Effect of Baicalin on Wound Healing in a Mouse Model of Pressure Ulcers
Abstract
1. Introduction
2. Results
2.1. Effect of Baicalin in Human Epidermal Keratinocytes (HEKs)
2.2. Topical Application of Baicalin Accelerates Wound Healing in Mice with Pressure Ulcers
2.3. Treatment with Baicalin Accelerates the Restoration of Skin Structures in Mice with Pressure Ulcers
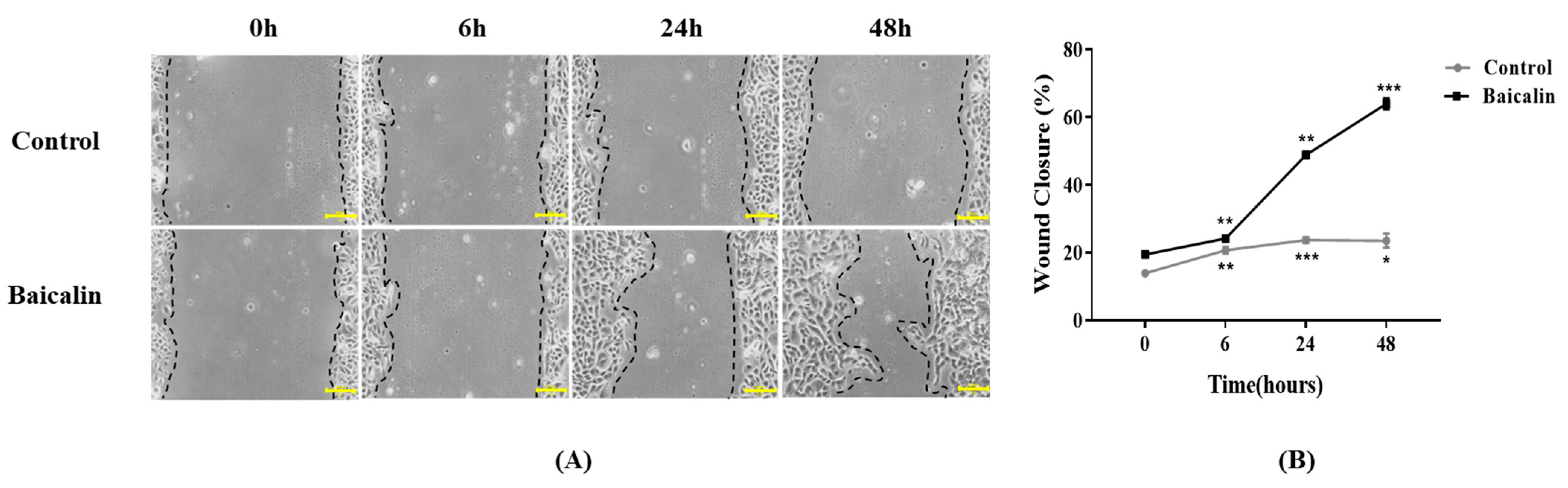
2.4. Effect of Baicalin on Keratinocyte Migration in Pressure Ulcer Wound Healing
2.5. Baicalin Alters Pro-Inflammatory and Anti-Inflammatory Cytokine Gene Expression in Mice with Pressure Ulcers
2.6. Effect of Baicalin on Angiogenesis-Related Gene Expression in Mice with Pressure Ulcers
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Cell Cytotoxicity Measurement
4.3. Wounding Healing Assay
4.4. Experimental Animals
4.5. Animal Experimental Design
4.6. Histological Analysis
4.7. Quantitative Reverse Transcription-Polymerase Chain Reaction (qRT-PCR)
4.8. Immunofluorescence Staining
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bluestein, D.; Javaheri, A. Pressure ulcers: Prevention, evaluation, and management. Am. Fam. Physician 2008, 78, 1186–1194. [Google Scholar] [PubMed]
- Thomas, D.R. Prevention and treatment of pressure ulcers. J. Am. Med. Dir. Assoc. 2006, 7, 46–59. [Google Scholar] [CrossRef] [PubMed]
- Swisher, S.L.; Lin, M.C.; Liao, A.; Leeflang, E.J.; Khan, Y.; Pavinatto, F.J.; Mann, K.; Naujokas, A.; Young, D.; Roy, S. Impedance sensing device enables early detection of pressure ulcers in vivo. Nat. Commun. 2015, 6, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Tang, N.; Zheng, Y.; Cui, D.; Haick, H. Multifunctional Dressing for Wound Diagnosis and Rehabilitation. Adv. Healthc. Mater. 2021, 10, 2101292. [Google Scholar] [CrossRef]
- Kim, S.-R.; Lee, S.; Kim, J.; Kim, E.; Kil, H.-J.; Yoo, J.-H.; Oh, J.-H.; Jeon, J.; Lee, E.-I.; Jeon, J.-W.; et al. A fabric-based multifunctional sensor for the early detection of skin decubitus ulcers. Biosens. Bioelectron. 2022, 215, 114555. [Google Scholar] [CrossRef]
- Shi, C.; Bonnett, L.J.; Dumville, J.C.; Cullum, N. Nonblanchable erythema for predicting pressure ulcer development: A systematic review with an individual participant data meta-analysis. Br. J. Derm. 2020, 182, 278–286. [Google Scholar] [CrossRef]
- Schultz, G.S.; Wysocki, A. Interactions between extracellular matrix and growth factors in wound healing. Wound Repair Regen. 2009, 17, 153–162. [Google Scholar] [CrossRef]
- Hewitt, H.; Wint, Y.; Talabere, L.; Lopez, S.; Bailey, E.; Parshad, O.; Weaver, S. The Use of Papaya on Pressure Ulcers: A natural alternative. AJN Am. J. Nurs. 2002, 102, 73–77. [Google Scholar]
- Charan, J.; Bhardwaj, P.; Dutta, S.; Kaur, R.; Bist, S.K.; Detha, M.D.; Kanchan, T.; Yadav, D.; Mitra, P.; Sharma, P. Use of complementary and alternative medicine (CAM) and home remedies by COVID-19 patients: A telephonic survey. Indian J. Clin. Biochem. 2021, 36, 108–111. [Google Scholar] [CrossRef]
- Yin, G.; Wang, Z.; Wang, Z.; Wang, X. Topical application of quercetin improves wound healing in pressure ulcer lesions. Exp. Dermatol. 2018, 27, 779–786. [Google Scholar] [CrossRef]
- Ghanadian, M.; Soltani, R.; Homayouni, A.; Khorvash, F.; Jouabadi, S.M.; Abdollahzadeh, M. The Effect of Plantago major Hydroalcoholic Extract on the Healing of Diabetic Foot and Pressure Ulcers: A Randomized Open-Label Controlled Clinical Trial. Int. J. Low. Extrem. Wounds 2022, 0, 15347346211070723. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.U.A.; Abd Razak, S.I.; Hassan, A.; Qureshi, S.; Stojanović, G.M. Multifunctional Arabinoxylan-functionalized-Graphene Oxide Based Composite Hydrogel for Skin Tissue Engineering. Front. Bioeng. Biotechnol. 2022, 10, 865059. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.U.A.; Iqbal, I.; Ansari, M.N.M.; Razak, S.I.A.; Raza, M.A.; Sajjad, A.; Jabeen, F.; Riduan Mohamad, M.; Jusoh, N. Development of antibacterial, degradable and ph-responsive chitosan/guar gum/polyvinyl alcohol blended hydrogels for wound dressing. Molecules 2021, 26, 5937. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, F.; Sverre, J.; Bustad, S. Pdb21 a cost-utility analysis of insulin glargine (Lantus®) in the treatment of patients with type 1 diabetes. Value Health 2003, 6, 682. [Google Scholar] [CrossRef][Green Version]
- Sun, S.-J.; Wu, X.-P.; Song, H.-L.; Li, G.-Q. Baicalin ameliorates isoproterenol-induced acute myocardial infarction through iNOS, inflammation, oxidative stress and P38MAPK pathway in rat. Int. J. Clin. Exp. Med. 2015, 8, 22063. [Google Scholar]
- Yu, Y.; Pei, M.; Li, L. Baicalin induces apoptosis in hepatic cancer cells in vitro and suppresses tumor growth in vivo. Int. J. Clin. Exp. Med. 2015, 8, 8958. [Google Scholar]
- Dinda, B.; Dinda, S.; DasSharma, S.; Banik, R.; Chakraborty, A.; Dinda, M. Therapeutic potentials of baicalin and its aglycone, baicalein against inflammatory disorders. Eur. J. Med. Chem. 2017, 131, 68–80. [Google Scholar] [CrossRef]
- Singh, S.; Meena, A.; Luqman, S. Baicalin mediated regulation of key signaling pathways in cancer. Pharmacol. Res. 2021, 164, 105387. [Google Scholar] [CrossRef]
- Bavarsad, N.; Hemmati, G.; Rezaie, A. The effect of topical quercetin loaded liposome on pressure ulcer healing in rats. Nanomed. J. 2021, 8, 187–199. [Google Scholar]
- Majtan, J.; Bohova, J.; Garcia-Villalba, R.; Tomas-Barberan, F.A.; Madakova, Z.; Majtan, T.; Majtan, V.; Klaudiny, J. Fir honeydew honey flavonoids inhibit TNF-α-induced MMP-9 expression in human keratinocytes: A new action of honey in wound healing. Arch. Dermatol. Res. 2013, 305, 619–627. [Google Scholar] [CrossRef]
- Ali, S.I.; Gopalakrishnan, B.; Venkatesalu, V. Pharmacognosy, phytochemistry and pharmacological properties of Achillea millefolium L.: A review. Phytother. Res. 2017, 31, 1140–1161. [Google Scholar] [CrossRef] [PubMed]
- Cavalcanti, A.M.; Baggio, C.H.; Freitas, C.S.; Rieck, L.; de Sousa, R.S.; Da Silva-Santos, J.E.; Mesia-Vela, S.; Marques, M.C.A. Safety and antiulcer efficacy studies of Achillea millefolium L. after chronic treatment in Wistar rats. J. Ethnopharmacol. 2006, 107, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Paudel, K.R.; Kim, D.-W. Microparticles-mediated vascular inflammation and its amelioration by antioxidant activity of baicalin. Antioxidants 2020, 9, 890. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Cheng, Z.-Y.; He, J.; Jia, L.-J.; Qiao, H.-L. Concentration-dependent inhibitory effects of baicalin on the metabolism of dextromethorphan, a dual probe of CYP2D and CYP3A, in rats. Chem. -Biol. Interact. 2013, 203, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Li, Z.; Li, B.; Wang, H. Baicalin regulates mRNA expression of VEGF-c, Ang-1/Tie2, TGF-β and Smad2/3 to inhibit wound healing in streptozotocin-induced diabetic foot ulcer rats. J. Biochem. Mol. Toxicol. 2021, 35, e22893. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Cao, X.; Zhang, R.; Li, Y.-X.; Xu, Z.-L.; Zhang, D.-G.; Wang, L.-S.; Wang, J.-Y. Protective effect of baicalin against experimental colitis via suppression of oxidant stress and apoptosis. Pharmacogn. Mag. 2016, 12, 225. [Google Scholar] [PubMed]
- Moll, R.; Pitz, S.; Levy, R.; Weikel, W.; Franke, W.W.; Czernobilsky, B. Complexity of expression of intermediate filament proteins, including glial filament protein, in endometrial and ovarian adenocarcinomas. Hum. Pathol. 1991, 22, 989–1001. [Google Scholar] [CrossRef] [PubMed]
- Proby, C.; Churchill, L.; Purkis, P.; Glover, M.; Sexton, C.; Leigh, I. Keratin 17 expression as a marker for epithelial transformation in viral warts. Am. J. Pathol. 1993, 143, 1667. [Google Scholar]
- Xing, F.; Yi, W.J.; Miao, F.; Su, M.Y.; Lei, T.C. Baicalin increases hair follicle development by increasing canonical Wnt/β-catenin signaling and activating dermal papillar cells in mice. Int. J. Mol. Med. 2018, 41, 2079–2085. [Google Scholar] [CrossRef]
- Werner, S.; Grose, R. Regulation of wound healing by growth factors and cytokines. Physiol. Rev. 2003, 83, 835–870. [Google Scholar] [CrossRef]
- Werner, S.; Krieg, T.; Smola, H. Keratinocyte–Fibroblast Interactions in Wound Healing. J. Investig. Dermatol. 2007, 127, 998–1008. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, S.; Mazumder, B.; Rajkonwar, J.; Pathak, M.P.; Patowary, P.; Chattopadhyay, P. bFGF and collagen matrix hydrogel attenuates burn wound inflammation through activation of ERK and TRK pathway. Sci. Rep. 2021, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Jimi, S.; Jaguparov, A.; Nurkesh, A.; Sultankulov, B.; Saparov, A. Sequential Delivery of Cryogel Released Growth Factors and Cytokines Accelerates Wound Healing and Improves Tissue Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 345. [Google Scholar] [CrossRef] [PubMed]
- Brodbeck, W.G.; Voskerician, G.; Ziats, N.P.; Nakayama, Y.; Matsuda, T.; Anderson, J.M. In vivo leukocyte cytokine mRNA responses to biomaterials are dependent on surface chemistry. J. Biomed. Mater. Res. Part A 2003, 64A, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Avraham-Davidi, I.; Yona, S.; Grunewald, M.; Landsman, L.; Cochain, C.; Silvestre, J.S.; Mizrahi, H.; Faroja, M.; Strauss-Ayali, D.; Mack, M. On-site education of VEGF-recruited monocytes improves their performance as angiogenic and arteriogenic accessory cells. J. Exp. Med. 2013, 210, 2611–2625. [Google Scholar] [CrossRef]
- Sasaki, T. The effects of basic fibroblast growth factor and doxorubicin on cultured human skin fibroblasts: Relevance to wound healing. J. Dermatol. 1992, 19, 664–666. [Google Scholar] [CrossRef]
- Powers, C.; McLeskey, S.; Wellstein, A. Fibroblast growth factors, their receptors and signaling. Endocr. -Relat. Cancer 2000, 7, 165–197. [Google Scholar] [CrossRef]
- Guo, J.; Hu, Z.; Yan, F.; Lei, S.; Li, T.; Li, X.; Xu, C.; Sun, B.; Pan, C.; Chen, L. Angelica dahurica promoted angiogenesis and accelerated wound healing in db/db mice via the HIF-1α/PDGF-β signaling pathway. Free Radic. Biol. Med. 2020, 160, 447–457. [Google Scholar] [CrossRef]
- Chen, J.S.; Longaker, M.T.; Gurtner, G.C. Murine models of human wound healing. In Wound Regeneration and Repair; Springer: Berlin/Heidelberg, Germany, 2013; pp. 265–274. [Google Scholar]
- Hariri, A.; Chen, F.; Moore, C.; Jokerst, J.V. Noninvasive staging of pressure ulcers using photoacoustic imaging. Wound Repair Regen. 2019, 27, 488–496. [Google Scholar] [CrossRef]
- Wassermann, E.; Van Griensven, M.; Gstaltner, K.; Oehlinger, W.; Schrei, K.; Redl, H. A chronic pressure ulcer model in the nude mouse. Wound Repair Regen. 2009, 17, 480–484. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, E.; Ham, S.; Jung, B.K.; Park, J.-W.; Kim, J.; Lee, J.H. Effect of Baicalin on Wound Healing in a Mouse Model of Pressure Ulcers. Int. J. Mol. Sci. 2023, 24, 329. https://doi.org/10.3390/ijms24010329
Kim E, Ham S, Jung BK, Park J-W, Kim J, Lee JH. Effect of Baicalin on Wound Healing in a Mouse Model of Pressure Ulcers. International Journal of Molecular Sciences. 2023; 24(1):329. https://doi.org/10.3390/ijms24010329
Chicago/Turabian StyleKim, Eunbin, Seoyoon Ham, Bok Ki Jung, Jin-Woo Park, Jihee Kim, and Ju Hee Lee. 2023. "Effect of Baicalin on Wound Healing in a Mouse Model of Pressure Ulcers" International Journal of Molecular Sciences 24, no. 1: 329. https://doi.org/10.3390/ijms24010329
APA StyleKim, E., Ham, S., Jung, B. K., Park, J.-W., Kim, J., & Lee, J. H. (2023). Effect of Baicalin on Wound Healing in a Mouse Model of Pressure Ulcers. International Journal of Molecular Sciences, 24(1), 329. https://doi.org/10.3390/ijms24010329





