Strigolactones Modulate Salicylic Acid-Mediated Disease Resistance in Arabidopsis thaliana
Abstract
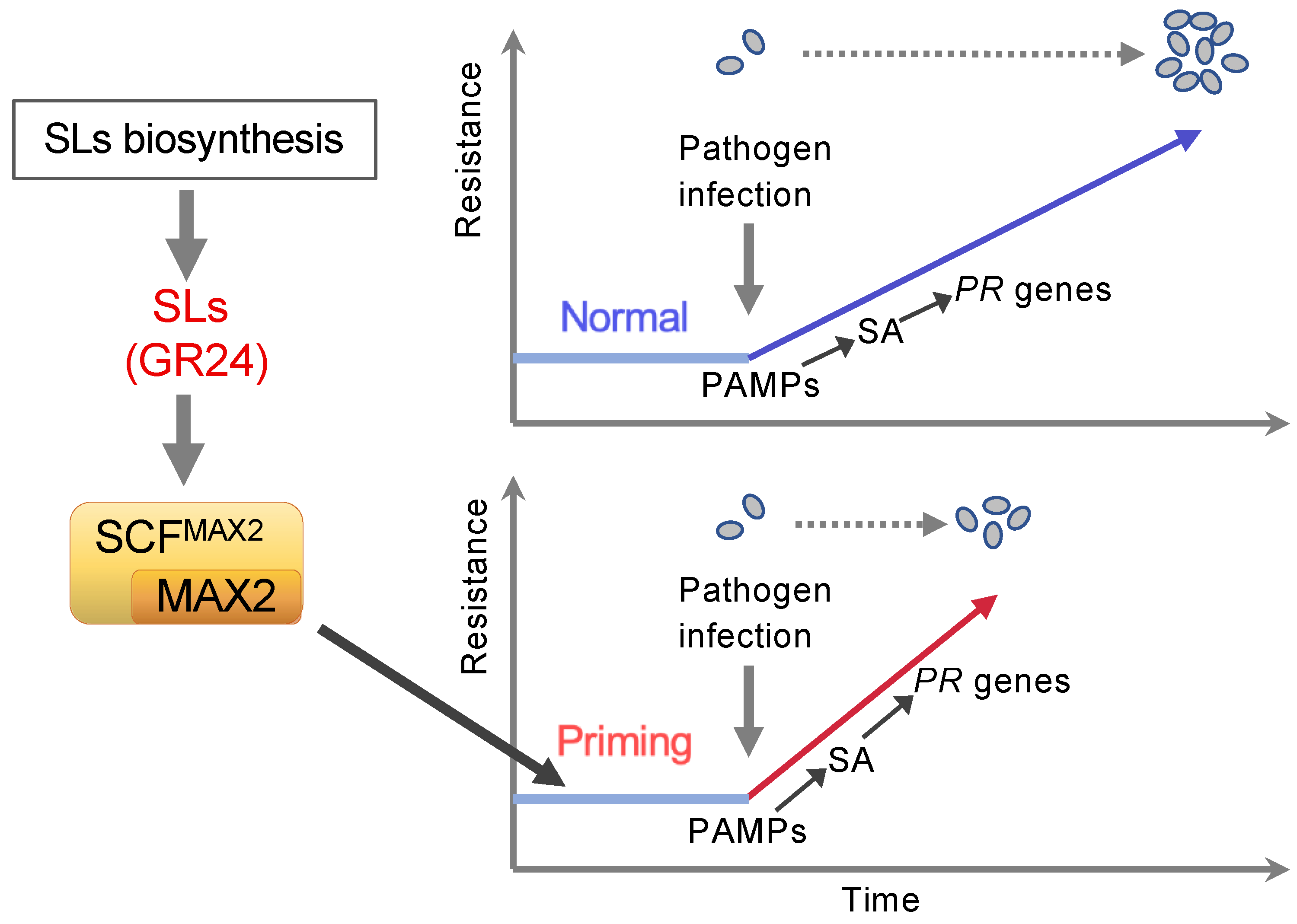
1. Introduction
2. Results
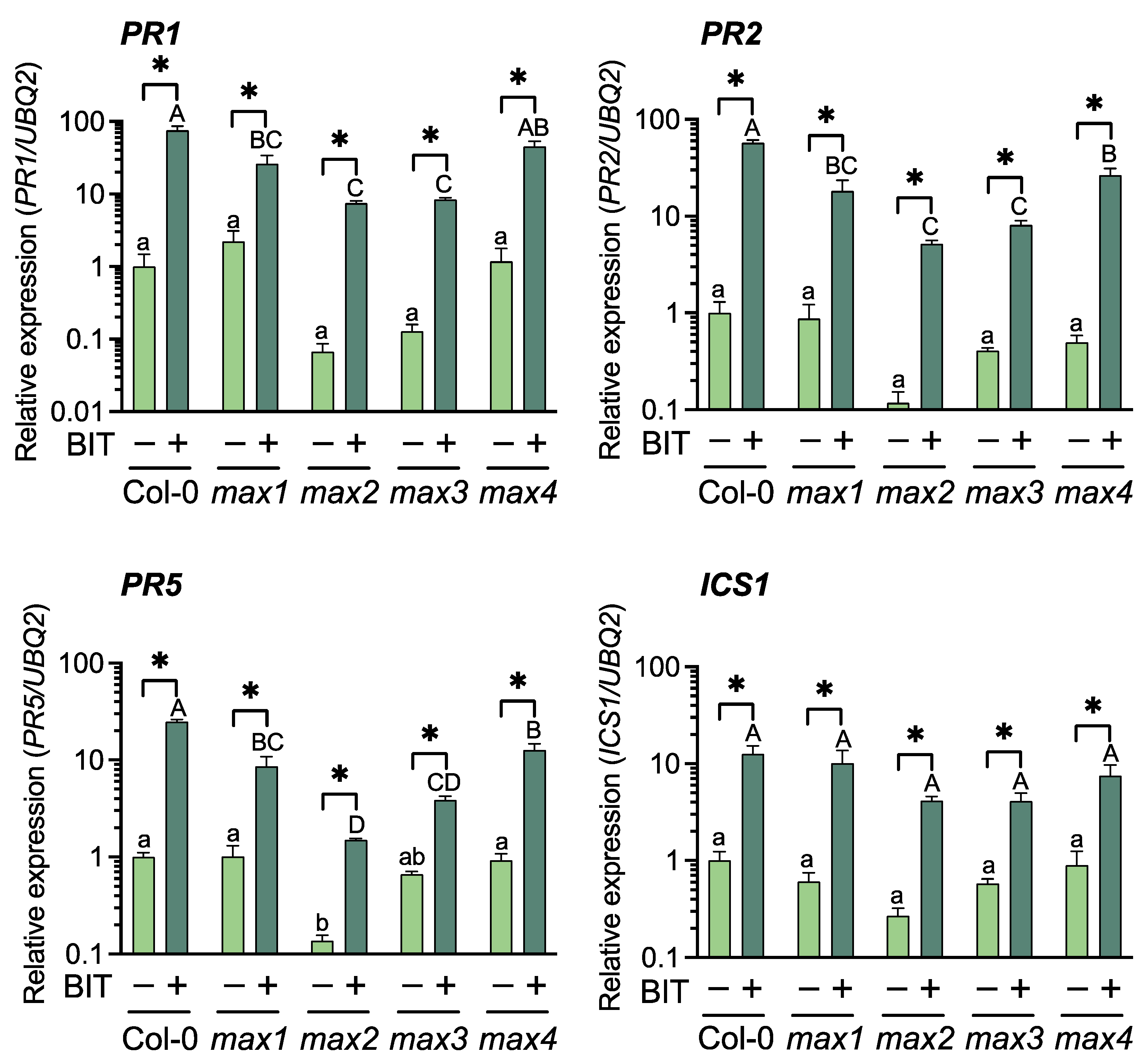
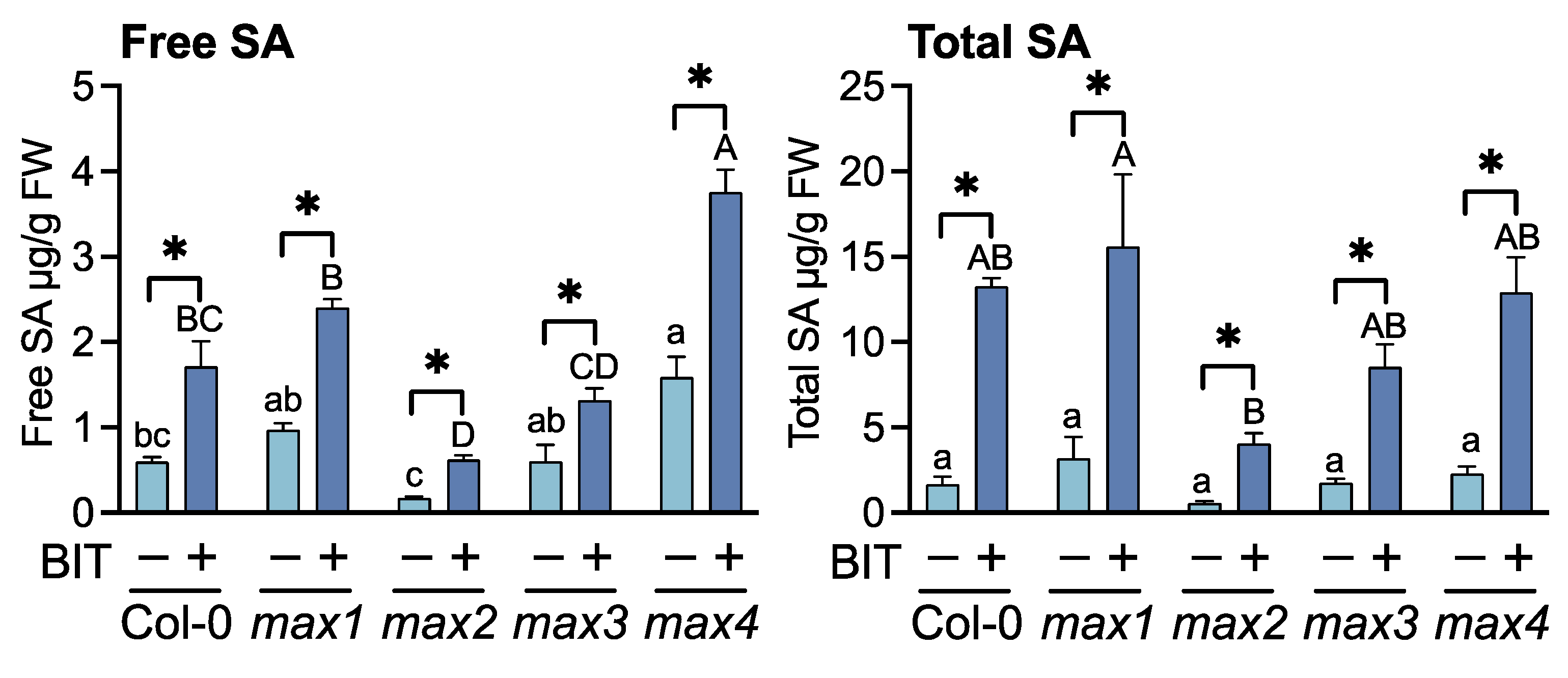
2.1. Relationship between SLs and SA Signaling Pathway in Arabidopsis
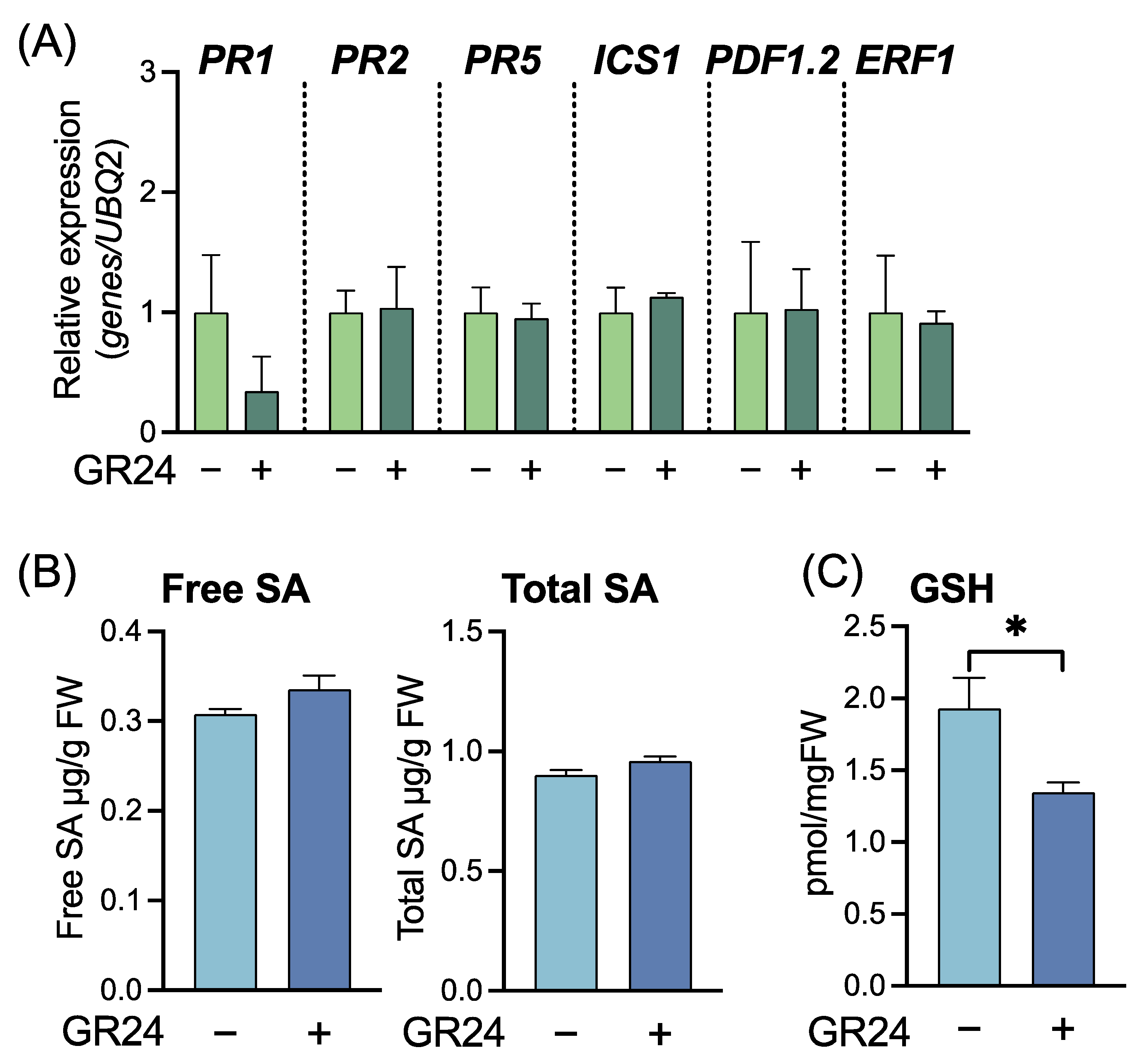
2.2. Effects of the Synthetic SL Analog GR24 on SA-Mediated Disease Resistance
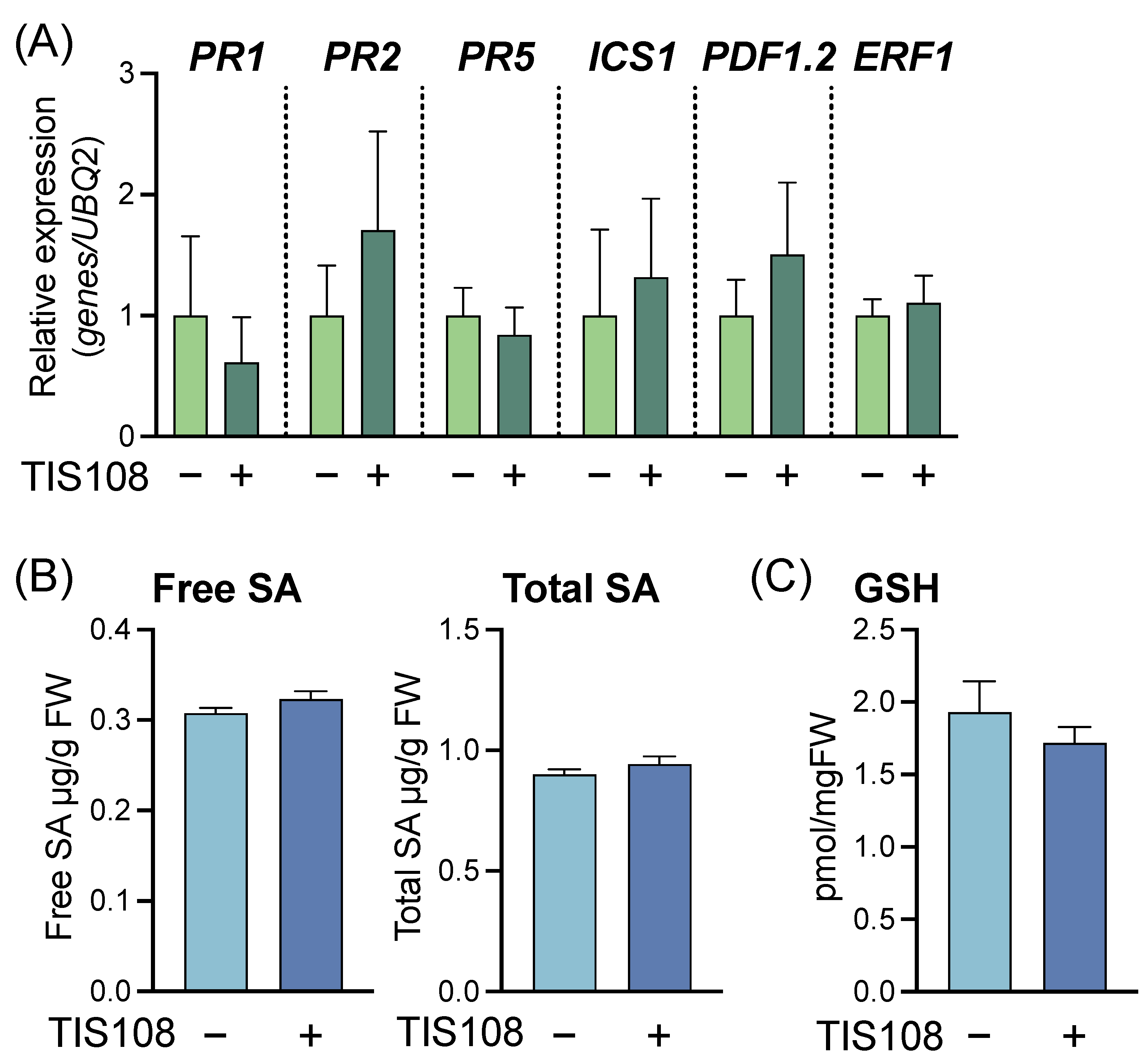
2.3. Effects of the SL Biosynthesis Inhibitor TIS108 on Disease Resistance
3. Discussion
4. Materials and Methods
4.1. Plant Material and Chemical Treatment
4.2. Pathogenicity Assays
4.3. Estimation of SA Levels
4.4. GSH Measurement
4.5. RT-qPCR
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, J.; Duan, G.; Li, C.; Liu, L.; Han, G.; Zhang, Y.; Wang, C. The Crosstalks between Jasmonic Acid and Other Plant Hormone Signaling Highlight the Involvement of Jasmonic Acid as a Core Component in Plant Response to Biotic and Abiotic Stresses. Front. Plant Sci. 2019, 10, 1349. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, M.; Ishikawa, A.; Jikumaru, Y.; Seki, M.; Umezawa, T.; Asami, T.; Maruyama-Nakashita, A.; Kudo, T.; Shinozaki, K.; Yoshida, S.; et al. Antagonistic Interaction between Systemic Acquired Resistance and the Abscisic Acid–mediated Abiotic Stress Response in Arabidopsis. Plant Cell 2008, 20, 1678–1692. [Google Scholar] [CrossRef] [PubMed]
- Kusajima, M.; Yasuda, M.; Kawashima, A.; Nojiri, H.; Yamane, H.; Nakajima, M.; Akutsu, K.; Nakashita, H. Suppressive Effect of Abscisic Acid on Systemic Acquired Resistance in Tobacco Plants. J. Gen. Plant Pathol. 2010, 76, 161–167. [Google Scholar] [CrossRef]
- Kusajima, M.; Okumura, Y.; Fujita, M.; Nakashita, H. Abscisic Acid Modulates Salicylic Acid Biosynthesis for Systemic Acquired Resistance in Tomato. Biosci. Biotechnol. Biochem. 2017, 81, 1850–1853. [Google Scholar] [CrossRef] [PubMed]
- Shigenaga, A.M.; Berens, M.L.; Tsuda, K.; Argueso, C.T. Towards Engineering of Hormonal Crosstalk in Plant Immunity. Curr. Opin. Plant Biol. 2017, 38, 164–172. [Google Scholar] [CrossRef]
- Lorenzo, O.; Piqueras, R.; Sánchez-Serrano, J.J.; Solano, R. ETHYLENE RESPONSE FACTOR1 Integrates Signals from Ethylene and Jasmonate Pathways in Plant Defense. Plant Cell 2003, 15, 165–178. [Google Scholar] [CrossRef]
- Schwessinger, B.; Zipfel, C. News from the Frontline: Recent Insights into PAMP-Triggered Immunity in Plants. Curr. Opin. Plant Biol. 2008, 11, 389–395. [Google Scholar] [CrossRef]
- Tripathi, D.; Raikhy, G.; Kumar, D. Chemical Elicitors of Systemic Acquired resistance—Salicylic Acid and its Functional Analogs. Curr. Plant Biol. 2019, 17, 48–59. [Google Scholar] [CrossRef]
- Wildermuth, M.C.; Dewdney, J.; Wu, G.; Ausubel, F.M. Isochorismate Synthase is Required to Synthesize Salicylic Acid for Plant Defence. Nature 2001, 414, 562–565. [Google Scholar] [CrossRef]
- Mou, Z.; Fan, W.; Dong, X. Inducers of Plant Systemic Acquired Resistance Regulate NPR1 Function through Redox Changes. Cell 2003, 113, 935–944. [Google Scholar] [CrossRef]
- Nakashita, H.; Yoshioka, K.; Yasuda, M.; Nitta, T.; Arai, Y.; Yoshida, S.; Yamaguchi, I. Probenazole Induces Systemic Acquired Resistance in Tobacco through Salicylic Acid Accumulation. Physiol. Mol. Plant Pathol. 2002, 61, 197–203. [Google Scholar] [CrossRef]
- Yoshioka, K.; Nakashita, H.; Klessig, D.F.; Yamaguchi, I. Probenazole Induces Systemic Acquired Resistance in Arabidopsis with a Novel Type of Action. Plant J. 2001, 25, 149–157. [Google Scholar] [CrossRef]
- Yasuda, M.; Nakashita, H.; Yoshida, S. Tiadinil, a Novel Class of Activator of Systemic Acquired Resistance, Induces Defense Gene Expression and Disease Resistance in Tobacco. J. Pest. Sci. 2004, 29, 46–49. [Google Scholar] [CrossRef]
- Umemura, K.; Satou, J.; Iwata, M.; Uozumi, N.; Koga, J.; Kawano, T.; Koshiba, T.; Anzai, H.; Mitomi, M. Contribution of Salicylic Acid Glucosyltransferase, OsSGT1, to Chemically Induced Disease Resistance in Rice Plants. Plant J. 2009, 57, 463–472. [Google Scholar] [CrossRef]
- Lawton, K.A.; Friedrich, L.; Hunt, M.; Weymann, K.; Delaney, T.; Kessmann, H.; Staub, T.; Ryals, J. Benzothiadiazole Induces Disease Resistance in Arabidopsis by Activation of the Systemic Acquired Resistance Signal Transduction Pathway. Plant J. Cell Mol. Biol. 1996, 10, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Nakashita, H. Studies on Regulation of Plant Physiology by Pesticides. J. Pest. Sci. 2021, 46, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.C.; Martinez-Medina, A.; Lopez-Raez, J.A.; Pozo, M.J. Mycorrhiza-Induced Resistance and Priming of Plant Defenses. J. Chem. Ecol. 2012, 38, 651–664. [Google Scholar] [CrossRef]
- Fujita, M.; Kusajima, M.; Fukagawa, M.; Okumura, Y.; Nakajima, M.; Akiyama, K.; Asami, T.; Yoneyama, K.; Kato, H.; Nakashita, H. Response of Tomatoes Primed by Mycorrhizal Colonization to Virulent and Avirulent Bacterial Pathogens. Sci. Rep. 2022, 12, 4686. [Google Scholar] [CrossRef]
- Pieterse, C.M.; Van Wees, S.C.; Hoffland, E.; Van Pelt, J.A.; Van Loon, L.C. Systemic Resistance in Arabidopsis Induced by Biocontrol Bacteria is Independent of Salicylic Acid Accumulation and Pathogenesis-Related Gene Expression. Plant Cell 1996, 8, 1225–1237. [Google Scholar]
- Fujita, M.; Kusajima, M.; Okumura, Y.; Nakajima, M.; Minamisawa, K.; Nakashita, H. Effects of Colonization of a Bacterial Endophyte, Azospirillum sp. B510, on Disease Resistance in Tomato. Biosci. Biotechnol. Biochem. 2017, 81, 1657–1662. [Google Scholar] [CrossRef]
- Buswell, W.; Schwarzenbacher, R.E.; Luna, E.; Sellwood, M.; Chen, B.; Flors, V.; Pétriacq, P.; Ton, J. Chemical Priming of Immunity without Costs to Plant Growth. New Phytol. 2018, 218, 1205–1216. [Google Scholar] [CrossRef] [PubMed]
- Cook, C.E.; Whichard, L.P.; Turner, B.; Wall, M.E.; Egley, G.H. Germination of Witchweed (Striga lutea Lour.): Isolation and Properties of a Potent Stimulant. Science 1966, 154, 1189–1190. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, K.; Matsuzaki, K.; Hayashi, H. Plant Sesquiterpenes Induce Hyphal Branching in Arbuscular Mycorrhizal Fungi. Nature 2005, 435, 824–827. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Roldan, V.; Fermas, S.; Brewer, P.B.; Puech-Pagès, V.; Dun, E.A.; Pillot, J.; Letisse, F.; Matusova, R.; Danoun, S.; Portais, J. Strigolactone Inhibition of Shoot Branching. Nature 2008, 455, 189–194. [Google Scholar] [CrossRef]
- Umehara, M.; Hanada, A.; Yoshida, S.; Akiyama, K.; Arite, T.; Takeda-Kamiya, N.; Magome, H.; Kamiya, Y.; Shirasu, K.; Yoneyama, K. Inhibition of Shoot Branching by New Terpenoid Plant Hormones. Nature 2008, 455, 195–200. [Google Scholar] [CrossRef]
- Matusova, R.; Rani, K.; Verstappen, F.W.; Franssen, M.C.; Beale, M.H.; Bouwmeester, H.J. The Strigolactone Germination Stimulants of the Plant-Parasitic Striga and Orobanche spp. are Derived from the Carotenoid Pathway. Plant Physiol. 2005, 139, 920–934. [Google Scholar] [CrossRef]
- Seto, Y.; Sado, A.; Asami, K.; Hanada, A.; Umehara, M.; Akiyama, K.; Yamaguchi, S. Carlactone is an Endogenous Biosynthetic Precursor for Strigolactones. Proc. Natl. Acad. Sci. USA 2014, 111, 1640–1645. [Google Scholar] [CrossRef]
- Booker, J.; Sieberer, T.; Wright, W.; Williamson, L.; Willett, B.; Stirnberg, P.; Turnbull, C.; Srinivasan, M.; Goddard, P.; Leyser, O. MAX1 Encodes a Cytochrome P450 Family Member that Acts Downstream of MAX3/4 to Produce a Carotenoid-Derived Branch-Inhibiting Hormone. Dev. Cell. 2005, 8, 443–449. [Google Scholar] [CrossRef]
- Stirnberg, P.; van De Sande, K.; Leyser, H.O. MAX1 and MAX2 Control Shoot Lateral Branching in Arabidopsis. Development 2002, 129, 1131–1141. [Google Scholar] [CrossRef]
- De Cuyper, C.; Struk, S.; Braem, L.; Gevaert, K.; De Jaeger, G.; Goormachtig, S. Strigolactones, Karrikins and Beyond. Plant Cell Environ. 2017, 40, 1691–1703. [Google Scholar] [CrossRef]
- Ito, S.; Umehara, M.; Hanada, A.; Yamaguchi, S.; Asami, T. Effects of Strigolactone-Biosynthesis Inhibitor TIS108 on Arabidopsis. Plant Signal. Behav. 2013, 8, e24193. [Google Scholar] [CrossRef] [PubMed]
- Mostofa, M.G.; Li, W.; Nguyen, K.H.; Fujita, M.; Tran, L.P. Strigolactones in Plant Adaptation to Abiotic Stresses: An Emerging Avenue of Plant Research. Plant Cell Environ. 2018, 41, 2227–2243. [Google Scholar] [CrossRef] [PubMed]
- Shirani Bidabadi, S.; Sharifi, P. Strigolactone and Methyl Jasmonate-Induced Antioxidant Defense and the Composition Alterations of Different Active Compounds in Dracocephalum Kotschyi Boiss Under Drought Stress. J. Plant Growth Regul. 2021, 40, 878–889. [Google Scholar] [CrossRef]
- Sharifi, P.; Bidabadi, S.S. Strigolactone could Enhances Gas-Exchange through Augmented Antioxidant Defense System in Salvia nemorosa, L. Plants Subjected to Saline Conditions Stress. Ind. Crops Prod. 2020, 151, 112460. [Google Scholar] [CrossRef]
- Cooper, J.W.; Hu, Y.; Beyyoudh, L.; Yildiz Dasgan, H.; Kunert, K.; Beveridge, C.A.; Foyer, C.H. Strigolactones Positively Regulate Chilling Tolerance in Pea and in Arabidopsis. Plant Cell Environ. 2018, 41, 1298–1310. [Google Scholar] [CrossRef]
- Van Ha, C.; Leyva-González, M.A.; Osakabe, Y.; Tran, U.T.; Nishiyama, R.; Watanabe, Y.; Tanaka, M.; Seki, M.; Yamaguchi, S.; Van Dong, N. Positive Regulatory Role of Strigolactone in Plant Responses to Drought and Salt Stress. Proc. Natl. Acad. Sci. USA 2014, 111, 851–856. [Google Scholar]
- Bu, Q.; Lv, T.; Shen, H.; Luong, P.; Wang, J.; Wang, Z.; Huang, Z.; Xiao, L.; Engineer, C.; Kim, T.H. Regulation of Drought Tolerance by the F-Box Protein MAX2 in Arabidopsis. Plant Physiol. 2014, 164, 424–439. [Google Scholar] [CrossRef]
- Torres-Vera, R.; García, J.M.; Pozo, M.J.; López-Ráez, J.A. Do Strigolactones Contribute to Plant Defence? Mol. Plant Pathol. 2014, 15, 211–216. [Google Scholar] [CrossRef]
- Nasir, F.; Tian, L.; Shi, S.; Chang, C.; Ma, L.; Gao, Y.; Tian, C. Strigolactones Positively Regulate Defense against Magnaporthe oryzae in Rice (Oryza sativa). Plant Physiol. Biochem. 2019, 142, 106–116. [Google Scholar] [CrossRef]
- Kalliola, M.; Jakobson, L.; Davidsson, P.; Pennanen, V.; Waszczak, C.; Yarmolinsky, D.; Zamora, O.; Palva, E.T.; Kariola, T.; Kollist, H. Differential Role of MAX2 and Strigolactones in Pathogen, Ozone, and Stomatal Responses. Plant Dir. 2020, 4, e00206. [Google Scholar] [CrossRef]
- Piisilä, M.; Keceli, M.A.; Brader, G.; Jakobson, L.; Jõesaar, I.; Sipari, N.; Kollist, H.; Palva, E.T.; Kariola, T. The F-Box Protein MAX2 Contributes to Resistance to Bacterial Phytopathogens in Arabidopsis thaliana. BMC Plant Biol. 2015, 15, 53. [Google Scholar] [CrossRef] [PubMed]
- Katagiri, F.; Thilmony, R.; He, S.Y. The Arabidopsis thaliana-Pseudomonas syringae Interaction. Am. Soc. Plant Biol. 2002, 1, e0039. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, Y.; Ali, A.M.; Yabuta, S.; Kinoshita, H.; Inanaga, S.; Itai, A. Germination Strategy of Striga hermonthica Involves Regulation of Ethylene Biosynthesis. Physiol. Plant. 2003, 119, 137–145. [Google Scholar] [CrossRef]
- Lee, H.Y.; Yoon, G.M. Strigolactone Elevates Ethylene Biosynthesis in Etiolated Arabidopsis Seedlings. Plant Signal. Behav. 2020, 15, 1805232. [Google Scholar] [CrossRef]
- Kapulnik, Y.; Resnick, N.; Mayzlish-Gati, E.; Kaplan, Y.; Wininger, S.; Hershenhorn, J.; Koltai, H. Strigolactones Interact with Ethylene and Auxin in Regulating Root-Hair Elongation in Arabidopsis. J. Exp. Bot. 2011, 62, 2915–2924. [Google Scholar] [CrossRef]
- De Vos, M.; Van Zaanen, W.; Koornneef, A.; Korzelius, J.P.; Dicke, M.; Van Loon, L.C.; Pieterse, C.M. Herbivore-Induced Resistance Against Microbial Pathogens in Arabidopsis. Plant Physiol. 2006, 142, 352–363. [Google Scholar] [CrossRef]
- Chen, H.; Xue, L.; Chintamanani, S.; Germain, H.; Lin, H.; Cui, H.; Cai, R.; Zuo, J.; Tang, X.; Li, X. ETHYLENE INSENSITIVE3 and ETHYLENE INSENSITIVE3-LIKE1 Repress SALICYLIC ACID INDUCTION DEFICIENT2 Expression to Negatively Regulate Plant Innate Immunity in Arabidopsis. Plant Cell 2009, 21, 2527–2540. [Google Scholar] [CrossRef]
- Campos-Soriano, L.; Bundó, M.; Bach-Pages, M.; Chiang, S.; Chiou, T.; San Segundo, B. Phosphate Excess Increases Susceptibility to Pathogen Infection in Rice. Mol. Plant Pathol. 2020, 21, 555–570. [Google Scholar] [CrossRef]
- Kusajima, M.; Fujita, M.; Yamakawa, H.; Ushiwatari, T.; Mori, T.; Tsukamoto, K.; Hayashi, H.; Maruyama-Nakashita, A.; Che, F.; Nakashita, H. Characterization of Plant Immunity-Activating Mechanism by a Pyrazole Derivative. Biosci. Biotechnol. Biochem. 2020, 84, 1427–1435. [Google Scholar] [CrossRef]
- Morikawa-Ichinose, T.; Kim, S.; Allahham, A.; Kawaguchi, R.; Maruyama-Nakashita, A. Glucosinolate Distribution in the Aerial Parts of Sel1-10, a Disruption Mutant of the Sulfate Transporter SULTR1; 2, in Mature Arabidopsis thaliana Plants. Plants 2019, 8, 95. [Google Scholar] [CrossRef]
- Maruyama-Nakashita, A.; Nakamura, Y.; Yamaya, T.; Takahashi, H. A Novel Regulatory Pathway of Sulfate Uptake in Arabidopsis Roots: Implication of CRE1/WOL/AHK4-mediated Cytokinin-dependent Regulation. Plant J. 2004, 38, 779–789. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kusajima, M.; Fujita, M.; Soudthedlath, K.; Nakamura, H.; Yoneyama, K.; Nomura, T.; Akiyama, K.; Maruyama-Nakashita, A.; Asami, T.; Nakashita, H. Strigolactones Modulate Salicylic Acid-Mediated Disease Resistance in Arabidopsis thaliana. Int. J. Mol. Sci. 2022, 23, 5246. https://doi.org/10.3390/ijms23095246
Kusajima M, Fujita M, Soudthedlath K, Nakamura H, Yoneyama K, Nomura T, Akiyama K, Maruyama-Nakashita A, Asami T, Nakashita H. Strigolactones Modulate Salicylic Acid-Mediated Disease Resistance in Arabidopsis thaliana. International Journal of Molecular Sciences. 2022; 23(9):5246. https://doi.org/10.3390/ijms23095246
Chicago/Turabian StyleKusajima, Miyuki, Moeka Fujita, Khamsalath Soudthedlath, Hidemitsu Nakamura, Koichi Yoneyama, Takahito Nomura, Kohki Akiyama, Akiko Maruyama-Nakashita, Tadao Asami, and Hideo Nakashita. 2022. "Strigolactones Modulate Salicylic Acid-Mediated Disease Resistance in Arabidopsis thaliana" International Journal of Molecular Sciences 23, no. 9: 5246. https://doi.org/10.3390/ijms23095246
APA StyleKusajima, M., Fujita, M., Soudthedlath, K., Nakamura, H., Yoneyama, K., Nomura, T., Akiyama, K., Maruyama-Nakashita, A., Asami, T., & Nakashita, H. (2022). Strigolactones Modulate Salicylic Acid-Mediated Disease Resistance in Arabidopsis thaliana. International Journal of Molecular Sciences, 23(9), 5246. https://doi.org/10.3390/ijms23095246





