VITASMART™ (Bridge to Life) | Hypothermia | Yes | Liver, kidney | ||||||
| LIFECRADLE™ (Bridge to Life) | Hypothermia | Yes | Heart | ||||||
| EVOSS™ (Bridge to Life) | Normothermia | Yes | Lung | ||||||
| Organ Care System—OCS™ Lung (transmedics) | s+ | Normothermia | Yes | Lung | |||||
| Organ Care System—OCS™ heart (transmedics) | + | Normothermia | Yes | Heart | |||||
| Organ Care System—OCS™ liver (transmedics) | + | Normothermia | Yes | Liver | |||||
| Steen Preservation Heart System (XVIVO) | Hypothermia | Heart | |||||||
| XVIVO XPS™—XVIVO LS™ (XVIVO) | Normothermia | Yes | Lung | ||||||
| Paragonix SherpaPak | + | Hypothermia | +/− | Heart, lung | |||||
| Other solutions suitable for infusion machines | |||||||||
| Solutions | K+ (mM) | Na+ (mM) | Buffer | Impermeants | Anti-oxidant | Colloid (g/L) | Organs | ||
| KPS-1® | 25 | 97.5 | (K)H2PO4 HEPES | + | + | HES (50 g/L) | Kidney | ||
| Belzer MPS® PERF-GEN® | 25 | 100 | (K)H2PO4 HEPES | + | + | HES (50 g/L) | Kidney, liver, pancreas | ||
| IGL2® | 25 | 125 | (K)H2PO4 Histidine | + | + | PEG 35 kDa (5g/L) | Liver, pancreas | ||
| OCS Lung Solution | 6 | 136 | Phosphate | + | Dextran 40 (50 g/L) | Lung | |||
| STEEN Solution ™ | “low” | (Na)H2PO4 | + | Human albumin Dextran 40 | Lung | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lepoittevin, M.; Giraud, S.; Kerforne, T.; Barrou, B.; Badet, L.; Bucur, P.; Salamé, E.; Goumard, C.; Savier, E.; Branchereau, J.; et al. Preservation of Organs to Be Transplanted: An Essential Step in the Transplant Process. Int. J. Mol. Sci. 2022, 23, 4989. https://doi.org/10.3390/ijms23094989
Lepoittevin M, Giraud S, Kerforne T, Barrou B, Badet L, Bucur P, Salamé E, Goumard C, Savier E, Branchereau J, et al. Preservation of Organs to Be Transplanted: An Essential Step in the Transplant Process. International Journal of Molecular Sciences. 2022; 23(9):4989. https://doi.org/10.3390/ijms23094989
Chicago/Turabian StyleLepoittevin, Maryne, Sébastien Giraud, Thomas Kerforne, Benoit Barrou, Lionel Badet, Petru Bucur, Ephrem Salamé, Claire Goumard, Eric Savier, Julien Branchereau, and et al. 2022. "Preservation of Organs to Be Transplanted: An Essential Step in the Transplant Process" International Journal of Molecular Sciences 23, no. 9: 4989. https://doi.org/10.3390/ijms23094989
APA StyleLepoittevin, M., Giraud, S., Kerforne, T., Barrou, B., Badet, L., Bucur, P., Salamé, E., Goumard, C., Savier, E., Branchereau, J., Battistella, P., Mercier, O., Mussot, S., Hauet, T., & Thuillier, R. (2022). Preservation of Organs to Be Transplanted: An Essential Step in the Transplant Process. International Journal of Molecular Sciences, 23(9), 4989. https://doi.org/10.3390/ijms23094989








