Murine Animal Models in Osteogenesis Imperfecta: The Quest for Improving the Quality of Life
Abstract
1. Introduction
2. Osteogenesis Imperfecta Murine Models Evaluated in Preclinical Research
2.1. Osteogenesis Imperfecta Mice (oim)
2.2. Heterozygotic G610C Mice (Amish Mice)
2.3. Brittle Mice (Brtl)
2.4. Jrt Heterozygous Mice
2.5. Heterozygous Abnormal Gait 2 (Aga2) Mice
2.6. Heterozygous Col1a1±365 OI Mouse
2.7. Crtap Mouse
2.8. IFITM5 Transgenic Mice
3. Revealing Parameters in Preclinical In Vivo Studies
3.1. Bone Microarchitecture
3.1.1. Microcomputed Tomography
3.1.2. Raman Spectroscopy
3.2. Bone Biomechanical Properties
3.2.1. Three- and Four-Point Bending
3.2.2. Torsional Loading to Failure
3.3. Markers in Biological Samples: Blood and Urine
3.4. Transcriptome Analysis
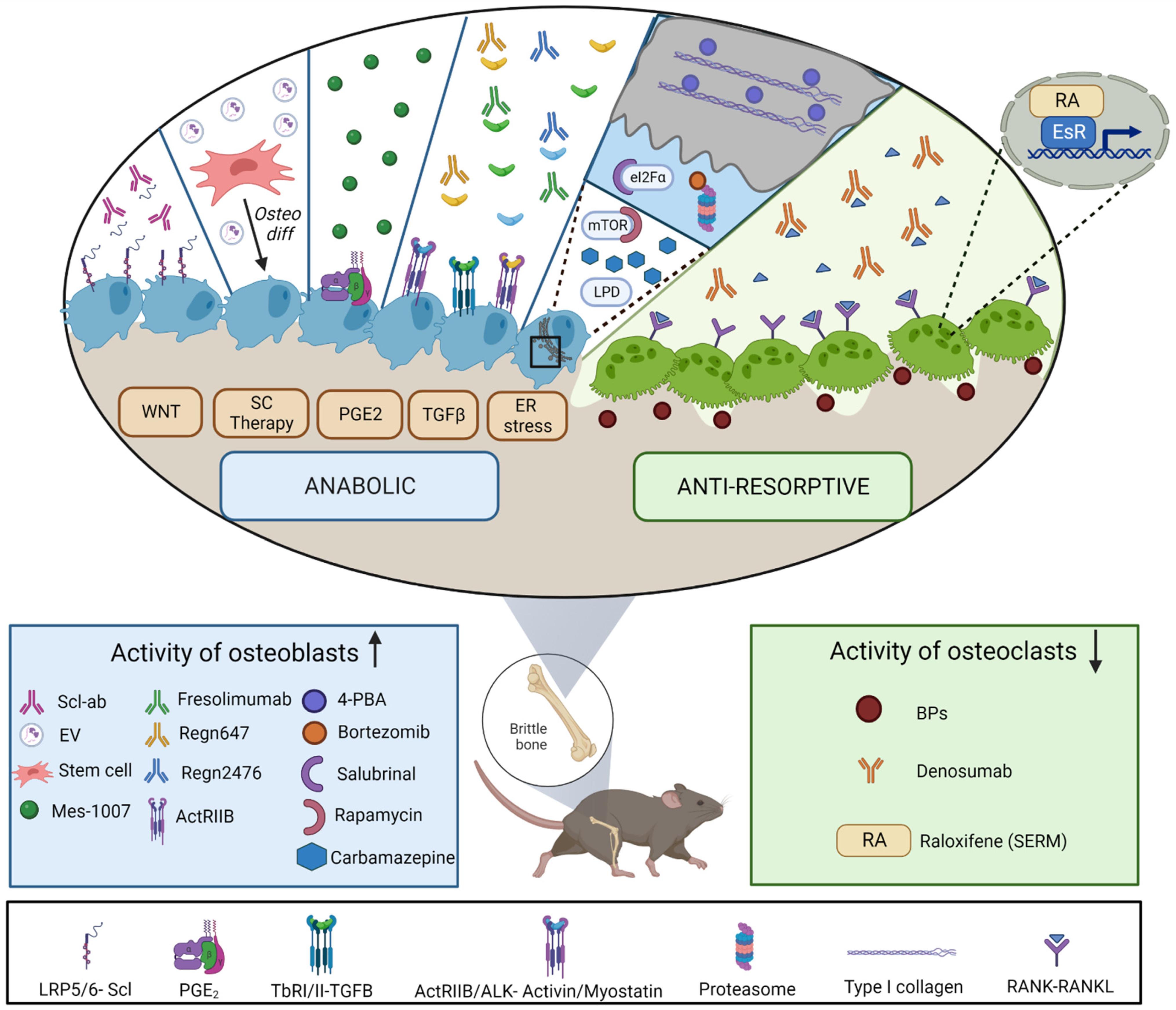
4. Treatments for Osteogenesis Imperfecta: In Vivo Studies
4.1. Antiresorptives
4.2. Anabolic Treatments
4.2.1. Targeting Wnt Signaling Pathway
4.2.2. TGF-β Superfamily Modulators
4.2.3. Targeting Cellular Stress
4.2.4. Prostaglandin E2 Receptor
4.2.5. Stem-Cell-Therapy Approaches
Moderate-Severe OI Mice Models and Cell Approaches
Mild-OI Mice Models and Cell Approaches
5. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Abbreviation | Definition |
| µCT | Microcomputed tomography |
| 2D | Two-dimensional |
| 3D | Three-dimensional |
| 4-PBA | 4-phenylbutyrate |
| N-terminal | Amino- terminal |
| ActRIIB | Activin receptor type IIB |
| ATP | Adenosine triphosphate |
| BM | Bone Marrow |
| BMD | Bone mineral density |
| BPs | Bisphosphonates |
| C-terminal | Carboxyl- terminal |
| CTX | Carboxyl-terminal cross-linking telopeptide of type I collagen |
| ECM | Extracellular matrix |
| ELISA | Enzyme-linked immunoassay |
| ER | Endoplasmic reticulum |
| EVs | Extracellular vesicles |
| FPP | Farnesyl Pyrophosphate |
| GFP | Green fluorescent protein |
| hfMSCs | Human fetal blood MSCs |
| ISR | Integrated stress response |
| IUT | In utero transplantation |
| LH2 | Lysyl hydroxylase 2 |
| LRP | Low density lipoprotein receptor-related protein |
| mAb | Monoclonal Antibody |
| MSCs | Mesenchymal stem cells |
| NGS | Next-generation sequencing |
| OI | Osteogenesis Imperfecta |
| OST | Osteocalcin |
| PGE2 | Prostaglandin E2 |
| RANKL | Receptor activator of nuclear factor kappa beta ligand |
| RNA | Ribonucleic acid |
| Scl-Ab | Anti-sclerostin antibody |
| SDF1 | Stromal cell-derived factor 1 |
| TGF-β | Transforming growth factor beta |
| TRAP | Tartrate-resistant acid phosphatase |
| WNT | Wingless-related integration site |
| WT | Wild type |
| YAP | Yes-associated protein |
References
- Marini, J.C.; Forlino, A.; Bächinger, H.P.; Bishop, N.J.; Byers, P.H.; Paepe, A.; Fassier, F.; Fratzl-Zelman, N.; Kozloff, K.M.; Krakow, D.; et al. Osteogenesis imperfecta. Nat. Rev. Dis. Primers 2017, 3, 17052. [Google Scholar] [CrossRef] [PubMed]
- Jovanovic, M.; Guterman-Ram, G.; Marini, J.C. Osteogenesis Imperfecta: Mechanisms and Signaling Pathways Connecting Classical and Rare OI Types. Endocr. Rev. 2022, 43, 61–90. [Google Scholar] [CrossRef] [PubMed]
- Garibaldi, N.; Besio, R.; Dalgleish, R.; Villani, S.; Barnes, A.M.; Marini, J.C.; Forlino, A. Dissecting the phenotypic variability of osteogenesis imperfecta. Dis. Model. Mech. 2022, 15, dmm049398. [Google Scholar] [CrossRef]
- Marom, R.; Rabenhorst, B.M.; Morello, R. Osteogenesis imperfecta: An update on clinical features and therapies. Eur. J. Endocrinol. 2020, 183, R95–R106. [Google Scholar] [CrossRef] [PubMed]
- Botor, M.; Fus-Kujawa, A.; Uroczynska, M.; Stepien, K.L.; Galicka, A.; Gawron, K.; Sieron, A.L. Osteogenesis Imperfecta: Current and Prospective Therapies. Biomolecules 2021, 11, 1493. [Google Scholar] [CrossRef] [PubMed]
- Dwan, K.; Phillipi, C.A.; Steiner, R.D.; Basel, D. Bisphosphonate therapy for osteogenesis imperfecta. Cochrane Database Syst. Rev. 2016, 10, CD005088. [Google Scholar] [CrossRef]
- Lu, L.; Zhang, J.; Li, J. Potential risks of rare serious adverse effects related to long-term use of bisphosphonates: An overview of systematic reviews. J. Clin. Pharm. Ther. 2020, 45, 45–51. [Google Scholar] [CrossRef]
- Castillo, H.; Samson-Fang, L.; American Academy for Cerebral Palsy and Developmental Medicine Treatment Outcomes Committee Review Panel. Effects of bisphosphonates in children with osteogenesis imperfecta: An AACPDM systematic review. Dev. Med. Child Neurol. 2009, 51, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Hoyer-Kuhn, H.; Franklin, J.; Allo, G.; Kron, M.; Netzer, C.; Eysel, P.; Hero, B.; Schoenau, E.; Semler, O. Safety and efficacy of denosumab in children with osteogenesis imperfect—A first prospective trial. J. Musculoskelet. Neuronal Interact. 2016, 16, 24–32. [Google Scholar]
- Bone, H.G.; Wagman, R.B.; Brandi, M.L.; Brown, J.P.; Chapurlat, R.; Cummings, S.R.; Czerwiński, E.; Fahrleitner-Pammer, A.; Kendler, D.L.; Lippuner, K.; et al. 10 years of denosumab treatment in postmenopausal women with osteoporosis: Results from the phase 3 randomised FREEDOM trial and open-label extension. Lancet Diabetes Endocrinol. 2017, 5, 513–523. [Google Scholar] [CrossRef]
- Trejo, P.; Rauch, F.; Ward, L. Hypercalcemia and hypercalciuria during denosumab treatment in children with osteogenesis imperfecta type VI. J. Musculoskelet. Neuronal Interact. 2018, 18, 76–80. [Google Scholar] [PubMed]
- Horiuchi, K.; Kobayashi, E.; Mizuno, T.; Susa, M.; Chiba, K. Hypercalcemia following discontinuation of denosumab therapy: A systematic review. Bone Rep. 2021, 15, 101148. [Google Scholar] [CrossRef] [PubMed]
- Ward, L.M.; Rauch, F. Anabolic Therapy for the Treatment of Osteoporosis in Childhood. Curr. Osteoporos. Rep. 2018, 16, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Leali, P.T.; Balsano, M.; Maestretti, G.; Brusoni, M.; Amorese, V.; Ciurlia, E.; Andreozzi, M.; Caggiari, G.; Doria, C. Efficacy of teriparatide. Clin. Cases Miner. Bone Metab. 2017, 14, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Vahle, J.L.; Sato, M.; Long, G.G.; Young, J.K.; Francis, P.C.; Engelhardt, J.A.; Westmore, M.S.; Linda, Y.; Nold, J.B. Skeletal changes in rats given daily subcutaneous injections of recombinant human parathyroid hormone (1-34) for 2 years and relevance to human safety. Toxicol. Pathol. 2002, 30, 312–321. [Google Scholar] [CrossRef]
- Enderli, T.A.; Burtch, S.R.; Templet, J.N.; Carriero, A. Animal models of osteogenesis imperfecta: Applications in clinical research. Orthop. Res. Rev. 2016, 8, 41–55. [Google Scholar] [CrossRef]
- Chipman, S.D.; Sweet, H.O.; McBride, D.J.; Davisson, M.T.; Marks, S.C.; Shuldiner, A.R.; Wenstrup, R.J.; Rowe, D.W.; Shapiro, J.R. Defective pro alpha 2(I) collagen synthesis in a recessive mutation in mice: A model of human osteogenesis imperfecta. Proc. Natl. Acad. Sci. USA 1993, 90, 1701–1705. [Google Scholar] [CrossRef]
- Lee, K.J.; Rambault, L.; Bou-Gharios, G.; Clegg, P.D.; Akhtar, R.; Czanner, G.; van ’t Hof, R.; Canty-Laird, E.G. Collagen (I) homotrimer potentiates the osteogenesis imperfecta (oim) mutant allele and reduces survival in male mice. Dis. Model. Mech. 2022, 15, dmm049428. [Google Scholar] [CrossRef]
- Pihlajaniemi, T.; Dickson, L.A.; Pope, F.M.; Korhonen, V.R.; Nicholls, A.; Prockop, D.J.; Myers, J.C. Osteogenesis imperfecta: Cloning of a pro-alpha 2(I) collagen gene with a frameshift mutation. J. Biol. Chem. 1984, 259, 12941–12944. [Google Scholar] [CrossRef]
- Pace, J.M.; Wiese, M.; Drenguis, A.S.; Kuznetsova, N.; Leikin, S.; Schwarze, U.; Chen, D.; Mooney, S.H.; Unger, S.; Byers, P.H. Defective C-propeptides of the proalpha2(I) chain of type I procollagen impede molecular assembly and result in osteogenesis imperfecta. J. Biol. Chem. 2008, 283, 16061–16067. [Google Scholar] [CrossRef]
- Nicholls, A.C.; Osse, G.; Schloon, H.G.; Lenard, H.G.; Deak, S.; Myers, J.C.; Prockop, D.J.; Weigel, W.R.; Fryer, P.; Pope, F.M. The clinical features of homozygous alpha 2(I) collagen deficient osteogenesis imperfecta. J. Med. Genet. 1984, 21, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Moffatt, P.; Boraschi-Diaz, I.; Marulanda, J.; Bardai, G.; Rauch, F. Calvaria Bone Transcriptome in Mouse Models of Osteogenesis Imperfecta. Int. J. Mol. Sci. 2021, 22, 5290. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, S.M.; Dimori, M.; Heard-Lipsmeyer, M.E.; Morello, R. The Osteocyte Transcriptome Is Extensively Dysregulated in Mouse Models of Osteogenesis Imperfecta. JBMR Plus 2019, 3, e10171. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Florez, N.; Garcia-Tunon, E.; Mukadam, Q.; Saiz, E.; Oldknow, K.J.; Farquharson, C.; Millán, J.L.; Boyde, A.; Shefelbine, S.J. An investigation of the mineral in ductile and brittle cortical mouse bone. J. Bone Miner. Res. 2015, 30, 786–795. [Google Scholar] [CrossRef]
- Maghsoudi-Ganjeh, M.; Samuel, J.; Ahsan, A.S.; Wang, X.; Zeng, X. Intrafibrillar mineralization deficiency and osteogenesis imperfecta mouse bone fragility. J. Mech. Behav. Biomed. Mater. 2021, 117, 104377. [Google Scholar] [CrossRef]
- Daley, E.; Streeten, E.A.; Sorkin, J.D.; Kuznetsova, N.; Shapses, S.A.; Carleton, S.M.; Shuldiner, A.R.; Marini, J.C.; Phillips, C.L.; Goldstein, S.A.; et al. Variable bone fragility associated with an Amish COL1A2 variant and a knock-in mouse model. J. Bone Miner. Res. 2010, 25, 247–261. [Google Scholar] [CrossRef]
- Kohler, R.; Tastad, C.A.; Creecy, A.; Wallace, J.M. Morphological and mechanical characterization of bone phenotypes in the Amish G610C murine model of osteogenesis imperfecta. PLoS ONE 2021, 16, e0255315. [Google Scholar] [CrossRef]
- Mirigian, L.S.; Makareeva, E.; Mertz, E.L.; Omari, S.; Roberts-Pilgrim, A.M.; Oestreich, A.K.; Phillips, C.L.; Leikin, S. Osteoblast Malfunction Caused by Cell Stress Response to Procollagen Misfolding in α2(I)-G610C Mouse Model of Osteogenesis Imperfecta. J. Bone Miner. Res. 2016, 31, 1608–1616. [Google Scholar] [CrossRef]
- Gorrell, L.; Makareeva, E.; Omari, S.; Otsuru, S.; Leikin, S. ER, Mitochondria, and ISR Regulation by mt-HSP70 and ATF5 upon Procollagen Misfolding in Osteoblasts. Adv. Sci. 2022, 9, e2201273. [Google Scholar] [CrossRef]
- Besio, R.; Maruelli, S.; Battaglia, S.; Leoni, L.; Villani, S.; Layrolle, P.; Rossi, A.; Trichet, V.; Forlino, A. Early Fracture Healing is Delayed in the Col1a2. Calcif. Tissue Int. 2018, 103, 653–662. [Google Scholar] [CrossRef]
- Forlino, A.; Porter, F.D.; Lee, E.J.; Westphal, H.; Marini, J.C. Use of the Cre/lox recombination system to develop a non-lethal knock-in murine model for osteogenesis imperfecta with an alpha1(I) G349C substitution. Variability in phenotype in BrtlIV mice. J. Biol. Chem. 1999, 274, 37923–37931. [Google Scholar] [CrossRef] [PubMed]
- Forlino, A.; Tani, C.; Rossi, A.; Lupi, A.; Campari, E.; Gualeni, B.; Bianchi, L.; Armini, A.; Cetta, G.; Bini, L.; et al. Differential expression of both extracellular and intracellular proteins is involved in the lethal or nonlethal phenotypic variation of BrtlIV, a murine model for osteogenesis imperfecta. Proteomics 2007, 7, 1877–1891. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, L.; Gagliardi, A.; Gioia, R.; Besio, R.; Tani, C.; Landi, C.; Cipriano, M.; Gimigliano, A.; Rossi, A.; Marini, J.C.; et al. Differential response to intracellular stress in the skin from osteogenesis imperfecta Brtl mice with lethal and non lethal phenotype: A proteomic approach. J. Proteom. 2012, 75, 4717–4733. [Google Scholar] [CrossRef] [PubMed]
- Uveges, T.E.; Collin-Osdoby, P.; Cabral, W.A.; Ledgard, F.; Goldberg, L.; Bergwitz, C.; Forlino, A.; Osdoby, P.; Gronowicz, G.A.; Marini, J.C. Cellular mechanism of decreased bone in Brtl mouse model of OI: Imbalance of decreased osteoblast function and increased osteoclasts and their precursors. J. Bone Miner. Res. 2008, 23, 1983–1994. [Google Scholar] [CrossRef] [PubMed]
- Wallace, J.M.; Orr, B.G.; Marini, J.C.; Holl, M.M. Nanoscale morphology of Type I collagen is altered in the Brtl mouse model of Osteogenesis Imperfecta. J. Struct. Biol. 2011, 173, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Blouin, S.; Fratzl-Zelman, N.; Roschger, A.; Cabral, W.A.; Klaushofer, K.; Marini, J.C.; Fratzl, P.; Roschger, P. Cortical bone properties in the Brtl/+ mouse model of Osteogenesis imperfecta as evidenced by acoustic transmission microscopy. J. Mech. Behav. Biomed. Mater. 2019, 90, 125–132. [Google Scholar] [CrossRef]
- Kozloff, K.M.; Carden, A.; Bergwitz, C.; Forlino, A.; Uveges, T.E.; Morris, M.D.; Marini, J.C.; Goldstein, S.A. Brittle IV mouse model for osteogenesis imperfecta IV demonstrates postpubertal adaptations to improve whole bone strength. J. Bone Miner. Res. 2004, 19, 614–622. [Google Scholar] [CrossRef]
- Forlino, A.; Kuznetsova, N.V.; Marini, J.C.; Leikin, S. Selective retention and degradation of molecules with a single mutant alpha1(I) chain in the Brtl IV mouse model of OI. Matrix Biol. 2007, 26, 604–614. [Google Scholar] [CrossRef]
- Chen, F.; Guo, R.; Itoh, S.; Moreno, L.; Rosenthal, E.; Zappitelli, T.; Zirngibl, R.A.; Flenniken, A.; Cole, W.; Grynpas, M.; et al. First mouse model for combined osteogenesis imperfecta and Ehlers-Danlos syndrome. J. Bone Miner. Res. 2014, 29, 1412–1423. [Google Scholar] [CrossRef]
- Eimar, H.; Tamimi, F.; Retrouvey, J.M.; Rauch, F.; Aubin, J.E.; McKee, M.D. Craniofacial and Dental Defects in the Col1a1Jrt/+ Mouse Model of Osteogenesis Imperfecta. J. Dent. Res. 2016, 95, 761–768. [Google Scholar] [CrossRef]
- Lisse, T.S.; Thiele, F.; Fuchs, H.; Hans, W.; Przemeck, G.K.; Abe, K.; Rathkolb, B.; Quintanilla-Martinez, L.; Hoelzlwimmer, G.; Helfrich, M.; et al. ER stress-mediated apoptosis in a new mouse model of osteogenesis imperfecta. PLoS Genet. 2008, 4, e7. [Google Scholar] [CrossRef] [PubMed]
- Thiele, F.; Cohrs, C.M.; Flor, A.; Lisse, T.S.; Przemeck, G.K.; Horsch, M.; Schrewe, A.; Gailus-Durner, V.; Ivandic, B.; Katus, H.A.; et al. Cardiopulmonary dysfunction in the Osteogenesis imperfecta mouse model Aga2 and human patients are caused by bone-independent mechanisms. Hum. Mol. Genet. 2012, 21, 3535–3545. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, J.; Liu, S.; Kuang, M.; Jing, Y.; Zhao, Y.; Wang, Z.; Li, G. A novel transgenic murine model with persistently brittle bones simulating osteogenesis imperfecta type I. Bone 2019, 127, 646–655. [Google Scholar] [CrossRef] [PubMed]
- Xiang, L.; Yu, H.; Zhang, X.; Wang, B.; Yuan, Y.; Zhang, Q.; Ye, R.; Gong, P.; Wu, Y. The versatile hippo pathway in oral-maxillofacial development and bone remodeling. Dev. Biol. 2018, 440, 53–63. [Google Scholar] [CrossRef]
- Marini, J.C.; Cabral, W.A.; Barnes, A.M.; Chang, W. Components of the collagen prolyl 3-hydroxylation complex are crucial for normal bone development. Cell Cycle 2007, 6, 1675–1681. [Google Scholar] [CrossRef]
- Morello, R.; Bertin, T.K.; Chen, Y.; Hicks, J.; Tonachini, L.; Monticone, M.; Castagnola, P.; Rauch, F.; Glorieux, F.H.; Vranka, J.; et al. CRTAP is required for prolyl 3- hydroxylation and mutations cause recessive osteogenesis imperfecta. Cell 2006, 127, 291–304. [Google Scholar] [CrossRef]
- Fratzl-Zelman, N.; Morello, R.; Lee, B.; Rauch, F.; Glorieux, F.H.; Misof, B.M.; Klaushofer, K.; Roschger, P. CRTAP deficiency leads to abnormally high bone matrix mineralization in a murine model and in children with osteogenesis imperfecta type VII. Bone 2010, 46, 820–826. [Google Scholar] [CrossRef]
- Lietman, C.D.; Marom, R.; Munivez, E.; Bertin, T.K.; Jiang, M.M.; Chen, Y.; Dawson, B.; Weis, M.A.; Eyre, D.; Lee, B. A transgenic mouse model of OI type V supports a neomorphic mechanism of the IFITM5 mutation. J. Bone Miner. Res. 2015, 30, 489–498. [Google Scholar] [CrossRef]
- Rauch, F.; Geng, Y.; Lamplugh, L.; Hekmatnejad, B.; Gaumond, M.H.; Penney, J.; Yamanaka, Y.; Moffatt, P. Crispr-Cas9 engineered osteogenesis imperfecta type V leads to severe skeletal deformities and perinatal lethality in mice. Bone 2018, 107, 131–142. [Google Scholar] [CrossRef]
- Tabeta, K.; Du, X.; Arimatsu, K.; Yokoji, M.; Takahashi, N.; Amizuka, N.; Hasegawa, T.; Crozat, K.; Maekawa, T.; Miyauchi, S.; et al. An ENU-induced splice site mutation of mouse Col1a1 causing recessive osteogenesis imperfecta and revealing a novel splicing rescue. Sci. Rep. 2017, 7, 11717. [Google Scholar] [CrossRef]
- Kasamatsu, A.; Uzawa, K.; Hayashi, F.; Kita, A.; Okubo, Y.; Saito, T.; Kimura, Y.; Miyamoto, I.; Oka, N.; Shiiba, M.; et al. Deficiency of lysyl hydroxylase 2 in mice causes systemic endoplasmic reticulum stress leading to early embryonic lethality. Biochem. Biophys. Res. Commun. 2019, 512, 486–491. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Terajima, M.; Taga, Y.; Hayashi, F.; Oshima, S.; Kasamatsu, A.; Okubo, Y.; Ito, C.; Toshimori, K.; Sunohara, M.; et al. Decrease of lysyl hydroxylase 2 activity causes abnormal collagen molecular phenotypes, defective mineralization and compromised mechanical properties of bone. Bone 2022, 154, 116242. [Google Scholar] [CrossRef] [PubMed]
- Barnes, A.M.; Cabral, W.A.; Weis, M.; Makareeva, E.; Mertz, E.L.; Leikin, S.; Eyre, D.; Trujillo, C.; Marini, J.C. Absence of FKBP10 in recessive type XI osteogenesis imperfecta leads to diminished collagen cross-linking and reduced collagen deposition in extracellular matrix. Hum. Mutat. 2012, 33, 1589–1598. [Google Scholar] [CrossRef] [PubMed]
- Joeng, K.S.; Lee, Y.C.; Jiang, M.M.; Bertin, T.K.; Chen, Y.; Abraham, A.M.; Ding, H.; Bi, X.; Ambrose, C.G.; Lee, B.H. The swaying mouse as a model of osteogenesis imperfecta caused by WNT1 mutations. Hum. Mol. Genet. 2014, 23, 4035–4042. [Google Scholar] [CrossRef] [PubMed]
- Fahiminiya, S.; Majewski, J.; Mort, J.; Moffatt, P.; Glorieux, F.H.; Rauch, F. Mutations in WNT1 are a cause of osteogenesis imperfecta. J. Med. Genet. 2013, 50, 345–348. [Google Scholar] [CrossRef]
- Vollersen, N.; Zhao, W.; Rolvien, T.; Lange, F.; Schmidt, F.N.; Sonntag, S.; Shmerling, D.; von Kroge, S.; Stockhausen, K.E.; Sharaf, A.; et al. The WNT1G177C mutation specifically affects skeletal integrity in a mouse model of osteogenesis imperfecta type XV. Bone Res. 2021, 9, 48. [Google Scholar] [CrossRef]
- Hedjazi, G.; Guterman-Ram, G.; Blouin, S.; Schemenz, V.; Wagermaier, W.; Fratzl, P.; Hartmann, M.A.; Zwerina, J.; Fratzl-Zelman, N.; Marini, J.C. Alterations of bone material properties in growing Ifitm5/BRIL p.S42 knock-in mice, a new model for atypical type VI osteogenesis imperfecta. Bone 2022, 162, 116451. [Google Scholar] [CrossRef]
- Gewartowska, O.; Aranaz-Novaliches, G.; Krawczyk, P.S.; Mroczek, S.; Kusio-Kobiałka, M.; Tarkowski, B.; Spoutil, F.; Benada, O.; Kofroňová, O.; Szwedziak, P.; et al. Cytoplasmic polyadenylation by TENT5A is required for proper bone formation. Cell Rep. 2021, 35, 109015. [Google Scholar] [CrossRef]
- Malhan, D.; Muelke, M.; Rosch, S.; Schaefer, A.B.; Merboth, F.; Weisweiler, D.; Heiss, C.; Arganda-Carreras, I.; El Khassawna, T. An Optimized Approach to Perform Bone Histomorphometry. Front. Endocrinol. 2018, 9, 666. [Google Scholar] [CrossRef]
- van’t Hof, R.J.; Rose, L.; Bassonga, E.; Daroszewska, A. Open source software for semi-automated histomorphometry of bone resorption and formation parameters. Bone 2017, 99, 69–79. [Google Scholar] [CrossRef]
- Xu, H.; Lenhart, S.A.; Chu, E.Y.; Chavez, M.B.; Wimer, H.F.; Dimori, M.; Somerman, M.J.; Morello, R.; Foster, B.L.; Hatch, N.E. Dental and craniofacial defects in the Crtap. Dev. Dyn. 2020, 249, 884–897. [Google Scholar] [CrossRef] [PubMed]
- Masci, M.; Wang, M.; Imbert, L.; Barnes, A.M.; Spevak, L.; Lukashova, L.; Huang, Y.; Ma, Y.; Marini, J.C.; Jacobsen, C.M.; et al. Bone mineral properties in growing Col1a2(+/G610C) mice, an animal model of osteogenesis imperfecta. Bone 2016, 87, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Stephens, M.; López-Linares, K.; Aldazabal, J.; Macias, I.; Ortuzar, N.; Bengoetxea, H.; Bulnes, S.; Alcorta-Sevillano, N.; Infante, A.; Lafuente, J.V.; et al. Murine femur micro-computed tomography and biomechanical datasets for an ovariectomy-induced osteoporosis model. Sci. Data 2021, 8, 240. [Google Scholar] [CrossRef] [PubMed]
- Kohler, R.; Tastad, C.A.; Stacy, A.J.; Swallow, E.A.; Metzger, C.E.; Allen, M.R.; Wallace, J.M. The Effect of Single Versus Group μCT on the Detection of Trabecular and Cortical Disease Phenotypes in Mouse Bones. JBMR Plus 2021, 5, e10473. [Google Scholar] [CrossRef]
- Bouxsein, M.L.; Boyd, S.K.; Christiansen, B.A.; Guldberg, R.E.; Jepsen, K.J.; Müller, R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J. Bone Miner. Res. 2010, 25, 1468–1486. [Google Scholar] [CrossRef]
- Mandair, G.S.; Morris, M.D. Contributions of Raman spectroscopy to the understanding of bone strength. Bonekey Rep. 2015, 4, 620. [Google Scholar] [CrossRef]
- Turner, C.H.; Wang, T.; Burr, D.B. Shear strength and fatigue properties of human cortical bone determined from pure shear tests. Calcif. Tissue Int. 2001, 69, 373–378. [Google Scholar] [CrossRef]
- Macías, I.; Alcorta-Sevillano, N.; Rodríguez, C.I.; Infante, A. Osteoporosis and the Potential of Cell-Based Therapeutic Strategies. Int. J. Mol. Sci. 2020, 21, 1653. [Google Scholar] [CrossRef]
- Boraschi-Diaz, I.; Tauer, J.T.; El-Rifai, O.; Guillemette, D.; Lefebvre, G.; Rauch, F.; Ferron, M.; Komarova, S.V. Metabolic phenotype in the mouse model of osteogenesis imperfecta. J. Endocrinol. 2017, 234, 279–289. [Google Scholar] [CrossRef]
- Wang, N.; Wang, L.; Wang, Z.; Cheng, L.; Wang, J. Solanum muricatum Ameliorates the Symptoms of Osteogenesis Imperfecta In Vivo. J. Food Sci. 2019, 84, 1646–1650. [Google Scholar] [CrossRef]
- Russell, R.G. Bisphosphonates: From bench to bedside. Ann. New York Acad. Sci. 2006, 1068, 367–401. [Google Scholar] [CrossRef]
- McCarthy, E.A.; Raggio, C.L.; Hossack, M.D.; Miller, E.A.; Jain, S.; Boskey, A.L.; Camacho, N.P. Alendronate treatment for infants with osteogenesis imperfecta: Demonstration of efficacy in a mouse model. Pediatr. Res. 2002, 52, 660–670. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.H.; Evans, K.D.; Oberbauer, A.M.; Martin, R.B. Bisphosphonate treatment in the oim mouse model alters bone modeling during growth. J. Biomech. 2008, 41, 3371–3376. [Google Scholar] [CrossRef] [PubMed]
- Misof, B.M.; Roschger, P.; Baldini, T.; Raggio, C.L.; Zraick, V.; Root, L.; Boskey, A.L.; Klaushofer, K.; Fratzl, P.; Camacho, N.P. Differential effects of alendronate treatment on bone from growing osteogenesis imperfecta and wild-type mouse. Bone 2005, 36, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Camacho, N.P.; Raggio, C.L.; Doty, S.B.; Root, L.; Zraick, V.; Ilg, W.A.; Toledano, T.R.; Boskey, A.L. A controlled study of the effects of alendronate in a growing mouse model of osteogenesis imperfecta. Calcif. Tissue Int. 2001, 69, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Powell, K.M.; Skaggs, C.; Pulliam, A.; Berman, A.; Allen, M.R.; Wallace, J.M. Zoledronate and Raloxifene combination therapy enhances material and mechanical properties of diseased mouse bone. Bone 2019, 127, 199–206. [Google Scholar] [CrossRef]
- Uveges, T.E.; Kozloff, K.M.; Ty, J.M.; Ledgard, F.; Raggio, C.L.; Gronowicz, G.; Goldstein, S.A.; Marini, J.C. Alendronate treatment of the brtl osteogenesis imperfecta mouse improves femoral geometry and load response before fracture but decreases predicted material properties and has detrimental effects on osteoblasts and bone formation. J. Bone Miner. Res. 2009, 24, 849–859. [Google Scholar] [CrossRef]
- Meganck, J.A.; Begun, D.L.; McElderry, J.D.; Swick, A.; Kozloff, K.M.; Goldstein, S.A.; Morris, M.D.; Marini, J.C.; Caird, M.S. Fracture healing with alendronate treatment in the Brtl/+ mouse model of osteogenesis imperfecta. Bone 2013, 56, 204–212. [Google Scholar] [CrossRef][Green Version]
- Delos, D.; Yang, X.; Ricciardi, B.F.; Myers, E.R.; Bostrom, M.P.; Camacho, N.P. The effects of RANKL inhibition on fracture healing and bone strength in a mouse model of osteogenesis imperfecta. J. Orthop. Res. 2008, 26, 153–164. [Google Scholar] [CrossRef]
- Bargman, R.; Huang, A.; Boskey, A.L.; Raggio, C.; Pleshko, N. RANKL inhibition improves bone properties in a mouse model of osteogenesis imperfecta. Connect. Tissue Res. 2010, 51, 123–131. [Google Scholar] [CrossRef]
- Zimmerman, S.M.; Heard-Lipsmeyer, M.E.; Dimori, M.; Thostenson, J.D.; Mannen, E.M.; O’Brien, C.A.; Morello, R. Loss of RANKL in osteocytes dramatically increases cancellous bone mass in the osteogenesis imperfecta mouse (oim). Bone Rep. 2018, 9, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Hoyer-Kuhn, H.; Netzer, C.; Koerber, F.; Schoenau, E.; Semler, O. Two years’ experience with denosumab for children with osteogenesis imperfecta type VI. Orphanet. J. Rare. Dis. 2014, 9, 145. [Google Scholar] [CrossRef] [PubMed]
- Berman, A.G.; Wallace, J.M.; Bart, Z.R.; Allen, M.R. Raloxifene reduces skeletal fractures in an animal model of osteogenesis imperfecta. Matrix Biol. 2016, 52, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Powell, K.M.; Brown, A.P.; Skaggs, C.G.; Pulliam, A.N.; Berman, A.G.; Deosthale, P.; Plotkin, L.I.; Allen, M.R.; Williams, D.R.; Wallace, J.M. 6’-Methoxy Raloxifene-analog enhances mouse bone properties with reduced estrogen receptor binding. Bone Rep. 2020, 12, 100246. [Google Scholar] [CrossRef]
- Long, F. Building strong bones: Molecular regulation of the osteoblast lineage. Nat. Rev. Mol. Cell Biol. 2011, 13, 27–38. [Google Scholar] [CrossRef]
- Duan, P.; Bonewald, L.F. The role of the wnt/β-catenin signaling pathway in formation and maintenance of bone and teeth. Int. J. Biochem. Cell Biol. 2016, 77, 23–29. [Google Scholar] [CrossRef]
- Yavropoulou, M.P.; Yovos, J.G. The role of the Wnt signaling pathway in osteoblast commitment and differentiation. Hormones 2007, 6, 279–294. [Google Scholar] [CrossRef]
- Semënov, M.; Tamai, K.; He, X. SOST is a ligand for LRP5/LRP6 and a Wnt signaling inhibitor. J. Biol. Chem. 2005, 280, 26770–26775. [Google Scholar] [CrossRef]
- Delgado-Calle, J.; Sato, A.Y.; Bellido, T. Role and mechanism of action of sclerostin in bone. Bone 2017, 96, 29–37. [Google Scholar] [CrossRef]
- Lin, C.; Jiang, X.; Dai, Z.; Guo, X.; Weng, T.; Wang, J.; Li, Y.; Feng, G.; Gao, X.; He, L. Sclerostin mediates bone response to mechanical unloading through antagonizing Wnt/beta-catenin signaling. J. Bone Miner. Res. 2009, 24, 1651–1661. [Google Scholar] [CrossRef]
- Kramer, I.; Loots, G.G.; Studer, A.; Keller, H.; Kneissel, M. Parathyroid hormone (PTH)-induced bone gain is blunted in SOST overexpressing and deficient mice. J. Bone Miner. Res. 2010, 25, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Sinder, B.P.; Salemi, J.D.; Ominsky, M.S.; Caird, M.S.; Marini, J.C.; Kozloff, K.M. Rapidly growing Brtl/+ mouse model of osteogenesis imperfecta improves bone mass and strength with sclerostin antibody treatment. Bone 2015, 71, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Sinder, B.P.; Eddy, M.M.; Ominsky, M.S.; Caird, M.S.; Marini, J.C.; Kozloff, K.M. Sclerostin antibody improves skeletal parameters in a Brtl/+ mouse model of osteogenesis imperfecta. J. Bone Miner. Res. 2013, 28, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Sinder, B.P.; White, L.E.; Salemi, J.D.; Ominsky, M.S.; Caird, M.S.; Marini, J.C.; Kozloff, K.M. Adult Brtl/+ mouse model of osteogenesis imperfecta demonstrates anabolic response to sclerostin antibody treatment with increased bone mass and strength. Osteoporos. Int. 2014, 25, 2097–2107. [Google Scholar] [CrossRef]
- Sinder, B.P.; Lloyd, W.R.; Salemi, J.D.; Marini, J.C.; Caird, M.S.; Morris, M.D.; Kozloff, K.M. Effect of anti-sclerostin therapy and osteogenesis imperfecta on tissue-level properties in growing and adult mice while controlling for tissue age. Bone 2016, 84, 222–229. [Google Scholar] [CrossRef]
- Scheiber, A.L.; Barton, D.K.; Khoury, B.M.; Marini, J.C.; Swiderski, D.L.; Caird, M.S.; Kozloff, K.M. Sclerostin Antibody-Induced Changes in Bone Mass Are Site Specific in Developing Crania. J. Bone Miner. Res. 2019, 34, 2301–2310. [Google Scholar] [CrossRef]
- Jacobsen, C.M.; Barber, L.A.; Ayturk, U.M.; Roberts, H.J.; Deal, L.E.; Schwartz, M.A.; Weis, M.; Eyre, D.; Zurakowski, D.; Robling, A.G.; et al. Targeting the LRP5 pathway improves bone properties in a mouse model of osteogenesis imperfecta. J. Bone Miner. Res. 2014, 29, 2297–2306. [Google Scholar] [CrossRef]
- Little, D.G.; Peacock, L.; Mikulec, K.; Kneissel, M.; Kramer, I.; Cheng, T.L.; Schindeler, A.; Munns, C. Combination sclerostin antibody and zoledronic acid treatment outperforms either treatment alone in a mouse model of osteogenesis imperfecta. Bone 2017, 101, 96–103. [Google Scholar] [CrossRef]
- Roschger, A.; Roschger, P.; Keplingter, P.; Klaushofer, K.; Abdullah, S.; Kneissel, M.; Rauch, F. Effect of sclerostin antibody treatment in a mouse model of severe osteogenesis imperfecta. Bone 2014, 66, 182–188. [Google Scholar] [CrossRef]
- Grafe, I.; Alexander, S.; Yang, T.; Lietman, C.; Homan, E.P.; Munivez, E.; Chen, Y.; Jiang, M.M.; Bertin, T.; Dawson, B.; et al. Sclerostin Antibody Treatment Improves the Bone Phenotype of Crtap(-/-) Mice, a Model of Recessive Osteogenesis Imperfecta. J. Bone Miner. Res. 2016, 31, 1030–1040. [Google Scholar] [CrossRef]
- Cardinal, M.; Tys, J.; Roels, T.; Lafont, S.; Ominsky, M.S.; Devogelaer, J.P.; Chappard, D.; Mabilleau, G.; Ammann, P.; Nyssen-Behets, C.; et al. Sclerostin antibody reduces long bone fractures in the oim/oim model of osteogenesis imperfecta. Bone 2019, 124, 137–147. [Google Scholar] [CrossRef]
- Cardinal, M.; Dessain, A.; Roels, T.; Lafont, S.; Ominsky, M.S.; Devogelaer, J.P.; Chappard, D.; Mabilleau, G.; Ammann, P.; Nyssen-Behets, C.; et al. Sclerostin-Antibody Treatment Decreases Fracture Rates in Axial Skeleton and Improves the Skeletal Phenotype in Growing oim/oim Mice. Calcif. Tissue Int. 2020, 106, 494–508. [Google Scholar] [CrossRef]
- Cardinal, M.; Chretien, A.; Roels, T.; Lafont, S.; Ominsky, M.S.; Devogelaer, J.P.; Manicourt, D.H.; Behets, C. Gender-Related Impact of Sclerostin Antibody on Bone in the Osteogenesis Imperfecta Mouse. Front. Genet. 2021, 12, 705505. [Google Scholar] [CrossRef]
- Glorieux, F.H.; Devogelaer, J.P.; Durigova, M.; Goemaere, S.; Hemsley, S.; Jakob, F.; Junker, U.; Ruckle, J.; Seefried, L.; Winkle, P.J. BPS804 Anti-Sclerostin Antibody in Adults With Moderate Osteogenesis Imperfecta: Results of a Randomized Phase 2a Trial. J. Bone Miner. Res. 2017, 32, 1496–1504. [Google Scholar] [CrossRef]
- Ishibashi, H.; Crittenden, D.B.; Miyauchi, A.; Libanati, C.; Maddox, J.; Fan, M.; Chen, L.; Grauer, A. Romosozumab increases bone mineral density in postmenopausal Japanese women with osteoporosis: A phase 2 study. Bone 2017, 103, 209–215. [Google Scholar] [CrossRef]
- Grafe, I.; Yang, T.; Alexander, S.; Homan, E.P.; Lietman, C.; Jiang, M.M.; Bertin, T.; Munivez, E.; Chen, Y.; Dawson, B.; et al. Excessive transforming growth factor-β signaling is a common mechanism in osteogenesis imperfecta. Nat. Med. 2014, 20, 670–675. [Google Scholar] [CrossRef]
- Tauer, J.T.; Abdullah, S.; Rauch, F. Effect of Anti-TGF-β Treatment in a Mouse Model of Severe Osteogenesis Imperfecta. J. Bone Miner. Res. 2019, 34, 207–214. [Google Scholar] [CrossRef]
- Song, I.W.; Nagamani, S.C.; Nguyen, D.; Grafe, I.; Sutton, V.R.; Gannon, F.H.; Munivez, E.; Jiang, M.M.; Tran, A.; Wallace, M.; et al. Targeting TGF-β for treatment of osteogenesis imperfecta. J. Clin. Investig. 2022, 132, e152571. [Google Scholar] [CrossRef]
- Greene, B.; Russo, R.J.; Dwyer, S.; Malley, K.; Roberts, E.; Serrielo, J.; Piepenhagen, P.; Cummings, S.; Ryan, S.; Zarazinski, C.; et al. Inhibition of TGF-β Increases Bone Volume and Strength in a Mouse Model of Osteogenesis Imperfecta. JBMR Plus 2021, 5, e10530. [Google Scholar] [CrossRef]
- Infante, A.; Gener, B.; Vázquez, M.; Olivares, N.; Arrieta, A.; Grau, G.; Llano, I.; Madero, L.; Bueno, A.M.; Sagastizabal, B.; et al. Reiterative infusions of MSCs improve pediatric osteogenesis imperfecta eliciting a pro-osteogenic paracrine response: TERCELOI clinical trial. Clin. Transl. Med. 2021, 11, e265. [Google Scholar] [CrossRef]
- Infante, A.; Cabodevilla, L.; Gener, B.; Rodríguez, C.I. Circulating TGF-β Pathway in Osteogenesis Imperfecta Pediatric Patients Subjected to MSCs-Based Cell Therapy. Front. Cell Dev. Biol. 2022, 10, 830928. [Google Scholar] [CrossRef]
- Kang, H.; Aryal Ac, S.; Barnes, A.M.; Martin, A.; David, V.; Crawford, S.E.; Marini, J.C. Antagonism Between PEDF and TGF-β Contributes to Type VI Osteogenesis Imperfecta Bone and Vascular Pathogenesis. J. Bone Miner. Res. 2022, 37, 925–937. [Google Scholar] [CrossRef]
- Hamrick, M.W.; McPherron, A.C.; Lovejoy, C.O. Bone mineral content and density in the humerus of adult myostatin-deficient mice. Calcif. Tissue Int. 2002, 71, 63–68. [Google Scholar] [CrossRef]
- Bowser, M.; Herberg, S.; Arounleut, P.; Shi, X.; Fulzele, S.; Hill, W.D.; Isales, C.M.; Hamrick, M.W. Effects of the activin A-myostatin-follistatin system on aging bone and muscle progenitor cells. Exp. Gerontol. 2013, 48, 290–297. [Google Scholar] [CrossRef]
- Lee, S.J.; Lehar, A.; Liu, Y.; Ly, C.H.; Pham, Q.M.; Michaud, M.; Rydzik, R.; Youngstrom, D.W.; Shen, M.M.; Kaartinen, V.; et al. Functional redundancy of type I and type II receptors in the regulation of skeletal muscle growth by myostatin and activin A. Proc. Natl. Acad. Sci. USA 2020, 117, 30907–30917. [Google Scholar] [CrossRef]
- Tauer, J.T.; Rauch, F. Novel ActRIIB ligand trap increases muscle mass and improves bone geometry in a mouse model of severe osteogenesis imperfecta. Bone 2019, 128, 115036. [Google Scholar] [CrossRef]
- Jeong, Y.; Daghlas, S.A.; Xie, Y.; Hulbert, M.A.; Pfeiffer, F.M.; Dallas, M.R.; Omosule, C.L.; Pearsall, R.S.; Dallas, S.L.; Phillips, C.L. Skeletal Response to Soluble Activin Receptor Type IIB in Mouse Models of Osteogenesis Imperfecta. J. Bone Miner. Res. 2018, 33, 1760–1772. [Google Scholar] [CrossRef]
- Omosule, C.L.; Gremminger, V.L.; Aguillard, A.M.; Jeong, Y.; Harrelson, E.N.; Miloscio, L.; Mastaitis, J.; Rafique, A.; Kleiner, S.; Pfeiffer, F.M.; et al. Impact of Genetic and Pharmacologic Inhibition of Myostatin in a Murine Model of Osteogenesis Imperfecta. J. Bone Miner. Res. 2021, 36, 739–756. [Google Scholar] [CrossRef]
- Omosule, C.L.; Joseph, D.; Weiler, B.; Gremminger, V.L.; Silvey, S.; Jeong, Y.; Rafique, A.; Krueger, P.; Kleiner, S.; Phillips, C.L. Combinatorial Inhibition of Myostatin and Activin A Improves Femoral Bone Properties in the G610C Mouse Model of Osteogenesis Imperfecta. J. Bone Miner. Res. 2022, 37, 938–953. [Google Scholar] [CrossRef]
- Kojima, T.; Miyaishi, O.; Saga, S.; Ishiguro, N.; Tsutsui, Y.; Iwata, H. The retention of abnormal type I procollagen and correlated expression of HSP 47 in fibroblasts from a patient with lethal osteogenesis imperfecta. J. Pathol. 1998, 184, 212–218. [Google Scholar] [CrossRef]
- Scheiber, A.L.; Guess, A.J.; Kaito, T.; Abzug, J.M.; Enomoto-Iwamoto, M.; Leikin, S.; Iwamoto, M.; Otsuru, S. Endoplasmic reticulum stress is induced in growth plate hypertrophic chondrocytes in G610C mouse model of osteogenesis imperfecta. Biochem. Biophys. Res. Commun. 2019, 509, 235–240. [Google Scholar] [CrossRef]
- Gioia, R.; Panaroni, C.; Besio, R.; Palladini, G.; Merlini, G.; Giansanti, V.; Scovassi, I.A.; Villani, S.; Villa, I.; Villa, A.; et al. Impaired osteoblastogenesis in a murine model of dominant osteogenesis imperfecta: A new target for osteogenesis imperfecta pharmacological therapy. Stem Cells 2012, 30, 1465–1476. [Google Scholar] [CrossRef] [PubMed]
- Duangchan, T.; Tawonsawatruk, T.; Angsanuntsukh, C.; Trachoo, O.; Hongeng, S.; Kitiyanant, N.; Supokawej, A. Amelioration of osteogenesis in iPSC-derived mesenchymal stem cells from osteogenesis imperfecta patients by endoplasmic reticulum stress inhibitor. Life Sci. 2021, 278, 119628. [Google Scholar] [CrossRef] [PubMed]
- Besio, R.; Garibaldi, N.; Leoni, L.; Cipolla, L.; Sabbioneda, S.; Biggiogera, M.; Mottes, M.; Aglan, M.; Otaify, G.A.; Temtamy, S.A.; et al. Cellular stress due to impairment of collagen prolyl hydroxylation complex is rescued by the chaperone 4-phenylbutyrate. Dis. Model. Mech. 2019, 12, dmm038521. [Google Scholar] [CrossRef] [PubMed]
- Besio, R.; Iula, G.; Garibaldi, N.; Cipolla, L.; Sabbioneda, S.; Biggiogera, M.; Marini, J.C.; Rossi, A.; Forlino, A. 4-PBA ameliorates cellular homeostasis in fibroblasts from osteogenesis imperfecta patients by enhancing autophagy and stimulating protein secretion. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 1642–1652. [Google Scholar] [CrossRef] [PubMed]
- Garibaldi, N.; Contento, B.M.; Babini, G.; Morini, J.; Siciliani, S.; Biggiogera, M.; Raspanti, M.; Marini, J.C.; Rossi, A.; Forlino, A.; et al. Targeting cellular stress in vitro improves osteoblast homeostasis, matrix collagen content and mineralization in two murine models of osteogenesis imperfecta. Matrix Biol. 2021, 98, 1–20. [Google Scholar] [CrossRef]
- Scheiber, A.L.; Wilkinson, K.J.; Suzuki, A.; Enomoto-Iwamoto, M.; Kaito, T.; Cheah, K.S.; Iwamoto, M.; Leikin, S.; Otsuru, S. 4PBA reduces growth deficiency in osteogenesis imperfecta by enhancing transition of hypertrophic chondrocytes to osteoblasts. JCI Insight 2022, 7, e149636. [Google Scholar] [CrossRef]
- Duran, I.; Zieba, J.; Csukasi, F.; Martin, J.H.; Wachtell, D.; Barad, M.; Dawson, B.; Fafilek, B.; Jacobsen, C.M.; Ambrose, C.G.; et al. 4-PBA Treatment Improves Bone Phenotypes in the Aga2 Mouse Model of Osteogenesis Imperfecta. J. Bone Miner. Res. 2022, 37, 675–686. [Google Scholar] [CrossRef]
- Takigawa, S.; Frondorf, B.; Liu, S.; Liu, Y.; Li, B.; Sudo, A.; Wallace, J.M.; Yokota, H.; Hamamura, K. Salubrinal improves mechanical properties of the femur in osteogenesis imperfecta mice. J. Pharmacol. Sci. 2016, 132, 154–161. [Google Scholar] [CrossRef]
- Bateman, J.F.; Sampurno, L.; Maurizi, A.; Lamandé, S.R.; Sims, N.A.; Cheng, T.L.; Schindeler, A.; Little, D.G. Effect of rapamycin on bone mass and strength in the α2(I)-G610C mouse model of osteogenesis imperfecta. J. Cell Mol. Med. 2019, 23, 1735–1745. [Google Scholar] [CrossRef]
- Hanagata, N.; Takemura, T.; Kamimura, K.; Koda, T. Effect of immunosuppressants on a mouse model of osteogenesis imperfecta type V harboring a heterozygous Ifitm5 c.-14C > T mutation. Sci. Rep. 2020, 10, 21197. [Google Scholar] [CrossRef] [PubMed]
- Blank, M.; McGregor, N.E.; Rowley, L.; Kung, L.H.W.; Crimeen-Irwin, B.; Poulton, I.J.; Walker, E.C.; Gooi, J.H.; Lamandé, S.R.; Sims, N.A.; et al. The effect of carbamazepine on bone structure and strength in control and osteogenesis imperfecta (Col1a2 +/p.G610C ) mice. J. Cell Mol. Med. 2022, 26, 4021–4031. [Google Scholar] [CrossRef] [PubMed]
- Mertz, E.L.; Makareeva, E.; Mirigian, L.S.; Koon, K.Y.; Perosky, J.E.; Kozloff, K.M.; Leikin, S. Makings of a brittle bone: Unexpected lessons from a low protein diet study of a mouse OI model. Matrix Biol. 2016, 52, 29–42. [Google Scholar] [CrossRef]
- Kawaguchi, H.; Pilbeam, C.C.; Harrison, J.R.; Raisz, L.G. The role of prostaglandins in the regulation of bone metabolism. Clin. Orthop. Relat. Res. 1995, 313, 36–46. [Google Scholar]
- Yoshida, K.; Oida, H.; Kobayashi, T.; Maruyama, T.; Tanaka, M.; Katayama, T.; Yamaguchi, K.; Segi, E.; Tsuboyama, T.; Matsushita, M.; et al. Stimulation of bone formation and prevention of bone loss by prostaglandin E EP4 receptor activation. Proc. Natl. Acad. Sci. USA 2002, 99, 4580–4585. [Google Scholar] [CrossRef]
- Li, M.; Thompson, D.D.; Paralkar, V.M. Prostaglandin E(2) receptors in bone formation. Int. Orthop. 2007, 31, 767–772. [Google Scholar] [CrossRef]
- Boraschi-Diaz, I.; Chen, G.; Polak-Nachumow, J.; Young, R.N.; Rauch, F. Effects of treatment with a bone-targeted prostaglandin E2 receptor 4 agonist C3 (Mes-1007) in a mouse model of severe osteogenesis imperfecta. Bone 2021, 145, 115867. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.F.; Halford, K.W.; O’Hara, M.D.; Leeper, D.B.; Sokolov, B.P.; Pollard, M.D.; Bagasra, O.; Prockop, D.J. Cultured adherent cells from marrow can serve as long-lasting precursor cells for bone, cartilage, and lung in irradiated mice. Proc. Natl. Acad. Sci. USA 1995, 92, 4857–4861. [Google Scholar] [CrossRef]
- Pereira, R.F.; O’Hara, M.D.; Laptev, A.V.; Halford, K.W.; Pollard, M.D.; Class, R.; Simon, D.; Livezey, K.; Prockop, D.J. Marrow stromal cells as a source of progenitor cells for nonhematopoietic tissues in transgenic mice with a phenotype of osteogenesis imperfecta. Proc. Natl. Acad. Sci. USA 1998, 95, 1142–1147. [Google Scholar] [CrossRef]
- Pereira, R.F.; Hume, E.L.; Halford, K.W.; Prockop, D.J. Bone fragility in transgenic mice expressing a mutated gene for type I procollagen (COL1A1) parallels the age-dependent phenotype of human osteogenesis imperfecta. J. Bone Miner. Res. 1995, 10, 1837–1843. [Google Scholar] [CrossRef]
- Millard, S.M.; Pettit, A.R.; Ellis, R.; Chan, J.K.Y.; Raggatt, L.J.; Khosrotehrani, K.; Fisk, N.M. Intrauterine Bone Marrow Transplantation in Osteogenesis Imperfecta Mice Yields Donor Osteoclasts and Osteomacs but Not Osteoblasts. Stem Cell Rep. 2015, 5, 682–689. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.R.; Peacock, L.; Ginn, S.L.; Cantrill, L.C.; Cheng, T.L.; Little, D.G.; Munns, C.F.; Schindeler, A. Bone Marrow Transplantation for Treatment of the Col1a2 +/G610C Osteogenesis Imperfecta Mouse Model. Calcif. Tissue Int. 2019, 104, 426–436. [Google Scholar] [CrossRef] [PubMed]
- Le Blanc, K.; Götherström, C.; Ringdén, O.; Hassan, M.; McMahon, R.; Horwitz, E.; Anneren, G.; Axelsson, O.; Nunn, J.; Ewald, U.; et al. Fetal mesenchymal stem-cell engraftment in bone after in utero transplantation in a patient with severe osteogenesis imperfecta. Transplantation 2005, 79, 1607–1614. [Google Scholar] [CrossRef] [PubMed]
- Götherström, C.; Westgren, M.; Shaw, S.W.; Aström, E.; Biswas, A.; Byers, P.H.; Mattar, C.N.; Graham, G.E.; Taslimi, J.; Ewald, U.; et al. Pre- and postnatal transplantation of fetal mesenchymal stem cells in osteogenesis imperfecta: A two-center experience. Stem Cells Transl. Med. 2014, 3, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, E.M.; Prockop, D.J.; Gordon, P.L.; Koo, W.W.; Fitzpatrick, L.A.; Neel, M.D.; McCarville, M.E.; Orchard, P.J.; Pyeritz, R.E.; Brenner, M.K. Clinical responses to bone marrow transplantation in children with severe osteogenesis imperfecta. Blood 2001, 97, 1227–1231. [Google Scholar] [CrossRef]
- Horwitz, E.M.; Gordon, P.L.; Koo, W.K.; Marx, J.C.; Neel, M.D.; McNall, R.Y.; Muul, L.; Hofmann, T. Isolated allogeneic bone marrow-derived mesenchymal cells engraft and stimulate growth in children with osteogenesis imperfecta: Implications for cell therapy of bone. Proc. Natl. Acad. Sci. USA 2002, 99, 8932–8937. [Google Scholar] [CrossRef]
- Otsuru, S.; Gordon, P.L.; Shimono, K.; Jethva, R.; Marino, R.; Phillips, C.L.; Hofmann, T.J.; Veronesi, E.; Dominici, M.; Iwamoto, M.; et al. Transplanted bone marrow mononuclear cells and MSCs impart clinical benefit to children with osteogenesis imperfecta through different mechanisms. Blood 2012, 120, 1933–1941. [Google Scholar] [CrossRef]
- Jones, G.N.; Moschidou, D.; Abdulrazzak, H.; Kalirai, B.S.; Vanleene, M.; Osatis, S.; Shefelbine, S.J.; Horwood, N.J.; Marenzana, M.; De Coppi, P.; et al. Potential of human fetal chorionic stem cells for the treatment of osteogenesis imperfecta. Stem Cells Dev. 2014, 23, 262–276. [Google Scholar] [CrossRef]
- Ranzoni, A.M.; Corcelli, M.; Hau, K.L.; Kerns, J.G.; Vanleene, M.; Shefelbine, S.; Jones, G.N.; Moschidou, D.; Dala-Ali, B.; Goodship, A.E.; et al. Counteracting bone fragility with human amniotic mesenchymal stem cells. Sci. Rep. 2016, 6, 39656. [Google Scholar] [CrossRef]
- Otsuru, S.; Desbourdes, L.; Guess, A.J.; Hofmann, T.J.; Relation, T.; Kaito, T.; Dominici, M.; Iwamoto, M.; Horwitz, E.M. Extracellular vesicles released from mesenchymal stromal cells stimulate bone growth in osteogenesis imperfecta. Cytotherapy 2018, 20, 62–73. [Google Scholar] [CrossRef]
- Wang, X.; Li, F.; Niyibizi, C. Progenitors systemically transplanted into neonatal mice localize to areas of active bone formation in vivo: Implications of cell therapy for skeletal diseases. Stem Cells 2006, 24, 1869–1878. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Wang, X.; Niyibizi, C. Distribution of single-cell expanded marrow derived progenitors in a developing mouse model of osteogenesis imperfecta following systemic transplantation. Stem Cells 2007, 25, 3183–3193. [Google Scholar] [CrossRef] [PubMed]
- Guillot, P.V.; Abass, O.; Bassett, J.H.; Shefelbine, S.J.; Bou-Gharios, G.; Chan, J.; Kurata, H.; Williams, G.R.; Polak, J.; Fisk, N.M. Intrauterine transplantation of human fetal mesenchymal stem cells from first-trimester blood repairs bone and reduces fractures in osteogenesis imperfecta mice. Blood 2008, 111, 1717–1725. [Google Scholar] [CrossRef] [PubMed]
- Vanleene, M.; Saldanha, Z.; Cloyd, K.L.; Jell, G.; Bou-Gharios, G.; Bassett, J.H.; Williams, G.R.; Fisk, N.M.; Oyen, M.L.; Stevens, M.M.; et al. Transplantation of human fetal blood stem cells in the osteogenesis imperfecta mouse leads to improvement in multiscale tissue properties. Blood 2011, 117, 1053–1060. [Google Scholar] [CrossRef]
- Jones, G.N.; Moschidou, D.; Lay, K.; Abdulrazzak, H.; Vanleene, M.; Shefelbine, S.J.; Polak, J.; de Coppi, P.; Fisk, N.M.; Guillot, P.V. Upregulating CXCR4 in human fetal mesenchymal stem cells enhances engraftment and bone mechanics in a mouse model of osteogenesis imperfecta. Stem Cells Transl. Med. 2012, 1, 70–78. [Google Scholar] [CrossRef]
- Ranzoni, A.M.; Corcelli, M.; Arnett, T.R.; Guillot, P.V. Micro-computed tomography reconstructions of tibiae of stem cell transplanted osteogenesis imperfecta mice. Sci. Data 2018, 5, 180100. [Google Scholar] [CrossRef]
- Cabral, W.A.; Marini, J.C. High proportion of mutant osteoblasts is compatible with normal skeletal function in mosaic carriers of osteogenesis imperfecta. Am. J. Hum. Genet. 2004, 74, 752–760. [Google Scholar] [CrossRef]
- Wynn, R.F.; Hart, C.A.; Corradi-Perini, C.; O’Neill, L.; Evans, C.A.; Wraith, J.E.; Fairbairn, L.J.; Bellantuono, I. A small proportion of mesenchymal stem cells strongly expresses functionally active CXCR4 receptor capable of promoting migration to bone marrow. Blood 2004, 104, 2643–2645. [Google Scholar] [CrossRef]
- Eggenhofer, E.; Benseler, V.; Kroemer, A.; Popp, F.C.; Geissler, E.K.; Schlitt, H.J.; Baan, C.C.; Dahlke, M.H.; Hoogduijn, M.J. Mesenchymal stem cells are short-lived and do not migrate beyond the lungs after intravenous infusion. Front. Immunol. 2012, 3, 297. [Google Scholar] [CrossRef]
- Sinder, B.P.; Novak, S.; Wee, N.K.Y.; Basile, M.; Maye, P.; Matthews, B.G.; Kalajzic, I. Engraftment of skeletal progenitor cells by bone-directed transplantation improves osteogenesis imperfecta murine bone phenotype. Stem Cells 2020, 38, 530–541. [Google Scholar] [CrossRef]
- Panaroni, C.; Gioia, R.; Lupi, A.; Besio, R.; Goldstein, S.A.; Kreider, J.; Leikin, S.; Vera, J.C.; Mertz, E.L.; Perilli, E.; et al. In utero transplantation of adult bone marrow decreases perinatal lethality and rescues the bone phenotype in the knockin murine model for classical, dominant osteogenesis imperfecta. Blood 2009, 114, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ju, M.; Wang, Z.; Li, J.; Shao, C.; Fu, T.; Jing, Y.; Zhao, Y.; Lv, Z.; Li, G. The synergistic effect of NELL1 and adipose-derived stem cells on promoting bone formation in osteogenesis imperfecta treatment. Biomed. Pharmacother. 2020, 128, 110235. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, Z.; Ju, M.; Zhao, Y.; Jing, Y.; Li, J.; Shao, C.; Fu, T.; Lv, Z.; Li, G. Modification of COL1A1 in Autologous Adipose Tissue-Derived Progenitor Cells Rescues the Bone Phenotype in a Mouse Model of Osteogenesis Imperfecta. J. Bone Miner. Res. 2021, 36, 1521–1534. [Google Scholar] [CrossRef]
- Jilka, R.L. The relevance of mouse models for investigating age-related bone loss in humans. J. Gerontol. A Biol. Sci. Med. Sci. 2013, 68, 1209–1217. [Google Scholar] [CrossRef]
- Maynard, R.D.; Ackert-Bicknell, C.L. Mouse Models and Online Resources for Functional Analysis of Osteoporosis Genome-Wide Association Studies. Front. Endocrinol. 2019, 10, 277. [Google Scholar] [CrossRef]
- Mackay, E.W.; Apschner, A.; Schulte-Merker, S. A bone to pick with zebrafish. Bonekey Rep. 2013, 2, 445. [Google Scholar] [CrossRef] [PubMed]
- Valenti, M.T.; Marchetto, G.; Mottes, M.; Dalle Carbonare, L. Zebrafish: A Suitable Tool for the Study of Cell Signaling in Bone. Cells 2020, 9, 1911. [Google Scholar] [CrossRef] [PubMed]
- Watson, C.J.; Monstad-Rios, A.T.; Bhimani, R.M.; Gistelinck, C.; Willaert, A.; Coucke, P.; Hsu, Y.H.; Kwon, R.Y. Phenomics-Based Quantification of CRISPR-Induced Mosaicism in Zebrafish. Cell Syst. 2020, 10, 275–286. [Google Scholar] [CrossRef]
- Gioia, R.; Tonelli, F.; Ceppi, I.; Biggiogera, M.; Leikin, S.; Fisher, S.; Tenedini, E.; Yorgan, T.A.; Schinke, T.; Tian, K.; et al. The chaperone activity of 4PBA ameliorates the skeletal phenotype of Chihuahua, a zebrafish model for dominant osteogenesis imperfecta. Hum. Mol. Genet. 2017, 26, 2897–2911. [Google Scholar] [CrossRef]
- Pfeiffer, B.J.; Franklin, C.L.; Hsieh, F.H.; Bank, R.A.; Phillips, C.L. Alpha 2(I) collagen deficient oim mice have altered biomechanical integrity, collagen content, and collagen crosslinking of their thoracic aorta. Matrix Biol. 2005, 24, 451–458. [Google Scholar] [CrossRef]
- Misof, K.; Landis, W.J.; Klaushofer, K.; Fratzl, P. Collagen from the osteogenesis imperfecta mouse model (oim) shows reduced resistance against tensile stress. J. Clin. Investig. 1997, 100, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Gentry, B.A.; Ferreira, J.A.; McCambridge, A.J.; Brown, M.; Phillips, C.L. Skeletal muscle weakness in osteogenesis imperfecta mice. Matrix Biol. 2010, 29, 638–644. [Google Scholar] [CrossRef] [PubMed]
- Phillips, C.L.; Pfeiffer, B.J.; Luger, A.M.; Franklin, C.L. Novel collagen glomerulopathy in a homotrimeric type I collagen mouse (oim). Kidney Int. 2002, 62, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Gautieri, A.; Uzel, S.; Vesentini, S.; Redaelli, A.; Buehler, M.J. Molecular and mesoscale mechanisms of osteogenesis imperfecta disease in collagen fibrils. Biophys. J. 2009, 97, 857–865. [Google Scholar] [CrossRef]
- Veilleux, L.N.; Trejo, P.; Rauch, F. Muscle abnormalities in osteogenesis imperfecta. J. Musculoskelet. Neuronal Interact. 2017, 17, 1–7. [Google Scholar]
- Boskey, A.L.; Marino, J.; Spevak, L.; Pleshko, N.; Doty, S.; Carter, E.M.; Raggio, C.L. Are Changes in Composition in Response to Treatment of a Mouse Model of Osteogenesis Imperfecta Sex-dependent? Clin. Orthop. Relat. Res. 2015, 473, 2587–2598. [Google Scholar] [CrossRef][Green Version]
- Yao, X.; Carleton, S.M.; Kettle, A.D.; Melander, J.; Phillips, C.L.; Wang, Y. Gender-dependence of bone structure and properties in adult osteogenesis imperfecta murine model. Ann. Biomed. Eng. 2013, 41, 1139–1149. [Google Scholar] [CrossRef]
- Lee, L.R.; Holman, A.E.; Li, X.; Vasiljevski, E.R.; O’Donohue, A.K.; Cheng, T.L.; Little, D.G.; Schindeler, A.; Biggin, A.; Munns, C.F. Combination treatment with growth hormone and zoledronic acid in a mouse model of Osteogenesis imperfecta. Bone 2022, 159, 116378. [Google Scholar] [CrossRef]
- Götherström, C.; David, A.L.; Walther-Jallow, L.; Åström, E.; Westgren, M. Mesenchymal Stem Cell Therapy for Osteogenesis Imperfecta. Clin. Obstet. Gynecol. 2021, 64, 898–903. [Google Scholar] [CrossRef]

| OI Mouse Models Used in Preclinical Studies | ||||
|---|---|---|---|---|
| Type OI In Humans | Mouse Model | Mutated Gene | Mutation | Consequences |
| III | oim | Col1a2 | 3983delG | Accumulation of αl homotrimeric collagen I |
| I/IV | Amish mice | Col1a2 | 610G>C | Structural: ER stress + osteblast malfunction |
| IV | Brtl | Col1a1 | 349G>C | Structural |
| IV + EDS | Jrt | Col1a1 | exon9del | Quantitative: collagen I ↓ |
| III/II | Aga2 | Col1a1 | C-terminal fsX | ER stress |
| I | Col1a1±365 | Col1a1 | exon 2-exon 5del | Quantitative: collagen I ↓ |
| VII | CRTAP | Crtap | KO | |
| V | IFITM5 | Ifitm5 | 14C>T | |
| Type of Treatment | Target | Treatment | Bone Parameters | oim | Amish | Brtl | Jrt | Aga2 | Col1a1±365 | crtap | Ifitm5 | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Anti-resorptive | ATP-dependent enzymes/FPP synthase | BPs | MST | ✓ | ✓ | NCT00159419, NCT00005901, NCT02303873, NCT00106028, NCT00131118, | ||||||
| BIOM | ✓ | ✓ | ||||||||||
| RANKL | Denosumab | MST | ✓ | NCT01799798, NCT02352753, NCT03638128 | ||||||||
| BIOM | ✓ | |||||||||||
| SERM | Raloxifene | MST | ✓ | |||||||||
| BIOM | ✓ | |||||||||||
| Anabolic | Wnt pathway | Scl-Ab | MST | ✓ | ✓ | ✓ | ✗ | ✓ | NCT01417091, NCT03118570 NCT05125809, NCT04545554 | |||
| BIOM | ✓ | ✓ | ✓ | ✗ | ✓ | |||||||
| TGF-β superfamily | α-TGF-β Ab | MST | ✓ | ✗ | ✓ | NCT03064074 | ||||||
| BIOM | ✓ | ✗ | ✓ | |||||||||
| ActRIIB | MST | ✓ | ✓ | ✗ | ||||||||
| BIOM | ✗ | ✓ | ✗ | |||||||||
| α–myost Ab | MST | ✗ | ||||||||||
| BIOM | ✗ | |||||||||||
| α–activin A + α–myost Ab | MST | ✓ | ||||||||||
| BIOM | ✓ | |||||||||||
| Cellular Stress | 4 PBA | MST | ✓ | ✓ | ||||||||
| BIOM | ✗ | ✗ | ||||||||||
| Bortezomib | MST | ✓ | ||||||||||
| BIOM | ||||||||||||
| Anabolic + Anti-resorptive | Salubrinal | MST | ✗ | |||||||||
| BIOM | ✓ | |||||||||||
| Anabolic | Rapamycin | MST | ✓ | |||||||||
| BIOM | ✗ | |||||||||||
| BMC | ✓ | |||||||||||
| Carbamazepine | MST | ✗ | ||||||||||
| BIOM | ✗ | |||||||||||
| Low protein diet | MST | ✗ | ||||||||||
| BIOM | ✗ | |||||||||||
| PGE2 | Mes-1007 | MST | * | ✗ | ||||||||
| BIOM | * | ✗ | ||||||||||
| SC Therapy | MSCs | MST | ✓ | ✓ | NCT03706482, NCT04623606, NCT01061099, NCT05559801, NCT02172885 | |||||||
| BIOM | ✓ | ✓ | ||||||||||
| EVs-MSCs | BG | ✓ | NCT00705120, NCT00187018 | |||||||||
| BM | MST | ✗ | ✓ | |||||||||
| BIOM | ✗ | ✓ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alcorta-Sevillano, N.; Infante, A.; Macías, I.; Rodríguez, C.I. Murine Animal Models in Osteogenesis Imperfecta: The Quest for Improving the Quality of Life. Int. J. Mol. Sci. 2023, 24, 184. https://doi.org/10.3390/ijms24010184
Alcorta-Sevillano N, Infante A, Macías I, Rodríguez CI. Murine Animal Models in Osteogenesis Imperfecta: The Quest for Improving the Quality of Life. International Journal of Molecular Sciences. 2023; 24(1):184. https://doi.org/10.3390/ijms24010184
Chicago/Turabian StyleAlcorta-Sevillano, Natividad, Arantza Infante, Iratxe Macías, and Clara I. Rodríguez. 2023. "Murine Animal Models in Osteogenesis Imperfecta: The Quest for Improving the Quality of Life" International Journal of Molecular Sciences 24, no. 1: 184. https://doi.org/10.3390/ijms24010184
APA StyleAlcorta-Sevillano, N., Infante, A., Macías, I., & Rodríguez, C. I. (2023). Murine Animal Models in Osteogenesis Imperfecta: The Quest for Improving the Quality of Life. International Journal of Molecular Sciences, 24(1), 184. https://doi.org/10.3390/ijms24010184






