The Functional Characterization of Carboxylesterases Involved in the Degradation of Volatile Esters Produced in Strawberry Fruits
Abstract
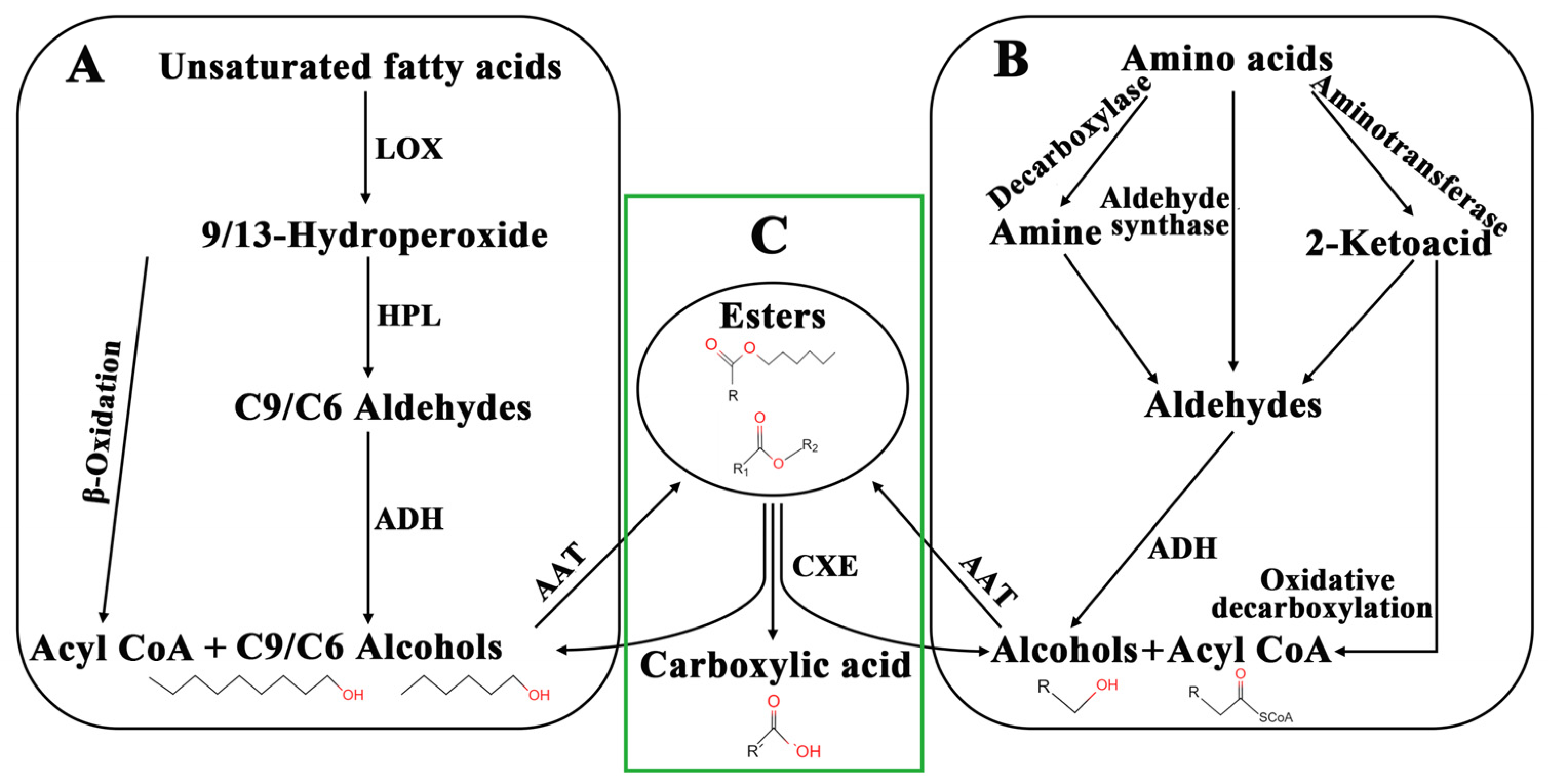
1. Introduction
2. Results
2.1. Volatile Esters Are the Basic Aroma Components in Some Fruits
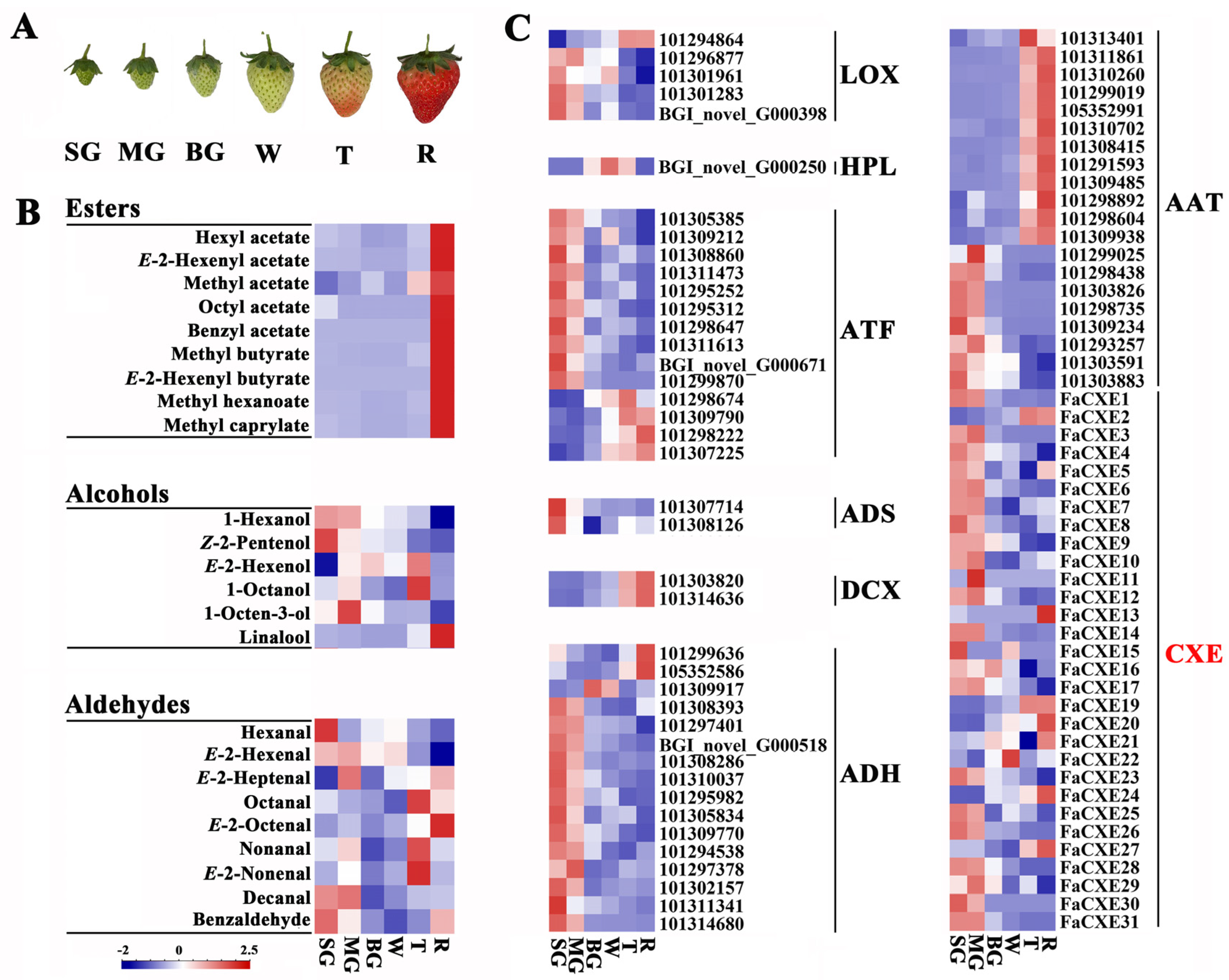
2.2. Correlation Analysis of Volatile Ester Components and Related Genes Expression during Strawberry Development
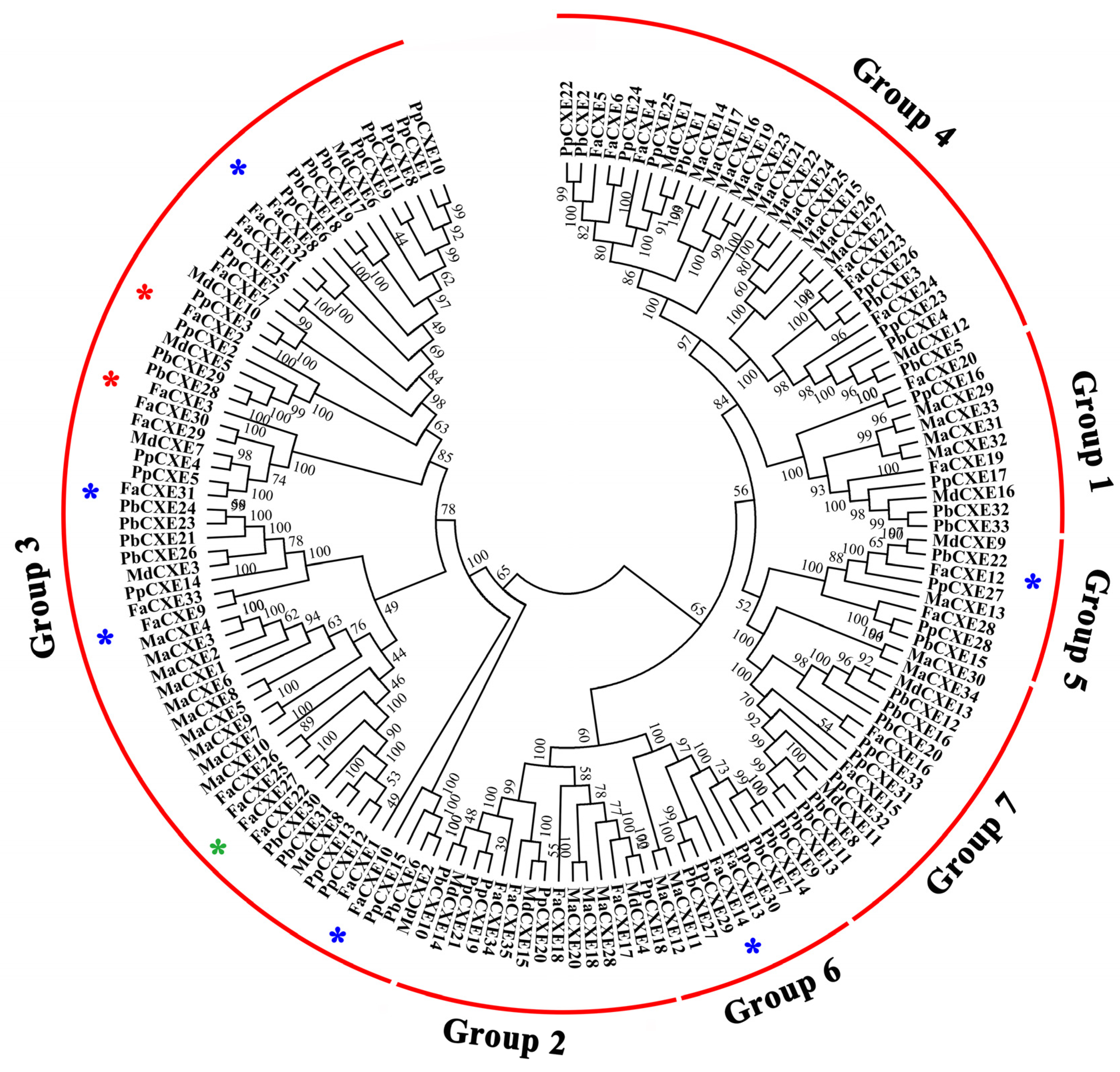
2.3. Identification of Carboxylesterases Involved in the Metabolism of Volatile Ester Compounds in Strawberries
2.4. Expression Pattern of FaCXEs in Strawberry Fruits
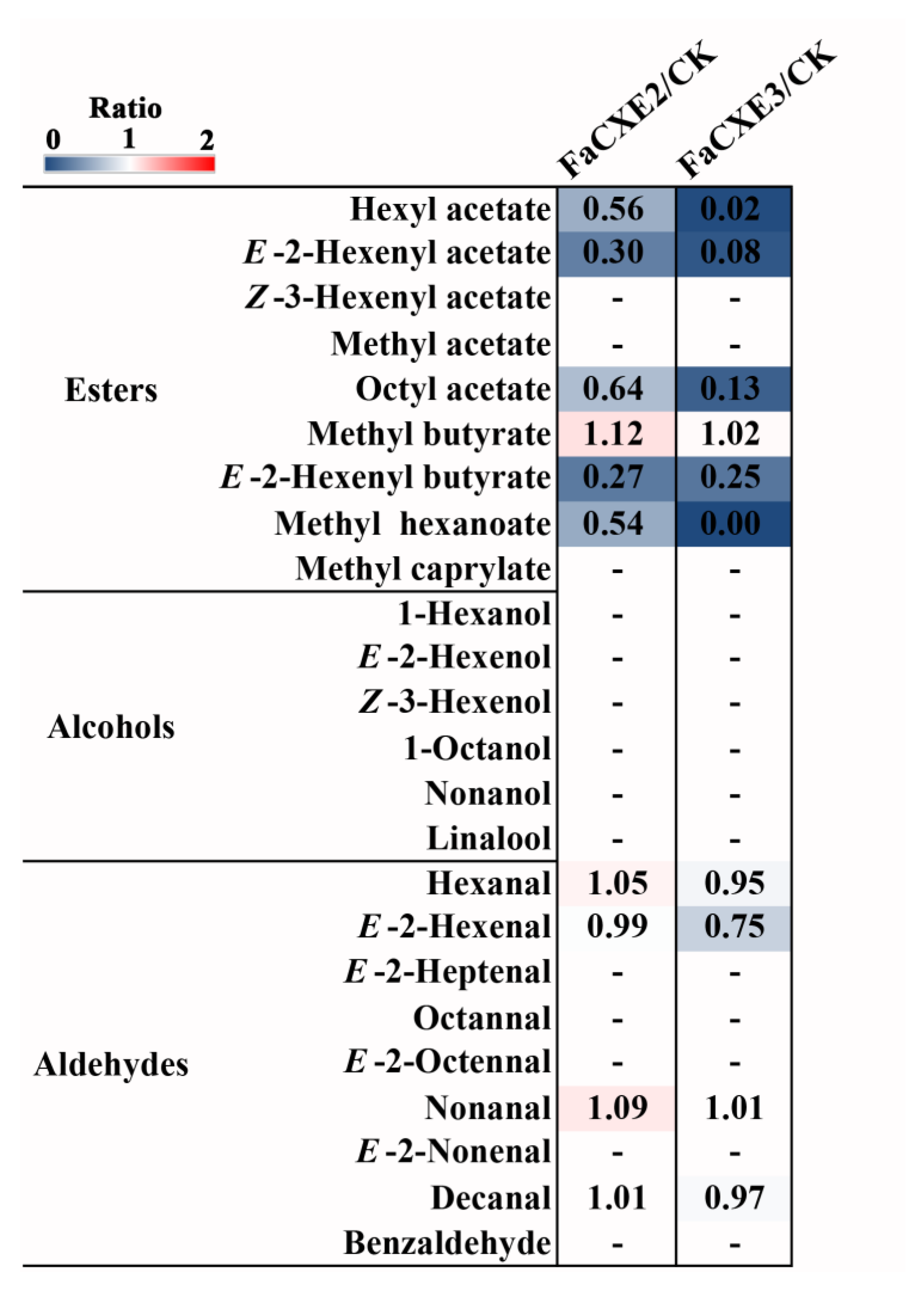
2.5. Transient Overexpression of FaCXEs in Strawberry Fruit
3. Discussion
4. Materials and Methods
4.1. Material
4.2. Preparation and Detection of Fruit Volatile Extracts
4.3. Bioinformatic Analysis of CXE Genes Family
4.4. Expression Analysis of CXE Genes
4.5. Cloning of FaCXE Genes
4.6. Construction of Prokaryotic Expression Vector and Expression of Recombinant Proteins
4.7. Construction of Binary Vectors and Transient Overexpression in Strawberry Fruits
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zabetakis, I.; Holden, M.A. Strawberry flavor: Analysis and biosynthesis. J. Sci. Food Agric. 1997, 74, 421–434. [Google Scholar] [CrossRef]
- Larsen, M.; Poll, L. Odour thresholds of some important aroma compounds in raspberries. Z. Lebensm.-Unters. Forsch. 1990, 191, 129–131. [Google Scholar] [CrossRef]
- Schieberle, P.; Hofmann, T. Evaluation of the character impact odorants in fresh strawberry juice by quantitative measurements and sensory studies on model mixtures. J. Agric. Food Chem. 1997, 45, 227–232. [Google Scholar] [CrossRef]
- Maarse, H. Volatile compounds in foods and beverages. Food Sci. Technol. 1991, 246, 514–518. [Google Scholar]
- Willhalm, B.S.M.; Thomas, A.F. 2,5-Dimethyl-4-hydroxy-2,3-dihydrofuran-3-one. Chem. Ind. 1965, 38, 1629–1630. [Google Scholar]
- Du, X.; Plotto, A.; Baldwin, E.; Rouseff, R. Evaluation of volatiles from two subtropical strawberry cultivars using GC-olfactometry, GC-MS odor activity values, and sensory analysis. J. Agric. Food Chem. 2011, 59, 12569–12577. [Google Scholar] [CrossRef]
- Jetti, R.R.; Yang, E.; Kurnianta, A.; Finn, C.; Qian, M.C. Quantification of selected aroma-ative compounds in strawberries by headspace solid-phase microextraction gas chromatography and correlation with sensory descriptive analysis. J. Food Sci. 2007, 72, S487–S496. [Google Scholar] [CrossRef]
- Dong, J.; Zhang, Y.; Tang, X.; Jin, W.; Han, Z. Differences in volatile ester composition between Fragaria × ananassa and F. vesca and implications for strawberry aroma patterns. Sci. Hortic. 2013, 150, 47–53. [Google Scholar] [CrossRef]
- Manríquez, D.; El-Sharkawy, I.; Flores, F.B.; El-Yahyaoui, F.; Regad, F.; Bouzayen, M.; Latché, A.; Pech, J.C. Two highly divergent alcohol dehydrogenases of melon exhibit fruit ripening-specific expression and distinct biochemical characteristics. Plant Mol. Biol. 2006, 61, 675–685. [Google Scholar] [CrossRef]
- Hirvi, T.; Honkanen, E. The volatiles of two new strawberry cultivars, ‘Annelie’ and ‘Alaska Pioneer’, obtained by backcrossing of cultivated strawberries with wild strawberries, fragaria vesca, rugen and fragaria virginiana. Z. Lebensm.-Unters. Forsch. 1982, 175, 113–116. [Google Scholar] [CrossRef]
- Wyllie, S.G.; Leach, D.N.; Nonhebel, H.N.; Lusunzi, I. Biochemical pathways for the formation of esters in ripening fruit. In Flavour Science; Woodhead Publishing: Cambridge, UK, 1996. [Google Scholar]
- Schaffer, R.J.; Friel, E.N.; Souleyre, E.J.; Bolitho, K.; Thodey, K.; Ledger, S.; Bowen, J.H.; Ma, J.H.; Nain, B.; Cohen, D.; et al. A genomics approach reveals that aroma production in apple is controlled by ethylene predominantly at the final step in each biosynthetic pathway. Plant Physiol. 2007, 144, 1899–1912. [Google Scholar] [CrossRef] [PubMed]
- Matsui, K.; Fukutomi, S.; Wilkinson, J.; Hiatt, B.; Knauf, V.; Kajwara, T. Effect of overexpression of fatty acid 9-hydroperoxide lyase in tomatoes (Lycopersicon esculentum Mill.). J. Agric. Food Chem. 2001, 49, 5418–5424. [Google Scholar] [CrossRef] [PubMed]
- González-Agüero, M.; Troncoso, S.; Gudenschwager, O.; Campos-Vargas, R.; Moya-León, M.A.; Defilippi, B.G. Differential expression levels of aroma-related genes during ripening of apricot (Prunus armeniaca L.). Plant Physiol. Biochem. 2009, 47, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Perez, A.G.; Rios, J.J.; Sanz, C.; Olias, J.M. Aroma Components and Free Amino Acids in Strawberry Variety Chandler during Ripening. J. Agric. Food Chem. 1992, 40, 2232. [Google Scholar] [CrossRef]
- Leone, A.; Bleve-Zacheo, T.; Gerardi, C.; Melillo, M.T.; Leo, L.; Zacheo, G. Lipoxygenase involvement in ripening strawberry. J. Agric. Food Chem. 2006, 54, 6835–6844. [Google Scholar] [CrossRef] [PubMed]
- Ozcan, G.; Barringer, S. Effect of enzymes on strawberry volatiles during storage, at different ripeness level, in different cultivars, and during eating. J. Food Sci. 2015, 76, 324–333. [Google Scholar] [CrossRef]
- Koutsompogeras, P.; Kyriacou, A.; Zabetakis, I. Characterizing NAD-dependent alcohol dehydrogenase enzymes of methylobacterium extorquens and strawberry (Fragaria × ananassa cv. Elsanta). J. Agric. Food Chem. 2006, 54, 235–242. [Google Scholar] [CrossRef]
- Goepfert, S.; Poirier, Y. β-Oxidation in fatty acid degradation and beyond. Curr. Opin. Plant Biol. 2007, 10, 245–251. [Google Scholar] [CrossRef]
- Tressl, R.; Drawert, F. Biogenesis of Banana Volatiles. J. Agric. Food Chem. 1973, 21, 560–565. [Google Scholar] [CrossRef]
- Olias, J.M.; Sanz, C.; Rios, J.J.; Pérez, A.G. Substrate Specificity of Alcohol Acyltransferase from Strawberry and Banana Fruits. ACS Natl. Meet. Book Abstr. 1995, 596, 134–141. [Google Scholar]
- Pérez, A.G.; Olías, R.; Luaces, P.; Sanz, C. Biosynthesis of strawberry aroma compounds through amino acid metabolism. J. Agric. Food Chem. 2002, 50, 4037–4042. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.; Radic, Z. The cholinesterases: From genes to proteins. Annu. Rev. Pharmacol. Toxicol. 1994, 34, 281–320. [Google Scholar] [CrossRef] [PubMed]
- Hemingway, J. The molecular basis of two contrasting metabolic mechanisms of insecticide resistance. Insect Biochem. Mol. Biol. 2000, 30, 1009–1015. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.M.; Larsen, N.A.; Basran, A.; Barbas, C.F.; Bruce, N.C.; Wilson, I.A.; Lerner, R.A. Biochemical characterization and structural analysis of a highly proficient cocaine esterase. Biochemistry 2002, 41, 12297–12307. [Google Scholar] [CrossRef] [PubMed]
- Marshall, S.D.; Putterill, J.J.; Plummer, K.M.; Newcomb, R.D. The carboxylesterase gene family from Arabidopsis thaliana. J. Mol. Evol. 2003, 57, 487–500. [Google Scholar] [PubMed]
- Cummins, I.; Landrum, M.; Steel, P.G.; Edwards, R. Structure activity studies with xenobiotic substrates using carboxylesterases isolated from Arabidopsis thaliana. Phytochemistry 2007, 68, 811–818. [Google Scholar] [CrossRef]
- Gershater, M.C.; Cummins, I.; Edwards, R. Role of a carboxylesterase in herbicide bioactivation in Arabidopsis thaliana. J. Biol. Chem. 2007, 282, 21460–21466. [Google Scholar] [CrossRef]
- Lee, S.; Hwang, S.; Seo, Y.W.; Jeon, W.B.; Oh, B.J. Molecular characterization of the AtCXE8 gene, which promotes resistance to Botrytis cinerea infection. Plant Biotechnol. Rep. 2012, 7, 109–119. [Google Scholar] [CrossRef]
- Goulet, C.; Mageroy, M.H.; Lam, N.B.; Floystad, A.; Tieman, D.M.; Klee, H.J. Role of an esterase in flavor volatile variation within the tomato clade. Proc. Natl. Acad. Sci. USA 2012, 109, 19009–19014. [Google Scholar] [CrossRef]
- Souleyre, E.J.; Marshall, S.D.; Oakeshott, J.G.; Russell, R.J.; Plummer, K.M.; Newcomb, R.D. Biochemical characterisation of MdCXE1, a carboxylesterase from apple that is expressed during fruit ripening. Phytochemistry 2011, 72, 564–571. [Google Scholar] [CrossRef]
- Cao, X.; Xie, K.; Duan, W.; Zhu, Y.; Liu, M.; Chen, K.; Klee, H.; Zhang, B. Peach carboxylesterase PpCXE1 is associated with catabolism of volatile esters. J. Agric. Food Chem. 2019, 67, 5189–5196. [Google Scholar] [CrossRef] [PubMed]
- Rivas, F.J.M.; Blanco-Portales, R.; Moyano, E.; Alseekh, S.; Caballero, J.L.; Schwab, W.; Fernie, A.R.; Muñoz-Blanco, J.; Molina-Hidalgo, F.J. Strawberry fruit FanCXE1 carboxylesterase is involved in the catabolism of volatile esters during the ripening process. Hortic. Res. 2022, 9, uhac095. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Duan, W.; Wei, C.; Chen, K.; Grierson, D.; Zhang, B. Genome-Wide Identification and Functional Analysis of Carboxylesterase and Methylesterase Gene Families in Peach (Prunus persica L. Batsch). Front. Plant Sci. 2019, 10, 1511. [Google Scholar] [CrossRef]
- Solís-Montero, L.; Cáceres-García, S.; Alavez-Rosas, D.; García-Crisóstomo, J.F.; Vega-Polanco, M.; Grajales-Conesa, J.; Cruz-López, L. Pollinator Preferences for Floral Volatiles Emitted by Dimorphic Anthers of a Buzz-Pollinated Herb. J. Chem. Ecol. 2018, 44, 1058–1067. [Google Scholar] [CrossRef] [PubMed]
- Schiestl, F.P. The evolution of floral scent and insect chemical communication. Ecol. Lett. 2010, 13, 643–656. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, C.C.; Yan, S.W.; Liu, Y.; Guo, M.B.; Dong, S.L.; Wang, G.R. An odorant receptor from the common cutworm (Spodoptera litura) exclusively tuned to the important plant volatile cis-3-Hexenyl acetate. Insect Mol. Biol. 2013, 22, 424–432. [Google Scholar] [CrossRef]
- Yan, S.W.; Zhang, J.; Liu, Y.; Li, G.Q.; Wang, G.R. An olfactory receptor from Apolygus lucorum (Meyer-Dur) mainly tuned to volatiles from flowering host plants. J. Insect Physiol. 2015, 79, 36–41. [Google Scholar] [CrossRef]
- Engelberth, J.; Alborn, H.T.; Schmelz, E.A.; Tumlinson, J.H. Airborne herbivore signals attack prime plants against insect. Proc. Natl. Acad. Sci. USA 2004, 101, 1781–1785. [Google Scholar] [CrossRef]
- De Moraes, C.M.; Mescher, M.C.; Tumlinson, J.H. Caterpillar-induced nocturnal plant volatiles repel conspecific females. Nature 2001, 410, 577–580. [Google Scholar] [CrossRef]
- Yan, J.W.; Ban, Z.J.; Lu, H.Y.; Li, D.; Poverenov, E.; Luo, Z.S.; Li, L. The aroma volatile repertoire in strawberry fruit: A review. J. Sci. Food Agric. 2018, 98, 4395–4402. [Google Scholar] [CrossRef]
- Li, D.; Han, D.; Zhang, H.; Zhang, C.; Li, M.; Wang, Y. Analysis of Aroma Volatile Compounds in Fuji Apple Using SPME with Different Fiber Coatings. Agric. Biotechnol. 2020, 9, 82–86. [Google Scholar]
- Shiota, H. Changes in the volatile composition of La France pear during maturation. J. Sci. Food Agric. 1990, 52, 421–429. [Google Scholar] [CrossRef]
- Defilippi, B.G.; Dandekar, A.M.; Kader, A.A. Relationship of ethylene biosynthesis to volatile production, related enzymes, and precursor availability in apple peel and flesh tissues. J. Agric. Food. Chem. 2005, 53, 3133–3141. [Google Scholar] [CrossRef] [PubMed]
- Dong, T.; Chen, X.J.; Wang, M.; Huang, Y.H.; Yi, G.J. Comparison of volatile aroma compounds in Dwarf Cavendish banana (Musa spp. AAA) grown under organic or traditional cultivation. J. Hortic. Sci. Biotechnol. 2014, 89, 441–447. [Google Scholar] [CrossRef]
- Fan, Z.; Hasing, T.; Johnson, T.S.; Garner, D.M.; Schwieterman, M.L.; Barbey, C.R.; Colquhoun, T.A.; Sims, C.A.; Resende, M.F.; Whitaker, V.M. Strawberry sweetness and consumer preference are enhanced by specific volatile compounds. Hortic. Res. 2021, 8, 870–884. [Google Scholar]
- Beekwilder, J.; Alvarez-Huerta, M.; Neef, E.; Verstappen, F.W.; Bouwmeester, H.J.; Aharoni, A. Functional characterization of enzymes forming volatile esters from strawberry and banana. Plant Physiol. 2004, 135, 1865–1878. [Google Scholar] [CrossRef]
- Potter, P.M. Carboxylesterases: General detoxifying enzymes. Chem. Biol. Interact. 2016, 259, 327–331. [Google Scholar]
- Souleyre, E.J.; Chagne, D.; Chen, X.; Tomes, S.; Turner, R.M.; Wang, M.Y.; Maddumage, R.; Hunt, M.B.; Winz, R.A.; Wiedow, C.; et al. The AAT1 locus is critical for the biosynthesis of esters contributing to ‘ripe apple’ flavour in ‘Royal Gala’ and ‘Granny Smith’ apples. Plant J. 2014, 78, 903–915. [Google Scholar] [CrossRef]
- Schwab, W.; Davidovich-Rikanati, R.; Lewinsohn, E. Biosynthesis of plant-derived flavor compounds. Plant J. 2008, 54, 712–732. [Google Scholar] [CrossRef]
- Jordán, M.J.; Tandon, K.; Shaw, P.E.; Goodner, K.L. Aromatic profile of aqueous banana essence and banana fruit by gas chromatography-mass spectrometry (GC-MS) and gas chromatography-olfactometry (GC-O). J. Agric. Food Chem. 2001, 49, 4813–4817. [Google Scholar] [CrossRef]
- Cumplido-Laso, G.; Medina-Puche, L.; Moyano, E.; Hoffmann, T.; Sinz, Q.; Ring, L.; Studart-Wittkowski, C.; Caballero, J.L.; Schwab, W.; Muñoz-Blanco, J.; et al. The fruit ripening-related gene FaAAT2 encodes an acyl transferase involved in strawberry aroma biogenesis. J. Exp. Bot. 2012, 63, 4275–4290. [Google Scholar] [CrossRef] [PubMed]
- Sussman, J.L.; Harel, M.; Frolow, F.; Oefner, C.; Goldman, A.; Toker, L.; Silman, I. Atomic structure of acetylcholinesterase from Torpedo californica: A prototypic acetylcholine-binding protein. Science 1991, 253, 872–879. [Google Scholar] [CrossRef] [PubMed]
- Oakeshott, J.G.; Claudianos, C.; Russell, R.J.; Robin, G.C. Carboxyl/cholinesterases: A case study of the evolution of a successful multigene family. BioEssays 1999, 21, 1031–1042. [Google Scholar] [CrossRef]
- Gasteiger, E.; Gattiker, A.; Hoogland, C.; Ivanyi, I.; Appel, R.D.; Bairoch, A. ExPASy: The proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Res. 2003, 31, 3784–3788. [Google Scholar] [CrossRef]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinf. 2011, 12, 323. [Google Scholar] [CrossRef]
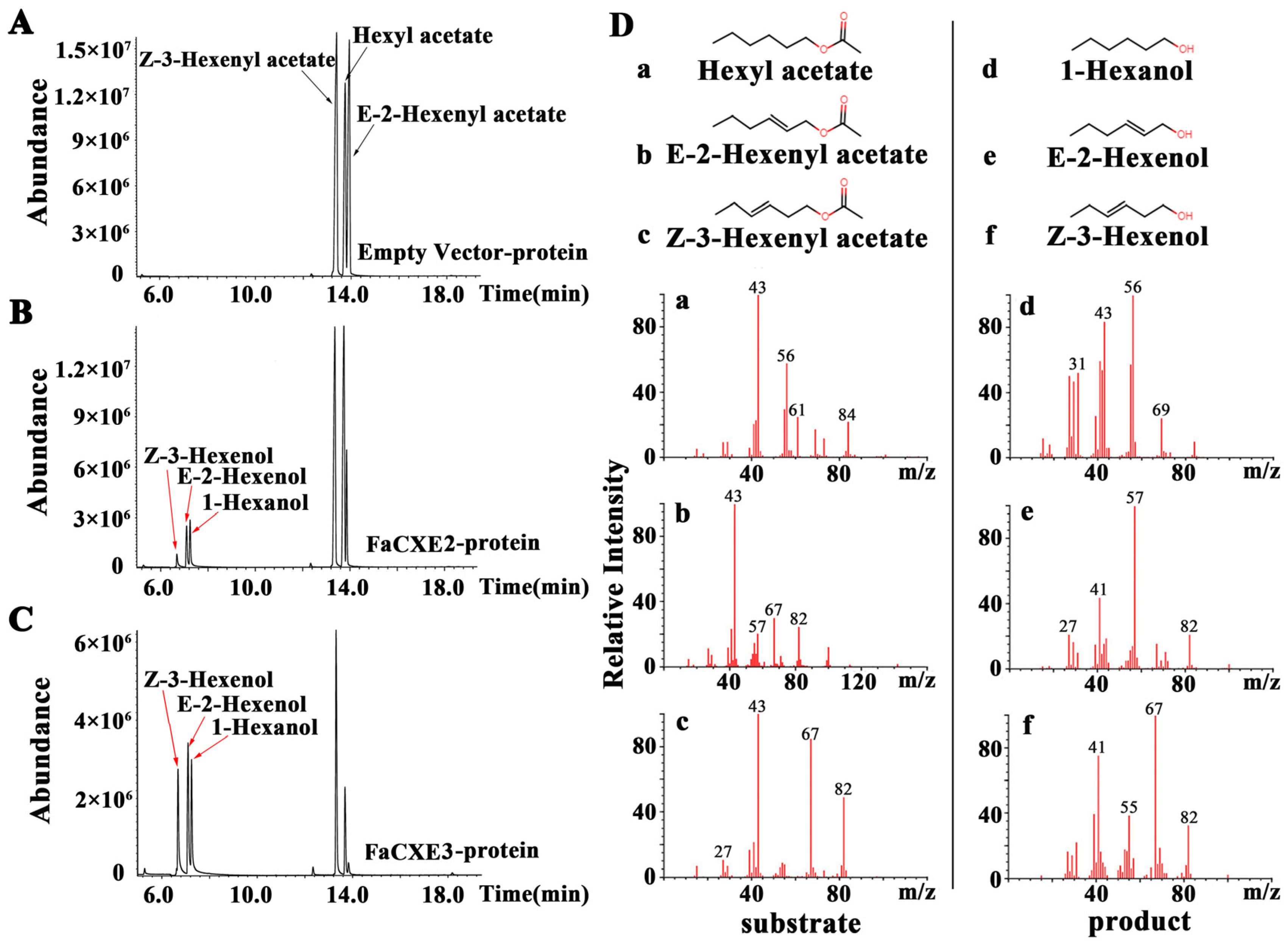
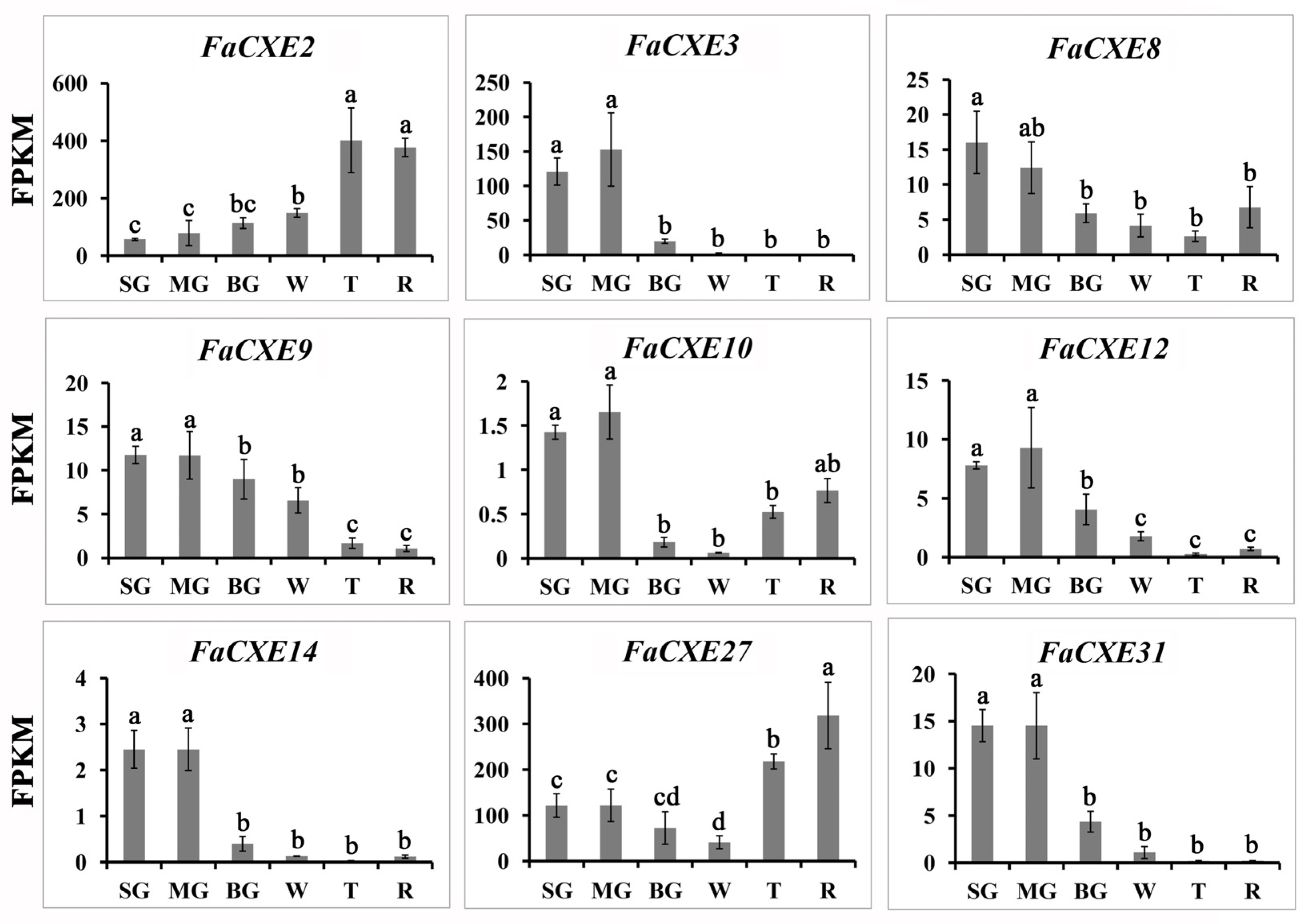
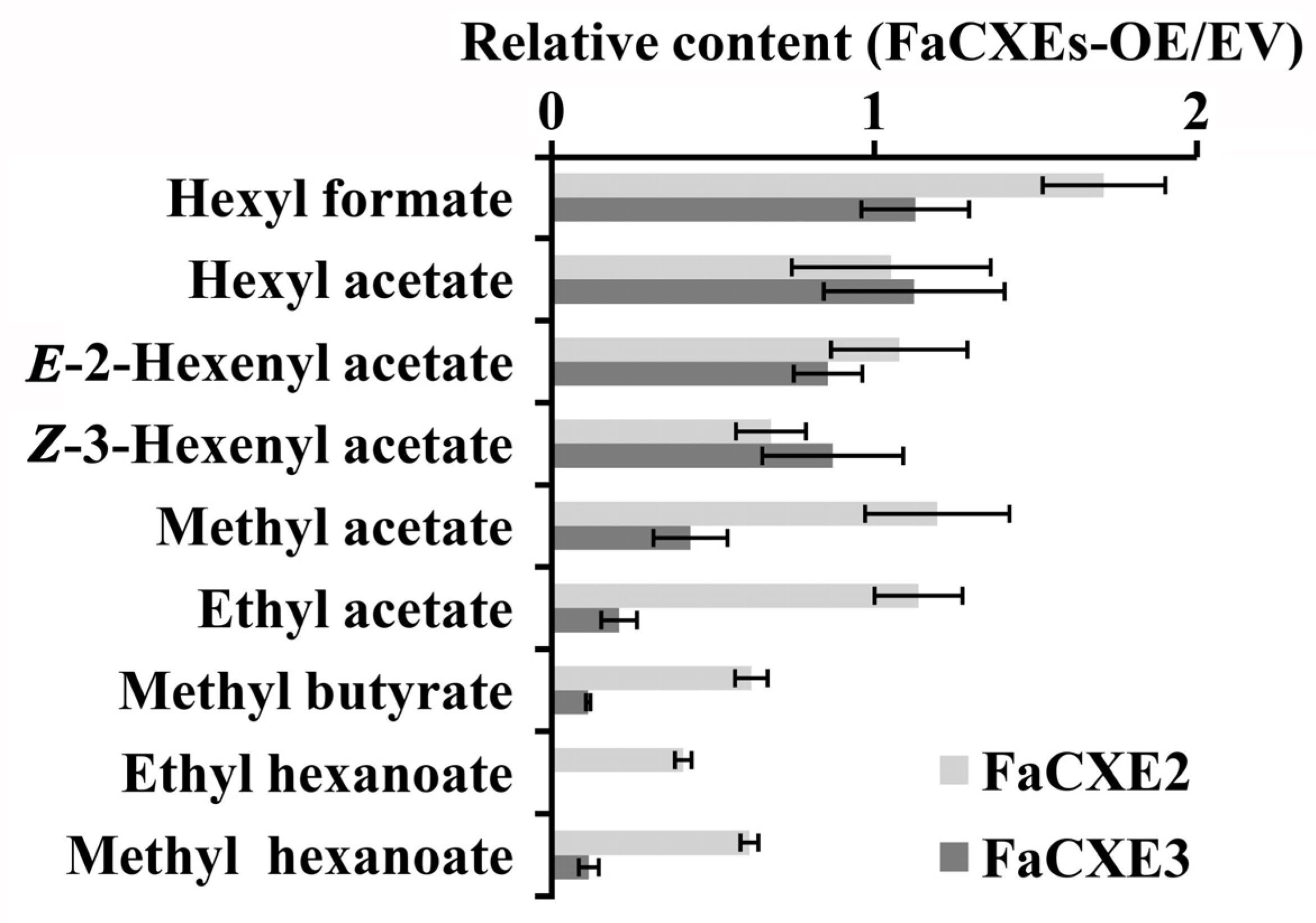
| Volatile Esters | Fa | Ma | Md | Pb | Pp | |
|---|---|---|---|---|---|---|
| 1 | Hexyl acetate | 4270 | 2940 | 2740 | 39.1 | 166 |
| 2 | Butyl butyrate | 1050 | 5060 | 277 | 38.4 | 69.4 |
| 3 | E-2-hexenyl acetate | 1110 | 241 | - | 11.8 | 72.4 |
| 4 | Hexyl butyrate | 2550 | 7600 | 693 | 15.3 | - |
| 5 | Butyl acetate | 940 | 1720 | 1680 | 39.1 | - |
| 6 | Methyl butyl caproate | 229 | 525 | 52.3 | 5.82 | 19.5 |
| 7 | 2-methyl-1-butanol acetate | 119 | 8070 | 4360 | 17.3 | - |
| 8 | Butyl isovalerate | 76.3 | 26,100 | 63.6 | - | - |
| 9 | E-2-hexenyl butyrate | 1030 | - | - | - | - |
| 10 | Octyl acetate | 25,500 | - | - | - | - |
| 11 | Methyl hexanoate | 390 | 102 | - | 26.3 | - |
| 12 | Butyl acrylate | - | - | 30.2 | 53.1 | 64.7 |
| 13 | Isobutyl acetate | 143 | 1040 | 155 | - | - |
| 14 | Ethyl hexanoate | 5230 | - | - | 1380 | 3350 |
| 15 | Heptyl acetate | 328 | 11,500 | - | 27.9 | - |
| 16 | Methyl acetate | - | - | - | 146 | - |
| 17 | Ethyl acetate | 613 | 271 | - | 23.1 | - |
| 18 | Ethyl propanoate | 59 | - | - | - | - |
| 19 | n-Propyl acetate | 65.8 | - | 184 | - | - |
| 20 | Methyl butyrate | 1470 | - | - | 14.3 | - |
| 21 | Methyl 2-methyl butyrate | 43.6 | - | - | - | - |
| 22 | Ethyl butyrate | 1760 | - | - | - | - |
| 23 | Propyl propionate | - | - | 178 | - | - |
| 24 | Isopropyl butyrate | 16.4 | - | - | - | - |
| 25 | 2-pentyl acetate | - | 7170 | - | - | - |
| 26 | Propyl butyrate | 79.8 | - | 284 | - | - |
| 27 | Amyl acetate | - | - | 283 | - | - |
| 28 | 2-Methylbut-2-en-1-yl acetate | 135 | - | 22 | - | - |
| 29 | Propyl 2-methylbutyrate | - | 635 | 160 | - | - |
| 30 | 2-Methylbutyl isobutyrate | - | 40.4 | - | - | - |
| 31 | Propyl 2-methyl butanoate | - | 2470 | - | - | - |
| 32 | 2-Pentanyl propanoate | - | - | 78.3 | - | - |
| 33 | Isopentyl butyrate | - | 159 | - | - | - |
| 34 | Z-3-hexenyl acetate | 455 | - | - | - | - |
| 35 | Isopentyl butyrate | - | 7380 | - | - | - |
| 36 | Ethyl butyrate | - | 654 | - | - | - |
| 37 | Hexyl 2-methylbutyrate | - | - | 281 | - | - |
| 38 | Hexyl 3-methylbutyrate | - | 471 | - | - | - |
| 39 | Isoamyl 2-methylbutyrate | - | 505 | - | - | - |
| 40 | 3-methylbut-2-enyl butanoate | - | 228 | - | - | - |
| 41 | Isoamyl isovalerate | - | 5420 | 128 | - | - |
| 42 | Methyl caprylate | 2420 | - | - | - | - |
| 43 | Ethyl 3-methylvalerate | - | 482 | - | - | - |
| 44 | Methyl phenylacetate | 7620 | - | - | - | - |
| 45 | Methyl salicylate | - | - | - | 10.9 | 87.8 |
| 46 | Ethyl caprylate | 4020 | - | - | - | - |
| 47 | Nonyl acetate | 3550 | - | - | - | - |
| 48 | Hexyl 3-methyl butanoate | - | 1300 | - | - | - |
| 49 | Pentyl hexanoate | - | - | 22.7 | - | - |
| 50 | Benzyl butyrate | 320 | - | - | - | - |
| 51 | Ethyl E-2-decenoate | 220 | - | - | - | - |
| 52 | Octyl butyrate | 1400 | - | - | - | - |
| 53 | Ethyl decanoate | 991 | - | - | - | - |
| 54 | Decyl acetate | 7290 | - | - | - | - |
| 55 | 2-Undecyl acetate | 1280 | - | - | - | - |
| 56 | Cinnamyl acetate | 797 | - | - | - | - |
| 57 | Hexadecyl decanoate | 625 | - | - | - | - |
| rFaCXE2 | rFaCXE3 | |||||
|---|---|---|---|---|---|---|
| Substrates | Km (mM) | Vmax (pKat μg−1) | Vmax/Km (pKat μg−1 mM−1) | Km (mM) | Vmax (pKat μg−1) | Vmax/Km (pKat μg−1 mM−1) |
| Hexyl acetate | 10.93 ± 0.84 | 129.00 ± 7.11 | 11.80 ± 1.04 | 0.84 ± 0.07 | 20.10 ± 2.35 | 24.00 ± 1.75 |
| E-2-Hexenyl acetate | 1.04 ± 0.05 | 5.46 ± 0.47 | 5.24 ± 0.22 | 0.98 ± 0.11 | 13.60 ± 1.21 | 13.80 ± 1.53 |
| Z-3-Hexenyl acetate | 2.50 ± 0.13 | 12.50 ± 1.21 | 5.00 ± 0.32 | 0.95 ± 0.05 | 18.50 ± 1.69 | 19.50 ± 2.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Zhou, K.; Wang, M.; Li, R.; Dai, X.; Liu, Y.; Jiang, X.; Xia, T.; Gao, L. The Functional Characterization of Carboxylesterases Involved in the Degradation of Volatile Esters Produced in Strawberry Fruits. Int. J. Mol. Sci. 2023, 24, 383. https://doi.org/10.3390/ijms24010383
Zhang L, Zhou K, Wang M, Li R, Dai X, Liu Y, Jiang X, Xia T, Gao L. The Functional Characterization of Carboxylesterases Involved in the Degradation of Volatile Esters Produced in Strawberry Fruits. International Journal of Molecular Sciences. 2023; 24(1):383. https://doi.org/10.3390/ijms24010383
Chicago/Turabian StyleZhang, Lingjie, Kang Zhou, Maohao Wang, Rui Li, Xinlong Dai, Yajun Liu, Xiaolan Jiang, Tao Xia, and Liping Gao. 2023. "The Functional Characterization of Carboxylesterases Involved in the Degradation of Volatile Esters Produced in Strawberry Fruits" International Journal of Molecular Sciences 24, no. 1: 383. https://doi.org/10.3390/ijms24010383
APA StyleZhang, L., Zhou, K., Wang, M., Li, R., Dai, X., Liu, Y., Jiang, X., Xia, T., & Gao, L. (2023). The Functional Characterization of Carboxylesterases Involved in the Degradation of Volatile Esters Produced in Strawberry Fruits. International Journal of Molecular Sciences, 24(1), 383. https://doi.org/10.3390/ijms24010383






