Molecular Pincers Using a Combination of N-H and C-H Donors for Anion Binding
Abstract
1. Introduction
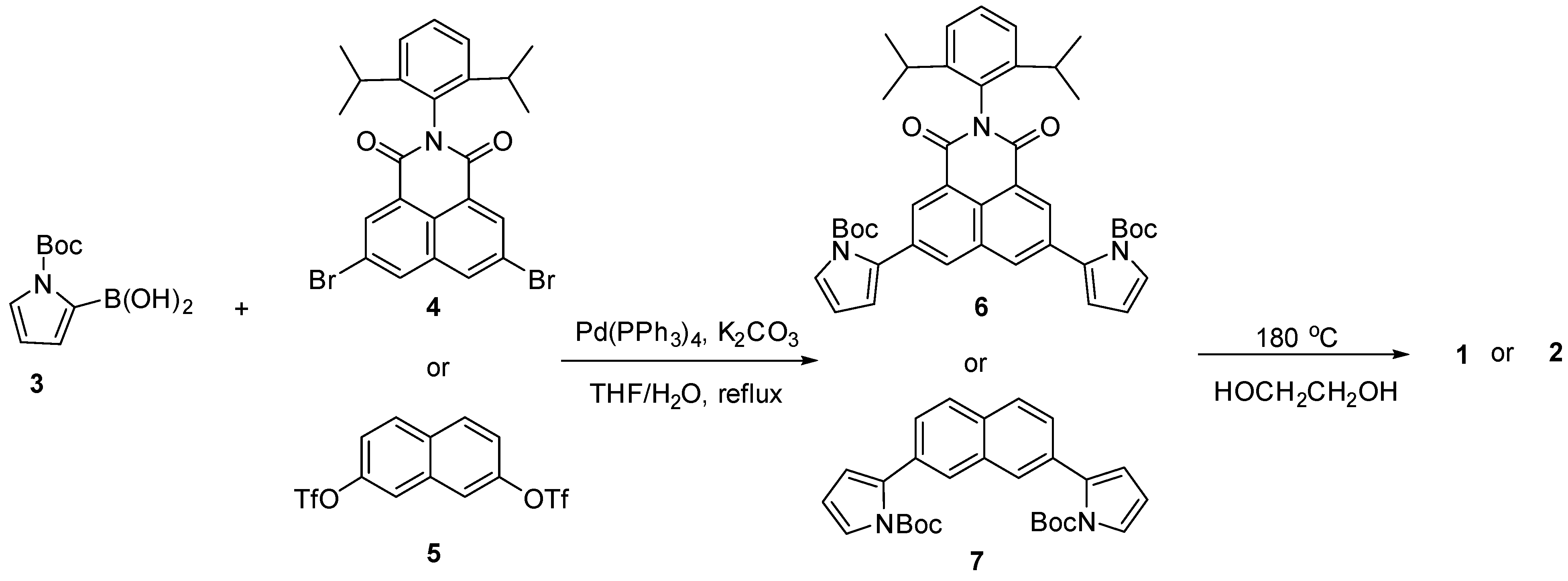
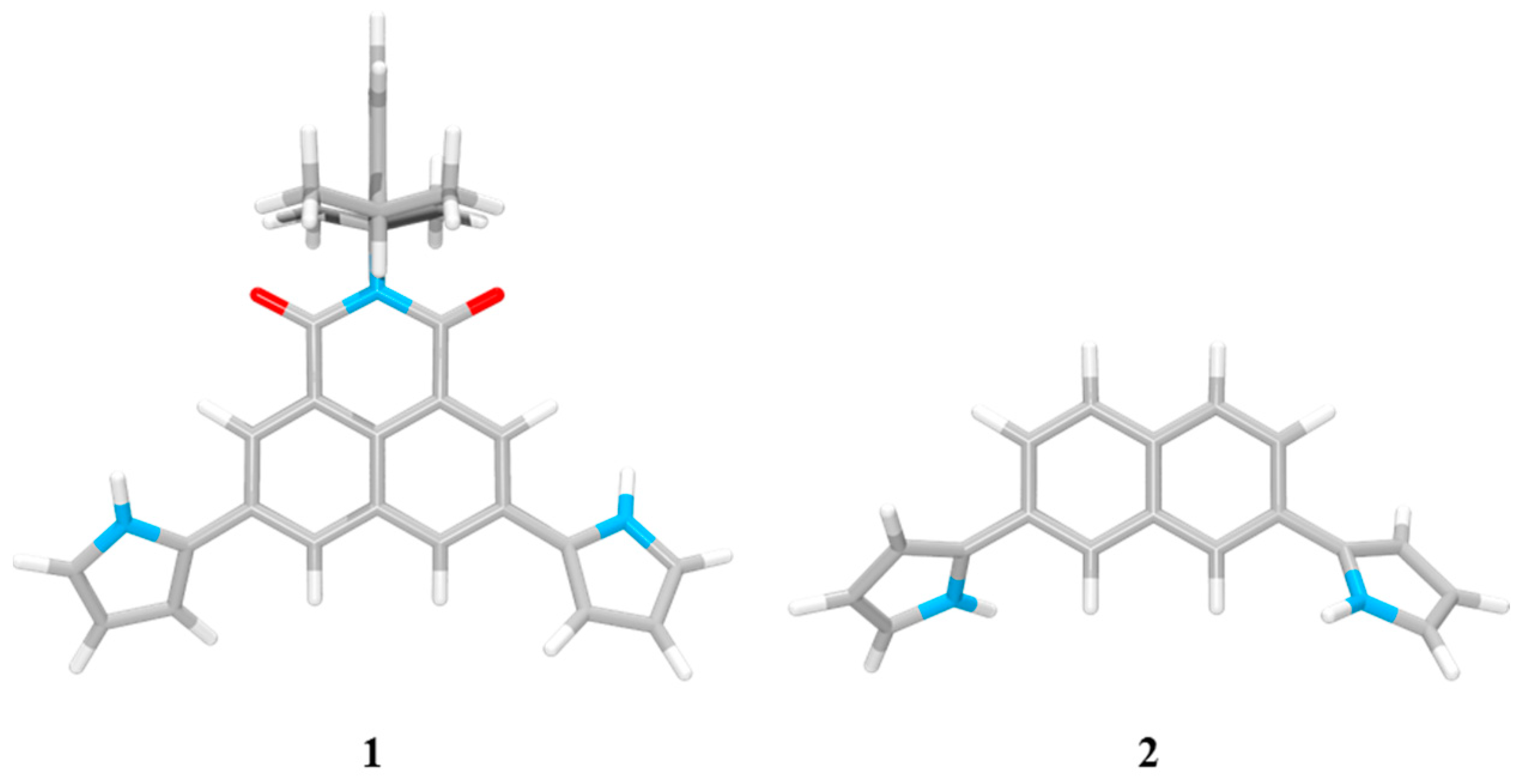
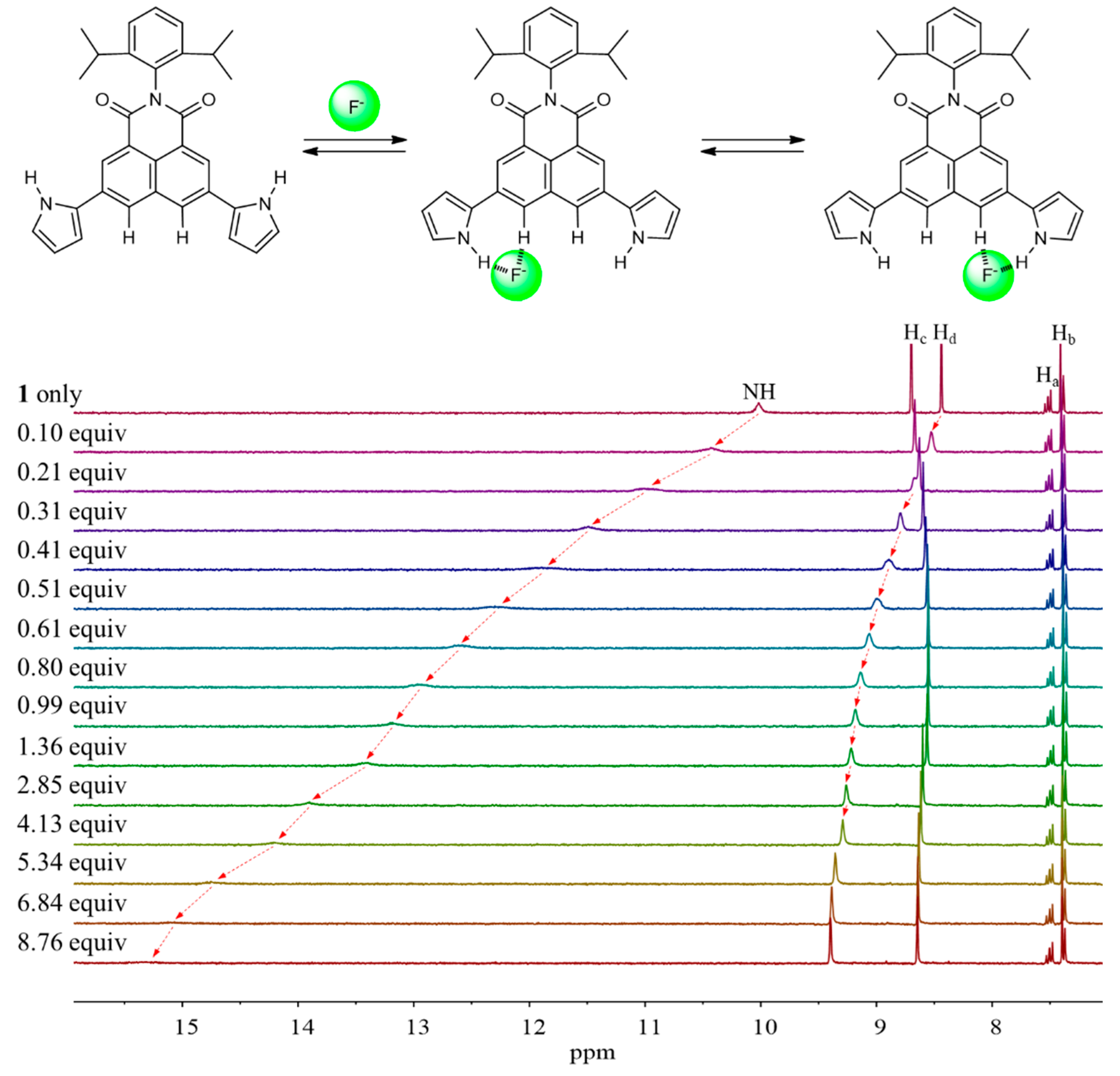
2. Results and Discussion
3. Materials and Methods
3.1. General Experimental
3.2. Synthesis of Compound 6
3.3. Synthesis of Compound 7
3.4. Synthesis of Compound 1
3.5. Synthesis of Compound 2
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MMFF94 | Merk Force Field 94 model |
| HRMS | High-Resolution Mass Spectrometry |
| THF | Tetrahydrofuran |
| TBA | Tetrabutylammonium |
| TEA | Tetraethylammonium |
| DMF | N,N-Dimethylformamide |
References
- Kollman, P.A.; Allen, L.C. The theory of the hydrogen bond. Chem. Rev. 1972, 72, 283–303. [Google Scholar] [CrossRef]
- Buckingham, A.D.; Del Bene, J.E.; McDowell, S.A.C. The hydrogen bond. Chem. Phys. Lett. 2008, 463, 1–10. [Google Scholar] [CrossRef]
- Steiner, T. The hydrogen bond in the solid state. Angew. Chem. Int. Ed. 2002, 41, 48–76. [Google Scholar] [CrossRef]
- Etter, M.C. Encoding and decoding hydrogen-bond patterns of organic compounds. Acc. Chem. Res. 1990, 23, 120–126. [Google Scholar] [CrossRef]
- Stahl, N.; Jencks, W.P. Hydrogen bonding between solutes in aqueous solution. J. Am. Chem. Soc. 1986, 108, 4196–4205. [Google Scholar] [CrossRef]
- Scheiner, S. The Hydrogen Bond: A Hundred Years and Counting. J. Indian Inst. Sci. 2020, 100, 61–76. [Google Scholar] [CrossRef]
- Kubik, S. Anion recognition in water. Chem. Soc. Rev. 2010, 39, 3648–3663. [Google Scholar] [CrossRef]
- Busschaert, N.; Caltagirone, C.; Rossom, W.V.; Gale, P.A. Applications of supramolecular anion recognition. Chem. Rev. 2015, 115, 8038–8155. [Google Scholar] [CrossRef]
- Beer, P.D.; Gale, P.A. Anion recognition and sensing the state of the art and future perspectives. Angew. Chem. Int. Ed. 2001, 40, 486–516. [Google Scholar] [CrossRef]
- Wu, X.; Gilchrist, A.M.; Gale, P.A. Prospects and challenges in anion recognition and transport. Chem 2022, 6, 1296–1309. [Google Scholar] [CrossRef]
- Jiang, L.H.; Caseley, E.A.; Muench, S.P.; Roger, S. Structural basis for the functional properties of the P2X7 receptor for extracellular ATP. Purinergic Signal. 2021, 17, 331–344. [Google Scholar] [CrossRef] [PubMed]
- Lane, A.N.; Jenkins, T.C. Structures and properties of multi-stranded nucleic acids. Curr. Org. Chem. 2001, 5, 845–869. [Google Scholar] [CrossRef]
- Watson, J.D.; Crick, F.H.C. Molecular structure of nucleic acids: A structure for deoxyribose nucleic acid. Nature 1953, 171, 737. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.C. Helical repeat of DNA in solution. Proc. Natl. Acad. Sci. USA 1979, 76, 200–203. [Google Scholar] [CrossRef]
- Pauling, L.; Corey, R.B. A Proposed structure for the nucleic acids. Proc. Natl. Acad. Sci. USA 1953, 39, 84–97. [Google Scholar] [CrossRef]
- Dobson, C.M. Protein folding and misfolding. Nature 2003, 426, 884–890. [Google Scholar] [CrossRef]
- Sun, T.; Zhou, B.; Lai, L.; Pei, J. Sequence-based prediction of protein interaction using a deep-learning algorithm. BMC Bioinform. 2017, 18, 277. [Google Scholar] [CrossRef]
- Hubbard, R.E.; Haider, M.K. Hydrogen bonds in proteins role and strength. In Encyclopedia of Life Sciences (ELS); John Wiley & Sons, Ltd.: Chichester, UK, 2010. [Google Scholar]
- Jones, S.; Thornton, J.M. Principles of protein-protein interactions. Proc. Natl. Acad. Sci. USA 1996, 93, 13–20. [Google Scholar] [CrossRef]
- Nooren, I.M.A.; Thoronton, J.M. Diversity of potein-protein interactions. EMBO J. 2003, 22, 3486–3492. [Google Scholar] [CrossRef]
- Sessler, J.L.; Gale, P.A.; Cho, W.S. Anion Receptor Chemistry; Stoddart, J.F., Ed.; Royal Society of Chemistry: Cambridge, UK, 2006; ISBN 978-0-85404-974-5. [Google Scholar]
- Lehn, J.M. Supramolecular Chemistry; VCH Press: Weinheim, Germany, 1995; ISBN 9783527607433. [Google Scholar]
- Desvergne, J.P.; Czarnik, A.W. Chemosensors of Ion and Molecular Recognition; NATO ASI Series C. Kluwer: Dordrecht, The Netherlands, 1997; ISBN 978-94-011-3973-1. [Google Scholar]
- Bianchi, A.; Bowman-James, K.; García-España, E. Supramolecular Chemistry of Anions; Willey: New York, NY, USA, 1997; ISBN 0-471-18622-8. [Google Scholar]
- Schmidtchen, F.P. Molekulare Wirte für Anionen. Nachr. Chem. Tech. Lab. 1988, 36, 8–17. [Google Scholar]
- Bowman-James, K.; Bianchi, A.; García-España, E. (Eds.) Anion Coordination Chemistry; Wiley-VCH: Weinheim, Germany, 2011; ISBN 9783527639502. [Google Scholar]
- Chen, L.; Berry, S.N.; Wu, X.; Howe, E.N.W.; Gale, P.A. Advances in anion receptor chemistry. Chem 2020, 6, 61–141. [Google Scholar] [CrossRef]
- Allison, S.J.; Bryk, J.; Clemett, C.J.; Faulkner, R.A.; Ginger, M.; Griffiths, H.B.S.; Harmer, J.; Owen-Lynch, P.J.; Pinder, E.; Wurdak, H.; et al. Self-assembly of an anion receptor with metal-dependent kinase inhibition and potent in vitro anti-cancer properties. Nat. Commun. 2021, 12, 3898. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, B.; Hosseini, M.W.; Lehn, J.M.; Sessions, R.B. Anion Receptor Molecules. Synthesis and anion-Binding properties of polyammonium macrocycles. J. Am. Chem. Soc. 1981, 103, 1282–1283. [Google Scholar] [CrossRef]
- Richard, D. Computational Chemistry of Solid State Materials a Guide for Materials Scientists, Chemists, Physicists and Others; Wiley-VCH: Weinheim, Germany, 2006; ISBN 9783527314102. [Google Scholar]
- Dydio, P.; Lichosyt, D.; Jurczak, J. Amide- and urea-functionalized pyrroles and benzopyrroles as synthetic, neutral anion receptors. Chem. Soc. Rev. 2011, 40, 2971–2985. [Google Scholar] [CrossRef] [PubMed]
- Gale, P.A.; Howe, E.N.W.; Wu, X. Anion receptor chemistry. Chem 2016, 1, 351–422. [Google Scholar] [CrossRef]
- Chang, K.J.; Kang, B.N.; Lee, M.H.; Jeong, K.S. Oligoindole-based foldamers with a helical conformation induced by chloride. J. Am. Chem. Soc. 2005, 127, 12214–12215. [Google Scholar] [CrossRef] [PubMed]
- Amendola, V.; Fabbrizzi, L.; Mosca, L. Anion recognition by hydrogen bonding: Urea-based receptors. Chem. Soc. Rev. 2010, 39, 3889–3915. [Google Scholar] [CrossRef]
- Bondy, C.R.; Loeb, S.J. Amide based receptors for anions. Coord. Chem. Rev. 2003, 240, 77–99. [Google Scholar] [CrossRef]
- Gale, P.A.; Sessler, J.L.; Král, V.; Lynch, V. Calix[4]pyrroles: Old yet new anion-binding agents. J. Am. Chem. Soc. 1996, 118, 5140–5141. [Google Scholar] [CrossRef]
- Gale, P.A.; Sessler, J.L.; Kral, V. Calixpyrroles. Chem. Commun. 1998, 1–8. [Google Scholar] [CrossRef]
- Lee, C.H.; Miyaji, H.; Yoon, D.W.; Sessler, J.L. Strapped and other topographically nonplanar calixpyrrole analogues. Improved anion receptors. Chem. Commun. 2008, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Gale, P.A.; Lee, C.-H. Calix[n]pyrroles as Anion and Ion-Pair Complexants. Top. Heterocycl. Chem. 2010, 24, 39–73. [Google Scholar]
- Peng, S.; He, Q.; Vargas-Zúñiga, G.I.; Qin, L.; Hwang, I.; Kim, S.K.; Heo, N.J.; Lee, C.H.; Dutta, R.; Sessler, J.L. Strapped calix[4]pyrroles: From syntheses to applications. Chem. Soc. Rev. 2020, 49, 865–907. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Lee, J.; Williams, N.J.; Lynch, V.M.; Hay, B.P.; Moyer, B.A.; Sessler, J.L. Bipyrrole-Strapped Calix[4]pyrroles: Strong anion receptors that extract the sulfate anion. J. Am. Chem. Soc. 2014, 136, 15079–15085. [Google Scholar] [CrossRef]
- Busschaert, N.; Kirby, I.L.; Young, S.; Coles, S.J.; Horton, P.N.; Light, M.E.; Gale, P.A. Squaramides as potent transmembrane anion transporters. Angew. Chem. Int. Ed. 2012, 51, 4426–4430. [Google Scholar] [CrossRef]
- Storer, R.I.; Aciro, C.; Jones, L.H. Squaramides: Physical properties, synthesis and applications. Chem. Soc. Rev. 2011, 40, 2330–2346. [Google Scholar] [CrossRef]
- Chauhan, P.; Mahajan, S.; Kaya, U.; Hack, D.; Enders, D. Bifunctional amine-squaramides: Powerful hydrogen-bonding organocatalysts for asymmetric domino/cascade reactions. Adv. Synth. Catal. 2015, 357, 253–281. [Google Scholar]
- Marchetti, L.A.; Kumawat, L.K.; Mao, N.; Stephens, J.C.; Elmes, R.B.P. The versatility of squaramides: From supramolecular chemistry to chemical biology. Chem 2019, 5, 1398–1485. [Google Scholar] [CrossRef]
- Lee, S.; Flood, A.H. Binding Anions in Rigid and Reconfigurable Triazole Receptors; Springer Berlin Heidelberg: Berlin/Heidelberg, Germeny, 2012; pp. 85–107. ISBN 978-3-642-29428-0. [Google Scholar]
- Ramabhadran, R.O.; Hua, Y.; Flood, A.H.; Raghavachari, K. From atomic to molecular anions: A neutral receptor captures cyanide using strong C–H hydrogen bonds. Chem. Eur. J. 2011, 17, 9123–9129. [Google Scholar]
- Zahran, E.M.; Hua, Y.; Lee, S.; Flood, A.H.; Bachas, L.G. Ion-selective electrodes based on a pyridyl-containing triazolophane: Altering halide selectivity by Combining dipole-promoted cooperativity with hydrogen bonding. Anal. Chem. 2011, 83, 3455–3461. [Google Scholar] [CrossRef]
- Hua, Y.; Ramabhadran, R.O.; Uduehi, E.O.; Karty, J.A.; Raghavachari, K.; Flood, A.H. Aromatic and aliphatic CH hydrogen bonds fight for chloride while competing alongside ion pairing within triazolophanes. Chem. Eur. J. 2011, 17, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Chen, C.H.; Flood, A.H. A pentagonal cyanostar macrocycle with cyanostilbene CH donors binds anions and forms dialkylphosphate [3]rotaxanes. Nat. Chem. 2013, 5, 704–710. [Google Scholar] [CrossRef] [PubMed]
- Majewski, M.A.; Hong, Y.; Lis, T.; Gregoliński, J.; Chmielewski, P.J.; Cybińska, J.; Kim, D.; Stępień, M. Octulene: A hyperbolic molecular belt that binds chloride anions. Angew. Chem. Int. Ed. 2016, 55, 14072–14076. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, W.; Chen, C.H.; Flood, A.H. Chloride capture using a C–H hydrogen bonding cage. Science 2019, 365, 159–161. [Google Scholar]
- Maeda, H.; Haketa, Y.; Nakanishi, T. Aryl-Substituted C3-bridged oligopyrroles as anion receptors for formation of supramolecular organogels. J. Am. Chem. Soc. 2007, 129, 13661–13674. [Google Scholar] [CrossRef]
- Ishi-i, T.; Ikeda, K.; Ogawa, M.; Kusakaki, Y. Light-emitting properties of donor–acceptor and donor–acceptor–donor dyes in solution, solid, and aggregated states: Structure–property relationship of emission behavior. RSC Adv. 2015, 5, 89171–89187. [Google Scholar] [CrossRef]
- Pal, A.; Karmakar, M.; Bhatta, S.R.; Thakur, A. A detailed insight into anion sensing based on intramolecular charge transfer (ICT) mechanism: A comprehensive review of the years 2016 to 2021. Coord. Chem. Rev. 2021, 448, 214167. [Google Scholar] [CrossRef]
- Meier, H. Conjugated oligomers with terminal donor–acceptor substitution. Angew. Chem. Int. Ed. 2005, 44, 2482–2506. [Google Scholar] [CrossRef]
- Panja, S.K.; Dwivedi, N.; Saha, S. Tuning the intramolecular charge transfer (ICT) process in push-pull systems: Effect of Nitro group. RSC Adv. 2016, 6, 105786–105794. [Google Scholar] [CrossRef]
- Xue, J.Y.; Nakanishi, W.; Tanimoto, D.; Hara, D.; Nakamura, Y.; Isobe, H. Convergent synthesis of hexameric naphthylene macrocycles with dicarboxylic imide appendages. Tetrahedron Lett. 2013, 54, 4963–4965. [Google Scholar] [CrossRef]
- Frigoli, M.; Moustrou, C.; Samat, A.; Guglielmetti, R. Synthesis of New thiophene-substituted 3,3-diphenyl-3H-naphtho[2,1-b]pyrans by cross-coupling reactions, precursors of photomodulated materials. Eur. J. Org. Chem. 2003, 15, 2799–2812. [Google Scholar] [CrossRef]
- Salman, H.; Abraham, Y.; Tal, S.; Meltzman, S.; Kapon, M.; Tessler, N.; Speiser, S.; Eichen, Y. 1,3-Di(2-pyrrolyl)azulene: An efficient luminescent probe for fluoride. Eur. J. Org. Chem. 2005, 2005, 2207–2212. [Google Scholar] [CrossRef]
- Weidauer, M.; Irran, E.; Enthaler, S. Synthesis and structural characterization of a trispyrrole iron(II) complex K(dme)4[tpaMesFe] and application in nitrous oxide dependent coupling reactions. Inorg. Chem. Commun. 2015, 54, 1–4. [Google Scholar] [CrossRef]
- Conformational Searches Performed with PCModel, Version 9.3; Serena Software: Bloomington, IN, USA, 2012.
- Halgren, T.A. Merck molecular force field. I. Basis, form, scope, parameterization, and performance of MMFF94. J. Comp. Chem. 1996, 17, 490–519. [Google Scholar] [CrossRef]
- Association Constants (Ka) Were Evaluated Using BindFit v5.0. Available online: http://app.supramolecular.org/bindfit/ (accessed on 28 October 2022).









| Anions | Receptor 1 c | Receptor 2 c |
|---|---|---|
| F− a Cl− a Br− a | (1.65 0.53) 103 | (1.96 0.20) 102 |
| (1.57 0.04) 102 | (4.33 0.06) 10 | |
| (2.54 0.02) 10 | (1.26 0.01) 10 | |
| I− a HSO4− a | 3.13 0.03 | 1.72 0.03 |
| (2.81 0.04) 10 | 5.72 0.13 | |
| H2PO4− a HP2O73− a HCO3− b | (1.33 0.08) 103 | (1.23 0.17) 103 |
| (1.53 0.59) 103 | -d | |
| (2.27 0.20) 103 | (9.46 0.99) 102 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Kim, S.H.; Heo, N.J.; Hay, B.P.; Kim, S.K. Molecular Pincers Using a Combination of N-H and C-H Donors for Anion Binding. Int. J. Mol. Sci. 2023, 24, 163. https://doi.org/10.3390/ijms24010163
Kim J, Kim SH, Heo NJ, Hay BP, Kim SK. Molecular Pincers Using a Combination of N-H and C-H Donors for Anion Binding. International Journal of Molecular Sciences. 2023; 24(1):163. https://doi.org/10.3390/ijms24010163
Chicago/Turabian StyleKim, Jaehyeon, Seung Hyeon Kim, Nam Jung Heo, Benjamin P. Hay, and Sung Kuk Kim. 2023. "Molecular Pincers Using a Combination of N-H and C-H Donors for Anion Binding" International Journal of Molecular Sciences 24, no. 1: 163. https://doi.org/10.3390/ijms24010163
APA StyleKim, J., Kim, S. H., Heo, N. J., Hay, B. P., & Kim, S. K. (2023). Molecular Pincers Using a Combination of N-H and C-H Donors for Anion Binding. International Journal of Molecular Sciences, 24(1), 163. https://doi.org/10.3390/ijms24010163






