Syntheses of Polypeptides and Their Biomedical Application for Anti-Tumor Drug Delivery
Abstract
:1. Introduction
2. Synthesis of Polypeptides through Polymerizations of Activated Amino Acid Monomers
2.1. Ring-Opening Polymerization (ROP) of α-Amino Acid N-Carboxy Anhydrides (NCAs)
2.2. Schemes of Ring-Opening Polymerization (ROP) of α-Amino Acid N-Thiocarboxyanhydrides (NTAs)
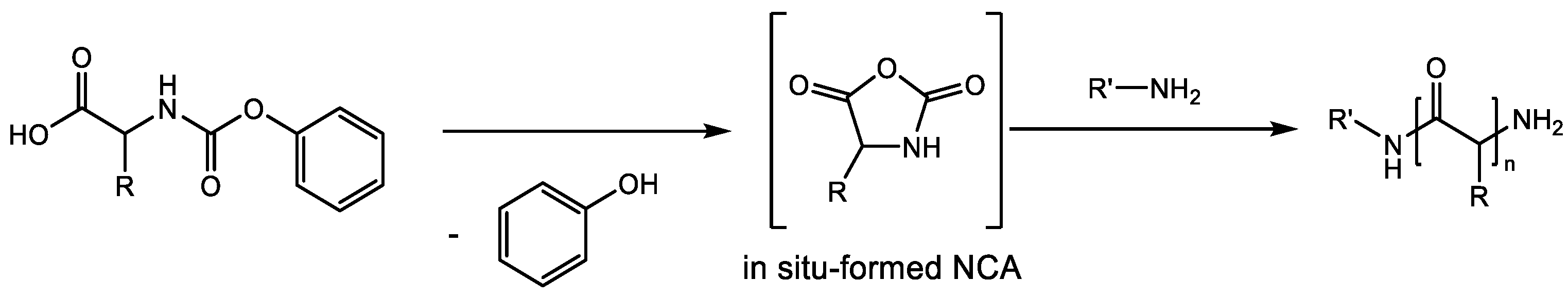
2.3. Polymerization of N-Phenoxycarbonyl Amino Acids (NPCs)
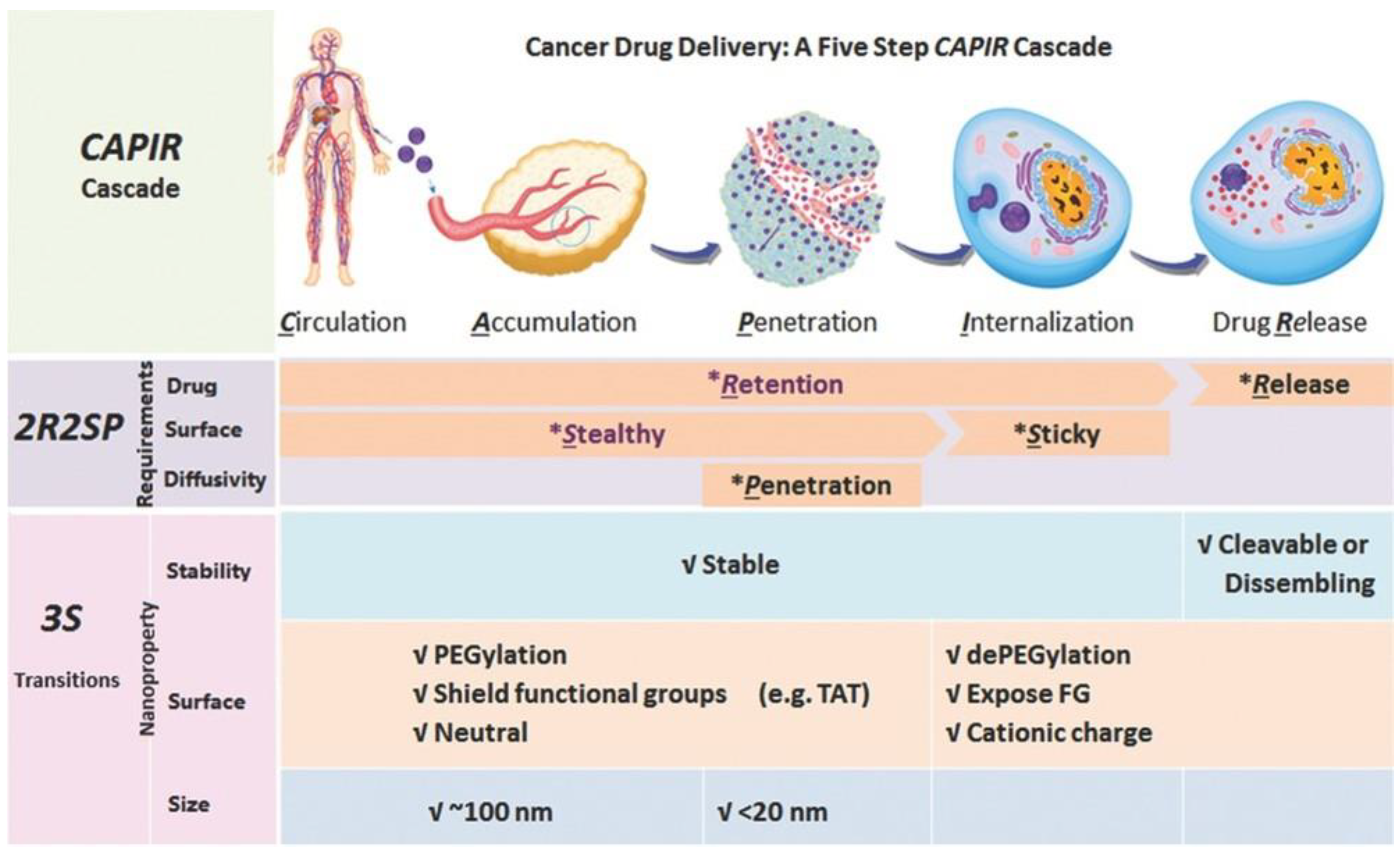
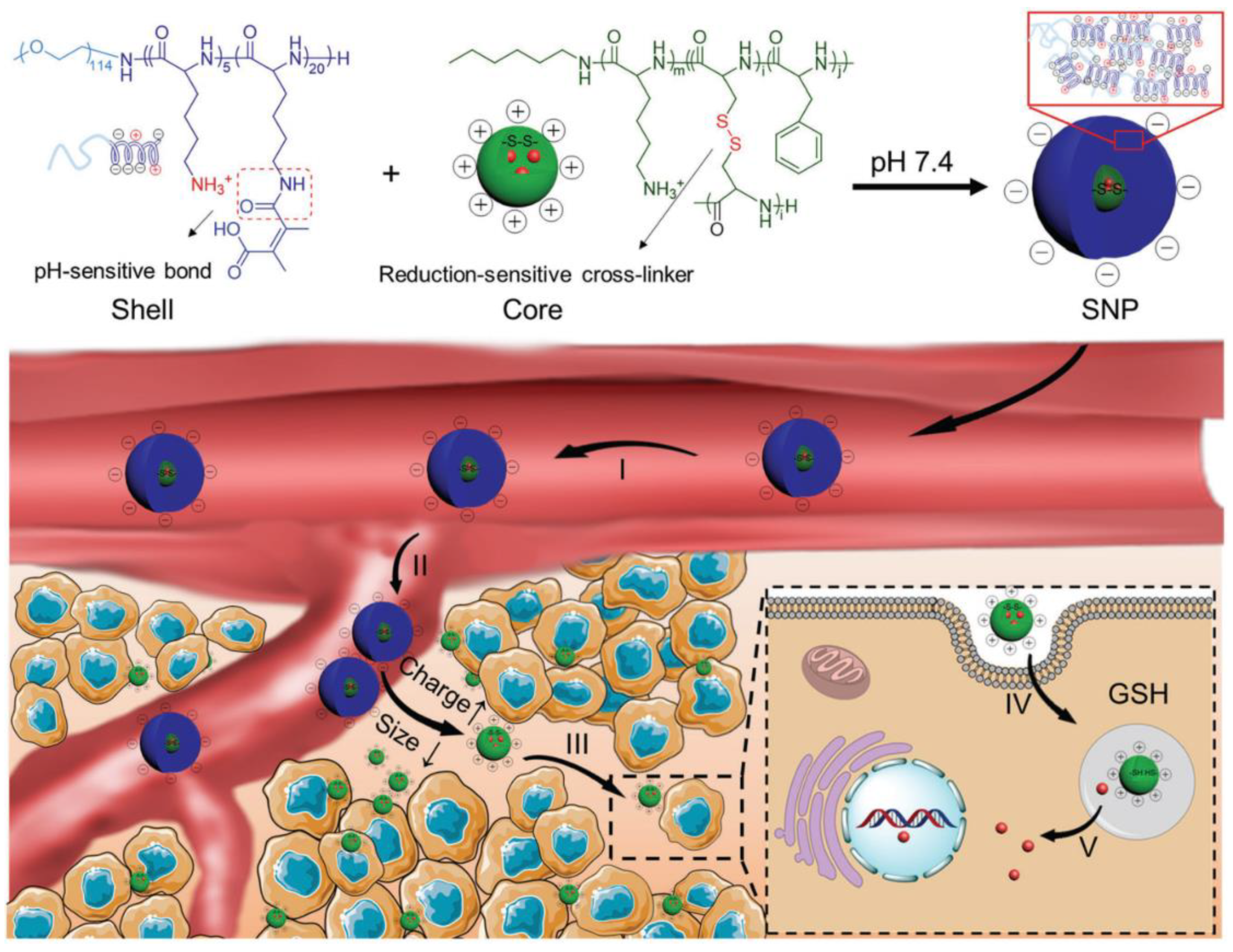
3. Polypeptides for Anticancer Drug Delivery
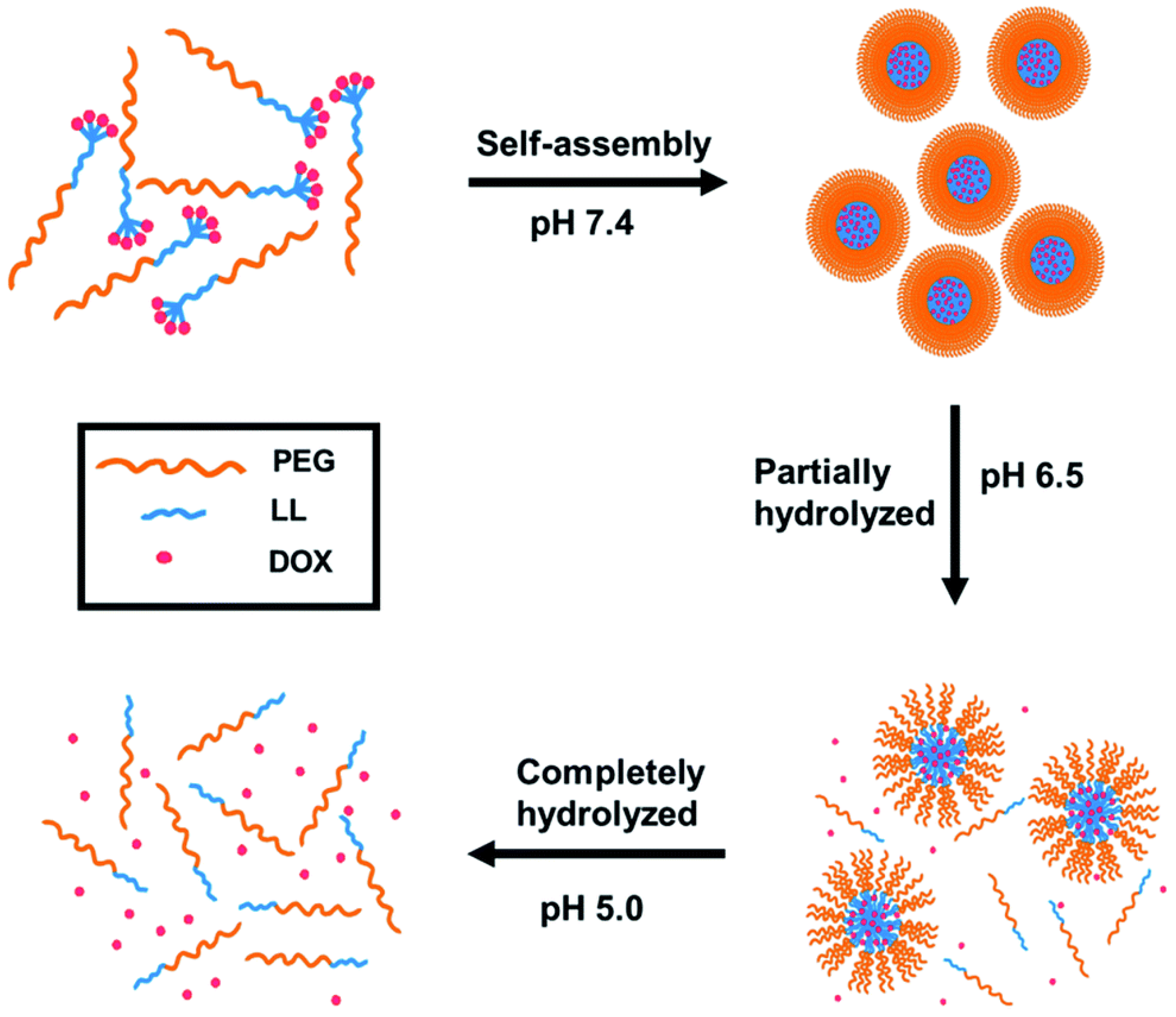
3.1. Stability Transition Polypeptide Systems
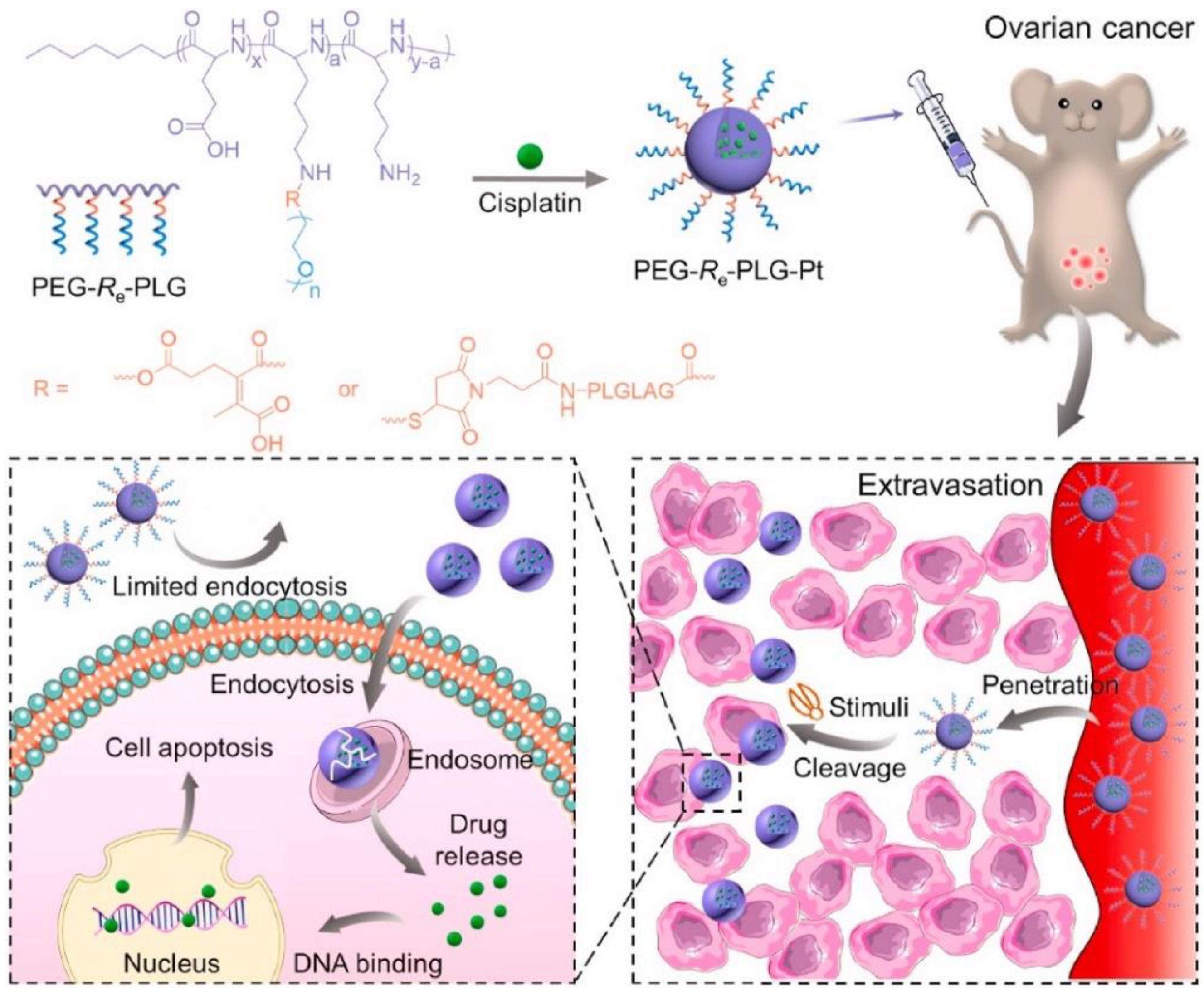
3.2. Surface Transition Polypeptide Systems
3.3. Size-Transition Polypeptide Systems
3.4. Polypeptide Systems Targeting Tumor Tissues
4. Critical Evaluation and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mura, S.; Nicolas, J.; Couvreur, P. Stimuli-responsive nanocarriers for drug delivery. Nat. Mater. 2013, 12, 991–1003. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Li, M.; Xiao, C.; Chen, X. Stimuli-responsive polypeptides for controlled drug delivery. Chem. Commun. 2021, 57, 9489–9503. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.Z.; Davis, E. Nanoplatforms for Targeted Stimuli-Responsive Drug Delivery: A Review of Platform Materials and Stimuli-Responsive Release and Targeting Mechanisms. Nanomaterials 2021, 11, 746. [Google Scholar] [CrossRef]
- Deming, T.J. Synthesis and Self-Assembly of Well-Defined Block Copolypeptides via Controlled NCA Polymerization. Adv. Polym. Sci. 2013, 262, 1–37. [Google Scholar]
- Feng, H.Y. Micro- and Nanocapsules Based on Artificial Peptides. Molecules 2022, 27, 1373. [Google Scholar] [CrossRef] [PubMed]
- Rasines Mazo, A.; Allison-Logan, S.; Karimi, F.; Chan, N.J.; Qiu, W.; Duan, W.; O’Brien-Simpson, N.M.; Qiao, G.G. Ring opening polymerization of alpha-amino acids: Advances in synthesis, architecture and applications of polypeptides and their hybrids. Chem. Soc. Rev. 2020, 49, 4737–4834. [Google Scholar] [CrossRef]
- Deming, T.J. Preparation and development of block copolypeptide vesicles and hydrogels for biological and medical applications. WIREs Nanomed. Nanobi. 2014, 6, 283–297. [Google Scholar] [CrossRef] [Green Version]
- Melnyk, T.; Dordevic, S.; Conejos-Sanchez, I.; Vicent, M.J. Therapeutic potential of polypeptide-based conjugates: Rational design and analytical tools that can boost clinical translation. Adv. Drug Deliv. Rev. 2020, 160, 136–169. [Google Scholar] [CrossRef]
- Kricheldorf, H.R. Polypeptides and 100 years of chemistry of alpha-amino acid N-carboxyanhydrides. Angew. Chem. Int. Ed. Engl. 2006, 45, 5752–5784. [Google Scholar] [CrossRef]
- Liu, Y.; Li, D.; Ding, J.; Chen, X. Controlled synthesis of polypeptides. Chin. Chem. Lett. 2020, 31, 3001–3014. [Google Scholar] [CrossRef]
- Tao, X.; Li, M.-H.; Ling, J. α-Amino acid N-thiocarboxyanhydrides: A novel synthetic approach toward poly(α-amino acid)s. Eur. Polym. J. 2018, 109, 26–42. [Google Scholar] [CrossRef]
- Zheng, B.; Bai, T.; Tao, X.; Ling, J. An Inspection into Multifarious Ways to Synthesize Poly(Amino Acid)s. Macromol. Rapid Commun. 2021, 42, 2100453. [Google Scholar] [CrossRef] [PubMed]
- Endo, T.; Sudo, A. Well-Defined Construction of Functional Macromolecular Architectures Based on Polymerization of Amino Acid Urethanes. Biomedicines 2020, 8, 317. [Google Scholar] [CrossRef]
- Klok, H.-A. Protein-Inspired Materials: Synthetic Concepts and Potential Applications. Angew. Chem. Int. Ed. 2002, 41, 1509–1513. [Google Scholar] [CrossRef]
- Vranken, D.V.; Weiss, G.A. Introduction to Bioorganic Chemistry and Chemical Biology; Garland Science: New York, NY, USA, 2012. [Google Scholar]
- Gaglione, R.; Pane, K.; Dell’Olmo, E.; Cafaro, V.; Pizzo, E.; Olivieri, G.; Notomista, E.; Arciello, A. Cost-effective production of recombinant peptides in Escherichia coli. New Biotechnol. 2019, 51, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Farthing, A.C.; Reynolds, R.J.W. Anhydro-N-carboxy-DL-β-phenylalanine. Nature 1950, 165, 647. [Google Scholar] [CrossRef]
- Feng, H.; Chu, D.; Li, Z.; Guo, Z.; Jin, L.; Fan, B.; Zhang, J.; Li, J. A DOX-loaded polymer micelle for effectively inhibiting cancer cells. RSC Adv. 2018, 8, 25949–25954. [Google Scholar] [CrossRef] [Green Version]
- Yu, M.; Nowak, A.P.; Deming, T.J.; Pochan, D.J. Methylated mono-and diethyleneglycol functionalized polylysines: Nonionic, α-helical, water-soluble polypeptides. J. Am. Chem. Soc. 1999, 121, 12210–12211. [Google Scholar] [CrossRef]
- Berger, A.; Noguchi, J.; Katchalski, E. Poly-L-cysteine. J. Am. Chem. Soc. 1956, 78, 4483–4488. [Google Scholar] [CrossRef]
- Szwarc, M. Living’polymers. Nature 1956, 178, 1168–1169. [Google Scholar] [CrossRef]
- Deming, T.J. Living polymerization of α-amino acid-N-carboxyanhydrides. J. Polym. Sci. Part A Polym. Chem. 2000, 38, 3011–3018. [Google Scholar] [CrossRef]
- Aubert, P.E.R.; Knott, E.B. Synthesis of thiazolid-2: 5-dione. Nature 1950, 166, 1039–1040. [Google Scholar] [CrossRef]
- Kricheldorf, H.R.; Sell, M.; Schwarz, G. Primary Amine-Initiated Polymerizations of α–Amino Acid N-Thiocarbonic Acid Anhydrosulfide. J. Macromol. Sci. Part A 2008, 45, 425–430. [Google Scholar] [CrossRef]
- Miao, Y.; Xie, F.; Cen, J.; Zhou, F.; Tao, X.; Luo, J.; Han, G.; Kong, X.; Yang, X.; Sun, J. Fe3+@polyDOPA-b-polysarcosine, a T1-Weighted MRI Contrast Agent via Controlled NTA Polymerization. ACS Macro. Lett. 2018, 7, 693–698. [Google Scholar] [CrossRef]
- Kamei, Y.; Nagai, A.; Sudo, A.; Nishida, H.; Kikukawa, K.; Endo, T. Convenient synthesis of poly(γ-benzyl-L-glutamate) from activated urethane derivatives of γ-benzyl-L-glutamate. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 2649–2657. [Google Scholar] [CrossRef]
- Kamei, Y.; Sudo, A.; Endo, T. Synthesis of Polypeptide Having Defined Terminal Structures Through Polymerization of Activated Urethane-Derivative of γ-Benzyl-l-glutamate. Macromolecules 2008, 41, 7913–7919. [Google Scholar] [CrossRef]
- Yamada, S.; Sudo, A.; Goto, M.; Endo, T. Phosgene-free synthesis of polypeptides using activated urethane derivatives of α-amino acids: An efficient synthetic approach to hydrophilic polypeptides. RSC Adv. 2014, 4, 29890–29896. [Google Scholar] [CrossRef]
- Yamada, S.; Koga, K.; Sudo, A.; Goto, M.; Endo, T. Phosgene-free synthesis of polypeptides: Useful synthesis for hydrophobic polypeptides through polycondensation of activated urethane derivatives of α-amino acids. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 3726–3731. [Google Scholar] [CrossRef]
- Sun, Q.; Radosz, M.; Shen, Y. Challenges in design of translational nanocarriers. J. Control. Release 2012, 164, 156–169. [Google Scholar] [CrossRef]
- Sun, Q.; Zhou, Z.; Qiu, N.; Shen, Y. Rational Design of Cancer Nanomedicine: Nanoproperty Integration and Synchronization. Adv. Mater. 2017, 29, 1606628. [Google Scholar] [CrossRef]
- Bae, Y.; Fukushima, S.; Harada, A.; Kataoka, K. Design of Environment-Sensitive Supramolecular Assemblies for Intracellular Drug Delivery: Polymeric Micelles that are Responsive to Intracellular pH Change. Angew. Chem. Int. Ed. 2003, 42, 4640–4643. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; He, Q.; Zhao, W.; Luo, J.; Gao, W. Tumor-homing, pH- and ultrasound-responsive polypeptide-doxorubicin nanoconjugates overcome doxorubicin resistance in cancer therapy. J. Control. Release 2017, 264, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Li, J.G.; Zhang, L.; Lin, Y.J.; Xiao, H.; Zuo, M.X.; Cheng, D.; Shuai, X.T. A pH-sensitive prodrug micelle self-assembled from multi-doxorubicin-tailed polyethylene glycol for cancer therapy. Rsc. Adv. 2016, 6, 9160–9163. [Google Scholar] [CrossRef]
- Teng, W.; Jia, F.; Han, H.; Qin, Z.; Jin, Q.; Ji, J. Polyamino acid-based gemcitabine nanocarriers for targeted intracellular drug delivery. Polym. Chem. 2017, 8, 2490–2498. [Google Scholar] [CrossRef]
- Li, Y.; Yang, H.; Yao, J.; Yu, H.; Chen, X.; Zhang, P.; Xiao, C. Glutathione-triggered dual release of doxorubicin and camptothecin for highly efficient synergistic anticancer therapy. Colloids Surf. B Biointerfaces 2018, 169, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yang, Y.; Peng, C.; Liu, H.; Yang, R.; Zheng, Y.; Cai, L.; Tan, H.; Fu, Q.; Ding, M. Drug-induced hierarchical self-assembly of poly(amino acid) for efficient intracellular drug delivery. Chin. Chem. Lett. 2020, 32, 1563–1566. [Google Scholar] [CrossRef]
- Wang, X.; Song, Z.; Wei, S.; Ji, G.; Zheng, X.; Fu, Z.; Cheng, J. Polypeptide-based drug delivery systems for programmed release. Biomaterials 2021, 275, 120913. [Google Scholar] [CrossRef]
- Li, Z.; Huang, J.; Wu, J. pH-Sensitive nanogels for drug delivery in cancer therapy. Biomater. Sci. 2021, 9, 574–589. [Google Scholar] [CrossRef]
- Gong, Z.Y.; Liu, X.Y.; Wu, J.L.; Li, X.C.; Tang, Z.Q.; Deng, Y.T.; Sun, X.M.; Chen, K.; Gao, Z.Q.; Bai, J.K. pH-triggered morphological change in a self-assembling amphiphilic peptide used as an antitumor drug carrier. Nanotechnology 2020, 31, 165601. [Google Scholar] [CrossRef]
- Agazzi, M.L.; Herrera, S.E.; Cortez, M.L.; Marmisoll, W.A.; Azzaroni, O. Self-assembled peptide dendrigraft supraparticles with potential application in pH/enzyme-triggered multistage drug release. Colloids Surf. B 2020, 190, 110895. [Google Scholar] [CrossRef]
- Zhang, P.; Yang, H.L.; Shen, W.; Liu, W.G.; Chen, L.; Xiao, C.S. Hypoxia-Responsive Polypeptide Nanoparticles Loaded with Doxorubicin for Breast Cancer Therapy. Acs. Biomater. Sci. Eng. 2020, 6, 2167–2174. [Google Scholar] [CrossRef] [PubMed]
- Sahu, A.; Choi, W.I.; Tae, G. Recent Progress in the Design of Hypoxia-Specific Nano Drug Delivery Systems for Cancer Therapy. Adv. Ther. 2018, 1, 1800026. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, Y.; Zhong, Z. Reduction-sensitive polymeric nanomedicines: An emerging multifunctional platform for targeted cancer therapy. Adv. Drug Deliv. Rev. 2018, 132, 16–32. [Google Scholar] [CrossRef] [PubMed]
- Hoang, Q.T.; Lee, D.; Choi, D.G.; Kim, Y.-C.; Shim, M.S. Efficient and selective cancer therapy using pro-oxidant drug-loaded reactive oxygen species (ROS)-responsive polypeptide micelles. J. Ind. Eng. Chem. 2020, 95, 101–108. [Google Scholar] [CrossRef]
- Mirhadi, E.; Mashreghi, M.; Maleki, M.F.; Alavizadeh, S.H.; Arabi, L.; Badiee, A.; Jaafari, M.R. Redox-sensitive nanoscale drug delivery systems for cancer treatment. Int. J. Pharm. 2020, 589, 119882. [Google Scholar] [CrossRef]
- Zhong, Y.; Huang, S.; Zheng, C.; Huang, J.; Li, B.; Han, S.; Xiao, H.; Wang, Y.; Shuai, X. A light and hypoxia-activated nanodrug for cascade photodynamic-chemo cancer therapy. Biomater. Sci. 2021, 9, 5218–5226. [Google Scholar] [CrossRef]
- Yallapu, M.M.; Foy, S.P.; Jain, T.K.; Labhasetwar, V. PEG-Functionalized Magnetic Nanoparticles for Drug Delivery and Magnetic Resonance Imaging Applications. Pharm. Res. 2010, 27, 2283–2295. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Z.; Feng, X.; Zou, H.; Xu, W.; Zhuang, X. Poly(l-glutamic acid)-cisplatin nanoformulations with detachable PEGylation for prolonged circulation half-life and enhanced cell internalization. Bioact. Mater. 2021, 6, 2688–2697. [Google Scholar] [CrossRef]
- Wu, Y.; Zhong, D.; Li, Y.; Wu, H.; Zhang, H.; Mao, H.; Yang, J.; Luo, K.; Gong, Q.; Gu, Z. A tumor-activatable peptide supramolecular nanoplatform for the delivery of dual-gene targeted siRNAs for drug-resistant cancer treatment. Nanoscale 2021, 13, 4887–4898. [Google Scholar] [CrossRef]
- Li, Z.; Gao, Y.; Li, W.; Li, Y.; Lv, H.; Zhang, D.; Peng, J.; Cheng, W.; Mei, L.; Chen, H.; et al. Charge-reversal nanomedicines as a smart bullet for deep tumor penetration. Smart Mater. Med. 2022, 3, 243–253. [Google Scholar] [CrossRef]
- Han, S.S.; Li, Z.Y.; Zhu, J.Y.; Han, K.; Zeng, Z.Y.; Hong, W.; Li, W.X.; Jia, H.Z.; Liu, Y.; Zhuo, R.X.; et al. Dual-pH Sensitive Charge-Reversal Polypeptide Micelles for Tumor-Triggered Targeting Uptake and Nuclear Drug Delivery. Small 2015, 11, 2543–2554. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.X.; Zhuang, W.H.; Wang, Y.A.; Luo, R.F.; Wang, Y.B. pH-sensitive doxorubicin-conjugated prodrug micelles with charge-conversion for cancer therapy. Acta Biomater. 2018, 70, 186–196. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Sun, N.; Cheng, R.; Zhao, C.; Liu, Z.; Li, X.; Liu, J.; Tian, Z. pH multistage responsive micellar system with charge-switch and PEG layer detachment for co-delivery of paclitaxel and curcumin to synergistically eliminate breast cancer stem cells. Biomaterials 2017, 147, 53–67. [Google Scholar] [CrossRef] [PubMed]
- Zhen, J.-R.; Tian, S.; Liu, Q.; Zheng, C.; Zhang, Z.; Ding, Y.; An, Y.; Liu, Y.; Shi, L. Nanocarriers responsive to a hypoxia gradient facilitate enhanced tumor penetration and improved anti-tumor efficacy. Biomater. Sci. 2019, 7, 2986–2995. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Ma, Y.; Du, C.; Wang, C.; Chen, T.; Wang, Y.; Wang, J.; Yao, Y.; Dong, C.-M. NO-releasing polypeptide nanocomposites reverse cancer multidrug resistance via triple therapies. Acta Biomater. 2021, 123, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Jing, T.; Li, T.; Ruan, Z.; Yan, L. pHe- and glutathione-stepwise-responsive polypeptide nanogel for smart and efficient drug delivery. J. Mater. Sci. 2018, 53, 14933–14943. [Google Scholar] [CrossRef]
- Kuang, Y.; Chen, H.; Chen, Z.Y.; Wan, L.H.; Liu, J.; Xu, Z.Q.; Chen, X.Q.; Jiang, B.B.; Li, C. Poly(amino acid)/ZnO/mesoporous silica nanoparticle based complex drug delivery system with a charge-reversal property for cancer therapy. Colloids Surf. B 2019, 181, 461–469. [Google Scholar] [CrossRef]
- Xu, C.; Song, R.J.; Lu, P.; Chen, J.C.; Zhou, Y.Q.; Shen, G.; Jiang, M.J.; Zhang, W. pH-triggered charge-reversal and redox-sensitive drug-release polymer micelles codeliver doxorubicin and triptolide for prostate tumor therapy. Int. J. Nanomed. 2018, 13, 7229–7249. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Yu, X.; Wang, Y.; Yuan, Y.; Xiao, H.; Cheng, D.; Shuai, X. A Reduction and pH Dual-Sensitive Polymeric Vector for Long-Circulating and Tumor-Targeted siRNA Delivery. Adv. Mater. 2014, 26, 8217–8224. [Google Scholar] [CrossRef]
- Qu, J.; Peng, S.; Wang, R.; Yang, S.T.; Zhou, Q.H.; Lin, J. Stepwise pH-sensitive and biodegradable polypeptide hybrid micelles for enhanced cellular internalization and efficient nuclear drug delivery. Colloids Surf. B 2019, 181, 315–324. [Google Scholar] [CrossRef]
- Qu, J.; Wang, R.; Peng, S.; Shi, M.; Yang, S.-T.; Luo, J.-B.; Lin, J.; Zhou, Q.-H. Stepwise dual pH and redox-responsive cross-linked polypeptide nanoparticles for enhanced cellular uptake and effective cancer therapy. J. Mater. Chem. B 2019, 7, 7129–7140. [Google Scholar] [CrossRef]
- Li, Z.; Shan, X.; Chen, Z.; Gao, N.; Zeng, W.; Zeng, X.; Mei, L. Applications of Surface Modification Technologies in Nanomedicine for Deep Tumor Penetration. Adv. Sci. 2020, 8, 2002589. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Xie, X.; Zhang, H.; Su, Q.; Yang, G.; Wei, X.; Li, N.; Li, T.; Qin, X.; Li, S.; et al. Multistage-responsive nanovehicle to improve tumor penetration for dual-modality imaging-guided photodynamic-immunotherapy. Biomaterials 2021, 275, 120990. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Zhang, G.D.; Mai, J.H.; Deng, X.Y.; Segura-Ibarra, V.; Wu, S.H.; Shen, J.L.; Liu, H.R.; Hu, Z.H.; Chen, L.X.; et al. An injectable nanoparticle generator enhances delivery of cancer therapeutics. Nat. Biotechnol. 2016, 34, 414–418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cun, X.; Li, M.; Wang, S.; Wang, Y.; Wang, J.; Lu, Z.; Yang, R.; Tang, X.; Zhang, Z.; He, Q. A size switchable nanoplatform for targeting the tumor microenvironment and deep tumor penetration. Nanoscale 2018, 10, 9935–9948. [Google Scholar] [CrossRef]
- Cao, M.W.; Lu, S.; Wang, N.N.; Xu, H.; Cox, H.; Li, R.H.; Waigh, T.; Han, Y.C.; Wang, Y.L.; Lu, J.R. Enzyme-Triggered Morphological Transition of Peptide Nanostructures for Tumor-Targeted Drug Delivery and Enhanced Cancer Therapy. Acs. Appl. Mater. Inter. 2019, 11, 16357–16366. [Google Scholar] [CrossRef]
- Cong, Y.; Ji, L.; Gao, Y.J.; Liu, F.H.; Cheng, D.B.; Hu, Z.Y.; Qiao, Z.Y.; Wang, H. Microenvironment-Induced In Situ Self-Assembly of Polymer-Peptide Conjugates That Attack Solid Tumors Deeply. Angew. Chem. Int. Ed. 2019, 58, 4632–4637. [Google Scholar] [CrossRef]
- Cong, Z.Q.; Zhang, L.; Ma, S.Q.; Lam, K.S.; Yang, F.F.; Liao, Y.H. Size-Transformable Hyaluronan Stacked Self-Assembling Peptide Nanoparticles for Improved Transcellular Tumor Penetration and Photo-Chemo Combination Therapy. Acs. Nano 2020, 14, 1958–1970. [Google Scholar] [CrossRef]
- Gao, Z.L.; Zhang, Z.H.; Guo, J.M.; Hao, J.C.; Zhang, P.Y.; Cui, J.W. Polypeptide Nanoparticles with pH-Sheddable PEGylation for Improved Drug Delivery. Langmuir 2020, 36, 13656–13662. [Google Scholar] [CrossRef]
- Chen, J.; Ding, J.; Wang, Y.; Cheng, J.; Ji, S.; Zhuang, X.; Chen, X. Sequentially Responsive Shell-Stacked Nanoparticles for Deep Penetration into Solid Tumors. Adv. Mater. 2017, 29, 1701170. [Google Scholar] [CrossRef]
- Liu, L.; Li, T.; Ruan, Z.; Yan, L. Polypeptide-based artificial erythrocytes conjugated with near infrared photosensitizers for imaging-guided photodynamic therapy. J. Mater. Sci. 2018, 53, 9368–9381. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, X.; Wan, K.; Zhou, N.; Wei, G.; Su, Z. Supramolecular peptide nano-assemblies for cancer diagnosis and therapy: From molecular design to material synthesis and function-specific applications. J. Nanobiotechnol. 2021, 19, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Ning, C.; Zhou, Z.; Yu, P.; Zhu, Y.; Tan, G.; Mao, C. Nanomaterials as photothermal therapeutic agents. Prog. Mater. Sci. 2018, 99, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.Z.; Gao, M.Y.; Fan, L.; Lai, Y.Y.; Fan, H.W.; Hua, Z.C. IR820 covalently linked with self-assembled polypeptide for photothermal therapy applications in cancer. Biomater. Sci. 2018, 6, 3410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, Q.L.; Abbas, M.; Zhao, L.Y.; Li, S.K.; Shen, G.Z.; Yan, X.H. Biological Photothermal Nanodots Based on Self-Assembly of Peptide Porphyrin Conjugates for Antitumor Therapy. J. Am. Chem. Soc. 2017, 139, 1921–1927. [Google Scholar] [CrossRef]
- Wan, Y.; Fu, L.; Li, C.; Lin, J.; Huang, P. Conquering the Hypoxia Limitation for Photodynamic Therapy. Adv. Mater. 2021, 33, 2103978. [Google Scholar] [CrossRef]
- Feng, H.; Linders, J.; Myszkowska, S.; Mayer, C. Capsules from synthetic diblock-peptides as potential artificial oxygen carriers. J. Microencapsul. 2021, 38, 276–284. [Google Scholar] [CrossRef]
- Conde, J. Above and Beyond Cancer Therapy: Translating Biomaterials into the Clinic. Trends Cancer 2020, 6, 730–732. [Google Scholar] [CrossRef]
- Faria, M.; Björnmalm, M.; Thurecht, K.J.; Kent, S.J.; Parton, R.G.; Kavallaris, M.; Johnston, A.P.R.; Gooding, J.J.; Corrie, S.R.; Boyd, B.J.; et al. Minimum information reporting in bio–nano experimental literature. Nat. Nanotechnol. 2018, 13, 777–785. [Google Scholar] [CrossRef]
- Mahmoudi, M. Antibody orientation determines corona mistargeting capability. Nat. Nanotechnol. 2018, 13, 775–776. [Google Scholar] [CrossRef]
- Ke, P.C.; Lin, S.; Parak, W.J.; Davis, T.P.; Caruso, F. A Decade of the Protein Corona. ACS Nano 2017, 11, 11773–11776. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, S.; Tavares, A.J.; Dai, Q.; Ohta, S.; Audet, J.; Dvorak, H.F.; Chan, W.C.W. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 2016, 1, 1–12. [Google Scholar] [CrossRef]
- de Lazaro, D.J. Mooney. A nanoparticle’s pathway into tumours. Nat. Mater. 2020, 19, 486–487. [Google Scholar] [CrossRef] [PubMed]
- Sindhwani, S.; Syed, A.M.; Ngai, J.; Kingston, B.R.; Maiorino, L.; Rothschild, J.; Macmillan, P.; Zhang, Y.; Rajesh, N.U.; Hoang, T.; et al. The entry of nanoparticles into solid tumours. Nat. Mater. 2020, 19, 566–575. [Google Scholar] [CrossRef]
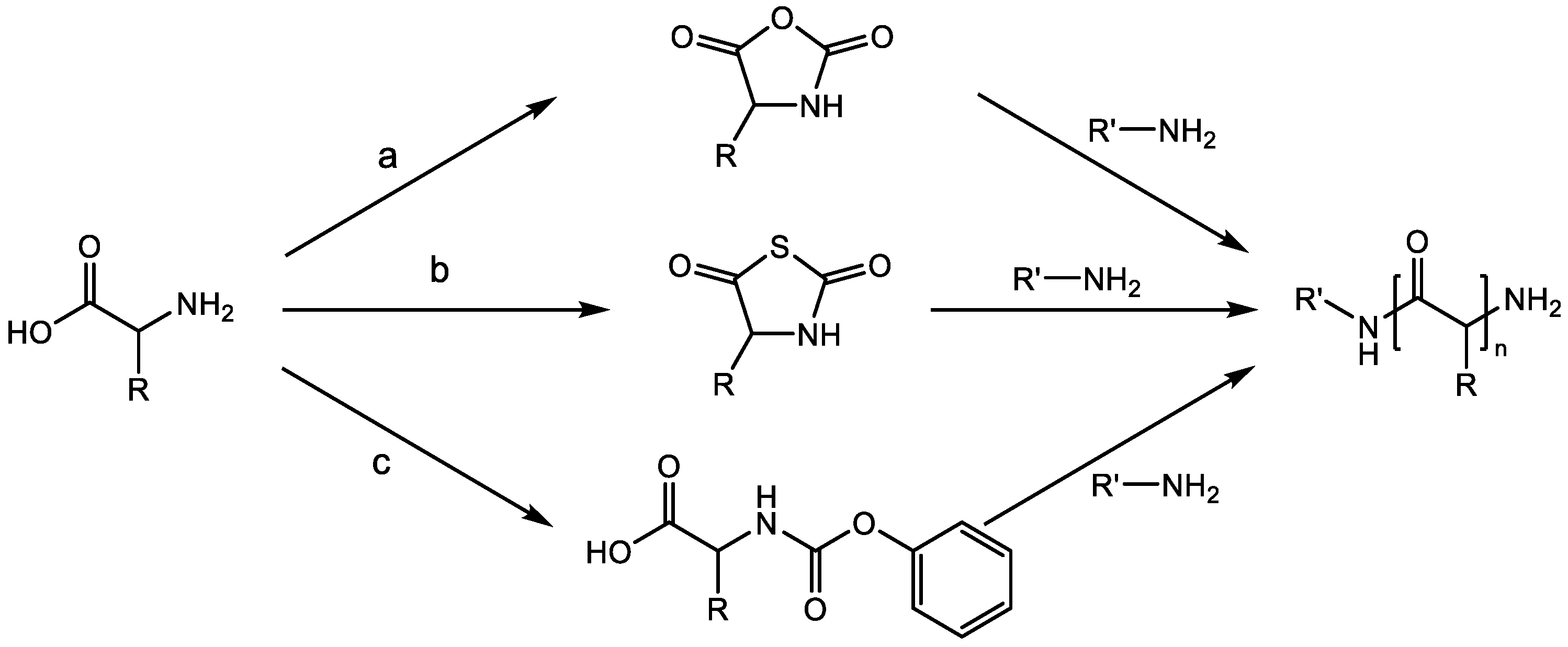
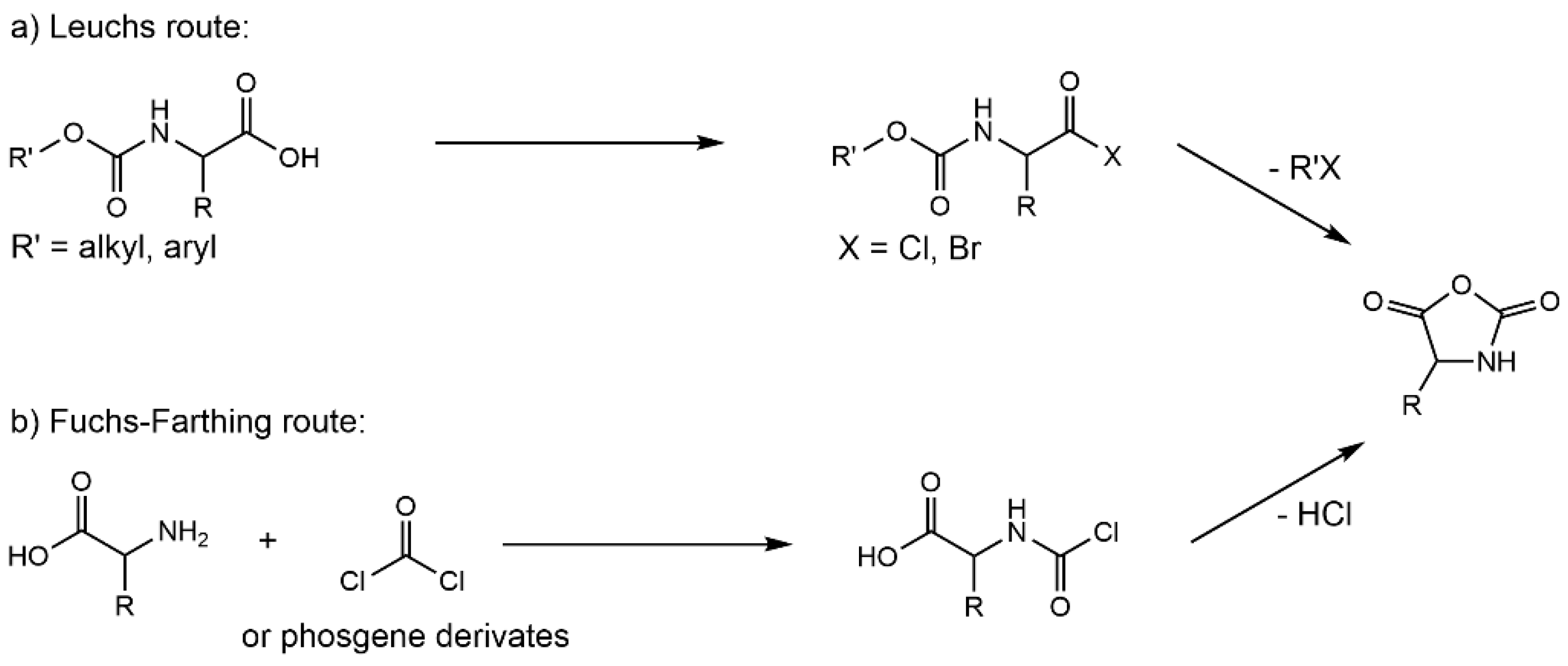
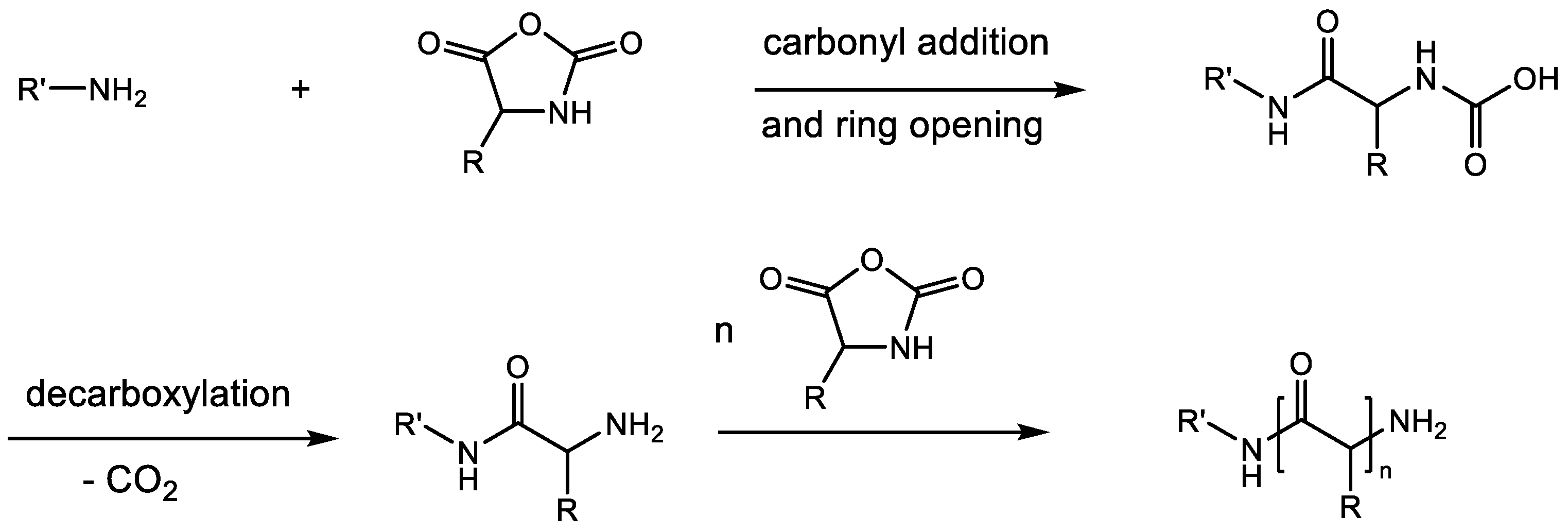

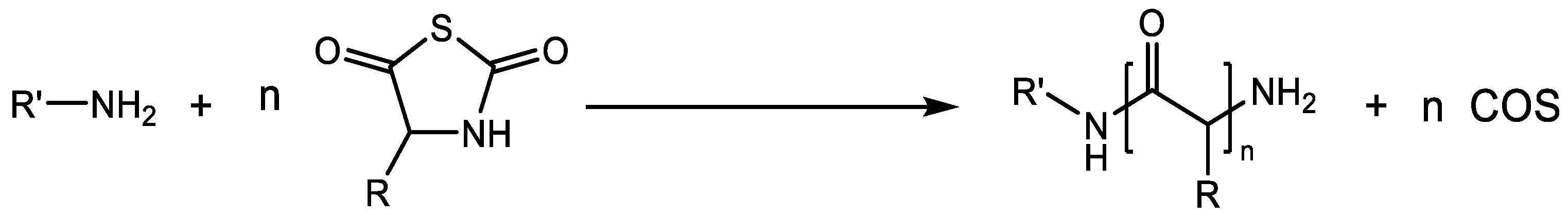

| Monomers | Advantages | Disadvantages |
|---|---|---|
| NCAs | High reactivity Easy synthesis | Phosgene derivatives used to synthesize NCAs are lethal or toxic. Unstable in moisture |
| NTAs | More stable under moisture and heat than NCAs. Easy isolation and storage. | Educts used to prepare NTAs are expensive. Toxic gaseous compound, carbonyl sulfide, is formed during polymerization. |
| NPCs | More stable under moisture and heat than NCAs. Easy synthesis, isolation, and storage. | The yield of polymerization is slightly lower than that of NCA. |
| Polypeptides | Drugs | Stimuli-Responsiveness | Transition Types | Ref. |
|---|---|---|---|---|
| polylysine | doxorubicin | pH | stability transition | [34] |
| polylysine | gemcitabine | GSH | stability transition | [35] |
| polyglutamic acid | camptothecin | GSH | stability transition | [36] |
| decapeptide consisting of leucine and lysine | doxorubicin | pH | stability transition | [40] |
| polymethionine | piperlongumine | ROS | stability transition | [45] |
| polyglutamate | doxorubicin | hypoxia | stability transition | [42] |
| polylysine dendrigraft | curcumin and doxorubicin | pH and enzyme | stability transition | [41] |
| polyaspartate and polyphenylalanine | tirapazamine | light and hypoxia | stability transition | [47] |
| polyglutamic acid | cisplatin | pH and enzyme | surface transition | [49] |
| polylysine and polycysteine | nitric oxide donor and doxorubicin | pH and light | surface transition | [56] |
| polyaspartate | siRNA | pH | surface transition | [60] |
| polylysine and polyleucine | doxorubicin | pH | surface transition | [52] |
| polylysine | doxorubicin | pH | surface transition | [58] |
| polyaspartate | paclitaxel and curcumin | pH | surface and size transition | [54] |
| polylysine | doxorubicin | pH | stability and surface transition | [53] |
| polylysine and polycysteine | doxorubicin | pH and GSH | stability and surface transition | [57] |
| polylysine | doxorubicin | pH and GSH | stability and surface transition | [61] |
| polylysine | triptolide and doxorubicin | pH and GSH | stability and surface transition | [59] |
| polylysine and polyglutamic acid | doxorubicin | pH and GSH | stability and surface transition | [62] |
| polyglutamic acid | doxorubicin | pH | size transition | [65] |
| polylysine | doxorubicin | enzyme | surface and size transition | [66] |
| hexadecapeptide consisting of lysine and glutamic acid | SN-38 | pH | size transition | [69] |
| polylysine and polycysteine | doxorubicin | pH and GSH | 3S transition | [71] |
| polylysine and polyglutamic acid | cisplatin | pH and GSH | 3S transition | [70] |
| polyaspartate | photosensitizers and hemoglobin | no stimuli | no transition | [72] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, H.; Fabrizi, J.; Li, J.; Mayer, C. Syntheses of Polypeptides and Their Biomedical Application for Anti-Tumor Drug Delivery. Int. J. Mol. Sci. 2022, 23, 5042. https://doi.org/10.3390/ijms23095042
Feng H, Fabrizi J, Li J, Mayer C. Syntheses of Polypeptides and Their Biomedical Application for Anti-Tumor Drug Delivery. International Journal of Molecular Sciences. 2022; 23(9):5042. https://doi.org/10.3390/ijms23095042
Chicago/Turabian StyleFeng, Huayang, Jonas Fabrizi, Jingguo Li, and Christian Mayer. 2022. "Syntheses of Polypeptides and Their Biomedical Application for Anti-Tumor Drug Delivery" International Journal of Molecular Sciences 23, no. 9: 5042. https://doi.org/10.3390/ijms23095042
APA StyleFeng, H., Fabrizi, J., Li, J., & Mayer, C. (2022). Syntheses of Polypeptides and Their Biomedical Application for Anti-Tumor Drug Delivery. International Journal of Molecular Sciences, 23(9), 5042. https://doi.org/10.3390/ijms23095042







