The Biological Implication of Semicarbazide-Sensitive Amine Oxidase (SSAO) Upregulation in Rat Systemic Inflammatory Response under Simulated Aerospace Environment
Abstract
1. Introduction
2. Results
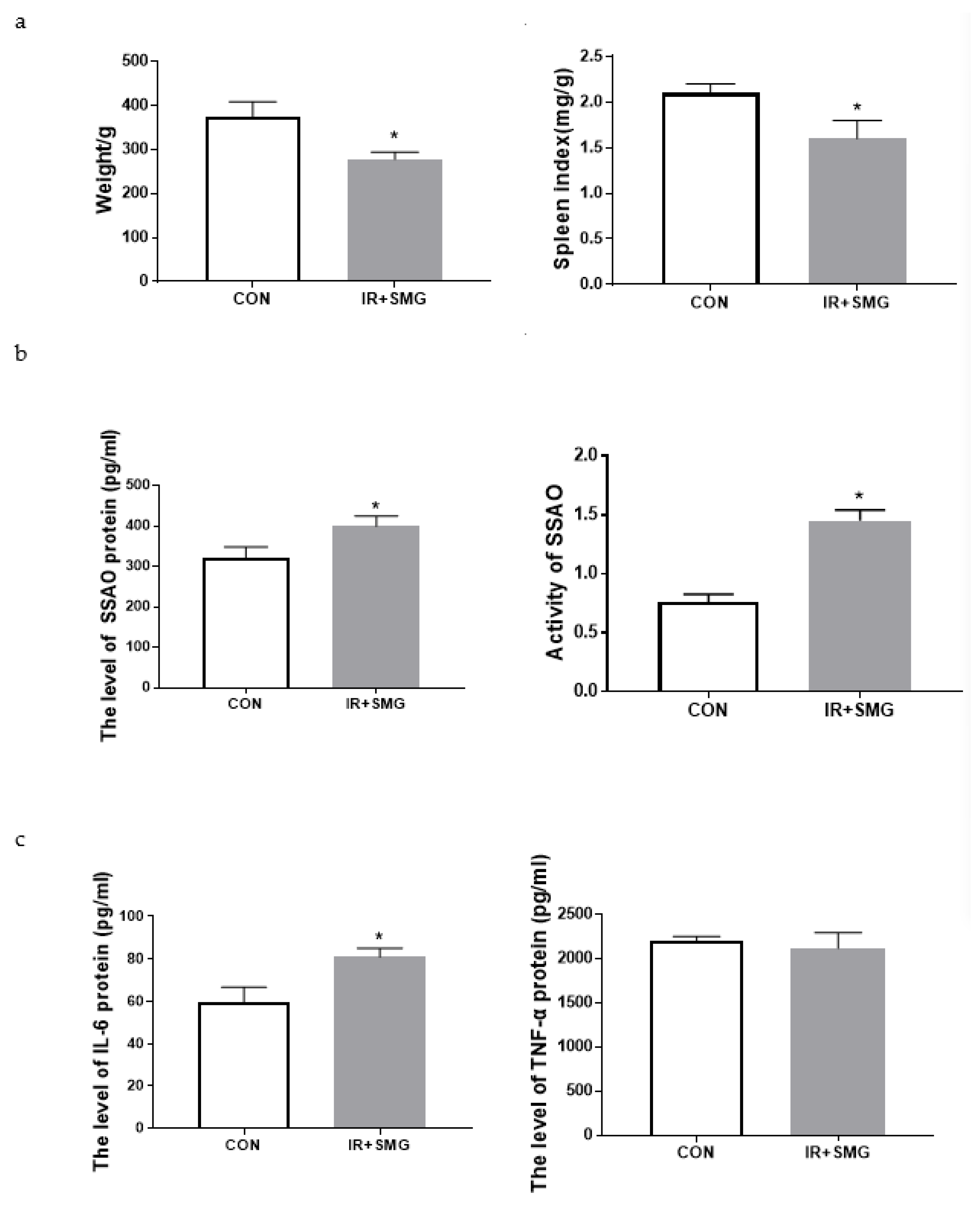
2.1. Simulated Space Environment Induced Changes of Physiological Characteristics and Inflammatory Factors of Serum in Rats
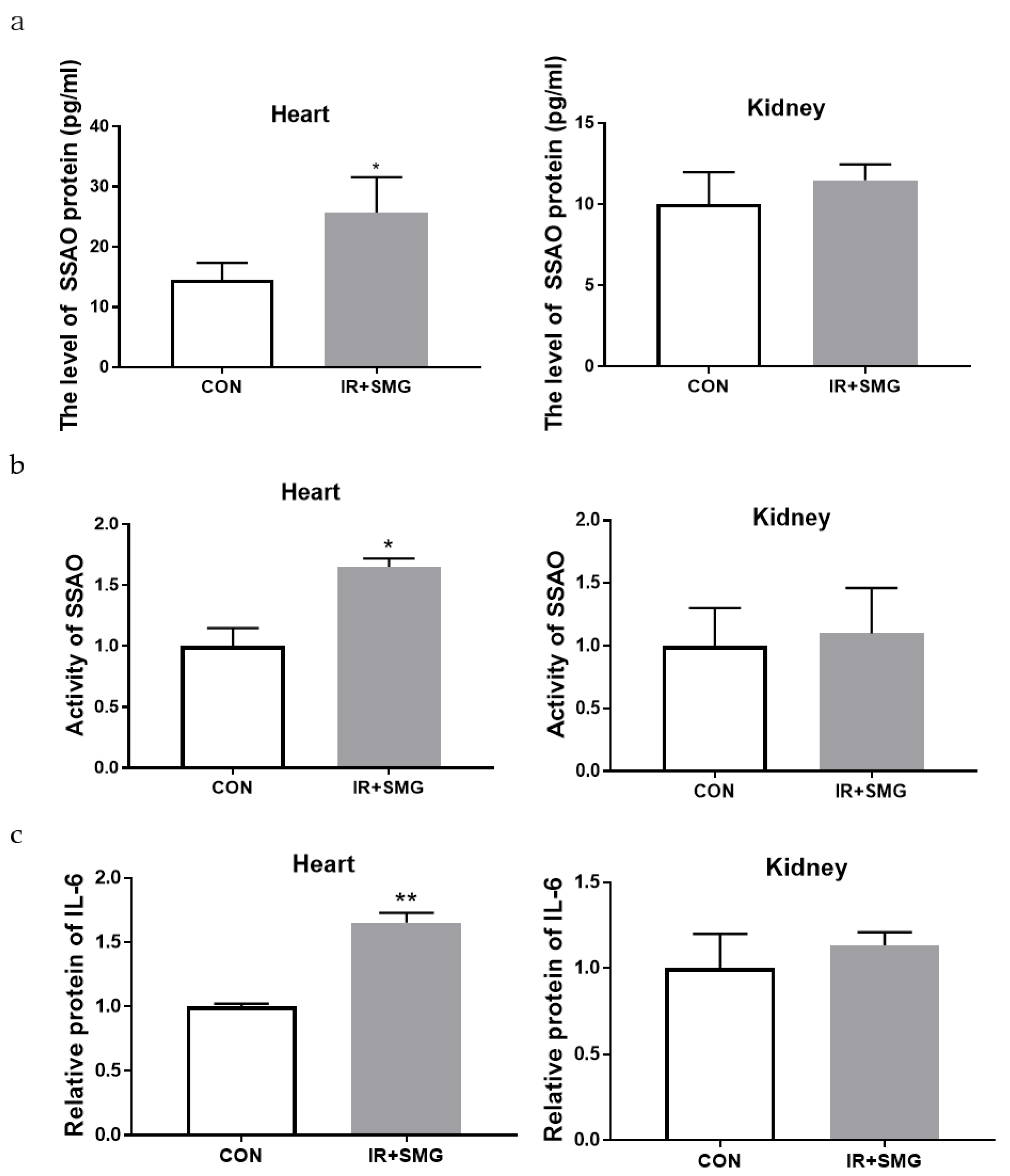
2.2. Changes of Different Tissues in Rats Induced by Simulated Space Environment
2.3. The Biological Effects of Space Environment on Rat Heart and Kidney Tissue Were Simulated
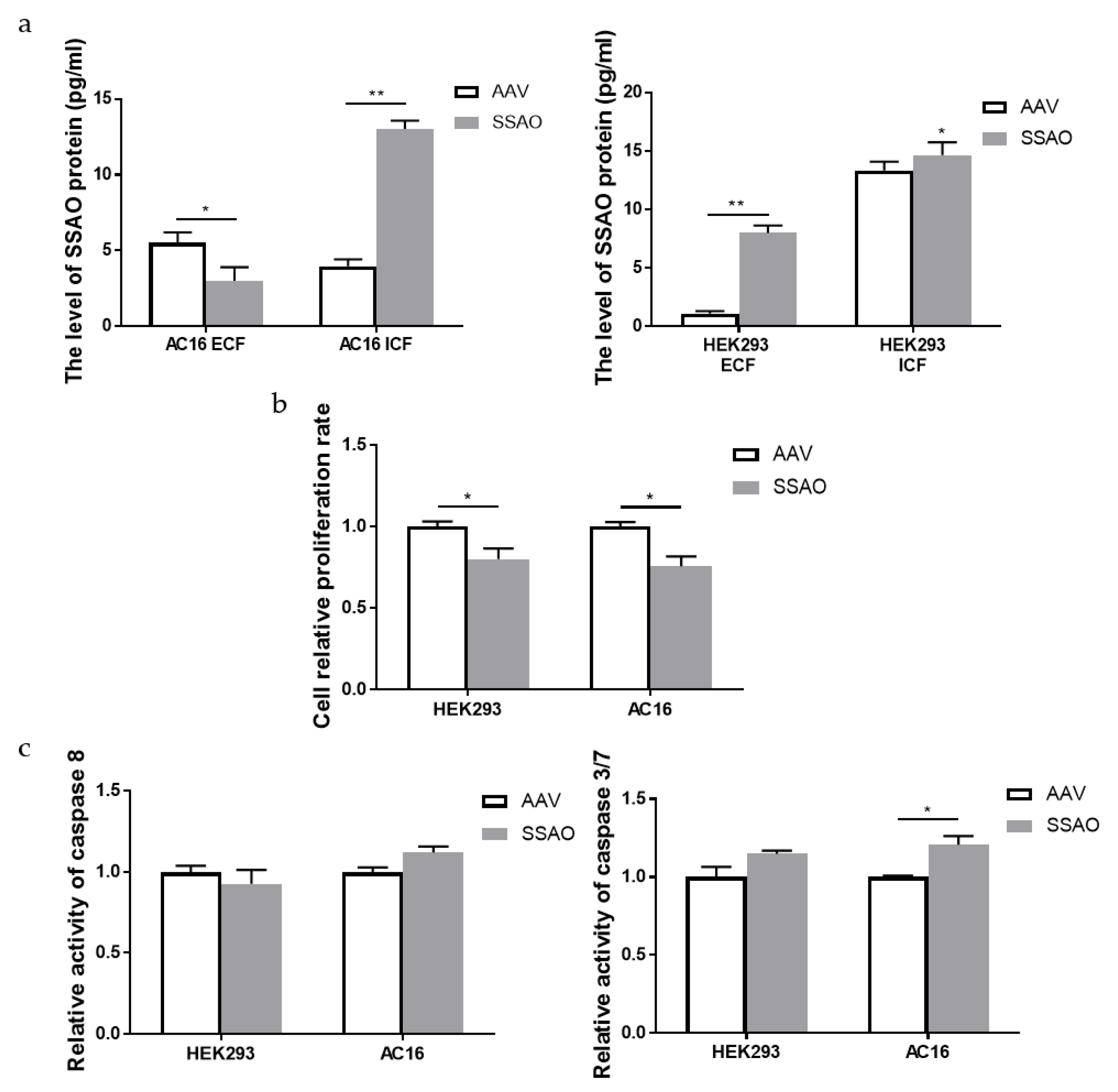
2.4. Bioactivity of Human Cardiomyocyte AC16 and Human Embryonic Kidney HEK293 Cells Overexpressed by SSAO
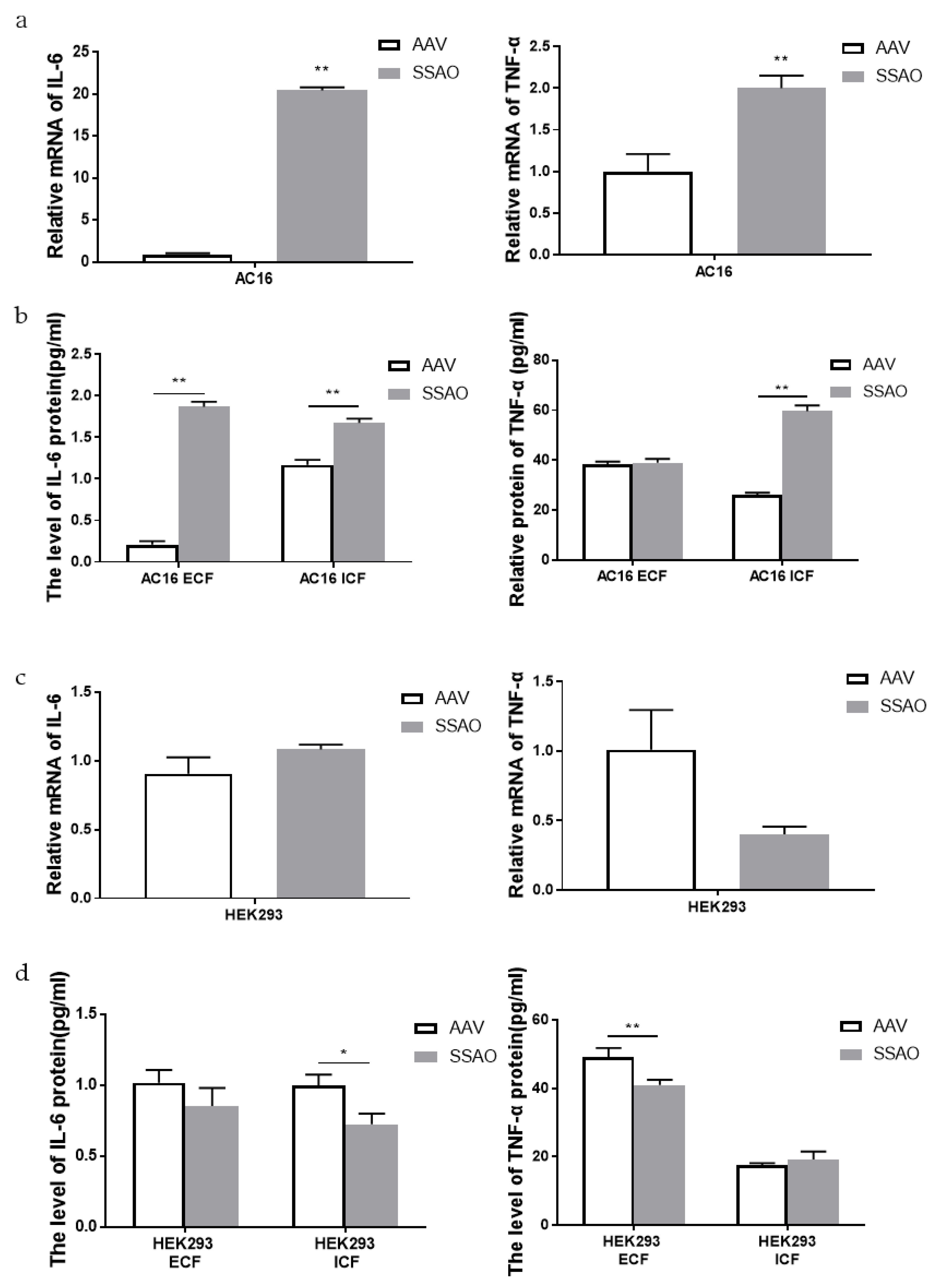
2.5. Effect of SSAO Overexpression on Inflammatory Response of Human Cardiomyocytes AC16 and Human Embryonic Kidney Cells Hek293
3. Discussion
4. Materials and Methods
4.1. Animal Groups and Treatment
4.2. H&E Staining
4.3. RT-PCR
4.4. ELISA
4.5. Packaging and Extraction of Adeno-Associated Virus
4.6. SSAO Activity Analysis
4.7. Cells and Culture
4.8. Caspase Activity Assays
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Virani, S.S.; Alonso, A.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2020 update: A report from the American Heart Association. Circulation 2020, 141, e139–e596. [Google Scholar] [PubMed]
- Zheng, Y.; Ley, S.H.; Hu, F.B. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat. Rev. Endocrinol. 2018, 14, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Goedert, M.; Spillantini, M.G. A century of Alzheimer’s disease. Science 2006, 314, 777–781. [Google Scholar] [CrossRef]
- Unzeta, M.; Hernàndez-Guillamon, M.; Sun, P.; Solé, M. SSAO/VAP-1 in cerebrovascular disorders: A potential therapeutic target for stroke and Alzheimer’s disease. Int. J. Mol. Sci. 2021, 22, 3365. [Google Scholar] [CrossRef]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef]
- Boomsma, F.; van Dijk, J.; Bhaggoe, U.M.; Bouhuizen, A.M.; Van den Meiracker, A.H. Variation in semicarbazide-sensitive amine oxidase activity in plasma and tissues of mammals. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2000, 126, 69–78. [Google Scholar] [CrossRef]
- Obata, T. Endogenous semicarbazide-sensitive amine oxidase (SSAO) inhibitor increases 1-methyl-4-phenylpyridinium ion (MPP+)-induced dopamine efflux by immobilization stress in rat striatum. Int. J. Dev. Neurosci. 2006, 24, 343–347. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Pei, S.; Wang, A.; Chen, Y.; Zhang, P.; Li, B.; Lodhi, A.F.; Ren, H.; Dai, R.; Deng, Y.; et al. Possible role of a dual regulator of neuroinflammation and autophagy in a simulated space environment. Acta Astronaut. 2021, 187, 181–189. [Google Scholar] [CrossRef]
- Lei, R.; Zhao, T.; Li, Q.; Wang, X.; Ma, H.; Deng, Y. Carbon ion irradiated neural injury induced the peripheral immune effects in vitro or in vivo. Int. J. Mol. Sci. 2015, 16, 28334–28346. [Google Scholar] [CrossRef]
- Zhang, P.; Chen, Y.; Zhu, H.; Yan, L.; Sun, C.; Pei, S.; Ma, H. The effect of gamma-ray-induced central nervous system injury on peripheral immune response: An in vitro and in vivo study. Radlat. Res. 2019, 192, 440–450. [Google Scholar] [CrossRef]
- Wang, Y.; Qin, P.; Hong, J.; Li, N.; Zhang, Y.; Deng, Y. Deep membrane proteome profiling of rat hippocampus in simulated complex space environment by SWATH. Space Sci. Technol. 2021, 2021, 9762372. [Google Scholar] [CrossRef]
- Smith, D.J.; Salmi, M.; Bono, P.; Hellman, J.; Leu, T.; Jalkanen, S. Cloning of Vascular Adhesion Protein 1 Reveals a Novel Multifunctional Adhesion Molecule. J. Exp. Med. 1998, 188, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Du, S.; Niu, P.; Gu, X.; Wang, J.; Zhao, Y. Vascular adhesion protein-1 (VAP-1)/Semicarbazide-sensitive amine oxidase (SSAO): A potential therapeutic target for atherosclerotic cardiovascular diseases. Front. Pharmacol. 2021, 12, 1748. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-Y.; Wei, J.-N.; Lin, M.-S.; Smith, D.J.; Vainio, J.; Lin, C.-H.; Chiang, F.-T.; Shih, S.-R.; Huang, C.-H.; Wu, M.-Y.; et al. Serum vascular adhesion protein-1 is increased in acute and chronic hyperglycemia. Clin. Chim. Acta 2009, 404, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Boomsma, F.; Meiracker, A.H.V.D.; Winkel, S.; Aanstoot, H.J.; Batstra, M.R.; Veld, A.J.M.I.; Bruining, G.J. Circulating semicarbazide-sensitive amine oxidase is raised both in type I (insulin-dependent), in type II (non-insulin-dependent) diabetes mellitus and even in childhood type I diabetes at first clinical diagnosis. Diabetologia 1999, 42, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Kurkijarvi, R.; Adams, D.H.; Leino, R.; Mottonen, T.; Jalkanen, S.; Salmi, M. Circulating form of human vascular adhesion protein-1 (VAP-1): Increased serum levels in inflammatory liver diseases. J. Immunol. 1998, 161, 1549–1557. [Google Scholar] [CrossRef] [PubMed]
- Aalto, K.; Maksimow, M.; Juonala, M.; Viikari, J.; Jula, A.; Kähönen, M.; Jalkanen, S.; Raitakari, O.T.; Salmi, M. Soluble vascular adhesion protein-1 correlates with cardiovascular risk factors and early atherosclerotic manifestations. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 523–532. [Google Scholar] [CrossRef]
- Li, H.Y.; Lin, M.S.; Wei, J.N.; Hung, C.S.; Chiang, F.T.; Lin, C.H.; Chuang, L.M. Change of serum vascular adhesion protein-1 after glucose loading correlates to carotid intima-medial thickness in non-diabetic subjects. Clin. Chim. Acta 2009, 403, 97–101. [Google Scholar] [CrossRef]
- Aalto, K.; Havulinna, A.S.; Jalkanen, S.; Salomaa, V.; Salmi, M. Soluble vascular adhesion protein-1 predicts incident major adverse cardiovascular events and improves reclassification in a Finnish prospective cohort study. Circ. Cardiovasc. Genet. 2014, 7, 529–535. [Google Scholar] [CrossRef]
- Mercier, N.; Pawelzik, S.-C.; Pirault, J.; Carracedo, M.; Persson, O.; Wollensack, B.; Franco-Cereceda, A.; Bäck, M. Semicarbazide-sensitive amine oxidase increases in calcific aortic valve stenosis and contributes to valvular interstitial cell calcification. Oxid. Med. Cell Longev. 2020, 2020, 5197376. [Google Scholar] [CrossRef]
- Li, H.-Y.; Jiang, Y.-D.; Chang, T.-J.; Wei, J.-N.; Lin, M.-S.; Lin, C.-H.; Chiang, F.-T.; Shih, S.-R.; Hung, C.S.; Hua, C.-H.; et al. Serum vascular adhesion protein-1 predicts 10-year cardiovascular and cancer mortality in individuals with type 2 diabetes. Diabetes 2011, 60, 993–999. [Google Scholar] [CrossRef] [PubMed]
- Rcheulishvili, N.; Papukashvili, D.; Deng, Z.; Wang, S.; Deng, Y. Simulated microgravity alters the expression of plasma SSAO and its enzymatic activity in healthy rats and increases the mortality in high-fat diet/streptozotocin-induced diabetes. Life Sci. Space Res. 2021, 30, 24–28. [Google Scholar] [CrossRef]
- Unzeta, M.; Sole, M.; Boada, M. Semicarbazide-sensitive amine oxidase (SSAO) and its possible contribution to vascular damage in Alzheimer’s disease. J. Neural. Transm. 2007, 114, 857–862. [Google Scholar] [CrossRef] [PubMed]
- Boomsma, F.; van Woerkens, L.J.; Man’t Veld, A.J.; Verdouw, P.D.; Schalekamp, M.A. High Activity of Semicarbazide-Sensitive Amine Oxidase (SSAO). J. Cardiovasc. Pharm. 1993, 22, 198–202. [Google Scholar] [CrossRef]
- Hughson Richard, L.; Alexander, H.; Marco, D. Heart in space: Effect of the extraterrestrial environment on the cardiovascular system. Nat. Rev. Cardiol. 2018, 15, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Kanapskyte, A.; Hawkins, E.M.; Liddell, L.C.; Bhardwaj, S.R.; Gentry, D.; Santa Maria, S.R. Space Biology Research and Biosensor Technologies: Past, Present, and Future. Biosensors 2021, 11, 38. [Google Scholar] [CrossRef]
- Dynan, W.S.; Chang, P.Y.; Sishc, B.J.; Elgart, S.R. Breaking the limit: Biological countermeasures for space radiation exposure to enable long-duration spaceflight. Life Sci. Space Res. 2022, 35, 1–3. [Google Scholar] [CrossRef]
- Stevens, A.H.; Kobs Nawotniak, S.E.; Garry, W.B.; Payler, S.J.; Brady, A.L.; Miller, M.J.; Lim, D.S. Tactical scientific decision-making during crewed astrobiology Mars missions. Astrobiology 2019, 19, 369–386. [Google Scholar] [CrossRef]
- Cui, Y.; Ding, Y.; Du, X. Research progress of cell apoptosis and radiation immune damage. Foreign Med. Radiol. J. Nucl. Med. 2002, 26, 271–274. [Google Scholar]
- Seki, H. Radioprotection of cytokines on apoptosis of peripheral lymphocyte subpopulations induced by radiation. Cell Immunol. 1995, 163, 30–36. [Google Scholar] [CrossRef]
- Yang, W.; Zhu, R. Radiation sensitivity of T and B lymphocytes. Foreign Med. Radiol. Nucl. Med. 1980, 3, 141–145. [Google Scholar]
- Benderitter, M.; Durand, V.; Caux, C.; Voisin, P. Clearance of radiation induced apoptotic lymphocytes: Ex vivo studies and an in vitro co-culture model. Radiat. Res. 2002, 158, 464–474. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.H.; Qu, L.N.; Chen, H.L. Space stress injury and related protective measures. Sheng Li Ke Xue Jin Zhan 2013, 44, 354–358. [Google Scholar] [PubMed]
- Sihver, L.; Mortazavi SM, J. Biological Protection in Deep Space Missions. J. Biomed. Phys. Eng. 2021, 11, 663. [Google Scholar] [CrossRef]
- Siddiqui, R.; Akbar, N.; Khan, N.A. Gut microbiome and human health under the space environment. J. Appl. Microbiol. 2021, 130, 14–24. [Google Scholar] [CrossRef]
- Garrett-Bakelman, F.E.; Darshi, M.; Green, S.J.; Gur, R.C.; Lin, L.; Macias, B.R.; Turek, F.W. The NASA Twins Study: A multidimensional analysis of a year-long human spaceflight. Science 2019, 364, eaau8650. [Google Scholar] [CrossRef]
- Kandarpa, K.; Schneider, V.; Ganapathy, K. Human health during space travel: An overview. Neurol. India 2019, 67, 176. [Google Scholar]
- Sekiguchi, C. Issues of health care under weightlessness. Acta physiologica scandinavica. Supplementum 1994, 616, 89–97. [Google Scholar]
- Debijadhi, R. Effect of weightlessness on human cardiovascular system. Srp. Arh. Celok. Lek. 1995, 123, 202–207. [Google Scholar]
- Bo, C.; Zhang, Y.S.; George, L.I.; Cho, J.-I.; Deng, Y.-L.; Li, Y.-J. The impacts of simulated microgravity on rat brain depended on durations and regions. Biomed. Environ. Sci. 2019, 32, 496–507. [Google Scholar]
- Whittle, R.S.; Diaz-Artiles, A. Modeling individual differences in cardiovascular response to gravitational stress using a sensitivity analysis. J. App. Physiol. 2021, 130, 1983–2001. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Zhao, X.C.; Jiao, B.; Yue, Z.J.; Yu, Z.B. Simulated microgravity hampers Notch signaling in the fight against myocardial ischemia-reperfusion injury. Mol. Med. Rep. 2018, 17, 5150–5158. [Google Scholar] [CrossRef] [PubMed]
- Morey-Holton, E.R.; Globus, R.K. Hindlimb unloading rodent model: Technical aspects. J. Appl. Physiol. 2002, 92, 1367–1377. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.P.; Tran, P.H.; Kim, K.S.; Yang, S.G. The effects of real and simulated microgravity on cellular mitochondrial function. NPJ Microgravity 2021, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hassler, D.M.; Zeitlin, C.; Wimmer-Schweingruber, R.F.; Ehresmann, B.; Rafkin, S.; Eigenbrode, J.L.; Berger, G. Mars’ surface radiation environment measured with the Mars Science Laboratory’s Curiosity rover. Science 2014, 343, 1244797. [Google Scholar] [CrossRef]
- Beheshti, A.; McDonald, J.T.; Hada, M.; Takahashi, A.; Mason, C.E.; Mognato, M. Genomic Changes Driven by Radiation-Induced DNA Damage and Microgravity in Human Cells. Int. J. Mol. Sci. 2021, 22, 10507. [Google Scholar] [CrossRef]
- Lizcano, J.M.; Unzeta, M. On the primary structure of membrane-bound semicarbazide-sensitive amine oxidase (SSAO). Neurobiology 2000, 8, 37–46. [Google Scholar]
- Zhao, B.X.; Chen, Y.H.; Liu, J.F.; Zhang, L.; Zhang, J.; Yang, Y.L.; Lv, Q.; Xie, M.X. Blood-brain barrier disruption induced by diagnostic ultrasound combined with microbubbles in mice. Oncotarget 2017, 9, 4897–4914. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, L.; Sun, C.; Zhang, Y.; Zhang, P.; Chen, Y.; Deng, Y.; Er, T.; Deng, Y.; Wang, Z.; Ma, H. The Biological Implication of Semicarbazide-Sensitive Amine Oxidase (SSAO) Upregulation in Rat Systemic Inflammatory Response under Simulated Aerospace Environment. Int. J. Mol. Sci. 2023, 24, 3666. https://doi.org/10.3390/ijms24043666
Yan L, Sun C, Zhang Y, Zhang P, Chen Y, Deng Y, Er T, Deng Y, Wang Z, Ma H. The Biological Implication of Semicarbazide-Sensitive Amine Oxidase (SSAO) Upregulation in Rat Systemic Inflammatory Response under Simulated Aerospace Environment. International Journal of Molecular Sciences. 2023; 24(4):3666. https://doi.org/10.3390/ijms24043666
Chicago/Turabian StyleYan, Liben, Chunli Sun, Yaxi Zhang, Peng Zhang, Yu Chen, Yifan Deng, Tianyi Er, Yulin Deng, Zhimin Wang, and Hong Ma. 2023. "The Biological Implication of Semicarbazide-Sensitive Amine Oxidase (SSAO) Upregulation in Rat Systemic Inflammatory Response under Simulated Aerospace Environment" International Journal of Molecular Sciences 24, no. 4: 3666. https://doi.org/10.3390/ijms24043666
APA StyleYan, L., Sun, C., Zhang, Y., Zhang, P., Chen, Y., Deng, Y., Er, T., Deng, Y., Wang, Z., & Ma, H. (2023). The Biological Implication of Semicarbazide-Sensitive Amine Oxidase (SSAO) Upregulation in Rat Systemic Inflammatory Response under Simulated Aerospace Environment. International Journal of Molecular Sciences, 24(4), 3666. https://doi.org/10.3390/ijms24043666





