Changes in Growth, Ionic Status, Metabolites Content and Antioxidant Activity of Two Ferns Exposed to Shade, Full Sunlight, and Salinity
Abstract
:1. Introduction
2. Results
2.1. Effect of Abiotic Stresses on Fern Growth Parameters
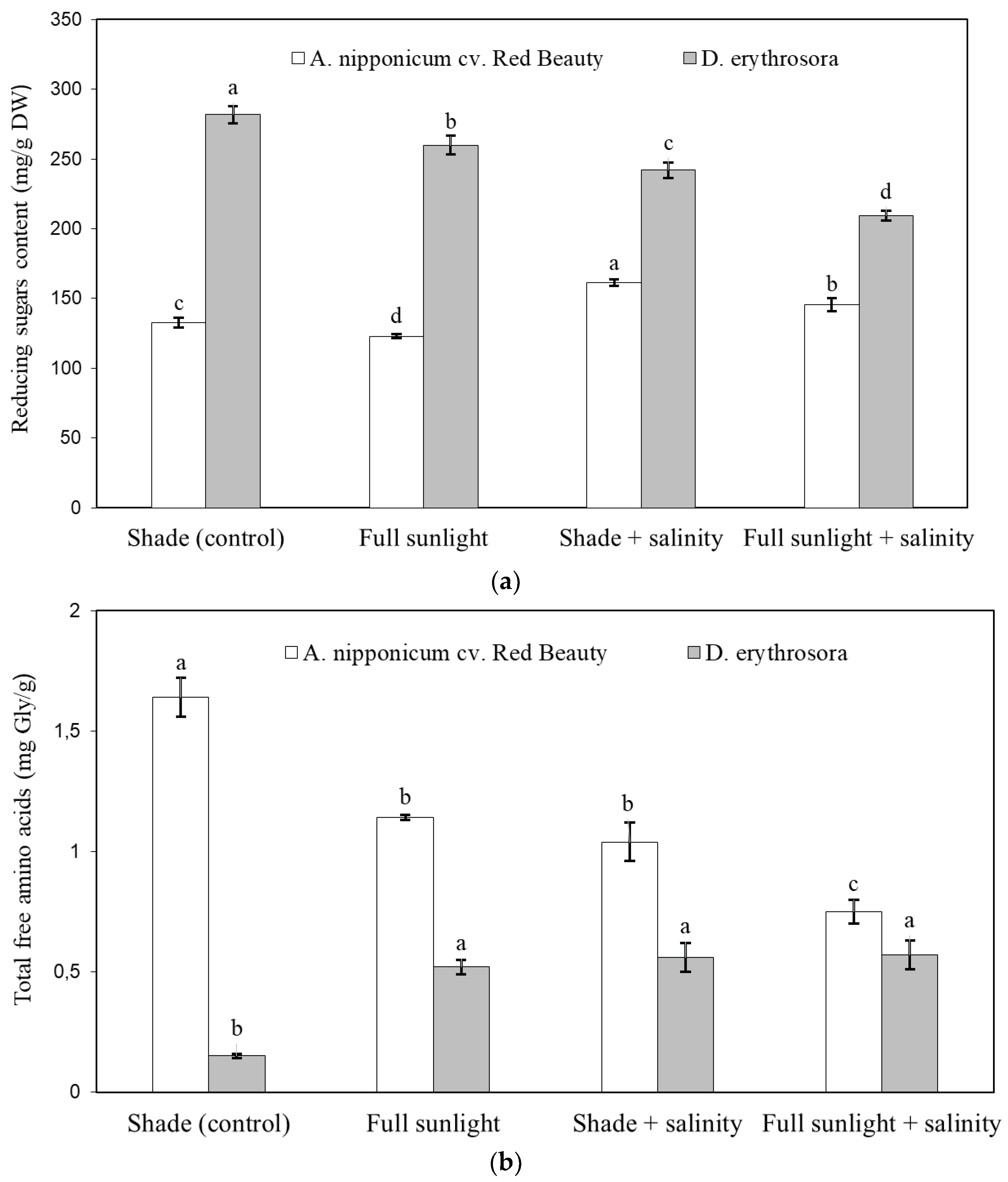
2.2. Effect of Abiotic Stresses on Fern Ion Balance, Chlorophyll Content, Carbohydrates, and Amino Acid Metabolism
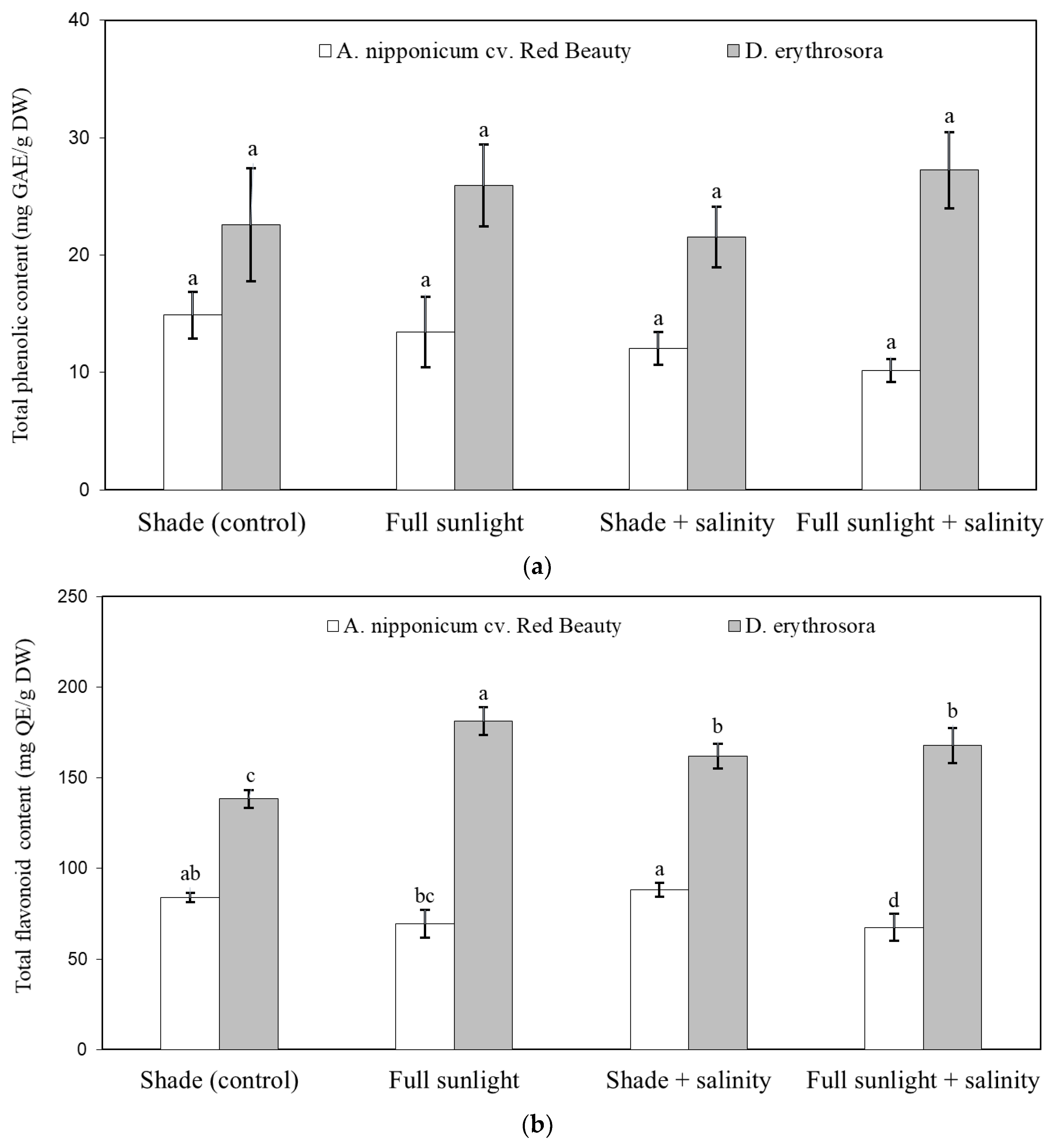
2.3. Effect of Abiotic Stresses on Antioxidant Compounds and Antioxidant Properties of Ferns
3. Discussion
3.1. Morphology
3.2. Physiological Responses
3.3. Antioxidant Responses
4. Materials and Methods
4.1. Plant Material, Treatments, and Growth Characteristics
4.2. Determination of Ionic Status
4.3. Determination of Photosynthetic Pigment Content
4.4. Preparation of Extracts
4.5. Determination of Total Reducing Sugars
4.6. Content of Total Free Amino Acids
4.7. Total Polyphenols and Total Flavonoids
4.8. 1,1-Diphenyl-2-Picryl-Hydrazyl (DPPH) and 2,2′-Azobis(3-Ethylbenzothiazoline-6-Sulfonate) (ABTS) Assays
4.9. Ferric Reducing Antioxidant Power (FRAP)
4.10. Reducing Power
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pittermann, J.; Brodersen, C.; Watkins, J. The Physiological resilience of fern sporophytes and gametophytes: Advances in water relations offer new insights into an old lineage. Front. Plant Sci. 2013, 4, 285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suissa, J.S.; Friedman, W.E. From cells to stems: The effects of primary vascular construction on drought-induced embolism in fern rhizomes. New Phytol. 2021, 232, 2238–2253. [Google Scholar] [CrossRef] [PubMed]
- Anderson, O.R. Physiological ecology of ferns: Biodiversity and conservation perspectives. Int. J. Biodivers. Conserv. 2021, 13, 49–63. [Google Scholar]
- Wang, Y.; Gao, S.; He, X.; Li, Y.; Li, P.; Zhang, Y.; Chen, W. Growth, Secondary Metabolites and Enzyme Activity Responses of Two Edible Fern Species to Drought Stress and Rehydration in Northeast China. Agronomy 2019, 9, 137. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.P.; Johari, D. Scope of ferns in horticulture and economic development. In Current Advances in Fern Research; Fernández, H., Ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 153–175. ISBN 978-3-319-75103-0. [Google Scholar]
- Salachna, P.; Siemińska, I.; Pietrak, A.; Zawadzińska, A.; Piechocki, R.; Dymek, R. Response of hardy ferns to drought stress. Biol. Life Sci. Forum 2021, 3, 8. [Google Scholar]
- Wang, X.; Wang, M.; Cao, J.; Wu, Y.; Xiao, J.; Wang, Q. Analysis of flavonoids and antioxidants in extracts of ferns from Tianmu Mountain in Zhejiang Province (China). Ind. Crops Prod. 2017, 97, 137–145. [Google Scholar] [CrossRef]
- Salachna, P.; Piechocki, R. Salinity tolerance of four hardy ferns from the genus Dryopteris Adans. grown under different light conditions. Agronomy 2021, 11, 49. [Google Scholar] [CrossRef]
- Yadav, R.K.; Chatrath, A.; Tripathi, K.; Gerard, M.; Ahmad, A.; Mishra, V.; Abraham, G. Salinity tolerance mechanism in the aquatic nitrogen fixing pteridophyte Azolla: A Review. Symbiosis 2021, 83, 129–142. [Google Scholar] [CrossRef]
- Cramer, G.R.; Urano, K.; Delrot, S.; Pezzotti, M.; Shinozaki, K. Effects of abiotic stress on plants: A systems biology perspective. BMC Plant Biology 2011, 11, 163. [Google Scholar] [CrossRef] [Green Version]
- Mittler, R. Abiotic stress, the field environment and stress combination. Trends Plant Sci. 2006, 11, 15–19. [Google Scholar] [CrossRef]
- Das, K.; Roychoudhury, A. Reactive oxygen species (ROS) and response of antioxidants as ros-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar] [CrossRef] [Green Version]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Parvin, K.; Bhuiyan, T.F.; Anee, T.I.; Nahar, K.; Hossen, M.S.; Zulfiqar, F.; Alam, M.M.; Fujita, M. Regulation of ROS Metabolism in Plants under Environmental Stress: A Review of Recent Experimental Evidence. Int. J. Mol. Sci. 2020, 21, 8695. [Google Scholar] [CrossRef]
- Zhang, H.; Sonnewald, U. Differences and commonalities of plant responses to single and combined stresses. Plant J. 2017, 90, 839–855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sack, L.; Buckley, T.N. Trait multi-functionality in plant stress response. Integr. Comp. Biol. 2020, 60, 98–112. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Ezzat, S.M.; Tsouh Fokou, P.V.; Albayrak, S.; Vlaisavljevic, S.; Sharifi-Rad, M.; Bhatt, I.D.; Sharifi-Rad, M.; Belwal, T.; Ayatollahi, S.A.; et al. Athyrium plants—Review on phytopharmacy properties. J. Tradit. Complement. Med. 2019, 9, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, X.; Wang, M.; Cao, J.; Xiao, J.; Wang, Q. Effects of different pretreatments on flavonoids and antioxidant activity of Dryopteris erythrosora leave. PLoS ONE 2019, 14, e0200174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, J.; Xia, X.; Chen, X.; Xiao, J.; Wang, Q. Characterization of flavonoids from Dryopteris erythrosora and evaluation of their antioxidant, anticancer and acetylcholinesterase inhibition activities. Food Chem. Toxicol. 2013, 51, 242–250. [Google Scholar] [CrossRef]
- Møller, I.S.; Tester, M. Salinity tolerance of Arabidopsis: A good model for cereals? Trends Plant Sci. 2007, 12, 534–540. [Google Scholar] [CrossRef]
- Shannon, M.C. Adaptation of plants to salinity. Adv. Agron. 1997, 60, 75–120. [Google Scholar]
- Alkharabsheh, H.M.; Seleiman, M.F.; Hewedy, O.A.; Battaglia, M.L.; Jalal, R.S.; Alhammad, B.A.; Schillaci, C.; Ali, N.; Al-Doss, A. Field Crop Responses and Management Strategies to Mitigate Soil Salinity in Modern Agriculture: A Review. Agronomy 2021, 11, 2299. [Google Scholar] [CrossRef]
- Hernández, J.A. Salinity Tolerance in Plants: Trends and Perspectives. Int. J. Mol. Sci. 2019, 20, 2408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Safdar, H.; Amin, A.; Shafiq, Y.; Ali, A.; Yasin, R.; Shoukat, A.; Hussan, M.U.; Sarwar, M.I. A review: Impact of salinity on plant growth. Nat. Sci. 2019, 17, 34–40. [Google Scholar]
- Salachna, P.; Zawadzińska, A.; Podsiadło, C. Response of Ornithogalum saundersiae Bak. to salinity stress. Acta Sci. Pol. Hortorum Cultus 2016, 15, 123–134. [Google Scholar]
- García-Caparrós, P.; Lao, M.T. The effects of salt stress on ornamental plants and integrative cultivation practices. Sci. Hortic. 2018, 240, 430–439. [Google Scholar] [CrossRef]
- Igliński, B.; Cichosz, M.; Kujawski, W.; Plaskacz-Dziuba, M.; Buczkowski, R. Helioenergy in Poland—Current state, surveys and prospects. Renew. Sustain. Energy Rev. 2016, 58, 862–870. [Google Scholar]
- Radić, S.; Prolić, M.; Pavlica, M.; Pevalek-Kozlina, B. Cytogenetic effects of osmotic stress on the root meristem cells of Centaurea ragusina L. Environ. Exp. Bot. 2005, 54, 213–218. [Google Scholar] [CrossRef]
- Porcelli, C.; Gutierrez, F.H.; Lavado, R. The K/Na and Ca/Na rations and rapeseed yield, under soil salinity or sodicity. Plant Soil 1995, 175, 251–255. [Google Scholar] [CrossRef]
- Muhammed, S.; Akbar, M.; Neue, H.U. Effect of Na/Ca and Na/K ratios in saline culture solution on the growth and mineral nutrition of rice (Oryza sativa L.). Plant Soil 1987, 104, 57–62. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; Adams, W.W. Photoprotection and other responses of plants to high light stress. Annu. Rev. Plant Biol. 1992, 43, 599–626. [Google Scholar] [CrossRef]
- Krause, G.H. Photoinhibition of photosynthesis. An evaluation of damaging and protective mechanisms. Physiol. Plant. 1988, 74, 566–574. [Google Scholar] [CrossRef]
- Bogdanović, M.; Ilić, M.; Živković, S.; Sabovljević, A.; Grubišić, D.; Sabovljević, M. Comparative study on the effects of NaCl on selected moss and fern representatives. Aust. J. Bot. 2012, 59, 734–740. [Google Scholar] [CrossRef]
- Kura-Hotta, M.; Satoh, K.; Katoh, S. Relationship between photosynthesis and chlorophyll content during leaf senescence of rice seedlings. Plant Cell Physiol. 1987, 28, 1321–1329. [Google Scholar]
- Hayden, D.B.; Baker, N.R.; Percival, M.P.; Beckwith, P.B. Modification of the photosystem II light-harvesting chlorophyll ab protein complex in maize during chillinduced photoinhibition. Biochim. Biophys. Acta—Bioenerg. 1986, 851, 86–92. [Google Scholar] [CrossRef]
- de Moraes, M.G.; de Oliveira, A.A.Q.; Santos, M.G. Sugars in ferns and lycophytes growing on rocky outcrops from southeastern brazilian coast. Biosci. J. 2014, 30, 1882–1884. [Google Scholar]
- Balibrea, M.E.; Dell’Amico, J.; Bolarín, M.C.; Pérez-Alfocea, F. Carbon partitioning and sucrose metabolism in tomato plants growing under salinity. Physiol. Plant. 2000, 110, 503–511. [Google Scholar] [CrossRef]
- van Kempen, M.M.L.; Smolders, A.J.P.; Bögemann, G.M.; Lamers, L.L.M.; Visser, E.J.W.; Roelofs, J.G.M. Responses of the Azolla filiculoides Stras.–Anabaena azollae Lam. association to elevated sodium chloride concentrations: Amino acids as indicators for salt stress and tipping point. Aquat. Bot. 2013, 106, 20–28. [Google Scholar] [CrossRef]
- Batista-Silva, W.; Heinemann, B.; Rugen, N.; Nunes-Nesi, A.; Araújo, W.L.; Braun, H.-P.; Hildebrandt, T.M. The role of amino acid metabolism during abiotic stress release. Plant Cell Environ. 2019, 42, 1630–1644. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Gao, S.; He, X.; Li, Y.; Zhang, Y.; Chen, W. Response of total phenols, flavonoids, minerals, and amino acids of four edible fern species to four shading treatments. PeerJ 2020, 8, e8354. [Google Scholar] [CrossRef]
- Xia, X.; Cao, J.; Zheng, Y.; Wang, Q.; Xiao, J. Flavonoid concentrations and bioactivity of flavonoid extracts from 19 species of ferns from China. Ind. Crops Prod. 2014, 58, 91–98. [Google Scholar] [CrossRef]
- Tattini, M.; Galardi, C.; Pinelli, P.; Massai, R.; Remorini, D.; Agati, G. Differential accumulation of flavonoids and hydroxycinnamates in leaves of Ligustrum vulgare under excess light and drought stress. New Phytol. 2004, 163, 547–561. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, M.; Kuiry, R.; Pal, P.K. Understanding the consequence of environmental stress for accumulation of secondary metabolites in medicinal and aromatic plants. J. Appl. Res. Med. Aromat. Pl. 2020, 18, 100255. [Google Scholar] [CrossRef]
- Piluzza, G.; Bullitta, S. Correlations between Phenolic Content and Antioxidant Properties in Twenty-Four Plant Species of Traditional Ethnoveterinary Use in the Mediterranean Area. Pharm. Biol. 2011, 49, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, T.; Vale, R.; Almeida, R.; Ferreira, T.; Guimarães, L. Antioxidant Potential and Its Correlation with the Contents of Phenolic Compounds and Flavonoids of Methanolic Extracts from Different Medicinal Plants. Rev. Virtual De Química 2017, 9, 1546–1559. [Google Scholar] [CrossRef]
- Stadtman, E.R.; Levine, R.L. Free radical-mediated oxidation of free amino acids and amino acid residues in proteins. Amino Acids 2003, 25, 207–218. [Google Scholar] [CrossRef]
- Visioli, F.; De La Lastra, C.A.; Andres-Lacueva, C.; Aviram, M.; Calhau, C.; Cassano, A.; D’Archivio, M.; Faria, A.; Favé, G.; Fogliano, V.; et al. Polyphenols and Human Health: A Prospectus. Crit. Rev. Food Sci. Nutr. 2011, 51, 524–546. [Google Scholar] [CrossRef]
- Ostrowska, A.; Gawliński, S.; Szczubiałka, Z. Methods for Analyzing and Assessing the Properties of Soil and Plants; Instytut Ochrony Środowiska: Warsaw, Poland, 1991; pp. 1–333. (In Polish) [Google Scholar]
- Grzeszczuk, M.; Salachna, P.; Meller, E. Changes in photosynthetic pigments, total phenolic content, and antioxidant activity of Salvia coccinea Buc’hoz ex Etl. Induced by exogenous salicylic acid and soil salinity. Molecules 2018, 23, 1296. [Google Scholar] [CrossRef] [Green Version]
- Łopusiewicz, Ł.; Drozłowska, E.; Siedlecka, P.; Mężyńska, M.; Bartkowiak, A.; Sienkiewicz, M.; Zielińska-Bliźniewska, H.; Kwiatkowski, P. Development, characterization, and bioactivity of non-dairy kefir-like fermented beverage based on flaxseed oil cake. Foods 2019, 8, 544. [Google Scholar] [CrossRef] [Green Version]
- Łopusiewicz, Ł.; Drozłowska, E.; Trocer, P.; Kwiatkowski, P.; Bartkowiak, A.; Gefrom, A.; Sienkiewicz, M. The Effect of fermentation with kefir grains on the physicochemical and antioxidant properties of beverages from blue lupin (Lupinus angustifolius L.) seeds. Molecules 2020, 25, 5791. [Google Scholar] [CrossRef]
- Tong, T.; Liu, Y.-J.; Kang, J.; Zhang, C.-M.; Kang, S.-G. Antioxidant activity and main chemical components of a novel fermented tea. Molecules 2019, 24, 2917. [Google Scholar] [CrossRef]
| Treatments | Plant Height (cm) | Frond Length (cm) | Fresh Weight (g) |
|---|---|---|---|
| A. nipponicum cv. Red Beauty | |||
| Shade (control) | 25.3 ± 1.07 a | 25.3 ± 1.06 ab | 8.90 ± 0.46 a |
| Full sunlight | 25.3 ± 0.96 a | 29.6 ± 3.92 a | 9.55 ± 0.42 a |
| Shade + salinity | 20.5 ± 1.50 b | 20.7 ± 1.47 c | 7.45 ± 0.18 b |
| Full sunlight + salinity | 18.8 ± 1.35 b | 24.1 ± 1.59 b | 7.47 ± 1.03 b |
| D. erythrosora | |||
| Shade (control) | 24.5 ± 0.50 b | 37.5 ± 1.81 a | 14.3 ± 0.75 b |
| Full sunlight | 29.3 ± 1.53 a | 36.9 ± 1.41 a | 19.5 ± 1.16 a |
| Shade + salinity | 15.3 ± 1.53 c | 27.1 ± 1.90 b | 8.34 ± 1.30 c |
| Full sunlight + salinity | 21.7 ± 2.52 b | 33.7 ± 1.02 a | 9.75 ± 0.99 c |
| Treatments | K+/Na+ Ratio | Ca2+/Na+ Ratio |
|---|---|---|
| A. nipponicum cv. Red Beauty | ||
| Shade (control) | 9.00 ± 0.07 a | 2.97 ± 0.64 a |
| Full sunlight | 6.23 ± 0.92 b | 2.47 ± 0.47 a |
| Shade + salinity | 1.07 ± 0.06 c | 0.60 ± 0.01 b |
| Full sunlight + salinity | 1.93 ± 0.15 c | 0.60 ± 0.17 b |
| D. erythrosora | ||
| Shade (control) | 13.47 ± 2.36 a | 2.97 ± 0.64 a |
| Full sunlight | 11.57 ± 1.27 a | 2.47 ± 0.47 a |
| Shade + salinity | 2.80 ± 0.20 b | 0.60 ± 0.01 b |
| Full sunlight + salinity | 2.77 ± 0.06 b | 0.60 ± 0.17 b |
| Treatments | Chlorophyll a mg/g DW | Chlorophyll b mg/g DW | Total Chlorophyll mg/g DW | Chlorophyll a/b Ratio |
|---|---|---|---|---|
| A. nipponicum cv. Red Beauty | ||||
| Shade (control) | 1.33 ± 0.05 b | 0.71 ± 0.03 b | 2.35 ± 0.09 b | 1.86 a ± 0.01 a |
| Full sunlight | 0.96 ± 0.03 c | 0.55 ± 0.02 c | 1.74 ± 0.06 c | 1.73 c ± 0.01 c |
| Shade + salinity | 1.52 ± 0.02 a | 0.86 ± 0.02 a | 2.75 ± 0.04 a | 1.78 b ± 0.01 b |
| Full sunlight + salinity | 0.80 ± 0.01 d | 0.47 ± 0.01 d | 1.46 ± 0.01 d | 1.71 c ± 0.03 c |
| D. erythrosora | ||||
| Shade (control) | 0.38 ± 0.03 a | 0.19 ± 0.02 ab | 0.63 ± 0.06 a | 2.01 ± 0.02 b |
| Full sunlight | 0.23 ± 0.01 b | 0.11 ± 0.01 c | 0.38 ± 0.02 b | 2.16 ± 0.03 a |
| Shade + salinity | 0.35 ± 0.01 a | 0.17 ± 0.00 b | 0.58 ± 0.02 a | 2.06 ± 0.02 b |
| Full sunlight + salinity | 0.39 ± 0.01 a | 0.20 ± 0.01 a | 0.66 ± 0.03 ab | 1.93 ± 0.02 c |
| Treatments | ABTS (%) | DPPH (%) | FRAP (mg AAE/g DW) | Reducing Power |
|---|---|---|---|---|
| A. nipponicum cv. Red Beauty | ||||
| Shade (control) | 80.77 ± 0.58 a | 28.44 ± 1.56 a | 12.60 ± 0.29 a | 1.55 ± 0.02 a |
| Full sunlight | 60.46 ± 0.62 c | 20.16 ± 0.16 b | 9.75 ± 0.57 b | 1.11 ± 0.01 c |
| Shade + salinity | 76.05 ± 1.34 b | 17.19 ± 1.88 b | 9.85 ± 0.24 b | 1.23 ± 0.02 b |
| Full sunlight + salinity | 56.41 ± 1.31 d | 17.97 ± 1.41 b | 10.50 ± 0.50 b | 1.17 ± 0.04 bc |
| D. erythrosora | ||||
| Shade (control) | 98.62 ± 0.09 ab | 77.66 ± 0.47 b | 31.20 ± 1.77 b | 2.47 ± 0.03 b |
| Full sunlight | 98.15 ± 0.39 b | 90.16 ± 0.16 a | 34.91 ± 1.16 a | 2.55 ± 0.02 a |
| Shade + salinity | 98.97 ± 0.27 a | 66.72 ± 0.94 c | 22.43 ± 1.33 d | 2.50 ± 0.02 ab |
| Full sunlight + salinity | 98.92 ± 0.09 a | 76.88 ± 0.31 b | 26.09 ± 0.36 c | 2.53 ± 0.04 ab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pietrak, A.; Salachna, P.; Łopusiewicz, Ł. Changes in Growth, Ionic Status, Metabolites Content and Antioxidant Activity of Two Ferns Exposed to Shade, Full Sunlight, and Salinity. Int. J. Mol. Sci. 2023, 24, 296. https://doi.org/10.3390/ijms24010296
Pietrak A, Salachna P, Łopusiewicz Ł. Changes in Growth, Ionic Status, Metabolites Content and Antioxidant Activity of Two Ferns Exposed to Shade, Full Sunlight, and Salinity. International Journal of Molecular Sciences. 2023; 24(1):296. https://doi.org/10.3390/ijms24010296
Chicago/Turabian StylePietrak, Anna, Piotr Salachna, and Łukasz Łopusiewicz. 2023. "Changes in Growth, Ionic Status, Metabolites Content and Antioxidant Activity of Two Ferns Exposed to Shade, Full Sunlight, and Salinity" International Journal of Molecular Sciences 24, no. 1: 296. https://doi.org/10.3390/ijms24010296








