Dose Rate Effects on the Selective Radiosensitization of Prostate Cells by GRPR-Targeted Gold Nanoparticles
Abstract
:1. Introduction
2. Results
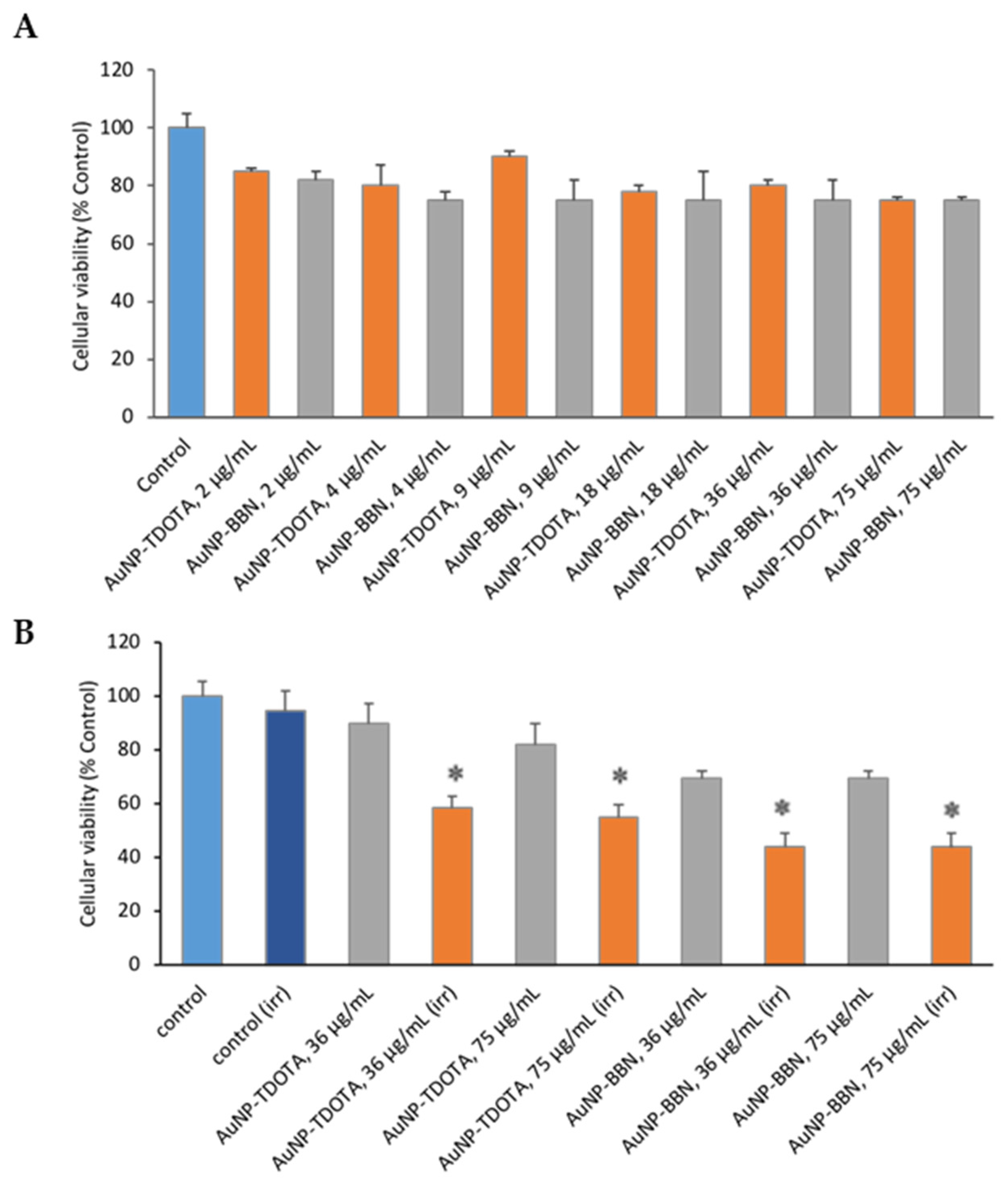
2.1. Cytotoxicity and Cellular Uptake Studies for GRPR-Targeted and Non-Targeted AuNPs: Selection of AuNP Concentration and Irradiation Dose
2.2. Irradiation Studies: Tumoral versus Non-Tumoral Cells and Dose Rate Effect
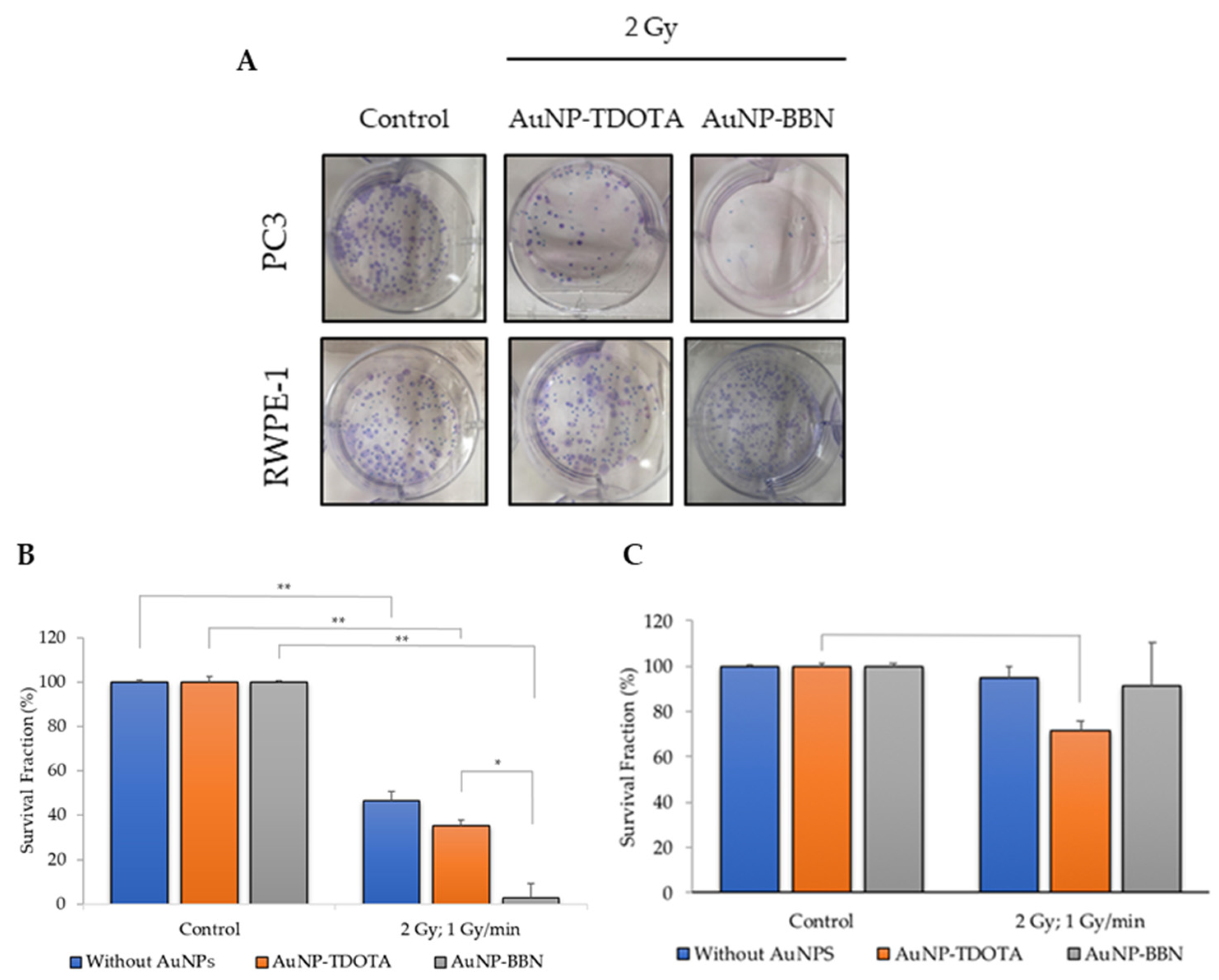
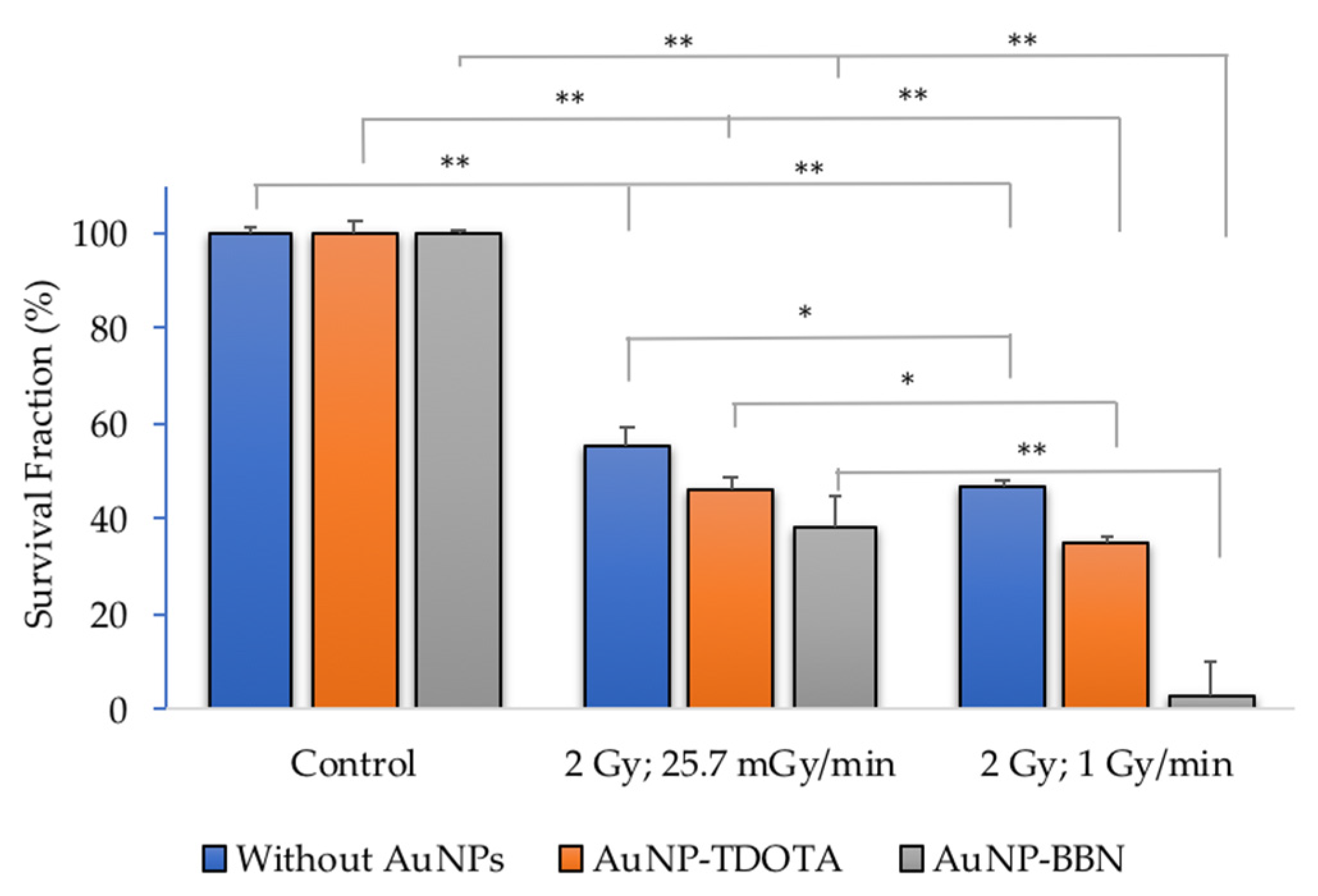
2.2.1. Cellular Survival—Colony Formation
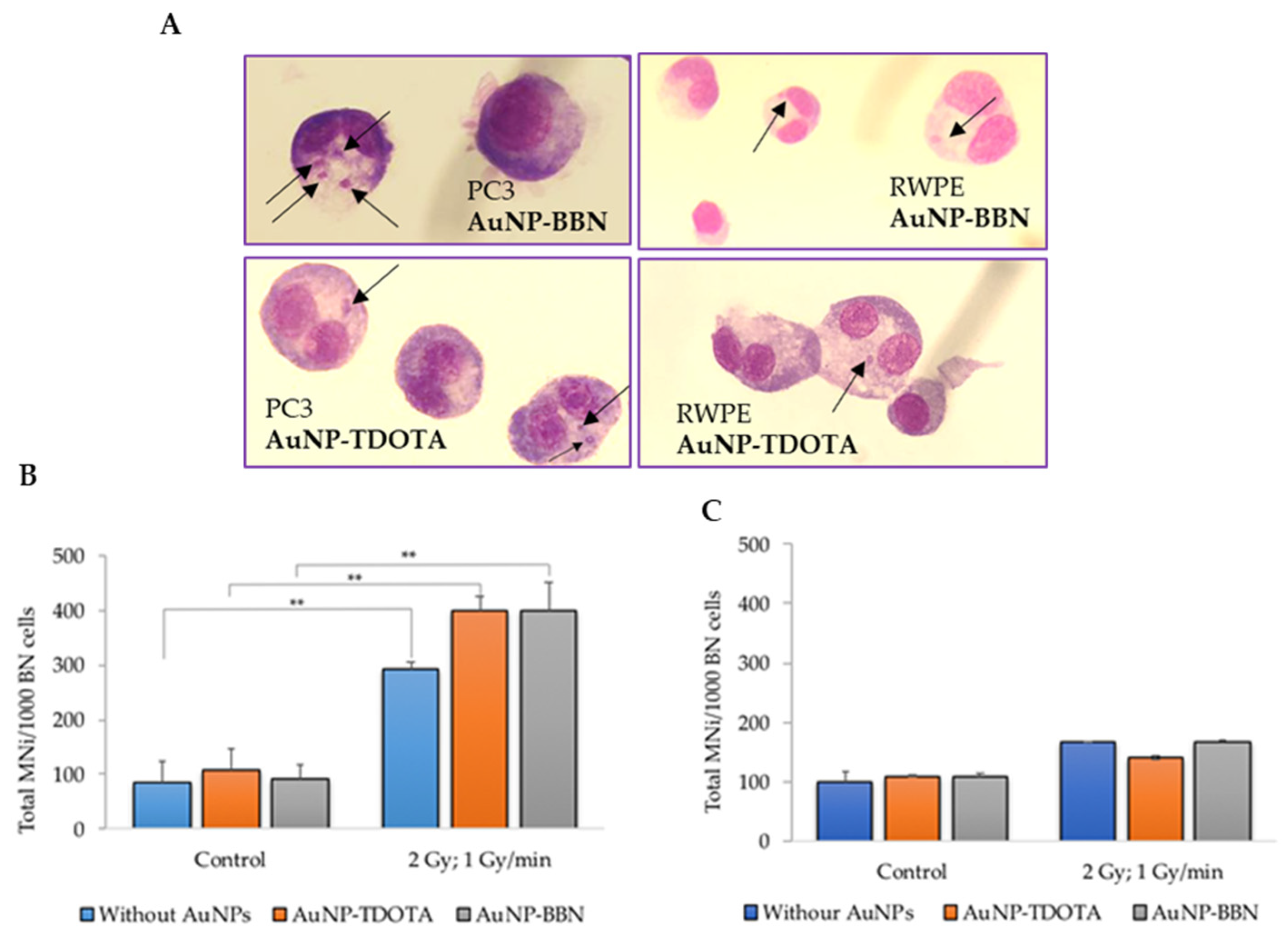
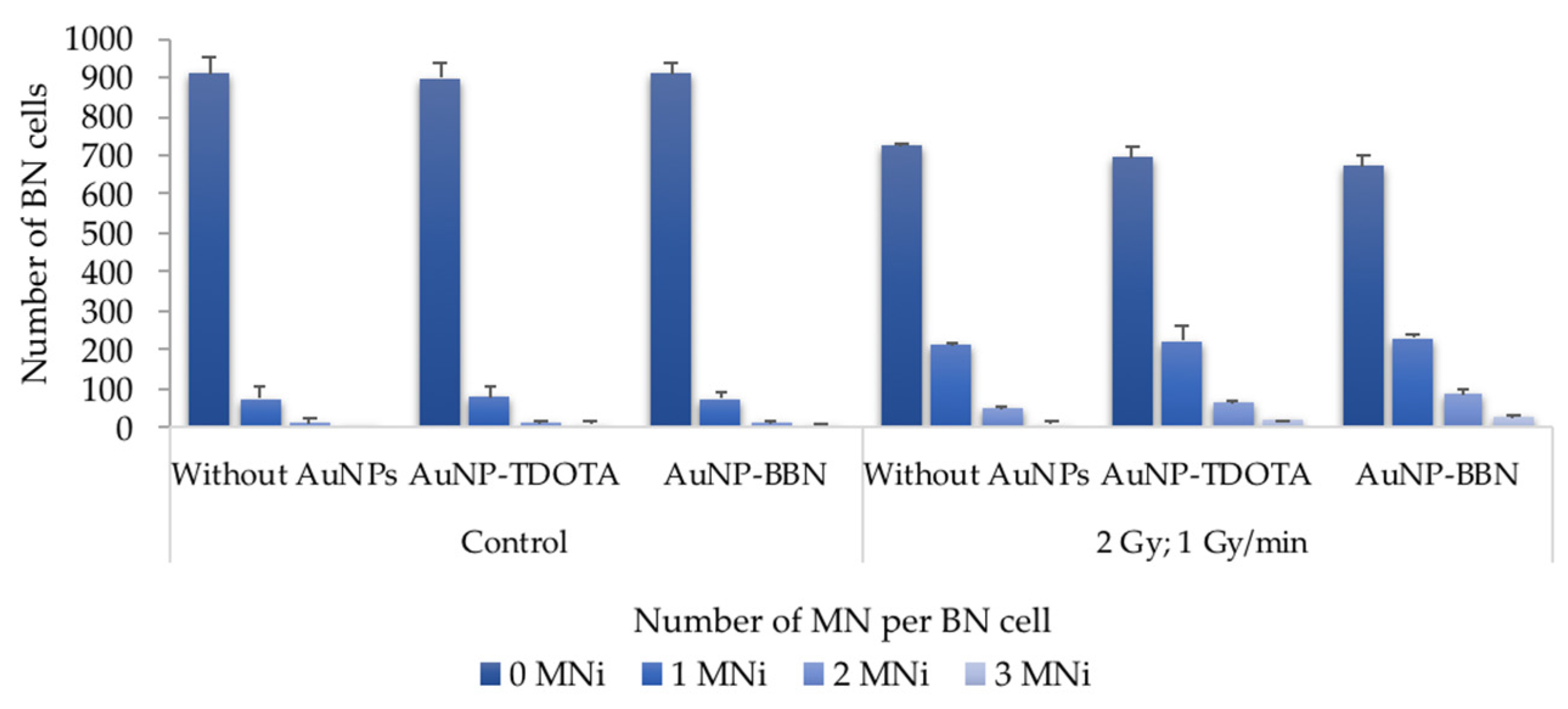
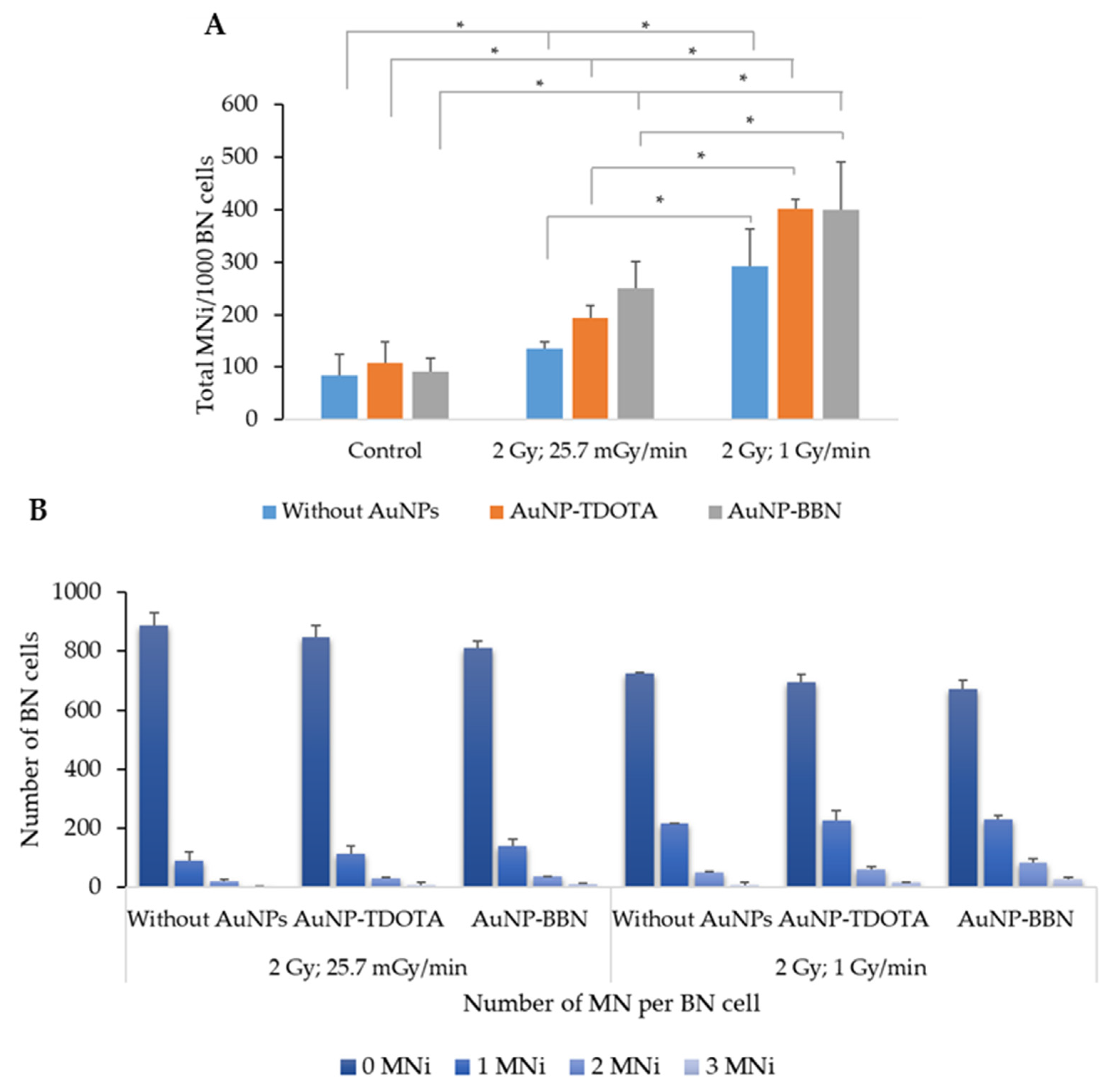
2.2.2. Induction of Genomic Instability
2.2.3. Generation of Reactive Oxygen Species (ROSs)
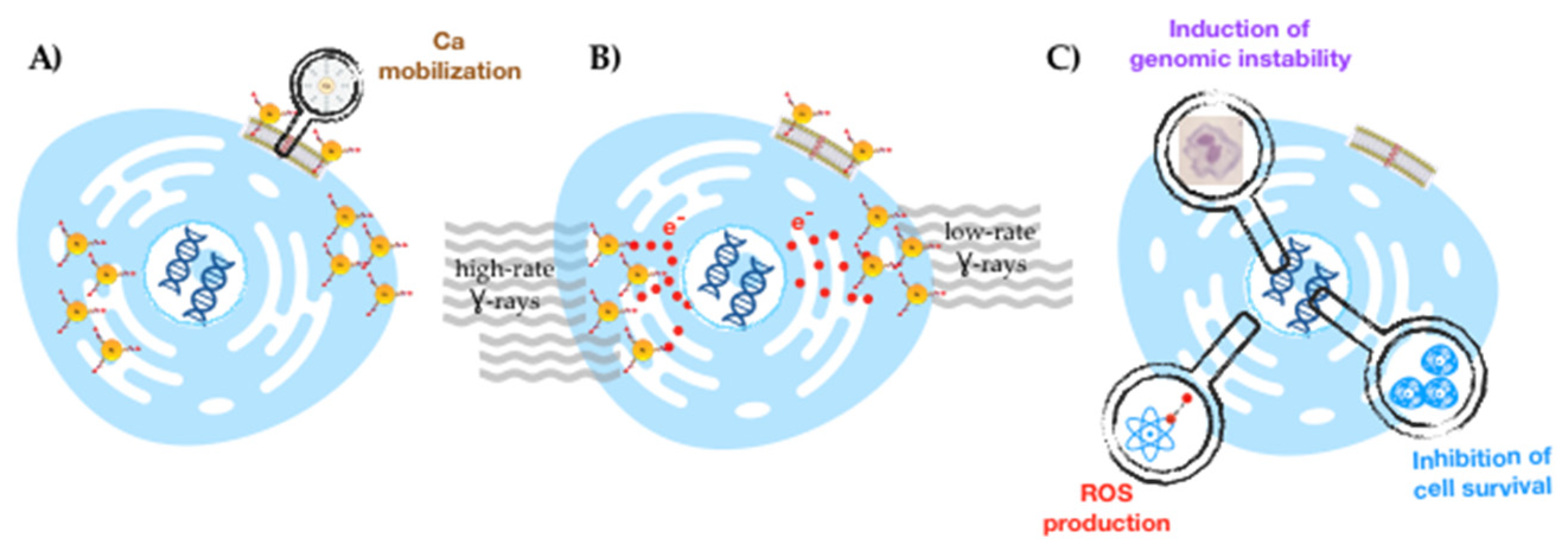
2.2.4. Dose Rate Effect
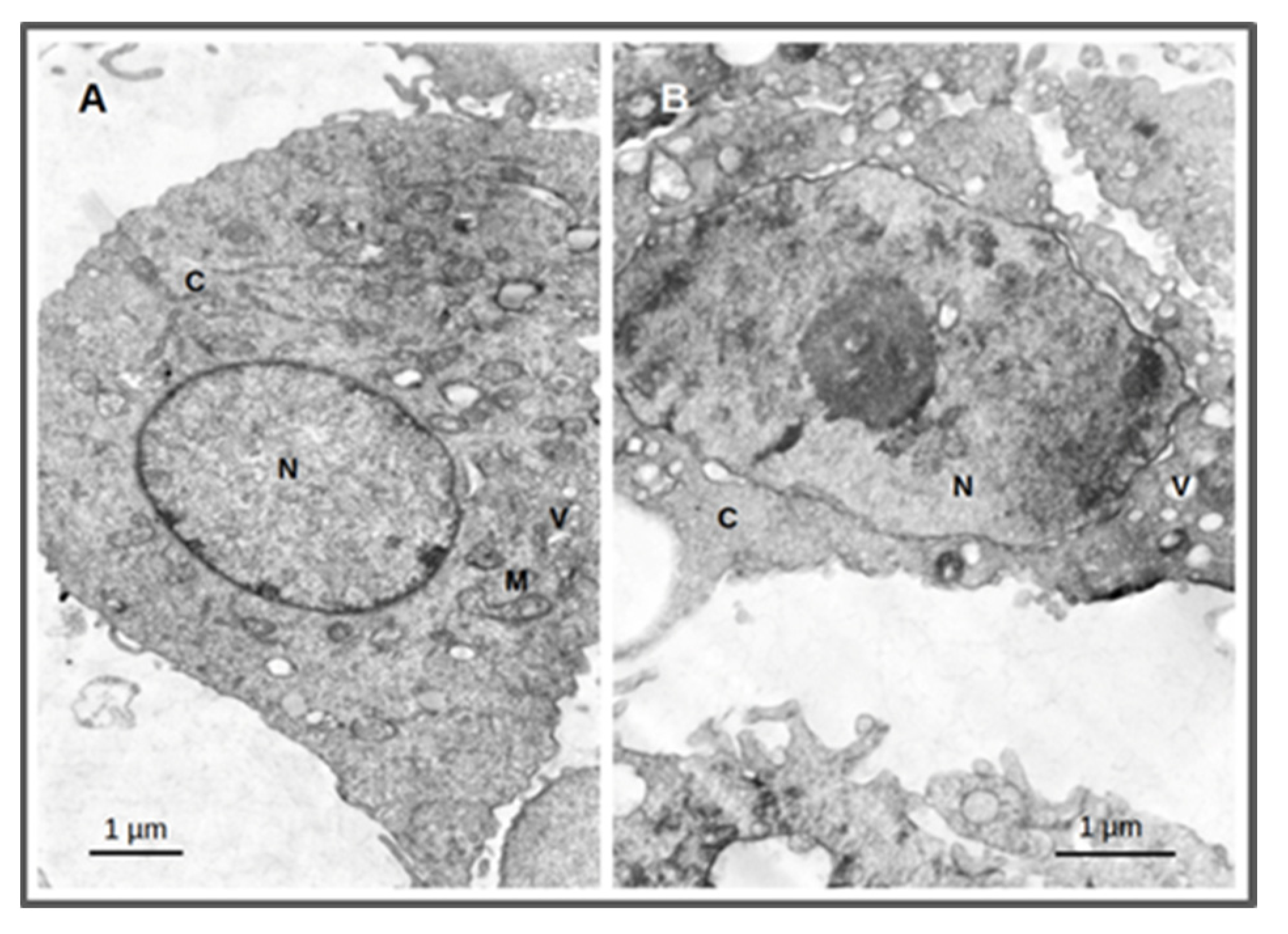
2.3. Cellular Morphological Alterations after Irradiation
3. Discussion
4. Materials and Methods
4.1. Synthesis and Dissolution of AuNPs
4.2. Irradiation Experiments
4.3. Cell Culture
4.4. MTT Assay
4.5. Cellular Uptake—PIXE
4.6. Cellular Proliferation and Colony Formation Assay
4.7. Cytokinesis-Blocked Micronucleus (CBMN) Assay
4.8. ROS Production
4.8.1. Superoxide Radical Production (NBT Assay)
4.8.2. Peroxide Production
4.8.3. Cellular Morphological Alterations by TEM
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Barton, M.; Jacob, S.; Shafiq, J.; Wong, K.; Thompson, S.R.; Hanna, T.; Delaney, G. Estimating the demand for radiotherapy from the evidence: A review of changes from 2003 to 2012. Radiother. Oncol. 2014, 112, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Baskar, R.; Lee, K.A.; Yeo, R.; Yeoh, K.-W. Cancer and Radiation Therapy: Current Advances and Future Directions. Int. J. Med. Sci. 2012, 9, 193–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gong, L.; Zhang, Y.; Liu, C.; Zhang, M.; Han, S. Application of Radiosensitizers in Cancer Radiotherapy. Int. J. Nanomed. 2021, 16, 1083–1102. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Gao, P.; Wu, T.; Pan, W.; Li, N.; Tang, B. Organelle-localized radiosensitizers. Chem. Commun. 2020, 56, 10621–10630. [Google Scholar] [CrossRef]
- Hainfeld, J.F.; Lin, L.; Slatkin, D.N.; Dilmanian, F.A.; Vadas, T.M.; Smilowitz, H.M. Gold nanoparticle hyperthermia reduces radiotherapy dose. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 1609–1617. [Google Scholar] [CrossRef] [Green Version]
- Rashid, R.A.; Abidin, S.Z.; Anuar, M.A.K.; Tominaga, T.; Akasaka, H.; Sasaki, R.; Kie, K.; Razak, K.A.; Pham, B.T.; Hawkett, B.; et al. Radiosensitization effects and ROS generation by high Z metallic nanoparticles on human colon carcinoma cell (HCT116) irradiated under 150 MeV proton beam. OpenNano 2019, 4, 100027. [Google Scholar] [CrossRef]
- Habiba, K.; Aziz, K.; Sanders, K.; Santiago, C.M.; Mahadevan, L.S.K.; Makarov, V.; Weiner, B.R.; Morell, G.; Krishnan, S. Enhancing Colorectal Cancer Radiation Therapy Efficacy using Silver Nanoprisms Decorated with Graphene as Radiosensitizers. Sci. Rep. 2019, 9, 17120. [Google Scholar] [CrossRef]
- Laprise-Pelletier, M.; Simão, T.; Fortin, M.-A. Gold Nanoparticles in Radiotherapy and Recent Progress in Nanobrachytherapy. Adv. Health Mater. 2018, 7, e1701460. [Google Scholar] [CrossRef]
- Alhussan, A.; Bozdoğan, E.; Chithrani, D. Combining Gold Nanoparticles with Other Radiosensitizing Agents for Unlocking the Full Potential of Cancer Radiotherapy. Pharmaceutics 2021, 13, 442. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, J.; Fu, S.; Wu, J. Gold Nanoparticles as Radiosensitizers in Cancer Radiotherapy. Int. J. Nanomed. 2020, 15, 9407–9430. [Google Scholar] [CrossRef]
- Bromma, K.; Cicon, L.; Beckham, W.; Chithrani, D.B. Gold nanoparticle mediated radiation response among key cell components of the tumour microenvironment for the advancement of cancer nanotechnology. Sci. Rep. 2020, 10, 12096. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Jung, K.O.; Graves, E.E.; Pratx, G. A gold nanoparticle system for the enhancement of radiotherapy and simultaneous monitoring of reactive-oxygen-species formation. Nanotechnology 2018, 29, 504001. [Google Scholar] [CrossRef] [PubMed]
- Schuemann, J.; Bagley, A.F.; Berbeco, R.; Bromma, K.; Butterworth, K.T.; Byrne, H.L.; Chithrani, B.D.; Cho, S.H.; Cook, J.R.; Favaudon, V.; et al. Roadmap for metal nanoparticles in radiation therapy: Current status, translational challenges, and future directions. Phys. Med. Biol. 2020, 65, 21RM02. [Google Scholar] [CrossRef]
- Penninckx, S.; Heuskin, A.-C.; Michiels, C.; Lucas, S. Gold Nanoparticles as a Potent Radiosensitizer: A Transdisciplinary Approach from Physics to Patient. Cancers 2020, 12, 2021. [Google Scholar] [CrossRef] [PubMed]
- Rosa, S.; Connolly, C.; Schettino, G.; Butterworth, K.T.; Prise, K.M. Biological mechanisms of gold nanoparticle radiosensitization. Cancer Nanotechnol. 2017, 8, 2. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Huang, F.; Ren, C.; Liu, J.; Yang, L.; Chen, S.; Chang, J.; Yang, C.; Wang, W.; Zhang, C.; et al. Enhanced Radiosensitization by Gold Nanoparticles with Acid-Triggered Aggregation in Cancer Radiotherapy. Adv. Sci. 2019, 6, 1801806. [Google Scholar] [CrossRef]
- Shi, Y.; Van Der Meel, R.; Chen, X.; Lammers, T. The EPR effect and beyond: Strategies to improve tumor targeting and cancer nanomedicine treatment efficacy. Theranostics 2020, 10, 7921–7924. [Google Scholar] [CrossRef]
- Popovtzer, A.; Mizrachi, A.; Motiei, M.; Bragilovski, D.; Lubimov, L.; Levi, M.; Hilly, O.; Ben-Aharon, I.; Popovtzer, R. Actively targeted gold nanoparticles as novel radiosensitizer agents: An in vivo head and neck cancer model. Nanoscale 2016, 8, 2678–2685. [Google Scholar] [CrossRef]
- Pallares, R.M.; Abergel, R.J. Nanoparticles for targeted cancer radiotherapy. Nano Res. 2020, 13, 2887–2897. [Google Scholar] [CrossRef]
- Palacios, D.A.; Miyake, M.; Rosser, C.J. Radiosensitization in prostate cancer: Mechanisms and targets. BMC Urol. 2013, 13, 4. [Google Scholar] [CrossRef] [Green Version]
- Deweese, T.L.; Shipman, J.M.; Dillehay, L.E.; Nelson, W.G. Sensitivity of Human Prostatic Carcinoma Cell Lines to Low Dose Rate Radiation Exposure. J. Urol. 1998, 159, 591–598. [Google Scholar] [CrossRef]
- Musielak, M.; Boś-Liedke, A.; Piotrowski, I.; Kozak, M.; Suchorska, W. The Role of Gold Nanorods in the Response of Prostate Cancer and Normal Prostate Cells to Ionizing Radiation—In Vitro Model. Int. J. Mol. Sci. 2021, 22, 16. [Google Scholar] [CrossRef]
- Chaiswing, L.; Weiss, H.L.; Jayswal, R.D.; Clair, D.K.S.; Kyprianou, N. Profiles of Radioresistance Mechanisms in Prostate Cancer. Crit. Rev. Oncog. 2018, 23, 39–67. [Google Scholar] [CrossRef] [PubMed]
- Ling, C.C.; Gerweck, L.E.; Zaider, M.; Yorke, E. Dose-rate effects in external beam radiotherapy redux. Radiother. Oncol. 2010, 95, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Bewes, J.M.; Suchowerska, N.; Jackson, M.; Zhang, M.; McKenzie, D. The radiobiological effect of intra-fraction dose-rate modulation in intensity modulated radiation therapy (IMRT). Phys. Med. Biol. 2008, 53, 3567–3578. [Google Scholar] [CrossRef]
- Esplen, N.; Mendonca, M.S.; Bazalova-Carter, M. Physics and biology of ultrahigh dose-rate (FLASH) radiotherapy: A topical review. Phys. Med. Biol. 2020, 65, 23TR03. [Google Scholar] [CrossRef]
- Morozov, K.V.; Kolyvanova, M.A.; Kartseva, M.E.; Shishmakova, E.M.; Dement’Eva, O.V.; Isagulieva, A.K.; Salpagarov, M.H.; Belousov, A.V.; Rudoy, V.M.; Shtil, A.A.; et al. Radiosensitization by Gold Nanoparticles: Impact of the Size, Dose Rate, and Photon Energy. Nanomaterials 2020, 10, 952. [Google Scholar] [CrossRef]
- Náfrádi, M.; Farkas, L.; Alapi, T.; Hernádi, K.; Kovács, K.; Wojnárovits, L.; Takács, E. Application of coumarin and coumarin-3-carboxylic acid for the determination of hydroxyl radicals during different advanced oxidation processes. Radiat. Phys. Chem. 2020, 170, 108610. [Google Scholar] [CrossRef]
- Cheng, N.N.; Starkewolf, Z.; Davidson, R.A.; Sharmah, A.; Lee, C.; Lien, J.; Guo, T. Chemical Enhancement by Nanomaterials under X-ray Irradiation. J. Am. Chem. Soc. 2012, 134, 1950–1953. [Google Scholar] [CrossRef]
- Sicard-Roselli, C.; Brun, E.; Gilles, M.; Baldacchino, G.; Kelsey, C.; McQuaid, H.; Polin, C.; Wardlow, N.; Currell, F. A New Mechanism for Hydroxyl Radical Production in Irradiated Nanoparticle Solutions. Small 2014, 10, 3338–3346. [Google Scholar] [CrossRef] [Green Version]
- Silva, F.; Zambre, A.; Campello, M.P.C.; Gano, L.; Santos, I.; Ferraria, A.M.; Ferreira, M.J.; Singh, A.; Upendran, A.; Paulo, A.; et al. Interrogating the Role of Receptor-Mediated Mechanisms: Biological Fate of Peptide-Functionalized Radiolabeled Gold Nanoparticles in Tumor Mice. Bioconjugate Chem. 2016, 27, 1153–1164. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.; Paulo, A.; Pallier, A.; Même, S.; Tóth, É.; Gano, L.; Marques, F.; Geraldes, C.F.; Castro, M.M.C.; Cardoso, A.M.; et al. Dual Imaging Gold Nanoplatforms for Targeted Radiotheranostics. Materials 2020, 13, 513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lechtman, E.; Chattopadhyay, N.; Cai, Z.; Mashouf, S.; Reilly, R.; Pignol, J.P. Implications on clinical scenario of gold nanoparticle radiosensitization in regards to photon energy, nanoparticle size, concentration and location. Phys. Med. Biol. 2011, 56, 4631–4647. [Google Scholar] [CrossRef]
- Zhang, X.D.; Wu, D.; Shen, X.; Chen, J.; Sun, Y.M.; Liu, P.X.; Liang, X.J. Size-dependent radiosensitization of PEG-coated gold nanoparticles for cancer radiation therapy. Biomaterials 2012, 33, 6408–6419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buch, K.; Peters, T.; Nawroth, T.; Sänger, M.; Schmidberger, H.; Langguth, P. Determination of cell survival after irradiation via clonogenic assay versus multiple MTT Assay—A comparative study. Radiat. Oncol. 2012, 7, 1. [Google Scholar] [CrossRef] [Green Version]
- Fenech, M. Cytokinesis-block micronucleus cytome assay. Nat. Protoc. 2007, 2, 1084–1104. [Google Scholar] [CrossRef] [Green Version]
- Behzadi, S.; Serpooshan, V.; Tao, W.; Hamaly, M.A.; Alkawareek, M.Y.; Dreaden, E.C.; Brown, D.; Alkilany, A.M.; Farokhzad, O.C.; Mahmoudi, M. Cellular uptake of nanoparticles: Journey inside the cell. Chem. Soc. Rev. 2017, 46, 4218–4244. [Google Scholar] [CrossRef]
- Heikkila, R.; Trepel, J.; Cuttitta, F.; Neckers, L.; Sausville, E. Bombesin-related peptides induce calcium mobilization in a subset of human small cell lung cancer cell lines. J. Biol. Chem. 1987, 262, 16456–16460. [Google Scholar] [CrossRef]
- Aprikian, A.G.; Han, K.; Chevalier, S.; Bazinet, M.; Viallet, J. Bombesin specifically induces intracellular calcium mobilization via gastrin-releasing peptide receptors in human prostate cancer cells. J. Mol. Endocrinol. 1996, 16, 297–306. [Google Scholar] [CrossRef]
- Kerkhofs, M.; Bittremieux, M.; Morciano, G.; Giorgi, C.; Pinton, P.; Parys, J.B.; Bultynck, G. Emerging molecular mechanisms in chemotherapy: Ca2+ signaling at the mitochondria-associated endoplasmic reticulum membranes. Cell Death Dis. 2018, 9, 334. [Google Scholar] [CrossRef]
- Wang, C.; Tian, L.-L.; Li, S.; Li, H.-B.; Zhou, Y.; Yang, Q.-Z.; Ma, L.-J.; Shang, D.-J.; Wang, H. Rapid Cytotoxicity of Antimicrobial Peptide Tempoprin-1CEa in Breast Cancer Cells through Membrane Destruction and Intracellular Calcium Mechanism. PLoS ONE 2013, 8, e60462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Liu, X.; Chen, S.; Liu, Z.; Zhang, X.; Liang, X.-J.; Li, L. Regulation of Ca2+ Signaling for Drug-Resistant Breast Cancer Therapy with Mesoporous Silica Nanocapsule Encapsulated Doxorubicin/siRNA Cocktail. ACS Nano 2019, 13, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Hempel, N.; Trebak, M. Crosstalk between calcium and reactive oxygen species signaling in cancer. Cell Calcium 2017, 63, 70–96. [Google Scholar] [CrossRef] [Green Version]
- Ślosarek, K.; Konopacka, M.; Rogoliński, J.; Latocha, M.; Sochanik, A. Effect of depth on radiation-induced cell damage in a water phantom. Rep. Pract. Oncol. Radiother. 2005, 10, 37–41. [Google Scholar] [CrossRef] [Green Version]
- Ślosarek, K.; Konopacka, M.; Rogoliński, J.; Sochanik, A. Effect of dose-rate and irradiation geometry on the biological response of normal cells and cancer cells under radiotherapeutic conditions. Mutat. Res. Toxicol. Environ. Mutagen. 2014, 773, 14–22. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, Z.; Lu, J.; Chen, Z.; Mao, A.; Teng, G.; Liu, F. A Comparison of the Biological Effects of 125I Seeds Continuous Low-Dose-Rate Radiation and 60Co High-Dose-Rate Gamma Radiation on Non-Small Cell Lung Cancer Cells. PLoS ONE 2015, 10, e0133728. [Google Scholar] [CrossRef]
- Sørensen, B.S.; Vestergaard, A.; Overgaard, J.; Præstegaard, L.H. Dependence of cell survival on instantaneous dose rate of a linear accelerator. Radiother. Oncol. 2011, 101, 223–225. [Google Scholar] [CrossRef] [PubMed]
- Verbakel, W.F.; Berg, J.V.D.; Slotman, B.; Sminia, P. Comparable cell survival between high dose rate flattening filter free and conventional dose rate irradiation. Acta Oncol. 2013, 52, 652–657. [Google Scholar] [CrossRef] [Green Version]
- Penninckx, S.; Heuskin, A.-C.; Michiels, C.; Lucas, S. The role of thioredoxin reductase in gold nanoparticle radiosensitization effects. Nanomedicine 2018, 13, 2917–2937. [Google Scholar] [CrossRef]
- Penninckx, S.; Heuskin, A.-C.; Michiels, C.; Lucas, S. Thioredoxin Reductase Activity Predicts Gold Nanoparticle Radiosensitization Effect. Nanomaterials 2019, 9, 295. [Google Scholar] [CrossRef] [Green Version]
- Ajdary, M.; Negahdary, M.; Chelongar, R.; Zadeh, S.K. The antioxidant effects of silver, gold, and zinc oxide nanoparticles on male mice in in vivo condition. Adv. Biomed. Res. 2015, 4, 69. [Google Scholar] [CrossRef] [PubMed]
- Daems, N.; Penninckx, S.; Nelissen, I.; Van Hoecke, K.; Cardinaels, T.; Baatout, S.; Michiels, C.; Lucas, S.; Aerts, A. Gold nanoparticles affect the antioxidant status in selected normal human cells. Int. J. Nanomed. 2019, 14, 4991–5015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belchior, A.; Botelho, M.; Peralta, L.; Vaz, P. Dose mapping of a 60Co irradiation facility using PENELOPE and MCNPX and its validation by chemical dosimetry. Appl. Radiat. Isot. 2008, 66, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Cudalbeanu, M.; Peitinho, D.; Silva, F.; Marques, R.; Pinheiro, T.; Ferreira, A.; Marques, F.; Paulo, A.; Soeiro, C.; Sousa, S.; et al. Sono-Biosynthesis and Characterization of AuNPs from Danube Delta Nymphaea alba Root Extracts and Their Biological Properties. Nanomaterials 2021, 11, 1562. [Google Scholar] [CrossRef]
- Barreiros, M.A.; Pinheiro, T.; Araujo, M.F.; Costa, M.; Palha, M.; da Silva, R. Quality assurance of X-ray spectrometry for chemical analysis. Spectrochim. Acta Part B At. Spectrosc. 2001, 56, 2095–2106. [Google Scholar] [CrossRef]
- Belchior, A.; Balásházy, I.; Gil, O.M.; Vaz, P.; Almeida, P. Does the Number of Irradiated Cells Influence the Spatial Distribution of Bystander Effects? Dose-Response 2014, 12, 525–539. [Google Scholar] [CrossRef] [Green Version]
- Belchior, A.; Balásházy, I.; Gil, O.M.; Vaz, P.; Almeida, P. Dose and Time Dependence of Targeted and Untargeted Effects after Very Low Doses of α-Particle Irradiation of Human Lung Cancer Cells. Dose-Response 2012, 11, 431–446. [Google Scholar] [CrossRef] [Green Version]
- Buşilă, M.; Tăbăcaru, A.; Muşsat, V.; Vasile, B.; Neaşu, I.A.; Pinheiro, T.; Roma-Rodrigues, C.; Baptista, P.V.; Fernandes, A.R.; Matos, A.P.; et al. Size-Dependent Biological Activities of Fluorescent Organosilane-Modified Zinc Oxide Nanoparticles. J. Biomed. Nanotechnol. 2020, 16, 137–152. [Google Scholar] [CrossRef]
- Javvaji, P.K.; Dhali, A.; Francis, J.R.; Kolte, A.P.; Mech, A.; Roy, S.C.; Mishra, A.; Bhatta, R. An Efficient Nitroblue Tetrazolium Staining and Bright-Field Microscopy Based Method for Detecting and Quantifying Intracellular Reactive Oxygen Species in Oocytes, Cumulus Cells and Embryos. Front. Cell Dev. Biol. 2020, 8, 764. [Google Scholar] [CrossRef]
- Rivas, F.; Medeiros, A.; Comini, M.; Suescun, L.; Arce, E.R.; Martins, M.; Pinheiro, T.; Marques, F.; Gambino, D. Pt-Fe ferrocenyl compounds with hydroxyquinoline ligands show selective cytotoxicity on highly proliferative cells. J. Inorg. Biochem. 2019, 199, 110779. [Google Scholar] [CrossRef]


| Compound | PC3 | RWPE1 |
|---|---|---|
| AuNP-TDOTA | 0.33 ± 0.02 | 0.24 ± 0.11 |
| AuNP-BBN | 4.81 ± 0.09 | 1.07 ± 0.08 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marques, A.; Belchior, A.; Silva, F.; Marques, F.; Campello, M.P.C.; Pinheiro, T.; Santos, P.; Santos, L.; Matos, A.P.A.; Paulo, A. Dose Rate Effects on the Selective Radiosensitization of Prostate Cells by GRPR-Targeted Gold Nanoparticles. Int. J. Mol. Sci. 2022, 23, 5279. https://doi.org/10.3390/ijms23095279
Marques A, Belchior A, Silva F, Marques F, Campello MPC, Pinheiro T, Santos P, Santos L, Matos APA, Paulo A. Dose Rate Effects on the Selective Radiosensitization of Prostate Cells by GRPR-Targeted Gold Nanoparticles. International Journal of Molecular Sciences. 2022; 23(9):5279. https://doi.org/10.3390/ijms23095279
Chicago/Turabian StyleMarques, Ana, Ana Belchior, Francisco Silva, Fernanda Marques, Maria Paula Cabral Campello, Teresa Pinheiro, Pedro Santos, Luis Santos, António P. A. Matos, and António Paulo. 2022. "Dose Rate Effects on the Selective Radiosensitization of Prostate Cells by GRPR-Targeted Gold Nanoparticles" International Journal of Molecular Sciences 23, no. 9: 5279. https://doi.org/10.3390/ijms23095279
APA StyleMarques, A., Belchior, A., Silva, F., Marques, F., Campello, M. P. C., Pinheiro, T., Santos, P., Santos, L., Matos, A. P. A., & Paulo, A. (2022). Dose Rate Effects on the Selective Radiosensitization of Prostate Cells by GRPR-Targeted Gold Nanoparticles. International Journal of Molecular Sciences, 23(9), 5279. https://doi.org/10.3390/ijms23095279







