Antibiotic Knockdown of Gut Bacteria Sex-Dependently Enhances Intravenous Fentanyl Self-Administration in Adult Sprague Dawley Rats
Abstract
:1. Introduction
2. Results
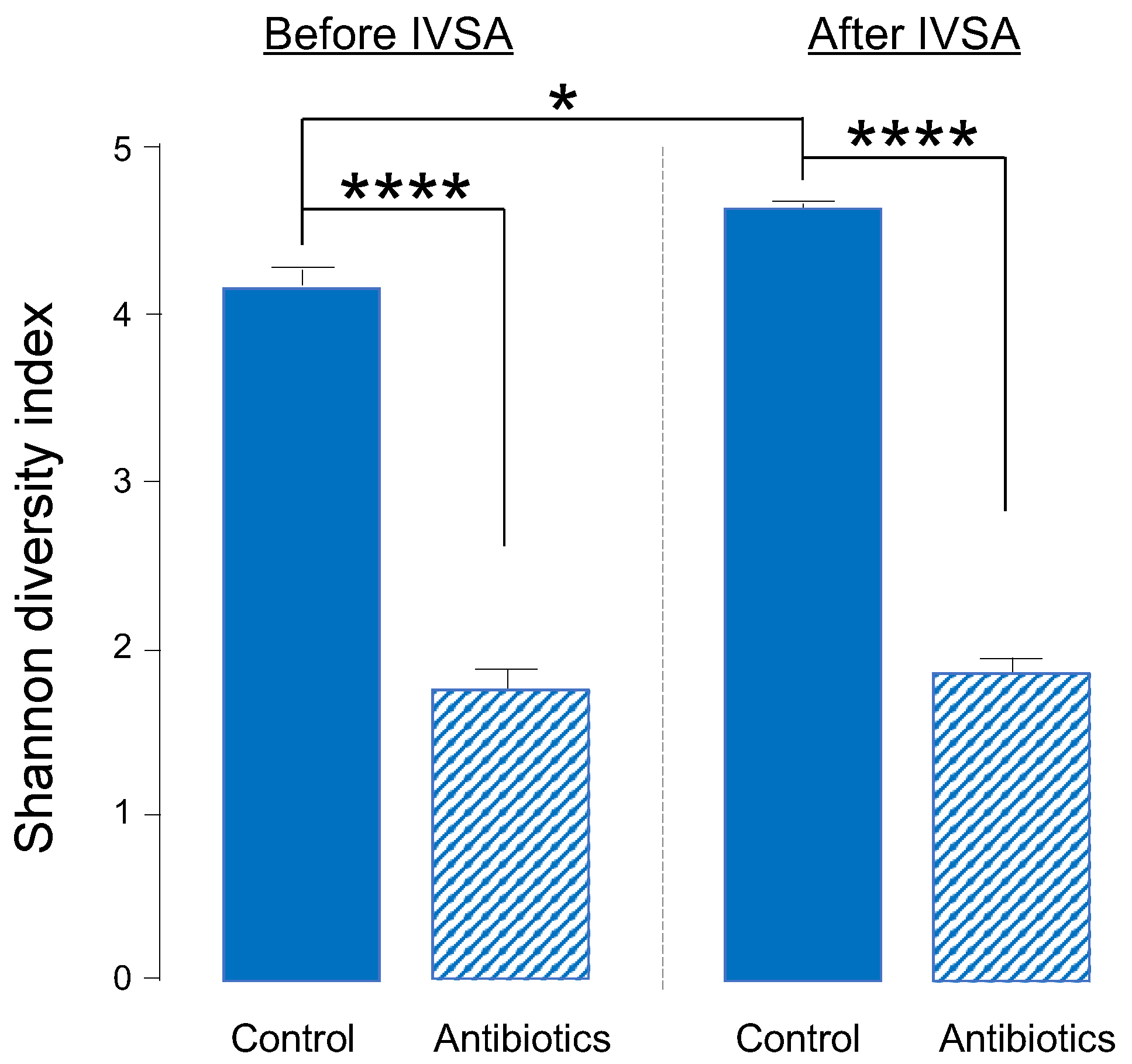
2.1. One-Week Oral Antibiotic Treatment Significantly Depletes Gut Bacteria (and Depletion Is Maintained throughout Experiment)
2.2. Antibiotic Treatment Causes Phylum- and Genus-Level Changes in Gut Bacteria
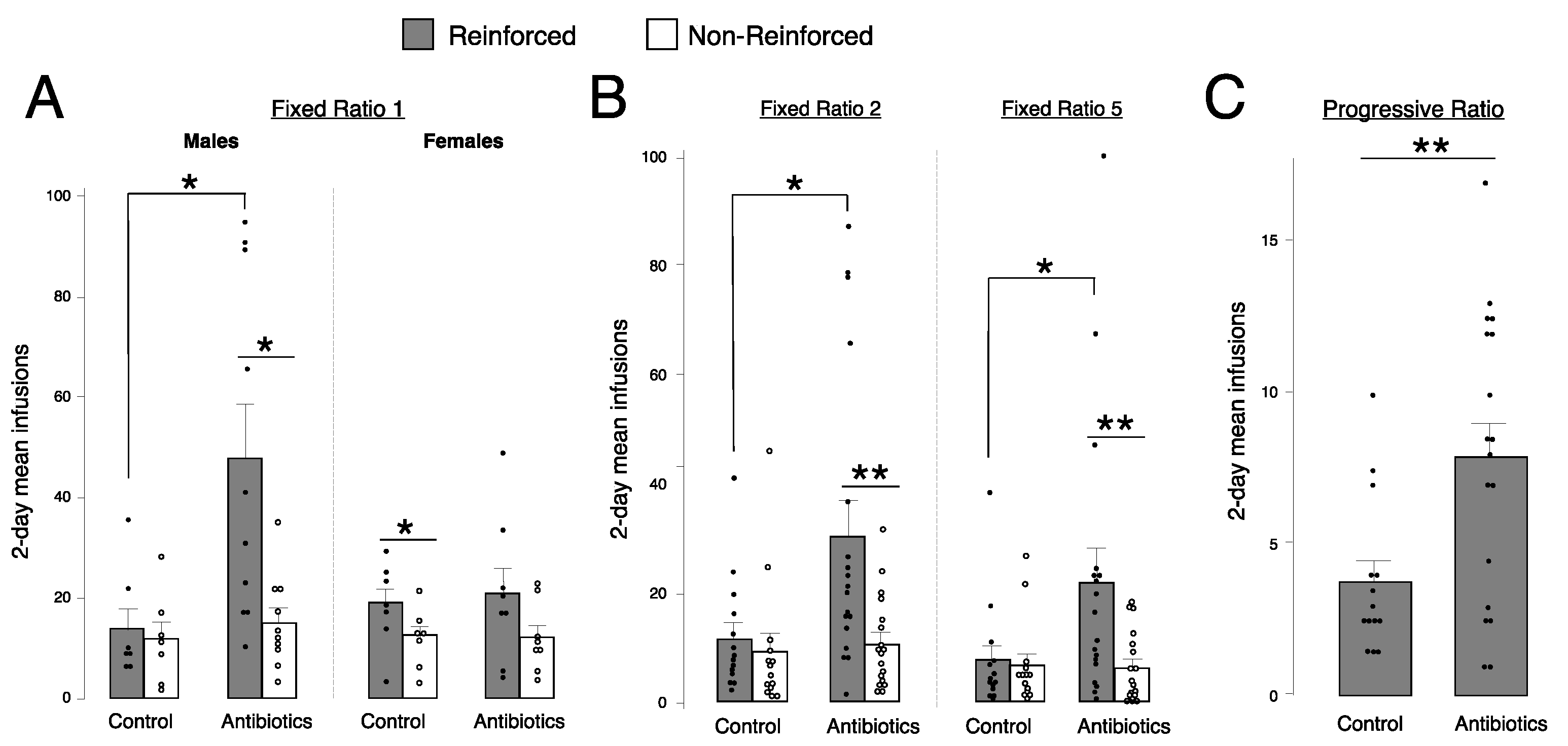
2.3. Antibiotic-Treated Males, but Not Females, Self-Administer More Fentanyl Than Controls at Fixed Ratio (FR) 1
2.4. Antibiotic-Treated Animals Self-Administer More Fentanyl Than Controls at FR2, FR5, and Progressive Ratio (PR)
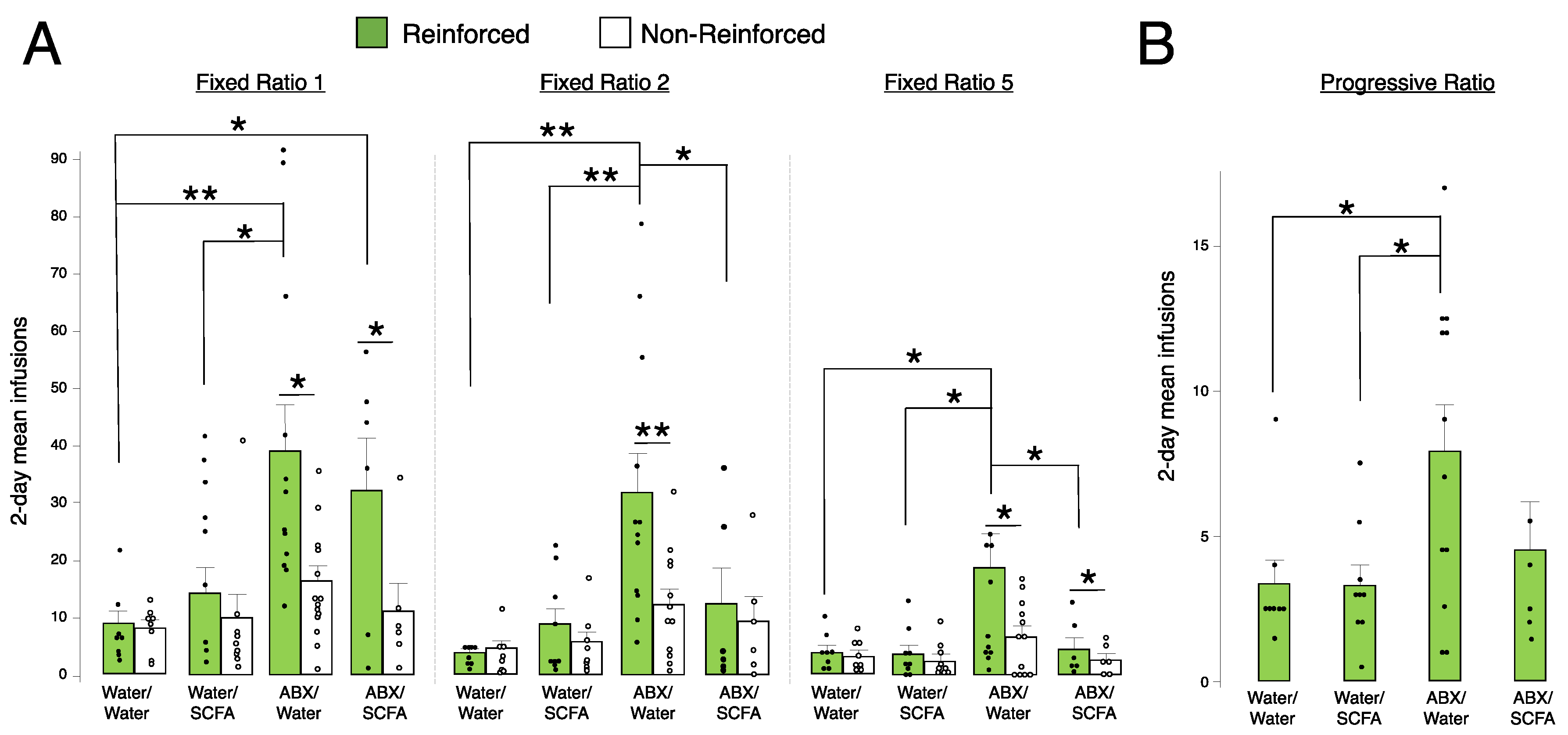
2.5. Short-Chain Fatty Acid (SCFA) Supplementation Blunts Fentanyl Self-Administration in Antibiotic-Treated Animals at FR2 and FR5
3. Discussion
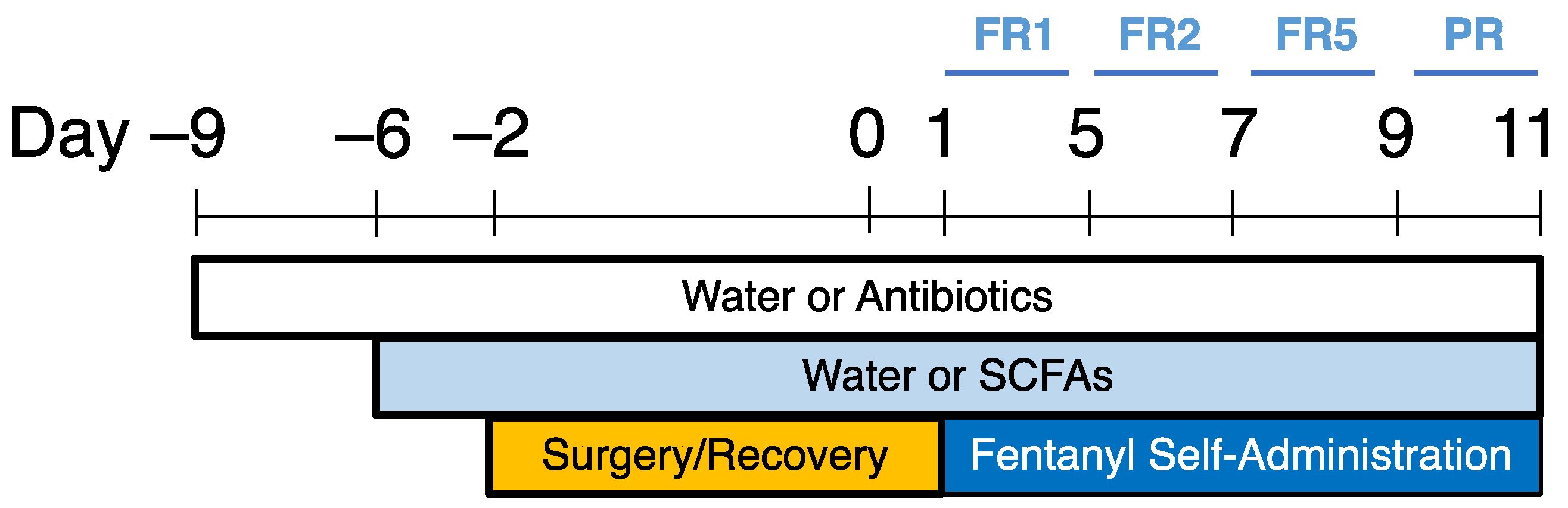
4. Materials and Methods
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rudd, R.A.; Seth, P.; David, F.; Scholl, L. Increases in Drug and Opioid-Involved Overdose Deaths—United States, 2010–2015. MMWR 2016, 65, 1445–1452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Substance Abuse and Mental Health Services Administration. Key Substance Use and Mental Health Indicators in the United States: Results from the 2019 National Survey on Drug Use and Health; HHS Publication: Rockville, MD, USA, 2020. [Google Scholar]
- Panchal, S.; Müller-Schwefe, P.; Wurzelmann, J. Opioid-induced Bowel Dysfunction: Prevalence, Pathophysiology and Burden. Int. J. Clin. Pract. 2007, 61, 1181–1187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holzer, P. Opioids and Opioid Receptors in the Enteric Nervous System: From a Problem in Opioid Analgesia to a Possible New Prokinetic Therapy in Humans. Neurosci. Lett. 2004, 361, 192–195. [Google Scholar] [CrossRef] [PubMed]
- Guarner, F.; Malagelada, J.-R. Gut Flora in Health and Disease. Lancet 2003, 361, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Iannone, L.F.; Preda, A.; Blottière, H.M.; Clarke, G.; Albani, D.; Belcastro, V.; Carotenuto, M.; Cattaneo, A.; Citraro, R.; Ferraris, C.; et al. Microbiota-Gut Brain Axis Involvement in Neuropsychiatric Disorders. Expert. Rev. Neurother. 2019, 19, 1037–1050. [Google Scholar] [CrossRef]
- Cryan, J.F.; O′Riordan, K.J.; Sandhu, K.; Peterson, V.; Dinan, T.G. The Gut Microbiome in Neurological Disorders. Lancet Neurol. 2020, 19, 179–194. [Google Scholar] [CrossRef]
- Puizillout, J.-J. Central Projections of Vagal Afferents; Editions Publibook: Paris, France, 2005; ISBN 2-7483-0895-6. [Google Scholar]
- Bercik, P.; Park, A.; Sinclair, D.; Khoshdel, A.; Lu, J.; Huang, X.; Deng, Y.; Blennerhassett, P.; Fahnestock, M.; Moine, D. The Anxiolytic Effect of Bifidobacterium Longum NCC3001 Involves Vagal Pathways for Gut–Brain Communication. Neurogastroenterol. Motil. 2011, 23, 1132–1139. [Google Scholar] [CrossRef] [Green Version]
- Ghia, J.-E.; Blennerhassett, P.; Collins, S.M. Impaired Parasympathetic Function Increases Susceptibility to Inflammatory Bowel Disease in a Mouse Model of Depression. J. Clin. Investig. 2008, 118, 2209–2218. [Google Scholar] [CrossRef]
- Bravo, J.A.; Forsythe, P.; Chew, M.V.; Escaravage, E.; Savignac, H.M.; Dinan, T.G.; Bienenstock, J.; Cryan, J.F. Ingestion of Lactobacillus Strain Regulates Emotional Behavior and Central GABA Receptor Expression in a Mouse via the Vagus Nerve. Proc. Natl. Acad. Sci. USA 2011, 108, 16050–16055. [Google Scholar] [CrossRef] [Green Version]
- Berthoud, H. Vagal and Hormonal Gut–Brain Communication: From Satiation to Satisfaction. Neurogastroenterol. Motil. 2008, 20, 64–72. [Google Scholar] [CrossRef] [Green Version]
- Mayer, E.A. Gut Feelings: The Emerging Biology of Gut–Brain Communication. Nat. Rev. Neurosci. 2011, 12, 453–466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharon, G.; Sampson, T.R.; Geschwind, D.H.; Mazmanian, S.K. The Central Nervous System and the Gut Microbiome. Cell 2016, 167, 915–932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bercik, P.; Denou, E.; Collins, J.; Jackson, W.; Lu, J.; Jury, J.; Deng, Y.; Blennerhassett, P.; Macri, J.; McCoy, K.D. The Intestinal Microbiota Affect Central Levels of Brain-Derived Neurotropic Factor and Behavior in Mice. Gastroenterology 2011, 141, 599–609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heijtz, R.D.; Wang, S.; Anuar, F.; Qian, Y.; Björkholm, B.; Samuelsson, A.; Hibberd, M.L.; Forssberg, H.; Pettersson, S. Normal Gut Microbiota Modulates Brain Development and Behavior. Proc. Natl. Acad. Sci. USA 2011, 108, 3047–3052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hegstrand, L.R.; Hine, R.J. Variations of Brain Histamine Levels in Germ-Free and Nephrectomized Rats. Neurochem. Res. 1986, 11, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Neufeld, K.; Kang, N.; Bienenstock, J.; Foster, J.A. Reduced Anxiety-like Behavior and Central Neurochemical Change in Germ-free Mice. Neurogastroenterol. Motil. 2011, 23, 255-e119. [Google Scholar] [CrossRef]
- Nobuyuki, S.; Yoichi, C.; Yuji, A.; Junko, S.; Naomi, O.; Xiao-Nian, Y.; Chiharu, K.; Yasuhiro, K. Postnatal Microbial Colonization Programs the Hypothalamic-Pituitary-Adrenal System for Stress Response in Mice. J. Physiol. 2004, 558, 263–275. [Google Scholar]
- Kelly, J.R.; Borre, Y.; O′Brien, C.; Patterson, E.; El Aidy, S.; Deane, J.; Kennedy, P.J.; Beers, S.; Scott, K.; Moloney, G. Transferring the Blues: Depression-Associated Gut Microbiota Induces Neurobehavioural Changes in the Rat. J. Psychiatr. Res. 2016, 82, 109–118. [Google Scholar] [CrossRef]
- Desbonnet, L.; Clarke, G.; Traplin, A.; O’Sullivan, O.; Crispie, F.; Moloney, R.D.; Cotter, P.D.; Dinan, T.G.; Cryan, J.F. Gut Microbiota Depletion from Early Adolescence in Mice: Implications for Brain and Behaviour. Brain Behav. Immun. 2015, 48, 165–173. [Google Scholar] [CrossRef]
- McKernan, D.; Fitzgerald, P.; Dinan, T.; Cryan, J. The Probiotic Bifidobacterium Infantis 35624 Displays Visceral Antinociceptive Effects in the Rat. Neurogastroenterol. Motil. 2010, 22, 1029-e268. [Google Scholar] [CrossRef]
- O′mahony, S.; Felice, V.; Nally, K.; Savignac, H.; Claesson, M.; Scully, P.; Woznicki, J.; Hyland, N.; Shanahan, F.; Quigley, E.M. Disturbance of the Gut Microbiota in Early-Life Selectively Affects Visceral Pain in Adulthood without Impacting Cognitive or Anxiety-Related Behaviors in Male Rats. Neuroscience 2014, 277, 885–901. [Google Scholar] [CrossRef] [PubMed]
- Kiraly, D.D.; Walker, D.M.; Calipari, E.S.; Labonte, B.; Issler, O.; Pena, C.J.; Ribeiro, E.A.; Russo, S.J.; Nestler, E.J. Alterations of the Host Microbiome Affect Behavioral Responses to Cocaine. Sci. Rep. 2016, 6, 35455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, M.; Mischel, R.A.; Bhave, S.; Komla, E.; Cho, A.; Huang, C.; Dewey, W.L.; Akbarali, H.I. The Effect of Gut Microbiome on Tolerance to Morphine Mediated Antinociception in Mice. Sci. Rep. 2017, 7, 42658. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Meng, J.; Zhang, L.; Johnson, T.; Chen, C.; Roy, S. Morphine Induces Changes in the Gut Microbiome and Metabolome in a Morphine Dependence Model. Sci. Rep. 2018, 8, 3596. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.; Vuong, H.E.; Nusbaum, D.J.; Hsiao, E.Y.; Evans, C.J.; Taylor, A.M. The Gut Microbiota Mediates Reward and Sensory Responses Associated with Regimen-Selective Morphine Dependence. Neuropsychopharmacology 2018, 43, 2606–2614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hofford, R.S.; Mervosh, N.L.; Euston, T.J.; Meckel, K.R.; Orr, A.T.; Kiraly, D.D. Alterations in Microbiome Composition and Metabolic Byproducts Drive Behavioral and Transcriptional Responses to Morphine. Neuropsychopharmacology 2021, 46, 2062–2072. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Tellez, L.A.; Perkins, M.H.; Perez, I.O.; Qu, T.; Ferreira, J.; Ferreira, T.L.; Quinn, D.; Liu, Z.-W.; Gao, X.-B. A Neural Circuit for Gut-Induced Reward. Cell 2018, 175, 665–678. [Google Scholar] [CrossRef] [Green Version]
- Thomaz, A.C.; Iyer, V.; Woodward, T.J.; Hohmann, A.G. Fecal Microbiota Transplantation and Antibiotic Treatment Attenuate Naloxone-Precipitated Opioid Withdrawal in Morphine-Dependent Mice. Exp. Neurol. 2021, 343, 113787. [Google Scholar] [CrossRef]
- Banerjee, S.; Sindberg, G.; Wang, F.; Meng, J.; Sharma, U.; Zhang, L.; Dauer, P.; Chen, C.; Dalluge, J.; Johnson, T.; et al. Opioid-Induced Gut Microbial Disruption and Bile Dysregulation Leads to Gut Barrier Compromise and Sustained Systemic Inflammation. Mucosal. Immunol. 2016, 9, 1418–1428. [Google Scholar] [CrossRef] [Green Version]
- Meng, J.; Yu, H.; Ma, J.; Wang, J.; Banerjee, S.; Charboneau, R.; Barke, R.A.; Roy, S. Morphine Induces Bacterial Translocation in Mice by Compromising Intestinal Barrier Function in a TLR-Dependent Manner. PLoS ONE 2013, 8, e54040. [Google Scholar] [CrossRef] [Green Version]
- Ren, M.; Lotfipour, S. Dose- and Sex-Dependent Bidirectional Relationship between Intravenous Fentanyl Self-Administration and Gut Microbiota. Microorganisms 2022, 10, 1127. [Google Scholar] [CrossRef] [PubMed]
- Acharya, C.; Betrapally, N.; Gillevet, P.; Sterling, R.; Akbarali, H.; White, M.; Ganapathy, D.; Fagan, A.; Sikaroodi, M.; Bajaj, J. Chronic Opioid Use Is Associated with Altered Gut Microbiota and Predicts Readmissions in Patients with Cirrhosis. Aliment Pharmacol. Ther. 2017, 45, 319–331. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yang, J.; Yang, C.; Chen, T.; Wang, Z.; Li, J.; Qin, F.; Deng, Q.; Zhang, X. Sensitivity to Morphine Reward Associates with Gut Dysbiosis in Rats with Morphine-Induced Conditioned Place Preference. Front. Psychiatry 2020, 11, 631. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Lebrón, A.; Johnson, R.; Mazahery, C.; Troyer, Z.; Joussef-Piña, S.; Quiñones-Mateu, M.E.; Strauch, C.M.; Hazen, S.L.; Levine, A.D. Chronic Opioid Use Modulates Human Enteric Microbiota and Intestinal Barrier Integrity. Gut Microbes 2021, 13, 1946368. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Deji, C.; Fan, J.; Chang, L.; Miao, X.; Xiao, Y.; Zhu, Y.; Li, S. Differential Alteration in Gut Microbiome Profiles during Acquisition, Extinction and Reinstatement of Morphine-Induced CPP. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2021, 104, 110058. [Google Scholar] [CrossRef]
- Burokas, A.; Arboleya, S.; Moloney, R.D.; Peterson, V.L.; Murphy, K.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Targeting the Microbiota-Gut-Brain Axis: Prebiotics Have Anxiolytic and Antidepressant-like Effects and Reverse the Impact of Chronic Stress in Mice. Biol. Psychiatry 2017, 82, 472–487. [Google Scholar] [CrossRef]
- Wong, M.-L.; Inserra, A.; Lewis, M.; Mastronardi, C.A.; Leong, L.; Choo, J.; Kentish, S.; Xie, P.; Morrison, M.; Wesselingh, S. Inflammasome Signaling Affects Anxiety-and Depressive-like Behavior and Gut Microbiome Composition. Mol. Psychiatry. 2016, 21, 797–805. [Google Scholar] [CrossRef] [Green Version]
- Neufeld, K.-A.M.; Kang, N.; Bienenstock, J.; Foster, J.A. Effects of Intestinal Microbiota on Anxiety-like Behavior. Commun. Integr. Biol. 2011, 4, 492–494. [Google Scholar] [CrossRef] [Green Version]
- Jalodia, R.; Abu, Y.F.; Oppenheimer, M.R.; Herlihy, B.; Meng, J.; Chupikova, I.; Tao, J.; Ghosh, N.; Dutta, R.K.; Kolli, U.; et al. Opioid Use, Gut Dysbiosis, Inflammation, and the Nervous System. J. Neuroimm. Pharmacol. 2022, 17, 76–93. [Google Scholar] [CrossRef]
- Ren, M.; Lotfipour, S. The Role of the Gut Microbiome in Opioid Use. Behav. Pharmacol. 2020, 31, 113. [Google Scholar] [CrossRef]
- O’Sullivan, S.J.; Malahias, E.; Park, J.; Srivastava, A.; Reyes, B.A.; Gorky, J.; Vadigepalli, R.; Van Bockstaele, E.J.; Schwaber, J.S. Single-Cell Glia and Neuron Gene Expression in the Central Amygdala in Opioid Withdrawal Suggests Inflammation with Correlated Gut Dysbiosis. Front. Neurosci. 2019, 13, 665. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Chen, S.; Liu, K.; Long, D.; Liu, D.; Jing, Z.; Huang, X. Differences in Gut Microbial Diversity Are Driven by Drug Use and Drug Cessation by Either Compulsory Detention or Methadone Maintenance Treatment. Microorganisms 2020, 8, 411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, J.; Sindberg, G.M.; Roy, S. Disruption of Gut Homeostasis by Opioids Accelerates HIV Disease Progression. Front. Microbiol. 2015, 6, 643. [Google Scholar] [CrossRef] [Green Version]
- Vincent, C.; Miller, M.A.; Edens, T.J.; Mehrotra, S.; Dewar, K.; Manges, A.R. Bloom and Bust: Intestinal Microbiota Dynamics in Response to Hospital Exposures and Clostridium Difficile Colonization or Infection. Microbiome 2016, 4, 12. [Google Scholar] [CrossRef] [Green Version]
- Stojanov, S.; Berlec, A.; Štrukelj, B. The Influence of Probiotics on the Firmicutes/Bacteroidetes Ratio in the Treatment of Obesity and Inflammatory Bowel Disease. Microorganisms 2020, 8, 1715. [Google Scholar] [CrossRef] [PubMed]
- Trompette, A.; Gollwitzer, E.S.; Yadava, K.; Sichelstiel, A.K.; Sprenger, N.; Ngom-Bru, C.; Blanchard, C.; Junt, T.; Nicod, L.P.; Harris, N.L.; et al. Gut Microbiota Metabolism of Dietary Fiber Influences Allergic Airway Disease and Hematopoiesis. Nat. Med. 2014, 20, 159–166. [Google Scholar] [CrossRef]
- Johnson, E.L.; Heaver, S.L.; Walters, W.A.; Ley, R.E. Microbiome and Metabolic Disease: Revisiting the Bacterial Phylum Bacteroidetes. J. Mol. Med. 2017, 95, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freedman, Z.G.; Kane, J.A.; King, T.S.; Graziane, N.M. The Effect of Prescribing Antibiotics with Opioids on the Development of Opioid Use Disorder: A National Database Study. J. Addict. Dis. 2022, 40, 62–70. [Google Scholar] [CrossRef]
- Mulder, M.; Radjabzadeh, D.; Kiefte-de Jong, J.C.; Uitterlinden, A.G.; Kraaij, R.; Stricker, B.H.; Verbon, A. Long-Term Effects of Antimicrobial Drugs on the Composition of the Human Gut Microbiota. Gut Microbes 2020, 12, 1791677. [Google Scholar] [CrossRef]
- Shao, H.; Zhang, C.; Xiao, N.; Tan, Z. Gut Microbiota Characteristics in Mice with Antibiotic-Associated Diarrhea. BMC Microbiol. 2020, 20, 313. [Google Scholar] [CrossRef]
- Ianiro, G.; Tilg, H.; Gasbarrini, A. Antibiotics as Deep Modulators of Gut Microbiota: Between Good and Evil. Gut 2016, 65, 1906. [Google Scholar] [CrossRef] [PubMed]
- Shin, N.-R.; Whon, T.W.; Bae, J.-W. Proteobacteria: Microbial Signature of Dysbiosis in Gut Microbiota. Trends Biotechnol. 2015, 33, 496–503. [Google Scholar] [CrossRef]
- Panda, S.; El Khader, I.; Casellas, F.; Lopez Vivancos, J.; Garcia Cors, M.; Santiago, A.; Cuenca, S.; Guarner, F.; Manichanh, C. Short-Term Effect of Antibiotics on Human Gut Microbiota. PLoS ONE 2014, 9, e95476. [Google Scholar] [CrossRef] [PubMed]
- Peterson, V.L.; Richards, J.B.; Meyer, P.J.; Cabrera-Rubio, R.; Tripi, J.A.; King, C.P.; Polesskaya, O.; Baud, A.; Chitre, A.S.; Bastiaanssen, T.F.S.; et al. Sex-Dependent Associations between Addiction-Related Behaviors and the Microbiome in Outbred Rats. eBioMedicine 2020, 55, 102769. [Google Scholar] [CrossRef] [PubMed]
- United Nations Office on Drugs. Crime World Drug Report; Boom Koninklijke Uitgevers: Amsterdam, The Netherlands, 2006; Volume 1, ISBN 92-1-148214-3. [Google Scholar]
- Becker, J.B.; Hu, M. Sex Differences in Drug Abuse. Front. Neuroendocrinol. 2008, 29, 36–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, B.; Han, S.; Kranzler, H.R.; Palmer, A.A.; Gelernter, J. Sex-specific Linkage Scans in Opioid Dependence. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2017, 174, 261–268. [Google Scholar] [CrossRef] [Green Version]
- Townsend, E.A.; Negus, S.S.; Caine, S.B.; Thomsen, M.; Banks, M.L. Sex Differences in Opioid Reinforcement under a Fentanyl vs. Food Choice Procedure in Rats. Neuropsychopharmacology 2019, 44, 2022–2029. [Google Scholar] [CrossRef]
- Cicero, T.J.; Aylward, S.C.; Meyer, E.R. Gender Differences in the Intravenous Self-Administration of Mu Opiate Agonists. Pharmacol. Biochem. Behav. 2003, 74, 541–549. [Google Scholar] [CrossRef]
- Taylor, A.M.; Castonguay, A.; Ghogha, A.; Vayssiere, P.; Pradhan, A.A.; Xue, L.; Mehrabani, S.; Wu, J.; Levitt, P.; Olmstead, M.C. Neuroimmune Regulation of GABAergic Neurons within the Ventral Tegmental Area during Withdrawal from Chronic Morphine. Neuropsychopharmacology 2016, 41, 949–959. [Google Scholar] [CrossRef] [Green Version]
- Gellner, C.A.; Belluzzi, J.D.; Leslie, F.M. Self-Administration of Nicotine and Cigarette Smoke Extract in Adolescent and Adult Rats. Neuropharmacology 2016, 109, 247–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roberts, D.; Loh, E.A.; Vickers, G. Self-Administration of Cocaine on a Progressive Ratio Schedule in Rats: Dose-Response Relationship and Effect of Haloperidol Pretreatment. Psychopharmacology 1989, 97, 535–538. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Huntley, J.; Fierer, N.; Owens, S.M.; Betley, J.; Fraser, L.; Bauer, M.; et al. Ultra-High-Throughput Microbial Community Analysis on the Illumina HiSeq and MiSeq Platforms. ISME J. 2012, 6, 1621–1624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walters, W.; Hyde, E.R.; Berg-Lyons, D.; Ackermann, G.; Humphrey, G.; Parada, A.; Gilbert, J.A.; Jansson, J.K.; Caporaso, J.G.; Fuhrman, J.A.; et al. Improved Bacterial 16S rRNA Gene (V4 and V4-5) and Fungal Internal Transcribed Spacer Marker Gene Primers for Microbial Community Surveys. MSystems 2016, 1, e00009-15. [Google Scholar] [CrossRef] [PubMed]



| Control | Antibiotics | |
|---|---|---|
| Males | 7 | 10 |
| Females | 7 | 8 |
| Control | Antibiotics | |
|---|---|---|
| Before IVSA | 5 | 8 |
| After IVSA | 4 | 6 |
| Water/Water | Water/SCFA | ABX/Water | ABX/SCFA | |
|---|---|---|---|---|
| Males | 8 | 9 | 12 | 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, M.; Lotfipour, S. Antibiotic Knockdown of Gut Bacteria Sex-Dependently Enhances Intravenous Fentanyl Self-Administration in Adult Sprague Dawley Rats. Int. J. Mol. Sci. 2023, 24, 409. https://doi.org/10.3390/ijms24010409
Ren M, Lotfipour S. Antibiotic Knockdown of Gut Bacteria Sex-Dependently Enhances Intravenous Fentanyl Self-Administration in Adult Sprague Dawley Rats. International Journal of Molecular Sciences. 2023; 24(1):409. https://doi.org/10.3390/ijms24010409
Chicago/Turabian StyleRen, Michelle, and Shahrdad Lotfipour. 2023. "Antibiotic Knockdown of Gut Bacteria Sex-Dependently Enhances Intravenous Fentanyl Self-Administration in Adult Sprague Dawley Rats" International Journal of Molecular Sciences 24, no. 1: 409. https://doi.org/10.3390/ijms24010409
APA StyleRen, M., & Lotfipour, S. (2023). Antibiotic Knockdown of Gut Bacteria Sex-Dependently Enhances Intravenous Fentanyl Self-Administration in Adult Sprague Dawley Rats. International Journal of Molecular Sciences, 24(1), 409. https://doi.org/10.3390/ijms24010409






