Antibacterial and Antibiofilm Properties of Self-Assembled Dipeptide Nanotubes
Abstract
:1. Introduction
2. Results
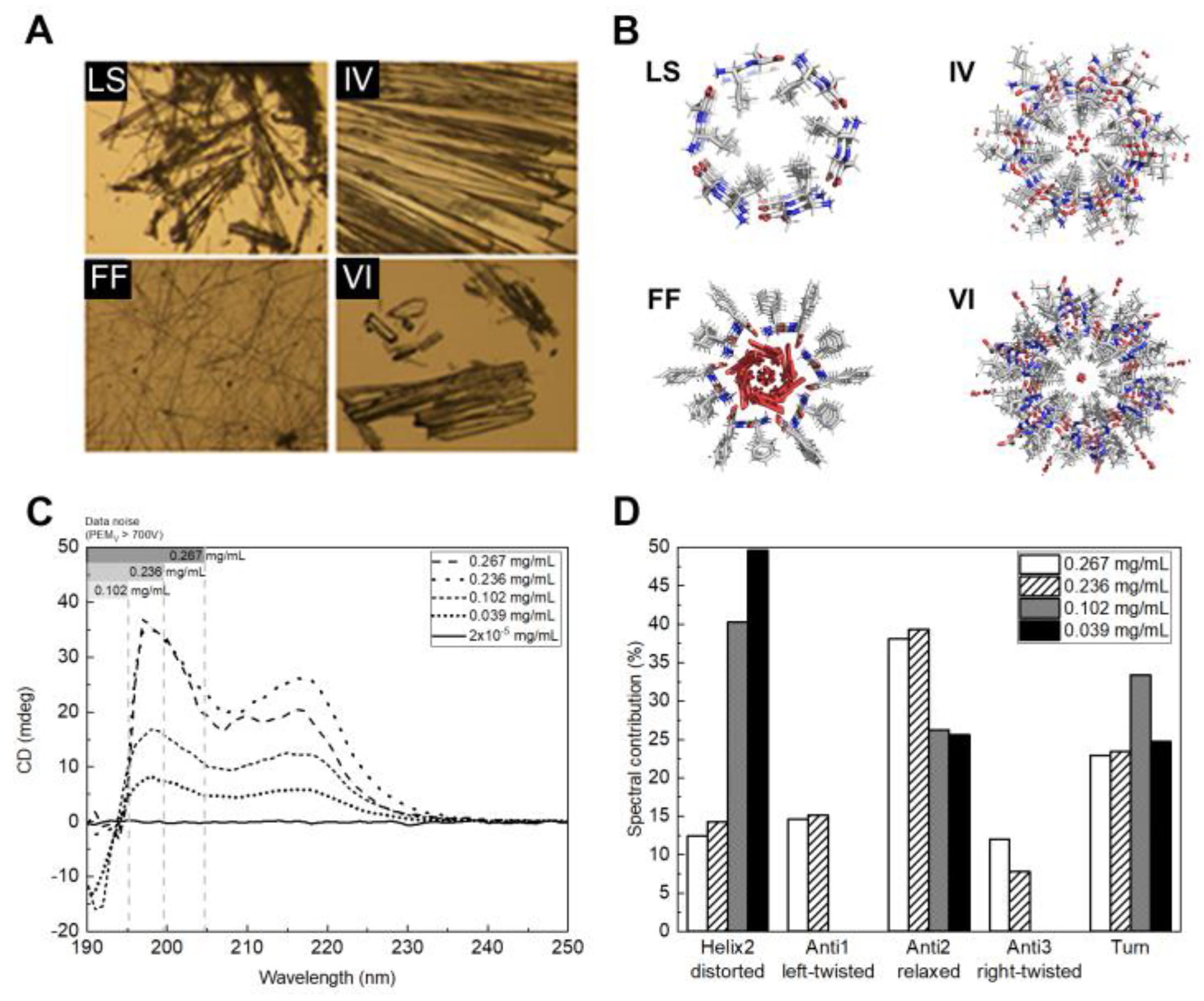
2.1. Dipeptide Nanotubes
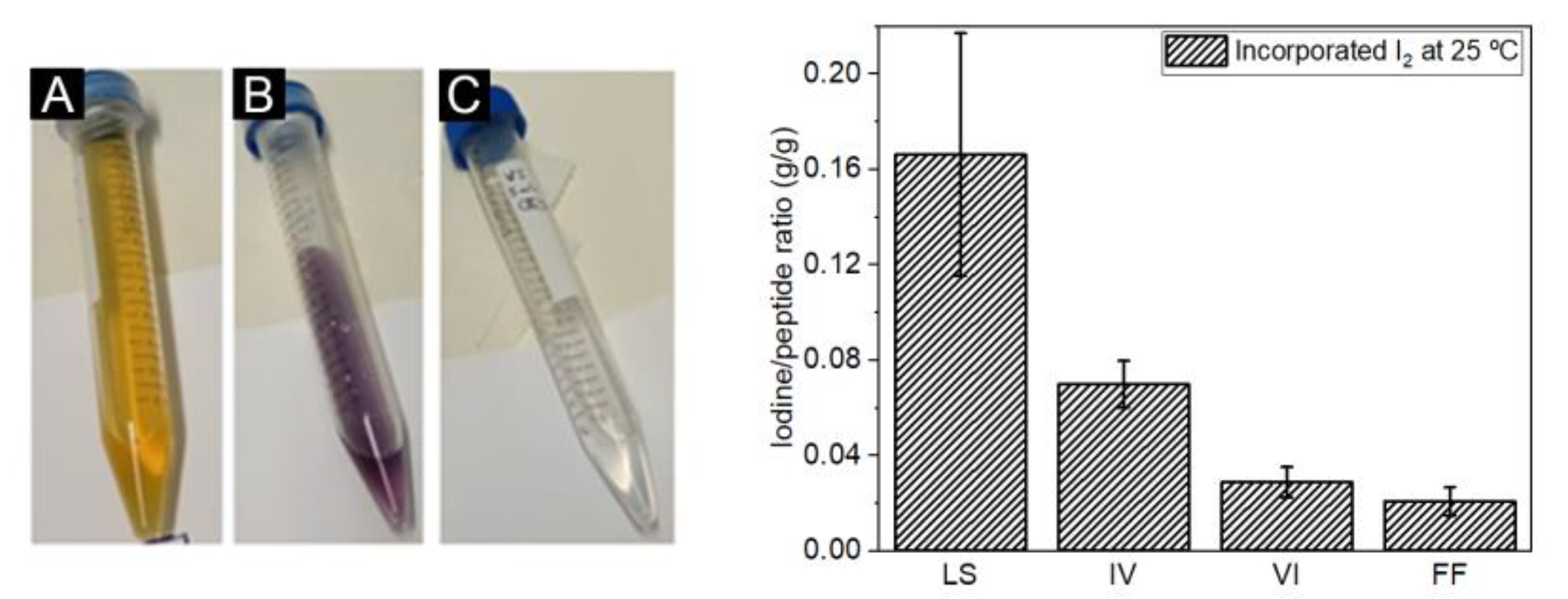
2.2. Iodine Incorporation
2.3. Antibacterial Activity of Dipeptide Nanotubes
2.4. Synergistic Association with Antibiotics
2.5. Effect in Membrane Permeability
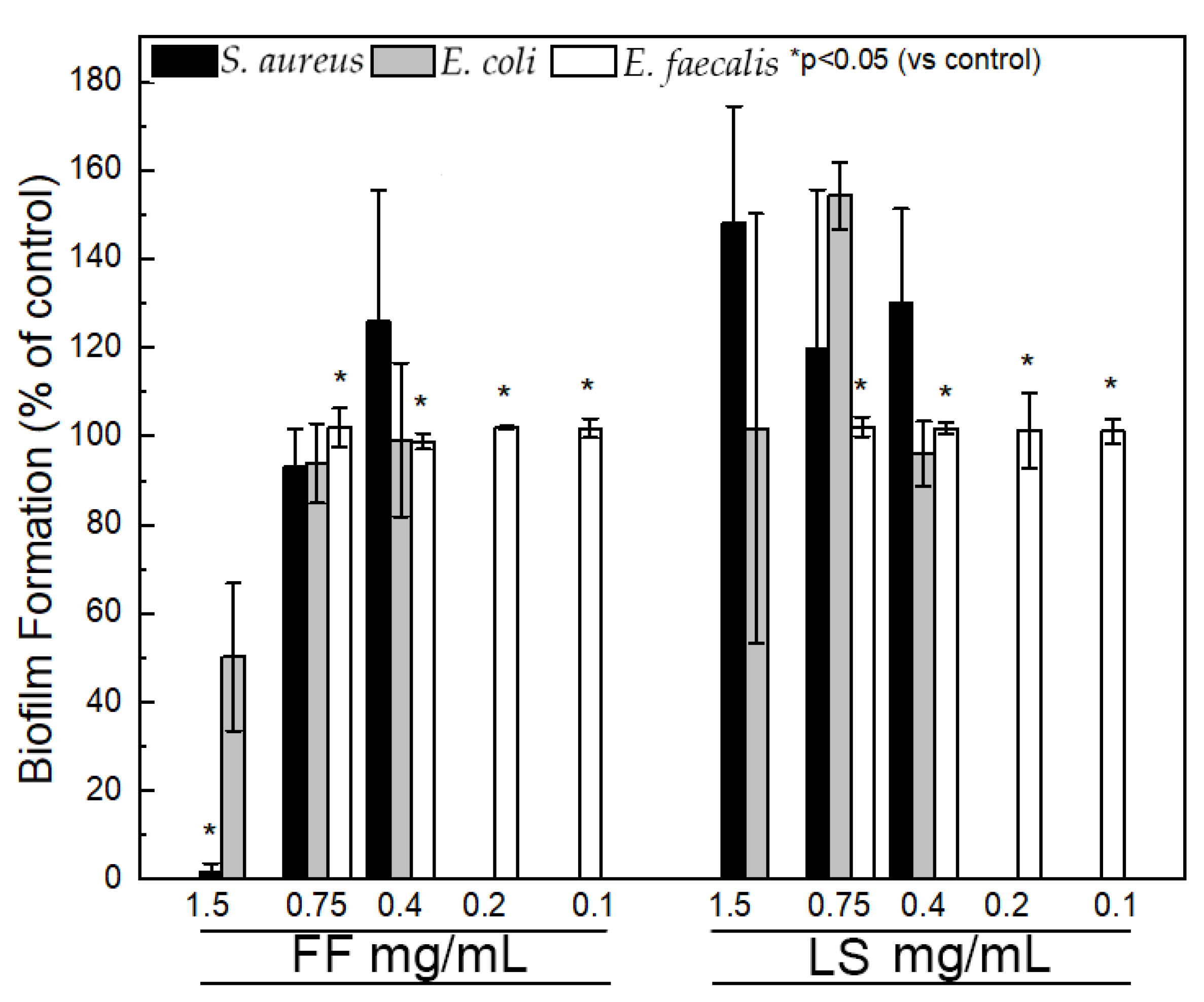
2.6. Inhibition of Biofilm Formation
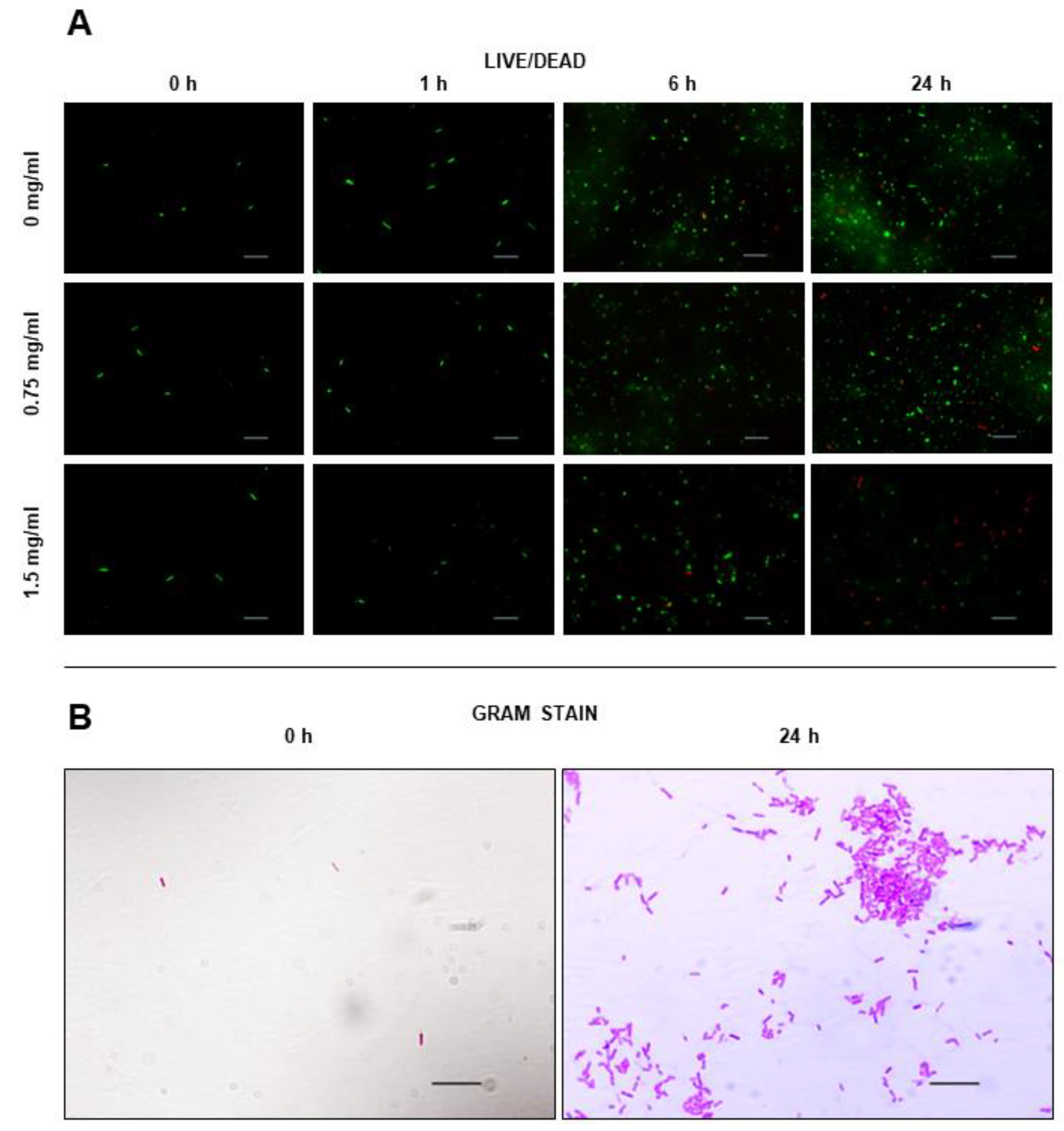
2.7. Effect on Cell Viability
3. Discussion
4. Materials and Methods
4.1. Crystal Growth of Nanotubular Dipeptides
4.2. Single Crystal X-ray Diffraction
4.3. Circular Dichroism
4.4. Iodine Incorporation
4.5. Bacterial Strains
4.6. Antibacterial Assays
4.7. Checkerboard Assay
4.8. Membrane Permeability Assay
4.9. Biofilm Formation Inhibition Assay
4.10. Live Dead Assay
4.11. Gram Staining
4.12. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rai, M.; Pandit, R.; Gaikwad, S.; Kövics, G. Antimicrobial peptides as natural bio-preservative to enhance the shelf-life of food. J. Food Sci. Technol. 2016, 53, 3381–3394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lü, F.; Ye, X.; Liu, D. Review of antimicrobial food packaging. Nongye Jixie Xuebao/Trans. Chin. Soc. Agric. Mach. 2009, 40, 138–142. [Google Scholar]
- Pasupuleti, M.; Schmidtchen, A.; Malmsten, M. Antimicrobial peptides: Key components of the innate immune system. Crit. Rev. Biotechnol. 2012, 32, 143–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anaya-López, J.L.; López-Meza, J.E.; Ochoa-Zarzosa, A. Bacterial resistance to cationic antimicrobial peptides. Crit. Rev. Microbiol. 2013, 39, 180–195. [Google Scholar] [CrossRef] [PubMed]
- Otto, M. Bacterial evasion of antimicrobial peptides by biofilm formation. Curr. Top. Microbiol. Immunol. 2006, 306, 251–258. [Google Scholar] [PubMed]
- Schnaider, L.; Brahmachari, S.; Schmidt, N.W.; Mensa, B.; Shaham-Niv, S.; Bychenko, D.; Adler-Abramovich, L.; Shimon, L.J.W.; Kolusheva, S.; Degrado, W.F.; et al. Self-assembling dipeptide antibacterial nanostructures with membrane disrupting activity. Nat. Commun. 2017, 8, 1365. [Google Scholar] [CrossRef] [Green Version]
- Lombardi, L.; Falanga, A.; Del Genio, V.; Galdiero, S. A new hope: Self-assembling peptides with antimicrobial activity. Pharmaceutics 2019, 11, 166. [Google Scholar] [CrossRef] [Green Version]
- Durão, J.; Gales, L. Peptide self-assembly for therapeutic applications. Curr. Org. Chem. 2015, 19, 1874–1881. [Google Scholar] [CrossRef]
- Leite, J.P.; Gimeno, A.; Taboada, P.; Jiménez-Barbero, J.J.; Gales, L. Dissection of the key steps of amyloid-β peptide 1–40 fibrillogenesis. Int. J. Biol. Macromol. 2020, 164, 2240–2246. [Google Scholar] [CrossRef]
- Selkoe, D.J.; Hardy, J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol. Med. 2016, 8, 595–608. [Google Scholar] [CrossRef]
- Li, F.; Liu, D.; Wang, T.; Hu, J.; Meng, F.; Sun, H.; Shang, Z.; Li, P.; Feng, W.; Li, W.; et al. J-aggregation in porphyrin nanoparticles induced by diphenylalanine. J. Solid State Chem. 2017, 252, 86–92. [Google Scholar] [CrossRef]
- Teixeira, R.; Andrade, S.M.; Vaz Serra, V.; Paulo, P.M.R.; Sánchez-Coronilla, A.; Neves, M.G.P.M.S.; Cavaleiro, J.A.S.; Costa, S.M.B. Reorganization of Self-Assembled Dipeptide Porphyrin J-Aggregates in Water–Ethanol Mixtures. J. Phys. Chem. B 2012, 116, 2396–2404. [Google Scholar] [CrossRef] [PubMed]
- Lutsyk, P.; Piryatinski, Y.; Shandura, M.; AlAraimi, M.; Tesa, M.; Arnaoutakis, G.E.; Melvin, A.A.; Kachkovsky, O.; Verbitsky, A.; Rozhin, A. Self-Assembly for Two Types of J-Aggregates: Cis-Isomers of Dye on the Carbon Nanotube Surface and Free Aggregates of Dye trans-Isomers. J. Phys. Chem. C 2019, 123, 19903–19911. [Google Scholar] [CrossRef]
- Lutsyk, P.; Piryatinski, Y.; AlAraimi, M.; Arif, R.; Shandura, M.; Kachkovsky, O.; Verbitsky, A.; Rozhin, A. Emergence of Additional Visible-Range Photoluminescence Due to Aggregation of Cyanine Dye: Astraphloxin on Carbon Nanotubes Dispersed with Anionic Surfactant. J. Phys. Chem. C 2016, 120, 20378–20386. [Google Scholar] [CrossRef] [Green Version]
- Afonso, R.; Mendes, A.; Gales, L. Peptide-based solids: Porosity and zeolitic behavior. J. Mater. Chem. 2012, 22, 1709–1723. [Google Scholar] [CrossRef]
- Emami, S.; Paz, F.A.A.; Mendes, A.; Gales, L. Toward the construction of 3D dipeptide-metal frameworks. Cryst. Growth Des. 2014, 14, 4777–4780. [Google Scholar] [CrossRef] [Green Version]
- Imaz, I.; Rubio-Martínez, M.; An, J.; Solé-Font, I.; Rosi, N.L.; Maspoch, D. Metal-biomolecule frameworks (MBioFs). Chem. Commun. 2011, 47, 7287–7302. [Google Scholar] [CrossRef]
- Rabone, J.; Yue, Y.F.; Chong, S.Y.; Stylianou, K.C.; Bacsa, J.; Bradshaw, D.; Darling, G.R.; Berry, N.G.; Khimyak, Y.Z.; Ganin, A.Y.; et al. An adaptable peptide-based porous material. Science 2010, 329, 1053–1057. [Google Scholar] [CrossRef]
- Görbitz, C.H. Nanotube formation by hydrophobic dipeptides. Chem.—Eur. J. 2001, 7, 5153–5159. [Google Scholar] [CrossRef]
- Afonso, R.; Mendes, A.; Gales, L. Hydrophobic dipeptide crystals: A promising Ag-free class of ultramicroporous materials showing argon/oxygen adsorption selectivity. Phys. Chem. Chem. Phys. 2014, 16, 19386–19393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Afonso, R.V.; Durão, J.; Mendes, A.; Damas, A.M.; Gales, L. Dipeptide crystals as excellent permselective materials: Sequential exclusion of argon, nitrogen, and oxygen. Angew. Chem.—Int. Ed. 2010, 49, 3034–3036. [Google Scholar] [CrossRef]
- Biernacki, K.; Sousa, S.F.; Gales, L.; Ramos, M.J.; Magalhães, A.L. Transport Properties of Light Gases in Nanochannels of L–Leu-L-Ser Dipeptide Crystals: A Comparative Study by Molecular Dynamics Simulations. ChemistrySelect 2018, 3, 5517–5525. [Google Scholar] [CrossRef]
- Comotti, A.; Bracco, S.; Distefano, G.; Sozzani, P. Methane, carbon dioxide and hydrogen storage in nanoporous dipeptide-based materials. Chem. Commun. 2009, 3, 284–286. [Google Scholar] [CrossRef] [PubMed]
- Durão, J.; Gales, L. Guest diffusion in dipeptide crystals. CrystEngComm 2013, 15, 1532–1535. [Google Scholar] [CrossRef]
- Biernacki, K.; Lopes, J.; Afonso, R.; Mendes, A.; Gales, L.; Magalhães, A.L. Selective adsorption and separation of light hydrocarbon gases in VI/IV dipeptide crystals. Microporous Mesoporous Mater. 2022, 345, 112284. [Google Scholar] [CrossRef]
- Durão, J.; Vale, N.; Gomes, S.; Gomes, P.; Barrias, C.C.; Gales, L. Nitric oxide release from antimicrobial peptide hydrogels for wound healing. Biomolecules 2019, 9, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bracco, S.; Asnaghi, D.; Negroni, M.; Sozzani, P.; Comotti, A. Porous dipeptide crystals as volatile-drug vessels. Chem. Commun. 2017, 54, 148–151. [Google Scholar] [CrossRef] [Green Version]
- Yan, X.; Zhu, P.; Li, J. Self-assembly and application of diphenylalanine-based nanostructures. Chem. Soc. Rev. 2010, 39, 1877–1890. [Google Scholar] [CrossRef]
- Marchesan, S.; Vargiu, A.V.; Styan, K.E. The Phe-Phe Motif for Peptide Self-Assembly in Nanomedicine. Molecules 2015, 20, 19775–19788. [Google Scholar] [CrossRef] [Green Version]
- Görbitz, C.H. Nanotubes from hydrophobic dipeptides: Pore size regulation through side chain substitution. New J. Chem. 2003, 27, 1789–1793. [Google Scholar] [CrossRef]
- Görbitz, C.H.; Nilsen, M.; Szeto, K.; Tangen, L.W. Microporous organic crystals: An unusual case for L-leucyl-L-serine. Chem. Commun. 2005, 34, 4288–4290. [Google Scholar] [CrossRef]
- Görbitz, C.H. Microporous organic materials from hydrophobic dipeptides. Chem.—Eur. J. 2007, 13, 1022–1031. [Google Scholar] [CrossRef]
- Görbitz, C.H. The structure of nanotubes formed by diphenylalanine, the core recognition motif of Alzheimer’s β-amyloid polypeptide. Chem. Commun. 2006, 22, 2332–2334. [Google Scholar] [CrossRef]
- Mason, T.O.; Chirgadze, D.Y.; Levin, A.; Adler-Abramovich, L.; Gazit, E.; Knowles, T.P.J.; Buell, A.K. Expanding the solvent chemical space for self-assembly of dipeptide nanostructures. ACS Nano 2014, 8, 1243–1253. [Google Scholar] [CrossRef] [Green Version]
- Eggers, M. Infectious Disease Management and Control with Povidone Iodine. Infect. Dis. Ther. 2019, 8, 581–593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pankey, G.A.; Sabath, L.D. Clinical relevance of bacteriostatic versus bactericidal mechanisms of action in the treatment of gram-positive bacterial infections. Clin. Infect. Dis. 2004, 38, 864–870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Humphries, R.M.; Ambler, J.; Mitchell, S.L.; Castanheira, M.; Dingle, T.; Hindler, J.A.; Koeth, L.; Sei, K. CLSI methods development and standardization working group best practices for evaluation of antimicrobial susceptibility tests. J. Clin. Microbiol. 2018, 56, e01934-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munita, J.M.; Arias, C.A. Mechanisms of antibiotic resistance. Microbiol. Spectr. 2016, 4. [Google Scholar] [CrossRef] [Green Version]
- Silva, R.F.; Araújo, D.R.; Silva, E.R.; Ando, R.A.; Alves, W.A. l-Diphenylalanine Microtubes As a Potential Drug-Delivery System: Characterization, Release Kinetics, and Cytotoxicity. Langmuir 2013, 29, 10205–10212. [Google Scholar] [CrossRef]
- Porter, S.L.; Coulter, S.M.; Pentlavalli, S.; Thompson, T.P.; Laverty, G. Self-assembling diphenylalanine peptide nanotubes selectively eradicate bacterial biofilm infection. Acta Biomater. 2018, 77, 96–105. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Z.; Gupta, K.M.; He, Z.; Jiang, J. Dipeptide Crystals as Reverse Osmosis Membranes for Water Desalination: Atomistic Simulation. J. Phys. Chem. C 2018, 122, 6026–6032. [Google Scholar] [CrossRef]
- Simões, M. Antimicrobial strategies effective against infectious bacterial biofilms. Curr. Med. Chem. 2011, 18, 2129–2145. [Google Scholar] [CrossRef]
- Flemming, H.C.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef]
- Kumar, D.K.; Choi, S.H.; Washicosky, K.J.; Eimer, W.A.; Tucker, S.; Ghofrani, J.; Lefkowitz, A.; McColl, G.; Goldstein, L.E.; Tanzi, R.E.; et al. Amyloid-β peptide protects against microbial infection in mouse and worm models of Alzheimer’s disease. Sci. Transl. Med. 2016, 8, 340ra72. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Simões, R.R.; Aires-de-Sousa, M.; Conceição, T.; Antunes, F.; da Costa, P.M.; de Lencastre, H. High prevalence of EMRSA-15 in Portuguese public buses: A worrisome finding. PLoS ONE 2011, 6, e17630. [Google Scholar] [CrossRef] [Green Version]
- Bessa, L.J.; Barbosa-Vasconcelos, A.; Mendes, Â.; Vaz-Pires, P.; Da Costa, P.M. High prevalence of multidrug-resistant Escherichia coli and Enterococcus spp. in river water, upstream and downstream of a wastewater treatment plant. J. Water Health 2014, 12, 426–435. [Google Scholar] [CrossRef]
- Odds, F.C. Synergy, antagonism, and what the chequerboard puts between them. J. Antimicrob. Chemother. 2003, 52, 1. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Faber, E.B.; Georg, G.I. Synthesis and Spectral Properties of 8-Anilinonaphthalene-1-sulfonic Acid (ANS) Derivatives Prepared by Microwave-Assisted Copper(0)-Catalyzed Ullmann Reaction. ACS Omega 2019, 4, 18472–18477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumla, D.; Dethoup, T.; Gales, L.; Pereira, J.A.; Freitas-Silva, J.; Costa, P.M.; Silva, A.M.S.; Pinto, M.M.M.; Kijjoa, A. Erubescensoic Acid, a new polyketide and a xanthonopyrone SPF-3059-26 From the culture of the marine sponge-associated fungus penicillium erubescens KUFA 0220 and Antibacterial activity evaluation of some of its constituents. Molecules 2019, 24, 208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stepanović, S.; Vuković, D.; Hola, V.; Di Bonaventura, G.; Djukić, S.; Ćirković, I.; Ruzicka, F. Quantification of biofilm in microtiter plates: Overview of testing conditions and practical recommendations for assessment of biofilm production by staphylococci. APMIS 2007, 115, 891–899. [Google Scholar] [CrossRef] [PubMed]

| E. coli ATCC 25922 | P.aeruginosa ATCC 27853 | E. faecalis ATCC 29212 | S. aureus ATCC 29213 | |||||
|---|---|---|---|---|---|---|---|---|
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | |
| FF | 1.5 | >1.5 | >1.5 | >1.5 | 0.4/0.75 | >1.5 | 1.5 | 1.5 |
| FF·I2 | >1.5 | >1.5 | >1.5 | >1.5 | 0.4/0.75 | 1.5 | 1.5 | 1.5 |
| LS | >1.5 | >1.5 | >1.5 | >1.5 | 0.4/0.75 | >1.5 | 1.5 | 1.5 |
| LS·I2 | 1.5 | 1.5 | >1.5 | >1.5 | 0.4/0.75 | >1.5 | 0.75/1.5 | >1.5 |
| IV | >1.5 | >1.5 | >1.5 | >1.5 | >1.5 | >1.5 | >1.5 | >1.5 |
| IV·I2 | >1.5 | >1.5 | >1.5 | >1.5 | >1.5 | >1.5 | >1.5 | >1.5 |
| VI | >1.5 | >1.5 | >1.5 | >1.5 | >1.5 | >1.5 | >1.5 | >1.5 |
| VI·I2 | >1.5 | >1.5 | >1.5 | >1.5 | >1.5 | >1.5 | >1.5 | >1.5 |
| Bacterial Strain | FF + Kanamycin | LS + Kanamycin | ||
|---|---|---|---|---|
| ΣFIC | Activity | ΣFIC | Activity | |
| E. faecalis ATCC 29212 | 0.26 | synergism | 0.26 | synergism |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soares, I.; Rodrigues, I.; da Costa, P.M.; Gales, L. Antibacterial and Antibiofilm Properties of Self-Assembled Dipeptide Nanotubes. Int. J. Mol. Sci. 2023, 24, 328. https://doi.org/10.3390/ijms24010328
Soares I, Rodrigues I, da Costa PM, Gales L. Antibacterial and Antibiofilm Properties of Self-Assembled Dipeptide Nanotubes. International Journal of Molecular Sciences. 2023; 24(1):328. https://doi.org/10.3390/ijms24010328
Chicago/Turabian StyleSoares, Iris, Inês Rodrigues, Paulo Martins da Costa, and Luís Gales. 2023. "Antibacterial and Antibiofilm Properties of Self-Assembled Dipeptide Nanotubes" International Journal of Molecular Sciences 24, no. 1: 328. https://doi.org/10.3390/ijms24010328
APA StyleSoares, I., Rodrigues, I., da Costa, P. M., & Gales, L. (2023). Antibacterial and Antibiofilm Properties of Self-Assembled Dipeptide Nanotubes. International Journal of Molecular Sciences, 24(1), 328. https://doi.org/10.3390/ijms24010328





