Role of Nitric Oxide-Derived Metabolites in Reactions of Methylglyoxal with Lysine and Lysine-Rich Protein Leghemoglobin
Abstract
1. Introduction
2. Results
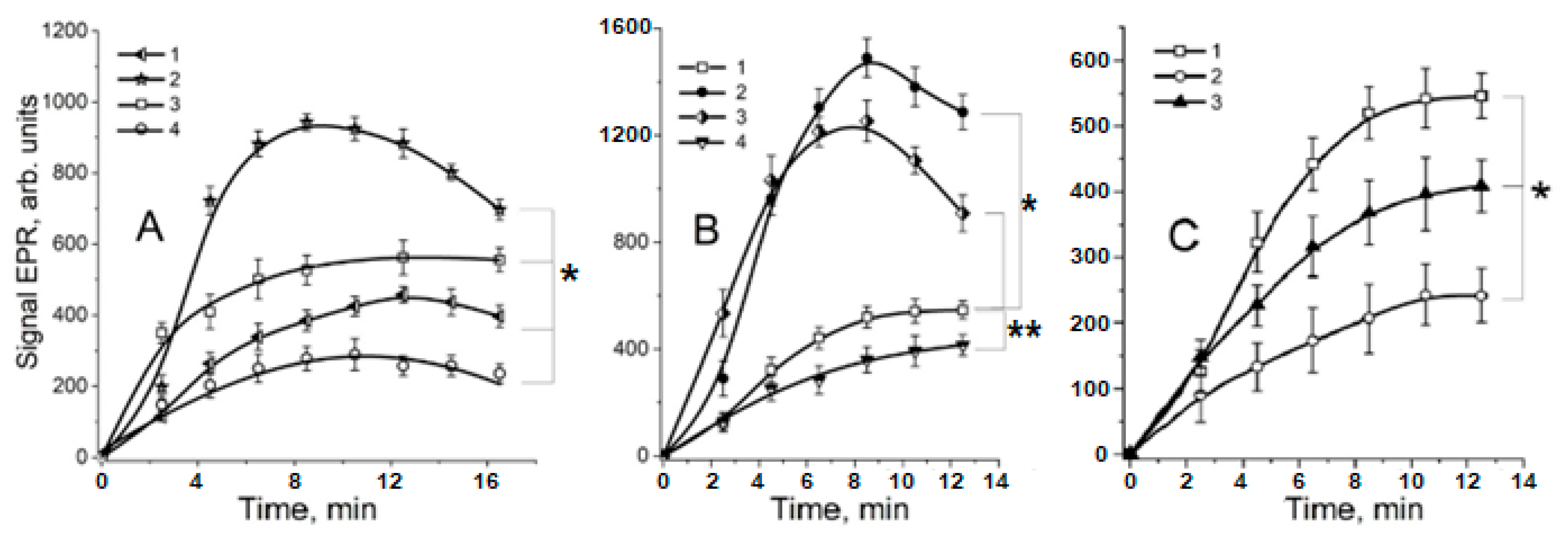
2.1. Effect of S-Nitrosothiols on the Formation of Free Radicals in the Maillard Reaction
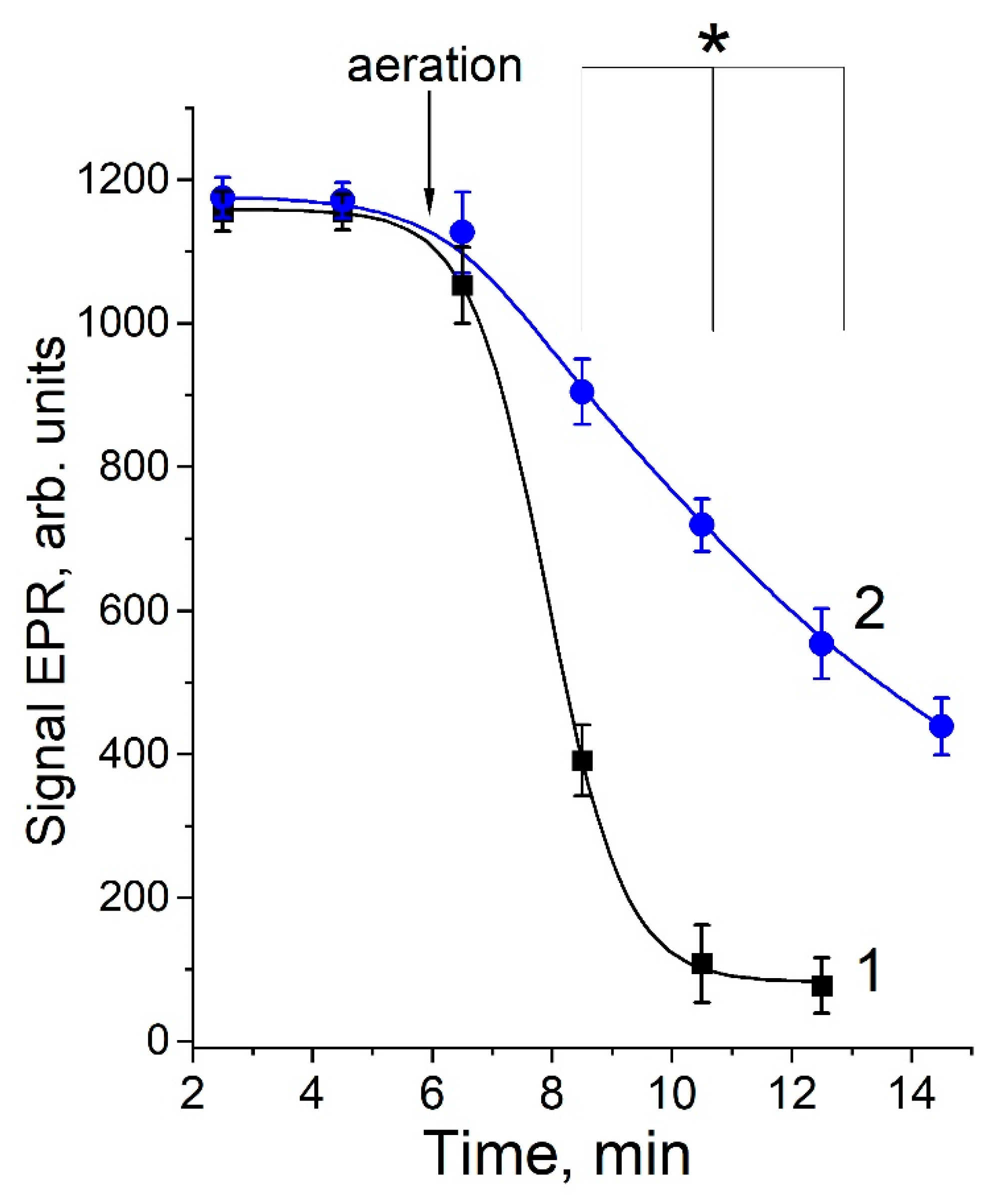
2.2. The Role of Dinitrosyl Iron Complexes in the Reaction of Non-Enzymatic Glycation of Lysine by Methylglyoxal
↑↓ ↑↓
[nL-Fe2+(NO,NO)] [nL-Fe+(NO+)2]
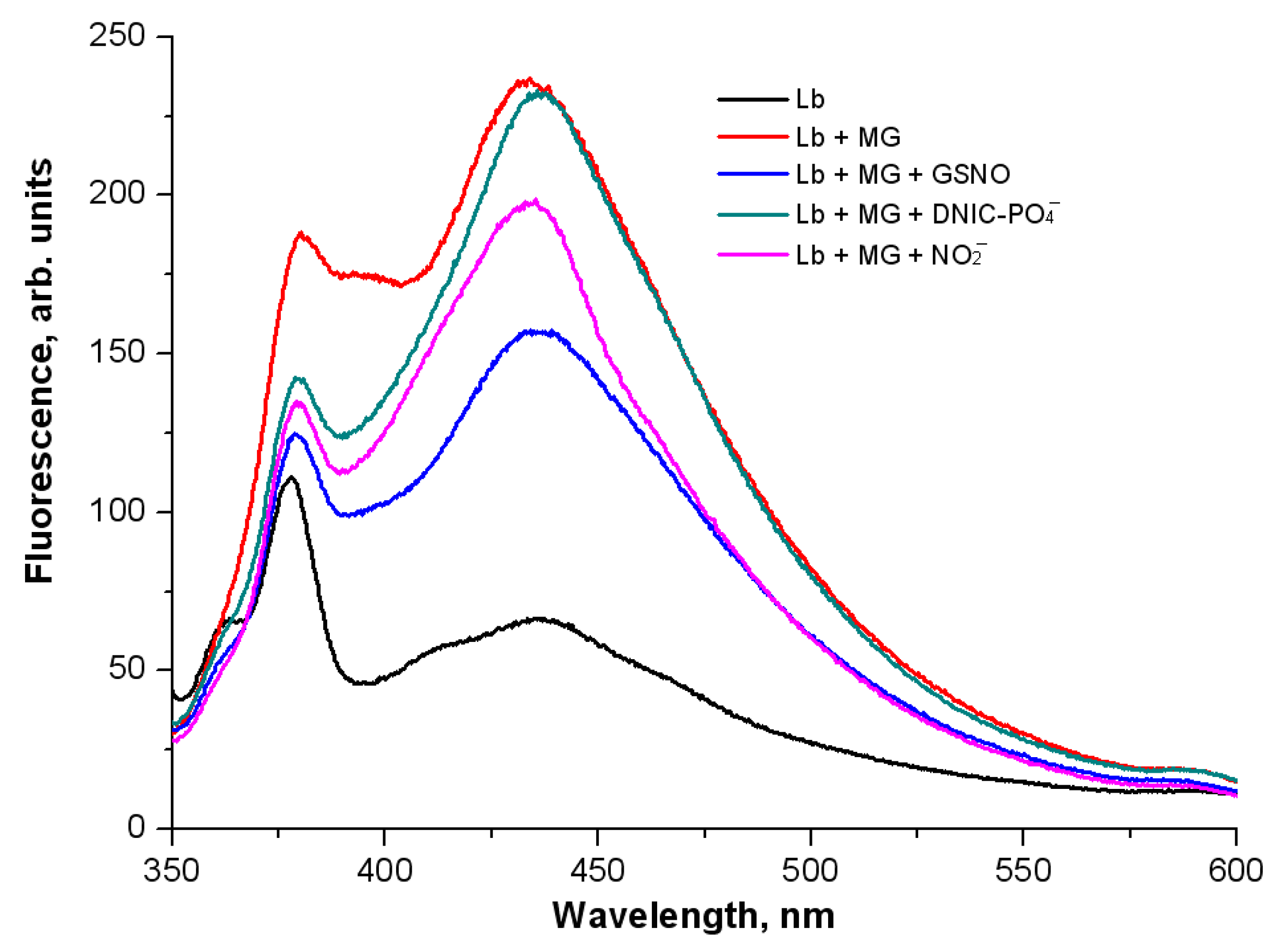
2.3. The Effect of •NO-Derived Metabolites on Non-Enzymatic Leghemoglobin Glycation
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. The Synthesis of RSNOs and DNICs
4.3. EPR Measurements
4.4. The Isolation and Purification of Lb from Bean Nodules
4.5. The Non-Enzymatic Glycation of Leghemoglobin
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, X.; Joshi, S.; Patil, S.; Khare, T.; Kumar, V. Reactive oxygen, nitrogen, carbonyl and sulfur species and their roles in plant abiotic stress responses and tolerance. J. Plant Growth Regul. 2021, 41, 119–142. [Google Scholar] [CrossRef]
- Massa, C.M.; Liu, Z.; Taylor, S.; Pettit, A.P.; Stakheyeva, M.N.; Korotkova, E.; Popova, V.; Atochina-Vasserman, E.N.; Gow, A.J. Biological mechanisms of S-nitrosothiol formation and degradation: How is specificity of S-nitrosylation achieved? Antioxidants 2021, 10, 1111. [Google Scholar] [CrossRef] [PubMed]
- Lehnert, N.; Kim, E.; Dong, H.T.; Harland, J.B.; Hunt, A.P.; Manickas, E.C.; Oakley, K.M.; Pham, J.; Reed, G.C.; Alfaro, V.S. The biologically relevant coordination chemistry of iron and nitric oxide: Electronic structure and reactivity. Chem. Rev. 2021, 121, 14682–14905. [Google Scholar] [CrossRef] [PubMed]
- Kosmachevskaya, O.V.; Shumaev, K.B.; Nasybullina, E.I.; Topunov, A.F. Formation of Nitri- and nitrosylhemoglobin in systems modeling the Maillard reaction. Clin. Chem. Lab. Med. 2014, 52, 161–168. [Google Scholar] [CrossRef]
- Truzzi, D.R.; Medeiros, N.M.; Augusto, O.; Ford, P.C. Dinitrosyl iron complexes (DNICs). From spontaneous assembly to biological roles. Inorg. Chem. 2021, 60, 15835–15845. [Google Scholar] [CrossRef] [PubMed]
- Goldin, A.; Beckman, J.A.; Schmidt, A.M.; Creager, M.A. Advanced glycation end products sparking the development of diabetic vascular injury. Circulation 2006, 114, 597–605. [Google Scholar] [CrossRef]
- Zhang, Q.; Ames, M.J.; Smith, R.D.; Baynes, J.W.; Metz, T.O. A Perspective on the Maillard Reaction and the analysis of protein glycation by mass spectrometry: Probing the pathogenesis of chronic disease. J. Proteome Res. 2009, 8, 754–769. [Google Scholar] [CrossRef]
- Rabbani, N.; Thornalley, P.J. Glycation research in amino acids: A place to call home. Amino Acids 2012, 42, 1087–1096. [Google Scholar] [CrossRef]
- Kosmachevskaya, O.V.; Novikova, N.N.; Topunov, A.F. Carbonyl stress in red blood cells and hemoglobin. Antioxidants 2021, 10, 253. [Google Scholar] [CrossRef]
- Rabbani, N.; Thornalley, P.J. The critical role of methylglyoxal and glyoxalase 1 in diabetic nephropathy diabetes. Diabetes 2014, 63, 50–52. [Google Scholar] [CrossRef]
- Vistoli, G.; De Maddis, D.; Cipak, A.; Zarkovic, N.; Carini, M.; Aldini, G. Advanced glycoxidation and lipoxidation end products (AGEs and ALEs): An overview of their mechanisms of formation. Free Radic. Res. 2013, 47, 3–27. [Google Scholar] [CrossRef] [PubMed]
- Shumaev, K.B.; Gubkina, S.A.; Kumskova, E.M.; Shepelkova, G.S.; Ruuge, E.K.; Lankin, V.Z. Superoxide formation as a result of interaction of L-lysine with dicarbonyl compounds and its possible mechanism. Biochemistry 2009, 74, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Desai, K.M.; Chang, T.; Wang, H.; Banigesh, A.; Dhar, A.; Liu, J.; Untereiner, A.; Wu, L. Oxidative stress and aging: Is methylglyoxal the hidden enemy? Can. J. Physiol. Pharmacol. 2010, 88, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.; Wu, L. Methylglyoxal, oxidative stress, and hypertension. Can. J. Physiol. Pharmacol. 2006, 84, 1229–1238. [Google Scholar] [CrossRef]
- Massari, J.; Tokikawa, R.; Zanolli, L.; Tavares, M.F.M.; Assunção, N.A.; Bechara, E.J.H. Acetyl radical production by the methylglyoxal-peroxynitrite system: A possible route for L-lysine acetylation. Chem. Res. Toxicol. 2010, 23, 1762–1770. [Google Scholar] [CrossRef]
- Mitsuda, B.H.; Yasumoto, K.; Yokoyama, K. Studies on the free radical in amino-carbonyl reaction. Agr. BioI. Chern. 1965, 29, 751–756. [Google Scholar] [CrossRef]
- Yim, H.-S.; Kang, S.-O.; Hah, Y.-C.; Chock, B.; Yim, M.B. Free radicals generated during the glycation reaction of amino acids by methylglyoxal. A model study of protein-cross-linked free radicals. J. Biol. Chem. 1995, 270, 28228–28233. [Google Scholar] [CrossRef]
- Lee, C.; Yim, M.B.P.; Chock, B.; Yim, H.S.; Kang, S.O. Oxidation-reduction properties of methylglyoxal-modified protein in relation to free radical generation. J. Biol. Chem. 1998, 273, 25272–25278. [Google Scholar] [CrossRef]
- Gascoyne, P.R.C.; Symons, M.C.R.; Mclaughlin, J.A. Spontaneous electron transfer in the reaction between methylglyoxal and methylamine. Int. J. Quantum Chem. 1983, 24, 123–132. [Google Scholar] [CrossRef]
- Asahi, K.; Ichimori, K.; Nakazawa, H.; Izuhara, Y.; Inagi, R.; Watanabe, T.; Miyata, T.; Kurokawa, K. Nitric oxide inhibits the formation of advanced glycation end products. Kidney Int. 2000, 58, 1780–1787. [Google Scholar] [CrossRef]
- Appleby, C.A. Leghemoglobin and Rhizobium respiration. Ann. Rev. Plant Physiol. 1984, 35, 443–478. [Google Scholar] [CrossRef]
- Layzell, D.B.; Atkins, C.A. The Physiology and Biochemistry of Legume N2 Fixation. In Plant Metabolism, 2nd ed.; Dennis, D.T., Turpin, D.H., Lefebvre, D.D., Layzell, D.B., Eds.; Longman: Harlow, UK, 1997; pp. 495–505. [Google Scholar]
- Singh, S.; Varma, A. Structure, Function, and Estimation of Leghemoglobin/Rhizobium Biology and Biotechnology; Part of the Soil Biology book series (SOILBIOL, volume 50); Springer: Cham, Switzerland, 2017; pp. 309–330. [Google Scholar] [CrossRef]
- Fraser, R.Z.; Shitut, M.; Agrawal, P.; Mendes, O.; Klapholz, S. Safety evaluation of soy leghemoglobin protein preparation derived from Pichia pastoris, intended for use as a flavor catalyst in plant-based meat. Int. J. Toxicol. 2018, 37, 241–262. [Google Scholar] [CrossRef] [PubMed]
- Reyes, T.F.; Chen, Y.; Fraser, R.Z.; Chan, T.; Li, X. Assessment of the potential allergenicity and toxicity of Pichia proteins in a novel leghemoglobin preparation. Regul. Toxicol. Pharmacol. 2021, 119, e104817. [Google Scholar] [CrossRef] [PubMed]
- UniProt. Available online: https://www.uniprot.org/uniprotkb?query=leghemoglobin (accessed on 5 December 2022).
- Kozlowski, L.P. Proteome-pI: Proteome isoelectric point database. Nucleic Acids Res. 2017, 45, D1112–D1116. [Google Scholar] [CrossRef] [PubMed]
- Matamoros, M.A.; Kim, A.; Peñuelas, M.; Ihling, C.; Griesser, E.; Hoffmann, R.; Fedorova, M.; Frolov, A.; Becana, M. Protein carbonylation and glycation in legume nodules. Plant Physiol. 2018, 177, 1510–1528. [Google Scholar] [CrossRef] [PubMed]
- Shumaev, K.B.; Gubkin, A.A.; Serezhenkov, V.A.; Lobysheva, I.I.; Kosmachevskaya, O.V.; Ruuge, E.K.; Lankin, V.Z.; Topunov, A.F.; Vanin, A.F. Interaction of reactive oxygen and nitrogen species with albumin and hemoglobin bound dinitrosyl iron complexes. Nitric Oxide 2008, 18, 37–46. [Google Scholar] [CrossRef]
- Shumaev, K.B.; Kosmachevskaya, O.V.; Timoshin, A.A.; Vanin, A.F.; Topunov, A.F. Dinitrosyl iron complexes bound with haemoglobin as markers of oxidative stress. Methods Enzymol. 2008, 436, 445–461. [Google Scholar] [CrossRef]
- Szaciłowski, K.; Stasicka, Z. S-Nitrosothiols: Materials, reactivity and mechanisms. Prog. React. Kinet. Mech. 2000, 26, 1–58. [Google Scholar] [CrossRef]
- Manoj, V.M.; Mohan, H.; Aravind, U.K.; Aravindakumar, C.T. One-electron reduction of S-nitrosothiols in aqueous medium. Free Rad. Biol. Med. 2006, 41, 1240–1246. [Google Scholar] [CrossRef]
- Lopez, B.E.; Shinyashiki, M.; Han, T.H.; Fukuto, J.M. Antioxidant actions of nitroxyl (HNO). Free Rad. Biol. Med. 2007, 42, 482–491. [Google Scholar] [CrossRef]
- Shoman, M.E.; Aly, O.M. Nitroxyl (HNO): A reduced form of nitric oxide with distinct chemical, pharmacological, and therapeutic properties. Oxid. Med. Cell. Longev. 2016, 2016, e4867124. [Google Scholar] [CrossRef] [PubMed]
- Pool, B.L.; Roper, H.; Roper, S.; Romruen, K. Mutagenicity studies on N-nitrosated products of the maillard browning reaction: N-nitroso-fructose-amino acids. Fd Chem. Toxic. 1984, 22, 797–801. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wu, B.; Ding, M.; Yang, Y.; Liu, Z.; Zhang, F.; Tang, E.; Duan, J. Aldehyde-mediated N-nitrosation of an amino acid. Arkivoc 2017, 2017, 12–19. [Google Scholar] [CrossRef]
- Hrable, J.A.; Srinivasan, A.; Clifford, G.; Keefer, L.K. Reaction of nitric oxide with the imine double bond of certain Schiff bases. Tetrahedron Lett. 1998, 39, 5933–5936. [Google Scholar] [CrossRef]
- Vanin, A.F. Dinitrosyl iron complexes with thiol-containing ligands as a “working form” of endogenous nitric oxide. Nitric Oxide 2016, 54, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Vanin, A.F. Physico-chemistry of dinitrosyl iron complexes as a determinant of their biological activity. Int. J. Mol. Sci. 2021, 22, 10356. [Google Scholar] [CrossRef]
- Shumaev, K.B.; Gubkin, A.A.; Gubkina, S.A.; Gudkov, L.L.; Sviryaeva, I.V.; Timoshin, A.A.; Topunov, A.F.; Vanin, A.F.; Ruuge, E.K. The interaction between dinitrosyl iron complexes and intermediates of oxidative stress. Biophysics 2006, 51, 423–428. [Google Scholar] [CrossRef]
- Toledo, J.C.; Bosworth, C.A.; Hennon, S.W.; Mahtani, H.A.; Bergonia, H.A.; Lancaster, J.R. Nitric oxide-induced conversion of cellular chelatable iron into macromolecule-bound paramagnetic dinitrosyl iron complexes. J. Biol. Chem. 2008, 283, 28926–28933. [Google Scholar] [CrossRef]
- Shumaev, K.B.; Dudylina, A.L.; Ivanova, M.V.; Pugachenko, I.S.; Ruuge, E.K. Dinitrosyl iron complexes: Formation and antiradical action in heart mitochondria. BioFactors 2018, 44, 237–244. [Google Scholar] [CrossRef]
- Shumaev, K.B.; Gubkina, S.A.; Vanin, A.F.; Burbaev, D.S.; Mokh, V.P.; Topunov, A.F.; Ruuge, E.K. Formation of a new type of dinitrosyl iron complexes bound to cysteine modified with methylglyoxal. Biophysics 2013, 58, 172–177. [Google Scholar] [CrossRef]
- Shumaev, K.B.; Kosmachevskaya, O.V.; Nasybullina, E.I.; Gromov, S.V.; Novikov, A.A.; Topunov, A.F. New dinitrosyl iron complexes bound with physiologically active dipeptide carnosine. J. Biol. Inorg. Chem. 2017, 22, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Qian, M.; Eaton, J.W. Glycochelates and the etiology of diabetic peripheral neuropathy. Free Radic. Biol. Med. 2000, 28, 652–656. [Google Scholar] [CrossRef]
- Monnier, V.M. Transition metals redox: Reviving an old plot for diabetic vascular disease. J. Clin. Invest. 2001, 107, 799–801. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tyagi, N.; Singh, O.; Singh, U.P.; Ghosh, K. Nitric oxide (NO) reactivity studies on mononuclear iron(ii) complexes supported by a tetradentate Schiff base ligand. RSC Adv. 2016, 6, 115326–115333. [Google Scholar] [CrossRef]
- Mishra, S.; Kumar, S.; Bhandari, A.; Das, A.; Mondal, P.; Hundal, G.; Olmstead, M.M.; Patra, A.K. Reactivity of nitric oxide and nitrosonium ion with copper(II/I) schiff base complexes: Mechanistic aspects of imine C═N bond cleavage and oxidation of pyridine-2-aldehyde to pyridine-2-carboxylic acid. Inorg. Chem. 2022, 61, 6421–6437. [Google Scholar] [CrossRef]
- Kosmachevskaya, O.V.; Nasybullina, E.I.; Shumaev, K.B.; Novikova, N.N.; Topunov, A.F. Protective effect of dinitrosyl iron complexes bound with hemoglobin on oxidative modification by peroxynitrite. Int. J. Mol. Sci. 2021, 22, 13649. [Google Scholar] [CrossRef]
- Li, Q.; Li, C.; Mahtani, H.K.; Du, J.; Patel, A.R.; Lancaster, J.R. Nitrosothiol formation and protection against fenton chemistry by nitric oxide-induced dinitrosyliron complex formation from anoxia-initiated cellular chelatable iron increase. J. Biol. Chem. 2014, 289, 19917–19927. [Google Scholar] [CrossRef] [PubMed]
- Price, D.L.; Rhett, P.M.; Thorpe, S.R.; Baynes, J.W. Chelating activity of advanced glycation end-product inhibitors. J. Biol. Chem. 2001, 276, 48967–48972. [Google Scholar] [CrossRef]
- Voziyan, P.A.; Khalifah, R.G.; Thibaudeau, C.; Yildiz, A.; Jacob, J.; Serianni, A.S.; Hudson, B.G. Modification of proteins in vitro by physiological levels of glucose: Pyridoxamine inhibits conversion of Amadori intermediate to advanced glycation end−products through binding of redox metal ions. J. Biol. Chem. 2003, 278, 46616–46624. [Google Scholar] [CrossRef]
- Nagai, R.; Murray, D.B.; Metz, T.O.; Baynes, J.W. Chelation: A fundamental mechanism of action of AGE Inhibitors, AGE breakers, and other inhibitors of diabetes complications. Diabetes 2012, 61, 549–559. [Google Scholar] [CrossRef]
- Ott, C.; Jacobs, K.; Haucke, E.; Santos, A.N.; Grune, T.; Simm, A. Role of advanced glycation end products in cellular signaling. Redox Biol. 2014, 2, 411–429. [Google Scholar] [CrossRef] [PubMed]
- Bucala, R.; Tracey, K.J.; Cerami, A. Advanced glycosylation products quench nitric oxide and mediate defective endo-thelium-dependent vasodilatation in experimental diabetes. J. Clin. Invest. 1991, 87, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.J.; Hebelstrup, K.H.; Mur, L.A.J.; Igamberdiev, A.U. Plant hemoglobins: Important players at the crossroads between oxygen and nitric oxide. FEBS Lett. 2011, 585, 3843–3849. [Google Scholar] [CrossRef]
- Berger, A.; Brouquisse, R.; Pathak, P.K.; Hichri, I.; Inderjit; Bhatia, S.; Boscari, A.; Igamberdiev, A.U.; Gupta, K.J. Pathways of nitric oxide metabolism and operation of phytoglobins in legume nodules: Missing links and future directions. Plant Cell Environ. 2018, 41, 2057–2068. [Google Scholar] [CrossRef]
- Kosmachevskaya, O.V.; Topunov, A.F. Formation of glycated recombinant leghemoglobin in Escherichia coli cells. Appl. Biochem. Microbiol. 2010, 46, 297–302. [Google Scholar] [CrossRef]
- Perrone, A.; Giovino, A.; Benny, J.; Martinelli, F. Advanced glycation end products (AGEs): Biochemistry, signaling, analytical methods, and epigenetic effects. Oxid. Med. Cell. Longev. 2020, 3, 1–18. [Google Scholar] [CrossRef]
- Villa, M.; Parravano, M.; Micheli, A.; Gaddini, L.; Matteucci, A.; Mallozzi, C.; Facchiano, F.; Malchiodi-Albedi, F.; Pricci, F. A quick, simple method for detecting circulating fluorescent advanced glycation end-products: Correlation with in vitro and in vivo non-enzymatic glycation. Metabolism 2017, 71, 64–69. [Google Scholar] [CrossRef]
- Kosmachevskaya, O.V.; Nasybullina, E.I.; Shumaev, K.B.; Topunov, A.F. Expressed soybean leghemoglobin: Effect on Escherichia coli at oxidative and nitrosative stress. Molecules 2021, 26, 7207. [Google Scholar] [CrossRef]
- Xu, X.; O’Callaghan, J.A.; Guarnero, Z.; Qiu, H.; Li, N.; Potocky, T.; Kamen, D.E.; Graham, K.S.; Shameem, M.; Yang, T.-C. Low pKa of Lys promotes glycation at one complementarity-determining region of a bispecific antibody. Biophys. J. 2022, 121, 1081–1093. [Google Scholar] [CrossRef]
- Shumilina, J.; Kusnetsova, A.; Tsarev, A.; Janse van Rensburg, H.C.; Medvedev, S.; Demidchik, V.; Van den Ende, W.; Frolov, A. Glycation of plant proteins: Regulatory roles and interplay with sugar signalling? Int. J. Mol. Sci. 2019, 20, 2366. [Google Scholar] [CrossRef]
- Rabbani, N.; Al-Motawa, M.; Thornalley, P.J. Protein glycation in plants-an under-researched field with much still to discover. Int. J. Mol. Sci. 2020, 21, 3942. [Google Scholar] [CrossRef] [PubMed]
- Warwicker, J.; Charonis, S.; Curtis, R.A. Lysine and arginine content of proteins: Computational analysis suggests a new tool for solubility design. Mol. Pharm. 2014, 11, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Azami-Movahed, M.; Meratan, A.A.; Ghasemi, A.; Ebrahim-Habibi, A.; Nemat-Gorgani, M. Acetylation of lysine residues in apomyoglobin: Structural changes, amyloid fibrillation, and role of surface charge. Int. J. Biol. Macromol. 2018, 107, 626–634. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zheng, Y.; Xu, P.; Zhu, X.; Zhou, C. L-Lysine and L-arginine inhibit myosin aggregation and interact with acidic amino acid residues of myosin: The role in increasing myosin solubility. Food Chem. 2018, 242, 22–28. [Google Scholar] [CrossRef]
- Ahmad, N.N.; Kamarudin, N.H.A.; Leow, A.T.C.; Abd. Rahman, R.N.Z.R. The role of surface exposed lysine in conformational stability and functional properties of lipase from Staphylococcus Family. Molecules 2020, 25, 3858. [Google Scholar] [CrossRef]
- Isogai, Y.; Nakae, S.; Sumi, T.; Takahashi, K.; Shirai, T. Common and unique strategies of myoglobin evolution for deep-sea adaptation of diving mammals. Iscience 2021, 24, 102920. [Google Scholar] [CrossRef]
- Verma, D.P.S.; Bal, A.K. Intracellular site of synthesis and localization of leghemoglobin in root nodules. Proc. Natl. Acad. Sci. USA 1976, 73, 3843–3847. [Google Scholar] [CrossRef]
- Davies, M.J.; Mathieu, C.; Puppo, A. Leghemoglobin: Properties and reactions. Adv. Inorg. Chem. 1999, 46, 495–542. [Google Scholar] [CrossRef]
- Raupbach, J.; Ott, C.; Koenig, J.; Grune, T. Proteasomal degradation of glycated proteins depends on substrate unfolding. Preferred degradation of moderately modified myoglobin. Free Radic. Biol. Med. 2022, 152, 516–524. [Google Scholar] [CrossRef]
- Banerjee, S.; Maity, S.; Chakraborti, A.S. Methylglyoxal-induced modification causes aggregation of myoglobin. Spectrochim. Acta A Mol. Biomol Spectrosc. 2016, 155, 1–10. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shumaev, K.B.; Kosmachevskaya, O.V.; Nasybullina, E.I.; Ruuge, E.K.; Topunov, A.F. Role of Nitric Oxide-Derived Metabolites in Reactions of Methylglyoxal with Lysine and Lysine-Rich Protein Leghemoglobin. Int. J. Mol. Sci. 2023, 24, 168. https://doi.org/10.3390/ijms24010168
Shumaev KB, Kosmachevskaya OV, Nasybullina EI, Ruuge EK, Topunov AF. Role of Nitric Oxide-Derived Metabolites in Reactions of Methylglyoxal with Lysine and Lysine-Rich Protein Leghemoglobin. International Journal of Molecular Sciences. 2023; 24(1):168. https://doi.org/10.3390/ijms24010168
Chicago/Turabian StyleShumaev, Konstantin B., Olga V. Kosmachevskaya, Elvira I. Nasybullina, Enno K. Ruuge, and Alexey F. Topunov. 2023. "Role of Nitric Oxide-Derived Metabolites in Reactions of Methylglyoxal with Lysine and Lysine-Rich Protein Leghemoglobin" International Journal of Molecular Sciences 24, no. 1: 168. https://doi.org/10.3390/ijms24010168
APA StyleShumaev, K. B., Kosmachevskaya, O. V., Nasybullina, E. I., Ruuge, E. K., & Topunov, A. F. (2023). Role of Nitric Oxide-Derived Metabolites in Reactions of Methylglyoxal with Lysine and Lysine-Rich Protein Leghemoglobin. International Journal of Molecular Sciences, 24(1), 168. https://doi.org/10.3390/ijms24010168





