Low Sulfur Amino Acid, High Polyunsaturated Fatty Acid Diet Inhibits Breast Cancer Growth
Abstract
:1. Introduction
2. Results
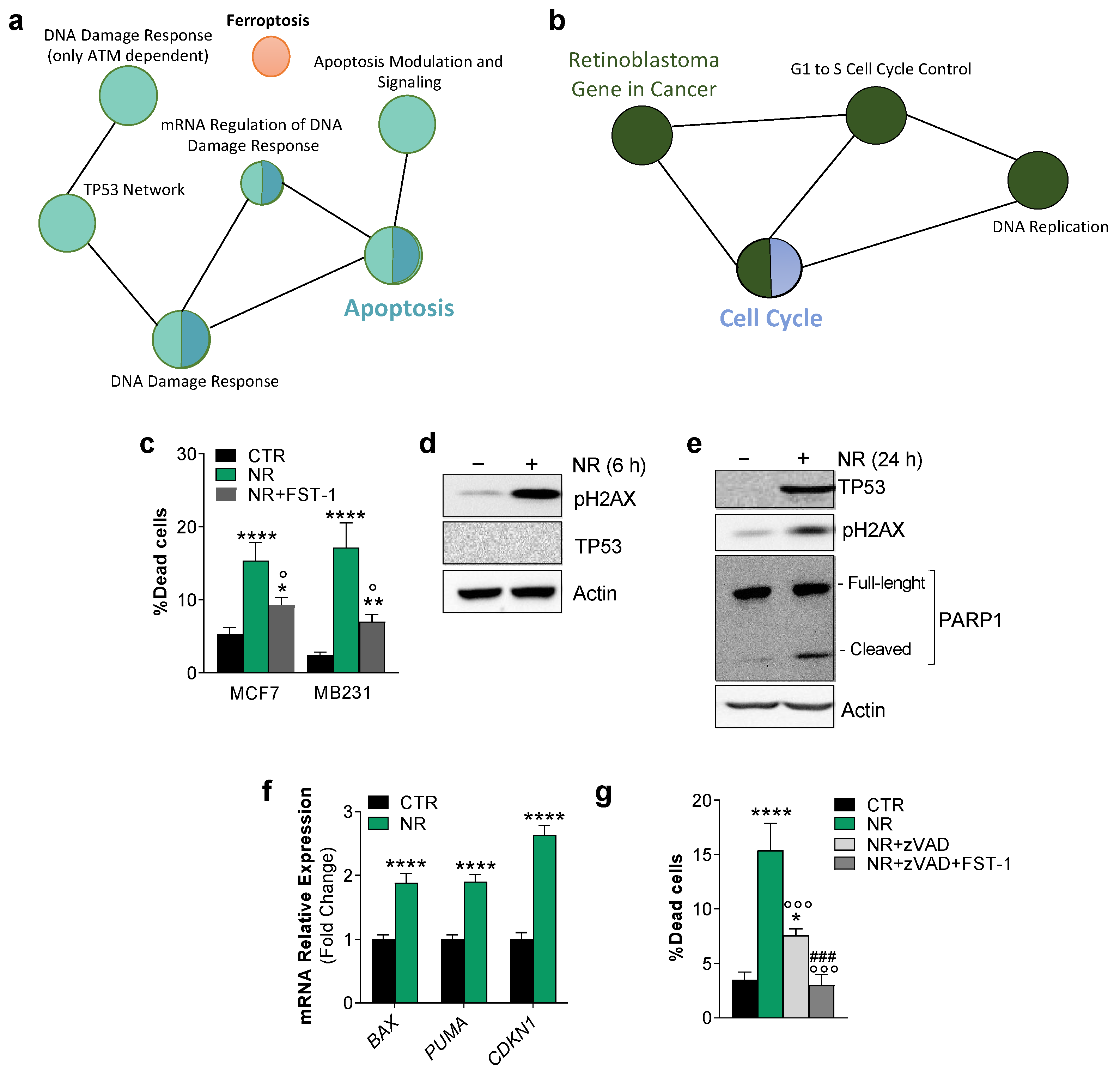
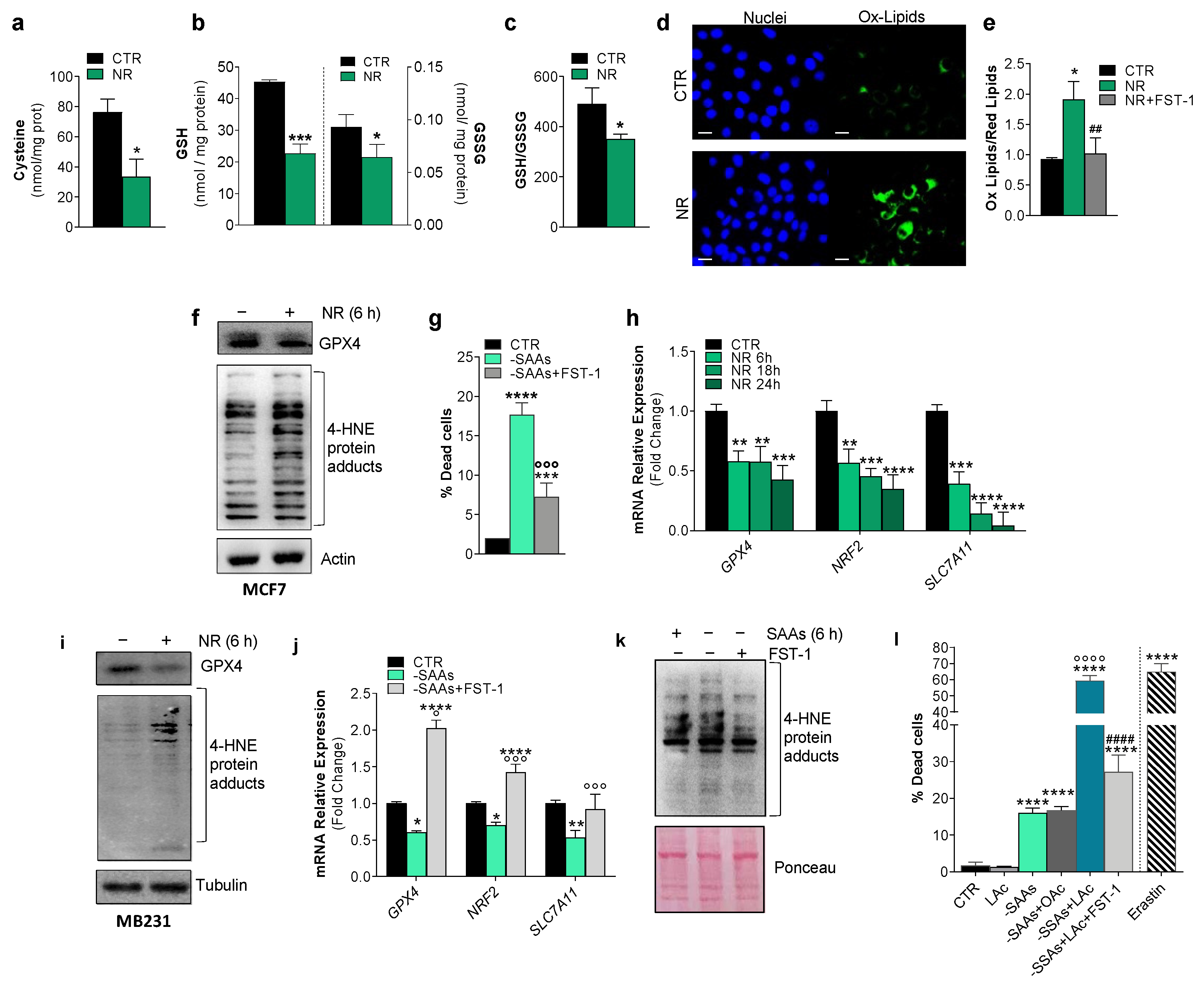
2.1. Human Breast Cancer Cells Are Susceptible to Apoptosis and Ferroptosis upon Nutrient Changes In Vitro
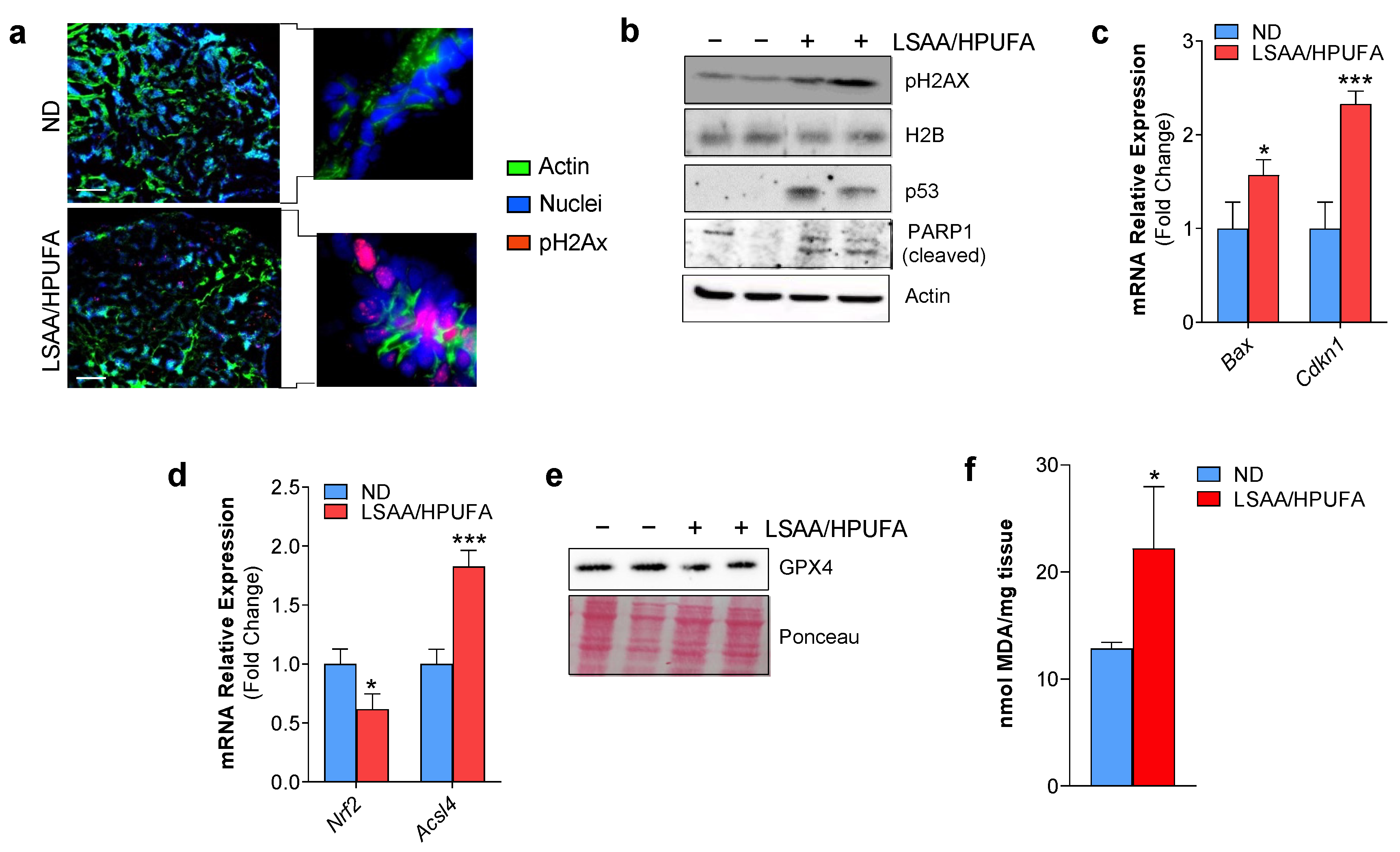
2.2. A Low SAA-High PUFA Diet Modulates Ferroptosis Marks and Restrains Breast Cancer Growth in Mice
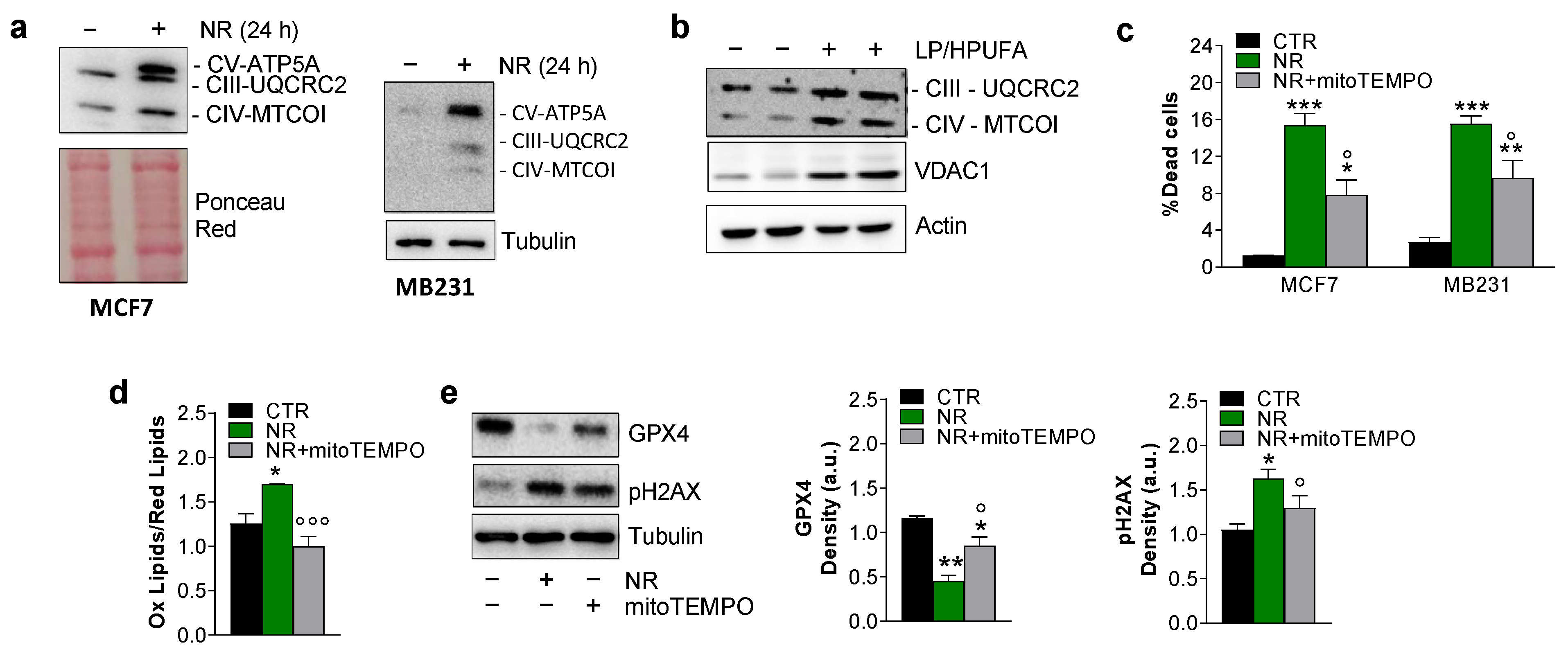
2.3. Ferroptosis in Breast Cancer Cells Is Mitochondria-Dependent
3. Discussion
4. Materials and Methods
4.1. Cells and Treatments
4.2. Mice and Treatments
4.3. Cysteine and Glutathione Determination
4.4. Bioinformatic Analysis
4.5. Western Blot Analysis
4.6. RT-qPCR
4.7. Microscope Analysis
4.8. Cytofluorimetric Analysis and Malondialdehyde Assay
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jan, R.; Chaudhry, G.-E. Understanding Apoptosis and Apoptotic Pathways Targeted Cancer Therapeutics. Adv. Pharm. Bull. 2019, 9, 205–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Kang, R.; Kroemer, G.; Tang, D. Broadening horizons: The role of ferroptosis in cancer. Nat. Rev. Clin. Oncol. 2021, 18, 280–296. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Cai, S.; Yu, S.; Lin, H. Metformin induces ferroptosis by targeting miR-324-3p/GPX4 axis in breast cancer. Acta Biochim. Biophys. Sin. 2021, 53, 333–341. [Google Scholar] [CrossRef]
- Zhang, Z.; Lu, M.; Chen, C.; Tong, X.; Li, Y.; Yang, K.; Lv, H.; Xu, J.; Qin, L. Holo-lactoferrin: The link between ferroptosis and radiotherapy in triple-negative breast cancer. Theranostics 2021, 11, 3167–3182. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Sui, S.; Wang, L.; Li, H.; Zhang, L.; Xu, S.; Zheng, X. Inhibition of tumor propellant glutathione peroxidase 4 induces ferroptosis in cancer cells and enhances anticancer effect of cisplatin. J. Cell. Physiol. 2020, 235, 3425–3437. [Google Scholar] [CrossRef] [PubMed]
- Fujihara, K.; Zhang, B.; Clemons, N. Opportunities for Ferroptosis in Cancer Therapy. Antioxidants 2021, 10, 986. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Zhao, B.; Zhou, L.; Zhang, Z.; Shen, Y.; Lv, H.; AlQudsy, L.H.H.; Shang, P. Ferroptosis, a novel pharmacological mechanism of anti-cancer drugs. Cancer Lett. 2020, 483, 127–136. [Google Scholar] [CrossRef]
- Lieu, E.L.; Nguyen, T.; Rhyne, S.; Kim, J. Amino acids in cancer. Exp. Mol. Med. 2020, 52, 15–30. [Google Scholar] [CrossRef]
- Ward, N.P.; DeNicola, G.M. Sulfur metabolism and its contribution to malignancy. Int. Rev. Cell Mol. Biol. 2019, 347, 39–103. [Google Scholar] [CrossRef]
- Yang, W.S.; Stockwell, B.R. Ferroptosis: Death by Lipid Peroxidation. Trends Cell Biol. 2016, 26, 165–176. [Google Scholar] [CrossRef]
- Xie, Y.; Hou, W.; Song, X.; Yu, Y.; Huang, J.; Sun, X.; Kang, R.; Tang, D. Ferroptosis: Process and function. Cell Death Differ. 2016, 23, 369–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Badgley, M.A.; Kremer, D.M.; Maurer, H.C.; DelGiorno, K.E.; Lee, H.-J.; Purohit, V.; Sagalovskiy, I.R.; Ma, A.; Kapilian, J.; Firl, C.E.M.; et al. Cysteine depletion induces pancreatic tumor ferroptosis in mice. Science 2020, 368, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Hens, J.R.; Sinha, I.; Perodin, F.; Cooper, T.; Sinha, R.; Plummer, J.; Perrone, C.E.; Orentreich, D. Methionine-restricted diet inhibits growth of MCF10AT1-derived mammary tumors by increasing cell cycle inhibitors in athymic nude mice. BMC Cancer 2016, 16, 349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daher, B.; Vučetić, M.; Pouysségur, J. Cysteine Depletion, a Key Action to Challenge Cancer Cells to Ferroptotic Cell Death. Front. Oncol. 2020, 10, 723. [Google Scholar] [CrossRef] [PubMed]
- D’Archivio, M.; Scazzocchio, B.; Vari, R.; Santangelo, C.; Giovannini, C.; Masella, R. Recent Evidence on the Role of Dietary PUFAs in Cancer Development and Prevention. Curr. Med. Chem. 2018, 25, 1818–1836. [Google Scholar] [CrossRef]
- Perez, M.A.; Magtanong, L.; Dixon, S.J.; Watts, J.L. Dietary Lipids Induce Ferroptosis in Caenorhabditiselegans and Human Cancer Cells. Dev. Cell 2020, 54, 447–454.e4. [Google Scholar] [CrossRef]
- Dierge, E.; Debock, E.; Guilbaud, C.; Corbet, C.; Mignolet, E.; Mignard, L.; Bastien, E.; Dessy, C.; Larondelle, Y.; Feron, O. Peroxidation of n-3 and n-6 polyunsaturated fatty acids in the acidic tumor environment leads to ferroptosis-mediated anticancer effects. Cell Metab. 2021, 33, 1701–1715.e5. [Google Scholar] [CrossRef]
- Lettieri-Barbato, D.; Minopoli, G.; Caggiano, R.; Izzo, R.; Santillo, M.; Aquilano, K.; Faraonio, R. Fasting Drives Nrf2-Related Antioxidant Response in Skeletal Muscle. Int. J. Mol. Sci. 2020, 21, 7780. [Google Scholar] [CrossRef]
- Lettieri-Barbato, D.; Aquilano, K. Pushing the Limits of Cancer Therapy: The Nutrient Game. Front. Oncol. 2018, 8, 148. [Google Scholar] [CrossRef] [Green Version]
- Tang, D.; Chen, X.; Kang, R.; Kroemer, G. Ferroptosis: Molecular mechanisms and health implications. Cell Res. 2021, 31, 107–125. [Google Scholar] [CrossRef]
- Liu, Y.; Gu, W. The complexity of p53-mediated metabolic regulation in tumor suppression. Semin. Cancer Biol. 2021, 85, 4–32. [Google Scholar] [CrossRef] [PubMed]
- Fragkos, M.; Jurvansuu, J.; Beard, P. H2AX Is Required for Cell Cycle Arrest via the p53/p21 Pathway. Mol. Cell. Biol. 2009, 29, 2828–2840. [Google Scholar] [CrossRef] [Green Version]
- Desroches, A.; Denault, J.-B. Caspase-7 uses RNA to enhance proteolysis of poly(ADP-ribose) polymerase 1 and other RNA-binding proteins. Proc. Natl. Acad. Sci. USA 2019, 116, 21521–21528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, D.; Tavana, O.; Chu, B.; Erber, L.; Chen, Y.; Baer, R.; Gu, W. NRF2 Is a Major Target of ARF in p53-Independent Tumor Suppression. Mol. Cell 2017, 68, 224–232.e4. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Ou, Z.; Chen, R.; Niu, X.; Chen, D.; Kang, R.; Tang, D. Activation of the p62-Keap1-NRF2 pathway protects against ferroptosis in hepatocellular carcinoma cells. Hepatology 2016, 63, 173–184. [Google Scholar] [CrossRef] [Green Version]
- Aquilano, K.; Baldelli, S.; Pagliei, B.; Cannata, S.M.; Rotilio, G.; Ciriolo, M.R. p53 Orchestrates the PGC-1α-Mediated Antioxidant Response Upon Mild Redox and Metabolic Imbalance. Antioxidants Redox Signal. 2013, 18, 386–399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, N.M.; Simon, M.C. The tumor microenvironment. Curr. Biol. 2020, 30, R921–R925. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhou, B.P. Epithelial-mesenchymal transition in breast cancer progression and metastasis. Chin. J. Cancer 2011, 30, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Kumar, L.D.; Kumar, A.; Golani, A. EMT in breast cancer metastasis: An interplay of microRNAs signaling pathways and circulating tumor cells. Front. Biosci. 2020, 25, 979–1010. [Google Scholar] [CrossRef]
- Matysiak, M.; Kapka-Skrzypczak, L.; Jodłowska-Jędrych, B.; Kruszewski, M. EMT promoting transcription factors as prognostic markers in human breast cancer. Arch. Gynecol. Obstet. 2017, 295, 817–825. [Google Scholar] [CrossRef]
- Gao, M.; Yi, J.; Zhu, J.; Minikes, A.; Monian, P.; Thompson, C.B.; Jiang, X. Role of Mitochondria in Ferroptosis. Mol. Cell 2019, 73, 354–363.e3. [Google Scholar] [CrossRef] [Green Version]
- Doll, S.; Proneth, B.; Tyurina, Y.; Panzilius, E.; Kobayashi, S.; Ingold, I.; Irmler, M.; Beckers, M.I.J.; Aichler, M.; Walch, M.A.A.; et al. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat. Chem. Biol. 2016, 13, 91–98. [Google Scholar] [CrossRef]
- Li, Z.; Chen, L.; Chen, C.; Zhou, Y.; Hu, D.; Yang, J.; Chen, Y.; Zhuo, W.; Mao, M.; Zhang, X.; et al. Targeting ferroptosis in breast cancer. Biomark. Res. 2020, 8, 58. [Google Scholar] [CrossRef]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An Iron-Dependent Form of Nonapoptotic Cell Death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef] [Green Version]
- Sakamoto, T.; Niiya, D.; Seiki, M. Targeting the Warburg Effect That Arises in Tumor Cells Expressing Membrane Type-1 Matrix Metalloproteinase. J. Biol. Chem. 2011, 286, 14691–14704. [Google Scholar] [CrossRef] [Green Version]
- Gatenby, R.A.; Gillies, R.J. Why do cancers have high aerobic glycolysis? Nat. Rev. Cancer 2004, 4, 891–899. [Google Scholar] [CrossRef]
- Liang, X.H.; Jackson, S.; Seaman, M.; Brown, K.; Kempkes, B.; Hibshoosh, H.; Levine, B. Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature 1999, 402, 672–676. [Google Scholar] [CrossRef]
- Chen, J.; Lu, L.; Feng, Y.; Wang, H.; Dai, L.; Li, Y.; Zhang, P. PKD2 mediates multi-drug resistance in breast cancer cells through modulation of P-glycoprotein expression. Cancer Lett. 2011, 300, 48–56. [Google Scholar] [CrossRef]
- Bebber, C.M.; Müller, F.; Prieto Clemente, L.; Weber, J.; Von Karstedt, S. Ferroptosis in Cancer Cell Biology. Cancers 2020, 12, 164. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Yu, C.; Luo, M.; Cen, C.; Qiu, J.; Zhang, S.; Hu, K. Ferroptosis in Cancer Treatment: Another Way to Rome. Front. Oncol. 2020, 10, 571127. [Google Scholar] [CrossRef]
- Kuwata, H.; Hara, S. Role of acyl-CoA synthetase ACSL4 in arachidonic acid metabolism. Prostaglandins Other Lipid Mediat. 2019, 144, 106363. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Fan, Y.; Liu, B.; Zhou, H.; Wang, J.; Chen, Q. ACSL4 suppresses glioma cells proliferation via activating ferroptosis. Oncol. Rep. 2020, 43, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Li, X.; Zhang, X.; Kang, R.; Tang, D. Identification of ACSL4 as a biomarker and contributor of ferroptosis. Biochem. Biophys. Res. Commun. 2016, 478, 1338–1343. [Google Scholar] [CrossRef] [PubMed]
- Lees, A.; Sessler, T.; McDade, S. Dying to Survive—The p53 Paradox. Cancers 2021, 13, 3257. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Xu, B.; Han, Q.; Zhou, H.; Xia, Y.; Gong, C.; Dai, X.; Li, Z.; Wu, G. Ferroptosis: A Novel Anti-tumor Action for Cisplatin. Cancer Res. Treat. 2018, 50, 445–460. [Google Scholar] [CrossRef] [Green Version]
- Louandre, C.; Ezzoukhry, Z.; Godin, C.; Barbare, J.-C.; Mazière, J.-C.; Chauffert, B.; Galmiche, A. Iron-dependent cell death of hepatocellular carcinoma cells exposed to sorafenib. Int. J. Cancer 2013, 133, 1732–1742. [Google Scholar] [CrossRef]
- Jelinek, A.; Heyder, L.; Daude, M.; Plessner, M.; Krippner, S.; Grosse, R.; Diederich, W.E.; Culmsee, C. Mitochondrial rescue prevents glutathione peroxidase-dependent ferroptosis. Free Radic. Biol. Med. 2018, 117, 45–57. [Google Scholar] [CrossRef]
- Neitemeier, S.; Jelinek, A.; Laino, V.; Hoffmann, L.; Eisenbach, I.; Eying, R.; Ganjam, G.K.; Dolga, A.M.; Oppermann, S.; Culmsee, C. BID links ferroptosis to mitochondrial cell death pathways. Redox Biol. 2017, 12, 558–570. [Google Scholar] [CrossRef]
- Nimni, M.E.; Han, B.; Cordoba, F. Are we getting enough sulfur in our diet? Nutr. Metab. 2007, 4, 24. [Google Scholar] [CrossRef] [Green Version]
- Patterson, E.; Wall, R.; Fitzgerald, G.F.; Ross, R.; Stanton, C. Health Implications of High Dietary Omega-6 Polyunsaturated Fatty Acids. J. Nutr. Metab. 2012, 2012, 539426. [Google Scholar] [CrossRef]
- Rovero, S.; Amici, A.; Di Carlo, E.; Bei, R.; Nanni, P.; Quaglino, E.; Porcedda, P.; Boggio, K.; Smorlesi, A.; Lollini, P.L.; et al. DNA vaccination against rat her-2/Neu p185 more effectively inhibits carcinogenesis than transplantable carcinomas in transgenic BALB/c mice. J. Immunol. 2000, 165, 5133–5142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reed, D.; Babson, J.; Beatty, P.; Brodie, A.; Ellis, W.; Potter, D. High-performance liquid chromatography analysis of nanomole levels of glutathione, glutathione disulfide, and related thiols and disulfides. Anal. Biochem. 1980, 106, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of Biomolecular Interaction Networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]

| Acsl4 (Mus musculus) | FW: 5′-AGCCAGCAATAAAGTACACTTACAG-3′ RV: 5′-CAGGGCTCCAAAAGTGACA-3′ |
| Bax (Mus musculus) | FW: 5′-TGGAGCTGCAGAGGATGATTG-3′ RV: 3′-AGCCACCCTGGTCTTGGA-3′ |
| BAX (Homo sapiens) | FW: 5′-CAGGGTTTCATCCAGGAT-3′ RV: 3′-AAACATGTCAGCTGCCAC-3′ |
| CDKN1 (Homo sapiens) | FW: 5′-GTGGCTATTTTGTCCTTGGGC-3′ RV: 3′- GTTCTGACATGGCGCCTGAA-3′ |
| Cdkn1 (Mus musculus) | FW: 5′-GCAGAATAAAAGGTGCCACAGG-3′ RV: 3′-AAAGTTCCACCGTTCTCGGG-3′ |
| GPX4 (Homo sapiens) | FW: 5′-GCCATCAAGTGGAACTTCACC-3′ RV: 5′-CTTCTCTACTACCAGGGGCTC-3′ |
| Gpx4 (Mus musculus) | FW: 5′-ATTGGTCGGCTGCGTGAG-3′ RV: 5′-ACACGAAACCCCTGTACTTATCC-3′ |
| Il1b(Mus musculus) | FW: 5′-TGCCACCTTTTGACAGTGATG-3′ RV: 5′-AAGGTCCACGGGAAAGACAC-3′ |
| Il6 (Mus musculus) | FW: 5′-GGATACCACTCCCAACAGACC-3′ RV: 5′-GCCATTGCACAACTCTTTTCTCA-3′ |
| Nos2 (Mus musculus) | FW: 5′-GCCTTCAACACCAAGGTTGTC-3′ RV: 5′-ACCACCAGCAGTAGTTGCTC-3′ |
| NRF2 (Homo sapiens) | FW: 5′-TGCCAACTACTCCCAGGTTG-3′ RV: 5′-AAGTGACTGAAACGTAGCCGA-3′ |
| Nrf2 (Mus musculus) | FW: 5′-CCTCTGCTGCAAGTAGCCTC-3′ RV: 5′-AATCCATGTCCTGCTGGGACT-3′ |
| PUMA (Homo sapiens) | FW: 5′-GAAATTTGGCATGGGGTCTGC-3′ RV: 3′-TCCCTGGGGCCACAAATCT-3′ |
| RPL8 (Homo sapiens) | FW: 5′-CACCATGCCTGAGGGTACAA-3′ RV: 5′-CGGGTCTTCTTGGTCTCAGG-3′ |
| Rpl8 (Mus musculus) | FW: 5′-GGAGCGACACGGCTACATTA-3′ RV: 5′-CCGATATTCAGCTGGGCCTT-3′ |
| SLC7A11 (Homo sapiens) | FW: 5′-CTTTCAAGGTGCCACTGTTCATC-3′ RV: 5′-AGATTGCCAAGATCTCAAGTCCA-3′ |
| Tnfa (Mus musculus) | FW: 5′-ATGGCCTCCCTCTCATCAGT-3′ RV: 5′-CTTGGTGGTTTGCTACGACG-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turchi, R.; Tortolici, F.; Benvenuto, M.; Punziano, C.; De Luca, A.; Rufini, S.; Faraonio, R.; Bei, R.; Lettieri-Barbato, D.; Aquilano, K. Low Sulfur Amino Acid, High Polyunsaturated Fatty Acid Diet Inhibits Breast Cancer Growth. Int. J. Mol. Sci. 2023, 24, 249. https://doi.org/10.3390/ijms24010249
Turchi R, Tortolici F, Benvenuto M, Punziano C, De Luca A, Rufini S, Faraonio R, Bei R, Lettieri-Barbato D, Aquilano K. Low Sulfur Amino Acid, High Polyunsaturated Fatty Acid Diet Inhibits Breast Cancer Growth. International Journal of Molecular Sciences. 2023; 24(1):249. https://doi.org/10.3390/ijms24010249
Chicago/Turabian StyleTurchi, Riccardo, Flavia Tortolici, Monica Benvenuto, Carolina Punziano, Anastasia De Luca, Stefano Rufini, Raffaella Faraonio, Roberto Bei, Daniele Lettieri-Barbato, and Katia Aquilano. 2023. "Low Sulfur Amino Acid, High Polyunsaturated Fatty Acid Diet Inhibits Breast Cancer Growth" International Journal of Molecular Sciences 24, no. 1: 249. https://doi.org/10.3390/ijms24010249









