Relationship between Neuronal Damage/Death and Astrogliosis in the Cerebral Motor Cortex of Gerbil Models of Mild and Severe Ischemia and Reperfusion Injury
Abstract
:1. Introduction
2. Results
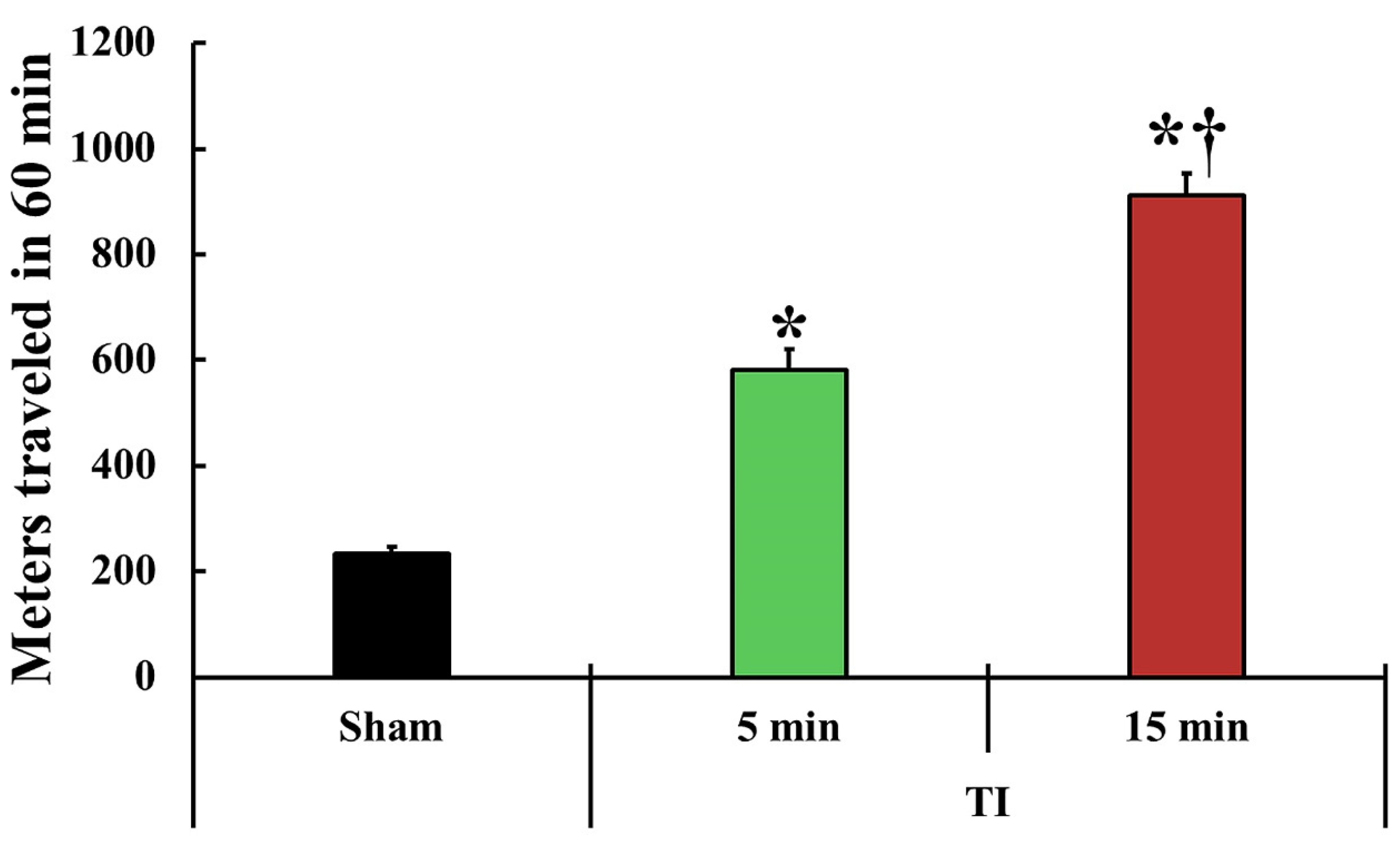
2.1. Change in Motor Behavior
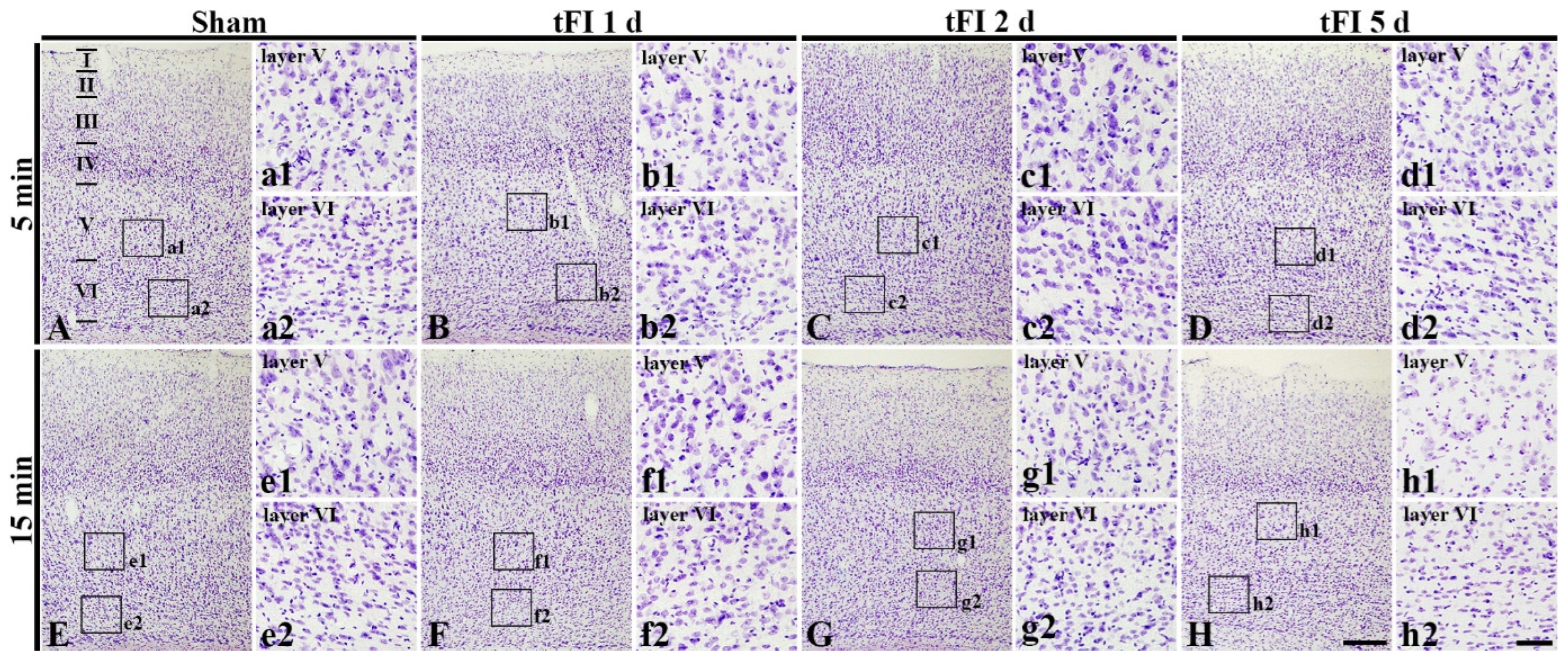
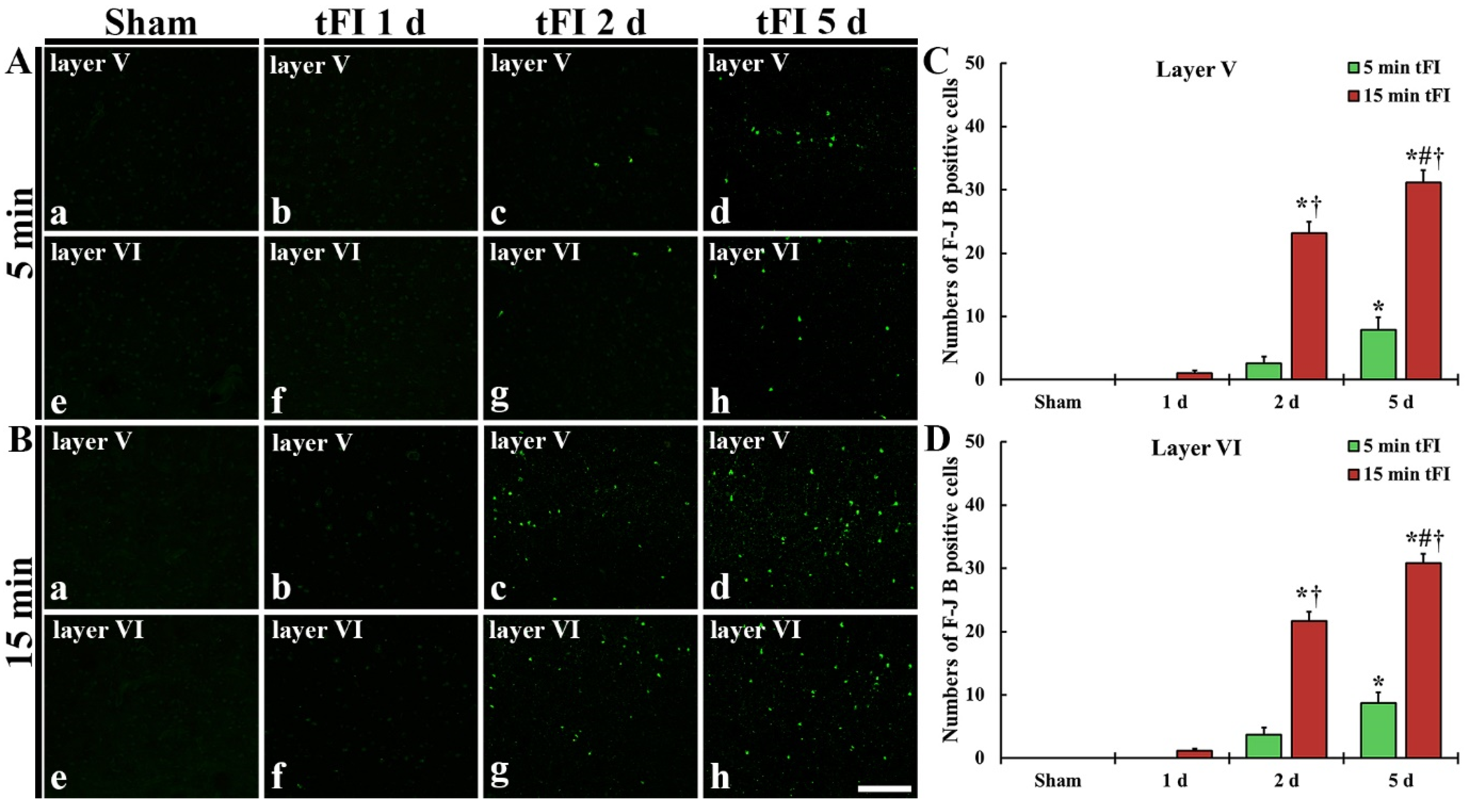
2.2. Neuronal Damage/Death (Loss)
2.2.1. Cresyl Violet (CV)-Stained Cells (CV-Cells)
2.2.2. Fluoro-Jade B (F-J B)-Positive Cells (F-J B-Cells)
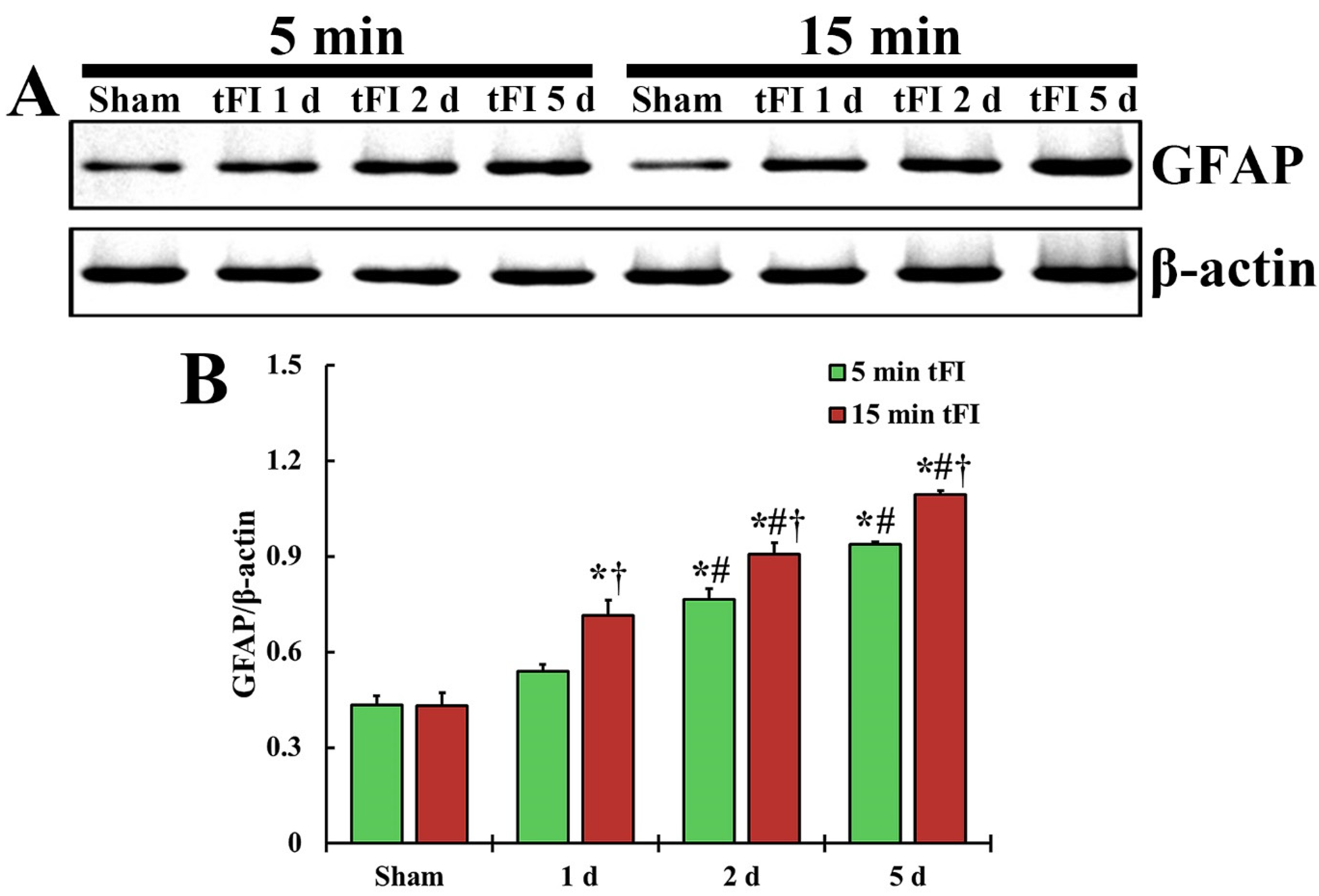
2.3. Astrogliosis
2.3.1. Glial Fibrillary Acidic Protein (GFAP)-Immunoreactive Astrocytes
2.3.2. GFAP Protein Level
2.3.3. S100-Immunoreactive Astrocytes
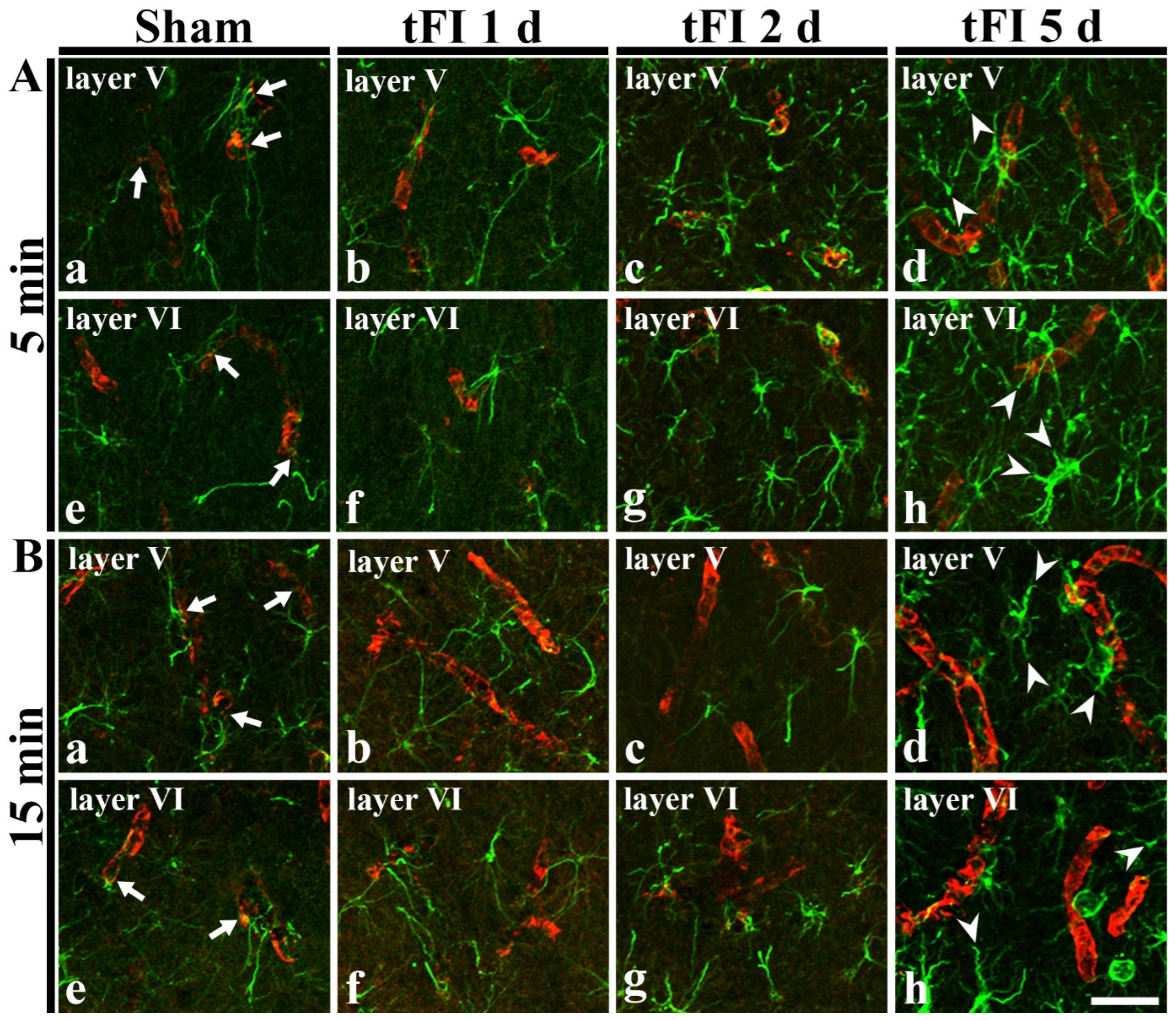
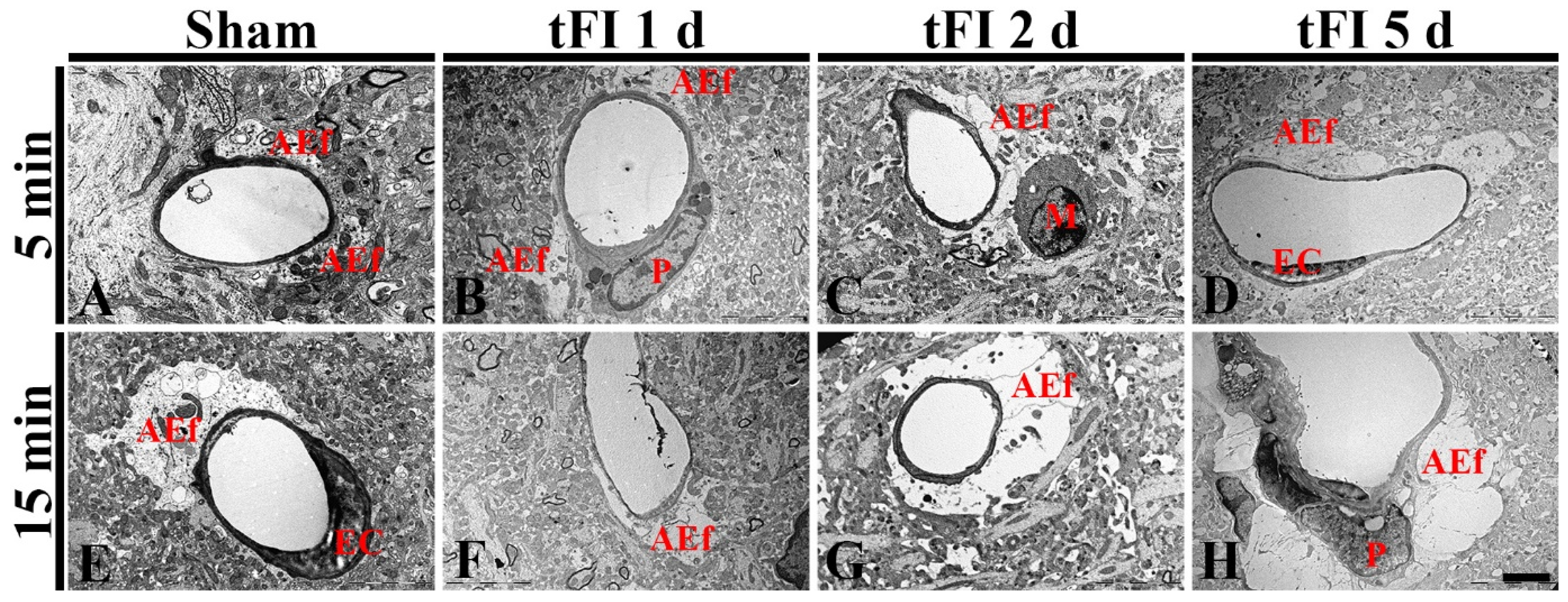
2.4. Damage to Astrocyte Endfoot
2.4.1. Double Immunofluorescence for GFAP and Glucose Transporter 1 (GLUT-1)
2.4.2. Ultrastructural Finding
3. Discussion
4. Materials and Methods
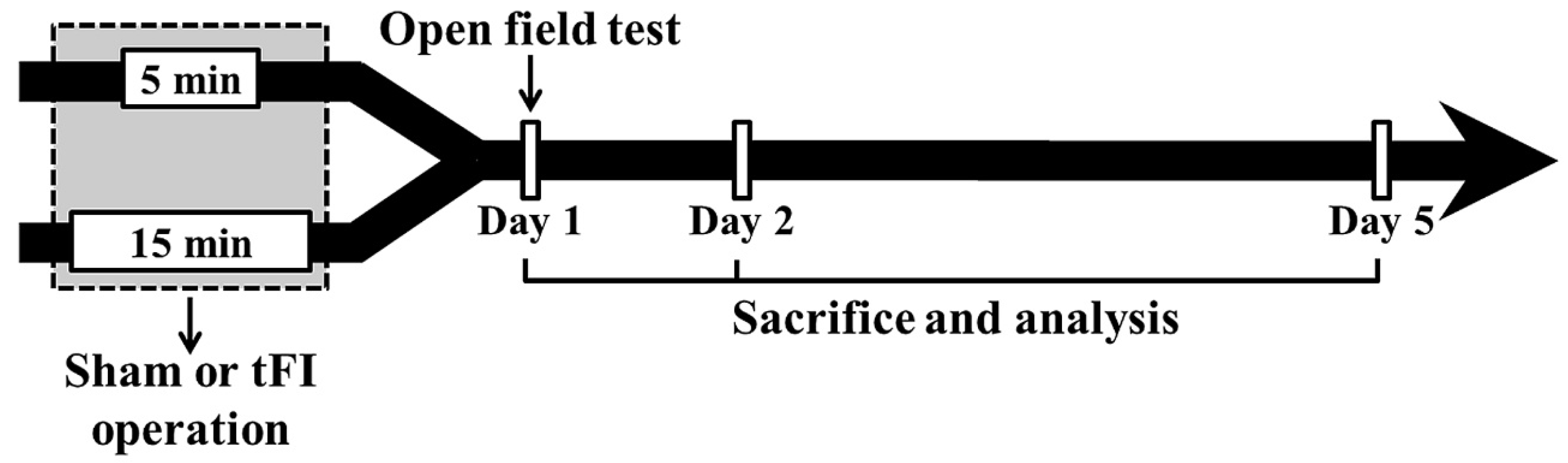
4.1. Animals
4.2. Induction of tFI
4.3. Open Field Test
4.4. Tissue Preparation for Histopathology
4.5. Stainings for Cell Damage/Death
4.5.1. CV Histochemistry
4.5.2. F-J B Histofluorescence
4.5.3. Immunohistochemistry for Astrocytes
4.6. Western Blotting of GFAP
4.7. Double Immunofluorescence for GFAP/GLUT-1
4.8. Ultrastructural Examination of Astrocyte Endfeet
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AEf | astrocyte endfoot |
| BBB | blood–brain barrier |
| CNS | central nervous system |
| F-J B | Fluoro-Jade B |
| GFAP | glial fibrillary acidic protein |
| GLUT-1 | glucose transporter 1 |
| IR | ischemia and reperfusion |
| ROD | relative optical density |
| SMA | spontaneous motor activity |
| tFI | transient forebrain ischemia |
| TEM | transmission electron microscope |
References
- Kondo, T.; Yoshida, S.; Nagai, H.; Takeshita, A.; Mino, M.; Morioka, H.; Nakajima, T.; Kusakabe, K.T.; Okada, T. Transient forebrain ischemia induces impairment in cognitive performance prior to extensive neuronal cell death in mongolian gerbil (meriones unguiculatus). J. Vet. Sci. 2018, 19, 505–511. [Google Scholar] [CrossRef]
- Lipton, P. Ischemic cell death in brain neurons. Physiol. Rev. 1999, 79, 1431–1568. [Google Scholar] [CrossRef]
- Radenovic, L.; Selakovic, V.; Olivan, S.; Calvo, A.C.; Rando, A.; Janac, B.; Osta, R. Neuroprotective efficiency of tetanus toxin c fragment in model of global cerebral ischemia in mongolian gerbils. Brain Res. Bull. 2014, 101, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.H.; Song, M.; Kim, H.; Lee, T.K.; Park, C.W.; Park, Y.E.; Lee, J.C.; Cho, J.H.; Kim, Y.M.; Hwang, I.K.; et al. Differential regional infarction, neuronal loss and gliosis in the gerbil cerebral hemisphere following 30 min of unilateral common carotid artery occlusion. Metab. Brain Dis. 2019, 34, 223–233. [Google Scholar] [CrossRef]
- de Araujo, F.L.; Bertolino, G.; Goncalves, R.B.; Marini Lde, C.; Coimbra, N.C.; de Araujo, J.E. Neuropathology and behavioral impairments after three types of global ischemia surgery in meriones unguiculatus: Evidence in motor cortex, hippocampal ca1 region and the neostriatum. J. Neurol. Sci. 2012, 312, 73–78. [Google Scholar] [CrossRef]
- Kirino, T.; Sano, K. Selective vulnerability in the gerbil hippocampus following transient ischemia. Acta Neuropathol. 1984, 62, 201–208. [Google Scholar] [CrossRef]
- Lee, J.C.; Ahn, J.H.; Lee, D.H.; Yan, B.C.; Park, J.H.; Kim, I.H.; Cho, G.S.; Kim, Y.M.; Lee, B.; Park, C.W.; et al. Neuronal damage and gliosis in the somatosensory cortex induced by various durations of transient cerebral ischemia in gerbils. Brain Res. 2013, 1510, 78–88. [Google Scholar] [CrossRef]
- Lee, T.K.; Lee, J.C.; Kim, J.D.; Kim, D.W.; Ahn, J.H.; Park, J.H.; Kim, H.I.; Cho, J.H.; Choi, S.Y.; Won, M.H.; et al. Populus tomentiglandulosa extract is rich in polyphenols and protects neurons, astrocytes, and the blood-brain barrier in gerbil striatum following ischemia-reperfusion injury. Molecules 2021, 26, 5430. [Google Scholar] [CrossRef]
- Kim, M.J.; Cho, J.H.; Cho, J.H.; Park, J.H.; Ahn, J.H.; Tae, H.J.; Cho, G.S.; Yan, B.C.; Hwang, I.K.; Lee, C.H.; et al. Impact of hyperthermia before and during ischemia-reperfusion on neuronal damage and gliosis in the gerbil hippocampus induced by transient cerebral ischemia. J. Neurol. Sci. 2015, 348, 101–110. [Google Scholar] [CrossRef]
- Liu, F.; McCullough, L.D. Interactions between age, sex, and hormones in experimental ischemic stroke. Neurochem. Int. 2012, 61, 1255–1265. [Google Scholar] [CrossRef] [Green Version]
- Manwani, B.; Liu, F.; Scranton, V.; Hammond, M.D.; Sansing, L.H.; McCullough, L.D. Differential effects of aging and sex on stroke induced inflammation across the lifespan. Exp. Neurol. 2013, 249, 120–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, B.; Friedman, B.; Cheng, Q.; Tsai, P.; Schim, E.; Kleinfeld, D.; Lyden, P.D. Severe blood-brain barrier disruption and surrounding tissue injury. Stroke 2009, 40, e666–e674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, T.K.; Kim, H.; Song, M.; Lee, J.C.; Park, J.H.; Ahn, J.H.; Yang, G.E.; Kim, H.; Ohk, T.G.; Shin, M.C.; et al. Time-course pattern of neuronal loss and gliosis in gerbil hippocampi following mild, severe, or lethal transient global cerebral ischemia. Neural. Regen. Res. 2019, 14, 1394–1403. [Google Scholar] [PubMed]
- An, H.; Lee, H.; Yang, S.; Won, W.; Lee, C.J.; Nam, M.H. Adenovirus-induced reactive astrogliosis exacerbates the pathology of parkinson’s disease. Exp. Neurobiol. 2021, 30, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.S.; Tsai, P.Y.; Yang, L.Y.; Lecca, D.; Luo, W.; Kim, D.S.; Hoffer, B.J.; Chiang, Y.H.; Greig, N.H.; Wang, J.Y. 3,6′-dithiopomalidomide ameliorates hippocampal neurodegeneration, microgliosis and astrogliosis and improves cognitive behaviors in rats with a moderate traumatic brain injury. Int. J. Mol. Sci. 2021, 22, 8276. [Google Scholar] [CrossRef] [PubMed]
- Pekny, M.; Pekna, M. Astrocyte reactivity and reactive astrogliosis: Costs and benefits. Physiol. Rev. 2014, 94, 1077–1098. [Google Scholar] [CrossRef]
- Sofroniew, M.V. Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci. 2009, 32, 638–647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ben Haim, L.; Rowitch, D.H. Functional diversity of astrocytes in neural circuit regulation. Nat. Rev. Neurosci. 2017, 18, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, N.B.; Attwell, D. Do astrocytes really exocytose neurotransmitters? Nat. Rev. Neurosci. 2010, 11, 227–238. [Google Scholar] [CrossRef]
- Sofroniew, M.V.; Vinters, H.V. Astrocytes: Biology and pathology. Acta Neuropathol. 2010, 119, 7–35. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Park, J.H.; Shin, M.C.; Cho, J.H.; Lee, T.K.; Kim, H.; Song, M.; Park, C.W.; Park, Y.E.; Lee, J.C.; et al. Fate of astrocytes in the gerbil hippocampus after transient global cerebral ischemia. Int. J. Mol. Sci. 2019, 20, 845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, D.; Smith, C.L.; Barone, F.C.; Ellison, J.A.; Lysko, P.G.; Li, K.; Simpson, I.A. Astrocytic demise precedes delayed neuronal death in focal ischemic rat brain. Brain Res. Mol. Brain Res. 1999, 68, 29–41. [Google Scholar] [CrossRef]
- Ouyang, Y.B.; Voloboueva, L.A.; Xu, L.J.; Giffard, R.G. Selective dysfunction of hippocampal ca1 astrocytes contributes to delayed neuronal damage after transient forebrain ischemia. J. Neurosci. 2007, 27, 4253–4260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Oliveira, J.L.; Crispin, P.; Duarte, E.C.; Marloch, G.D.; Gargioni, R.; Trentin, A.G.; Alvarez-Silva, M. Histopathology of motor cortex in an experimental focal ischemic stroke in mouse model. J. Chem. Neuroanat. 2014, 57–58, 1–9. [Google Scholar] [CrossRef]
- Kitabatake, T.T.; Marini, L.C.; Goncalves, R.B.; Bertolino, G.; de Souza, H.C.D.; de Araujo, J.E. Behavioral effects and neural changes induced by continuous and not continuous treadmill training, post bilateral cerebral ischemia in gerbils. Behav. Brain Res. 2015, 291, 20–25. [Google Scholar] [CrossRef]
- Bertolino, G.; De Araujo, F.L.; Souza, H.C.; Coimbra, N.C.; De Araujo, J.E. Neuropathology and behavioral impairments after bilateral global ischemia surgery and exposure to static magnetic field: Evidence in the motor cortex, the hippocampal ca1 region and the neostriatum. Int. J. Radiat. Biol. 2013, 89, 595–601. [Google Scholar] [CrossRef]
- Zhu, H.; Yoshimoto, T.; Imajo-Ohmi, S.; Dazortsava, M.; Mathivanan, A.; Yamashima, T. Why are hippocampal ca1 neurons vulnerable but motor cortex neurons resistant to transient ischemia? J. Neurochem. 2012, 120, 574–585. [Google Scholar] [CrossRef]
- Guo, H.; Zhu, L.; Tang, P.; Chen, D.; Li, Y.; Li, J.; Bao, C. Carthamin yellow improves cerebral ischemiareperfusion injury by attenuating inflammation and ferroptosis in rats. Int. J. Mol. Med. 2021, 47, 52. [Google Scholar] [CrossRef]
- Kumar, G.; Mukherjee, S.; Paliwal, P.; Singh, S.S.; Birla, H.; Singh, S.P.; Krishnamurthy, S.; Patnaik, R. Neuroprotective effect of chlorogenic acid in global cerebral ischemia-reperfusion rat model. Naunyn Schmiedebergs Arch. Pharmacol. 2019, 392, 1293–1309. [Google Scholar] [CrossRef]
- Thal, S.C.; Thal, S.E.; Plesnila, N. Characterization of a 3-vessel occlusion model for the induction of complete global cerebral ischemia in mice. J. Neurosci. Methods 2010, 192, 219–227. [Google Scholar] [CrossRef]
- Murakami, K.; Kondo, T.; Kawase, M.; Chan, P.H. The development of a new mouse model of global ischemia: Focus on the relationships between ischemia duration, anesthesia, cerebral vasculature, and neuronal injury following global ischemia in mice. Brain Res. 1998, 780, 304–310. [Google Scholar] [CrossRef]
- Shen, X.Y.; Gao, Z.K.; Han, Y.; Yuan, M.; Guo, Y.S.; Bi, X. Activation and role of astrocytes in ischemic stroke. Front. Cell Neurosci. 2021, 15, 755955. [Google Scholar] [CrossRef] [PubMed]
- Sofroniew, M.V. Astrogliosis. Cold Spring Harb. Perspect. Biol. 2014, 7, a020420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patabendige, A.; Singh, A.; Jenkins, S.; Sen, J.; Chen, R. Astrocyte activation in neurovascular damage and repair following ischaemic stroke. Int. J. Mol. Sci. 2021, 22, 4280. [Google Scholar] [CrossRef] [PubMed]
- Ito, U.; Hakamata, Y.; Kawakami, E.; Oyanagi, K. Degeneration of astrocytic processes and their mitochondria in cerebral cortical regions peripheral to the cortical infarction: Heterogeneity of their disintegration is closely associated with disseminated selective neuronal necrosis and maturation of injury. Stroke 2009, 40, 2173–2181. [Google Scholar]
- Barreto, G.E.; Sun, X.; Xu, L.; Giffard, R.G. Astrocyte proliferation following stroke in the mouse depends on distance from the infarct. PLoS ONE 2011, 6, e27881. [Google Scholar] [CrossRef] [Green Version]
- Shimada, I.S.; Borders, A.; Aronshtam, A.; Spees, J.L. Proliferating reactive astrocytes are regulated by notch-1 in the peri-infarct area after stroke. Stroke 2011, 42, 3231–3237. [Google Scholar] [CrossRef] [Green Version]
- Sims, N.R.; Yew, W.P. Reactive astrogliosis in stroke: Contributions of astrocytes to recovery of neurological function. Neurochem. Int. 2017, 107, 88–103. [Google Scholar] [CrossRef]
- Lin, C.Y.; Chang, C.; Cheung, W.M.; Lin, M.H.; Chen, J.J.; Hsu, C.Y.; Chen, J.H.; Lin, T.N. Dynamic changes in vascular permeability, cerebral blood volume, vascular density, and size after transient focal cerebral ischemia in rats: Evaluation with contrast-enhanced magnetic resonance imaging. J. Cereb. Blood Flow Metab. 2008, 28, 1491–1501. [Google Scholar] [CrossRef] [Green Version]
- Ma, F.; Sun, P.; Zhang, X.; Hamblin, M.H.; Yin, K.J. Endothelium-targeted deletion of the mir-15a/16-1 cluster ameliorates blood-brain barrier dysfunction in ischemic stroke. Sci. Signal. 2020, 13, 5686. [Google Scholar] [CrossRef]
- Lee, T.K.; Kang, I.J.; Sim, H.; Lee, J.C.; Ahn, J.H.; Kim, D.W.; Park, J.H.; Lee, C.H.; Kim, J.D.; Won, M.H.; et al. Therapeutic effects of decursin and angelica gigas nakai root extract in gerbil brain after transient ischemia via protecting bbb leakage and astrocyte endfeet damage. Molecules 2021, 26, 2126. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Leng, Y.; Tsai, L.K.; Leeds, P.; Chuang, D.M. Valproic acid attenuates blood-brain barrier disruption in a rat model of transient focal cerebral ischemia: The roles of hdac and mmp-9 inhibition. J. Cereb. Blood Flow Metab. 2011, 31, 52–57. [Google Scholar] [CrossRef]
- Zhang, H.; Park, J.H.; Maharjan, S.; Park, J.A.; Choi, K.S.; Park, H.; Jeong, Y.; Ahn, J.H.; Kim, I.H.; Lee, J.C.; et al. Sac-1004, a vascular leakage blocker, reduces cerebral ischemia-reperfusion injury by suppressing blood-brain barrier disruption and inflammation. J. Neuroinflam. 2017, 14, 122. [Google Scholar] [CrossRef] [PubMed]
- Seo, W.J.; Ahn, J.H.; Lee, T.K.; Kim, B.; Lee, J.C.; Park, J.H.; Yoo, Y.H.; Shin, M.C.; Cho, J.H.; Won, M.H.; et al. High fat diet accelerates and exacerbates microgliosis and neuronal damage/death in the somatosensory cortex after transient forebrain ischemia in gerbils. Lab. Anim. Res. 2020, 36, 28. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.K.; Hong, J.; Lee, J.W.; Kim, S.S.; Sim, H.; Lee, J.C.; Kim, D.W.; Lim, S.S.; Kang, I.J.; Won, M.H. Ischemia-induced cognitive impairment is improved via remyelination and restoration of synaptic density in the hippocampus after treatment with cog-up((r)) in a gerbil model of ischemic stroke. Vet. Sci. 2021, 8, 321. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.S.; Dhar, A.; Kaundal, R.K. Fetpps protects against global cerebral ischemic-reperfusion injury in gerbils. Pharmacol. Res. 2007, 55, 335–342. [Google Scholar] [CrossRef]
- Ahn, J.H.; Chen, B.H.; Park, J.H.; Shin, B.N.; Lee, T.K.; Cho, J.H.; Lee, J.C.; Park, J.R.; Yang, S.R.; Ryoo, S.; et al. Early iv-injected human dermis-derived mesenchymal stem cells after transient global cerebral ischemia do not pass through damaged blood-brain barrier. J. Tissue Eng. Regen. Med. 2018, 12, 1646–1657. [Google Scholar] [CrossRef]


| Primary Antibodies | Dilution | Suppliers |
|---|---|---|
| Mouse anti-glial fibrillary acidic protein (GFAP) | 1:800 | Merck-Millipore, Burlington, MA, USA |
| Rabbit anti-S100 protein (S100) | 1:1000 | Abcam, Cambridge, UK |
| Secondary Antibodies | Dilution | Suppliers |
| Biotinylated horse anti-mouse IgG | 1:250 | Vector Laboratories Inc., Burlingame, CA, USA |
| Biotinylated goat anti-rabbit IgG | 1:250 | Vector Laboratories Inc., Burlingame, CA, USA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, C.-H.; Lee, T.-K.; Kim, D.W.; Lim, S.S.; Kang, I.J.; Ahn, J.H.; Park, J.H.; Lee, J.-C.; Kim, C.-H.; Park, Y.; et al. Relationship between Neuronal Damage/Death and Astrogliosis in the Cerebral Motor Cortex of Gerbil Models of Mild and Severe Ischemia and Reperfusion Injury. Int. J. Mol. Sci. 2022, 23, 5096. https://doi.org/10.3390/ijms23095096
Lee C-H, Lee T-K, Kim DW, Lim SS, Kang IJ, Ahn JH, Park JH, Lee J-C, Kim C-H, Park Y, et al. Relationship between Neuronal Damage/Death and Astrogliosis in the Cerebral Motor Cortex of Gerbil Models of Mild and Severe Ischemia and Reperfusion Injury. International Journal of Molecular Sciences. 2022; 23(9):5096. https://doi.org/10.3390/ijms23095096
Chicago/Turabian StyleLee, Choong-Hyun, Tae-Kyeong Lee, Dae Won Kim, Soon Sung Lim, Il Jun Kang, Ji Hyeon Ahn, Joon Ha Park, Jae-Chul Lee, Choong-Hyo Kim, Yoonsoo Park, and et al. 2022. "Relationship between Neuronal Damage/Death and Astrogliosis in the Cerebral Motor Cortex of Gerbil Models of Mild and Severe Ischemia and Reperfusion Injury" International Journal of Molecular Sciences 23, no. 9: 5096. https://doi.org/10.3390/ijms23095096






