VANL-100 Attenuates Beta-Amyloid-Induced Toxicity in SH-SY5Y Cells
Abstract
1. Introduction
2. Results
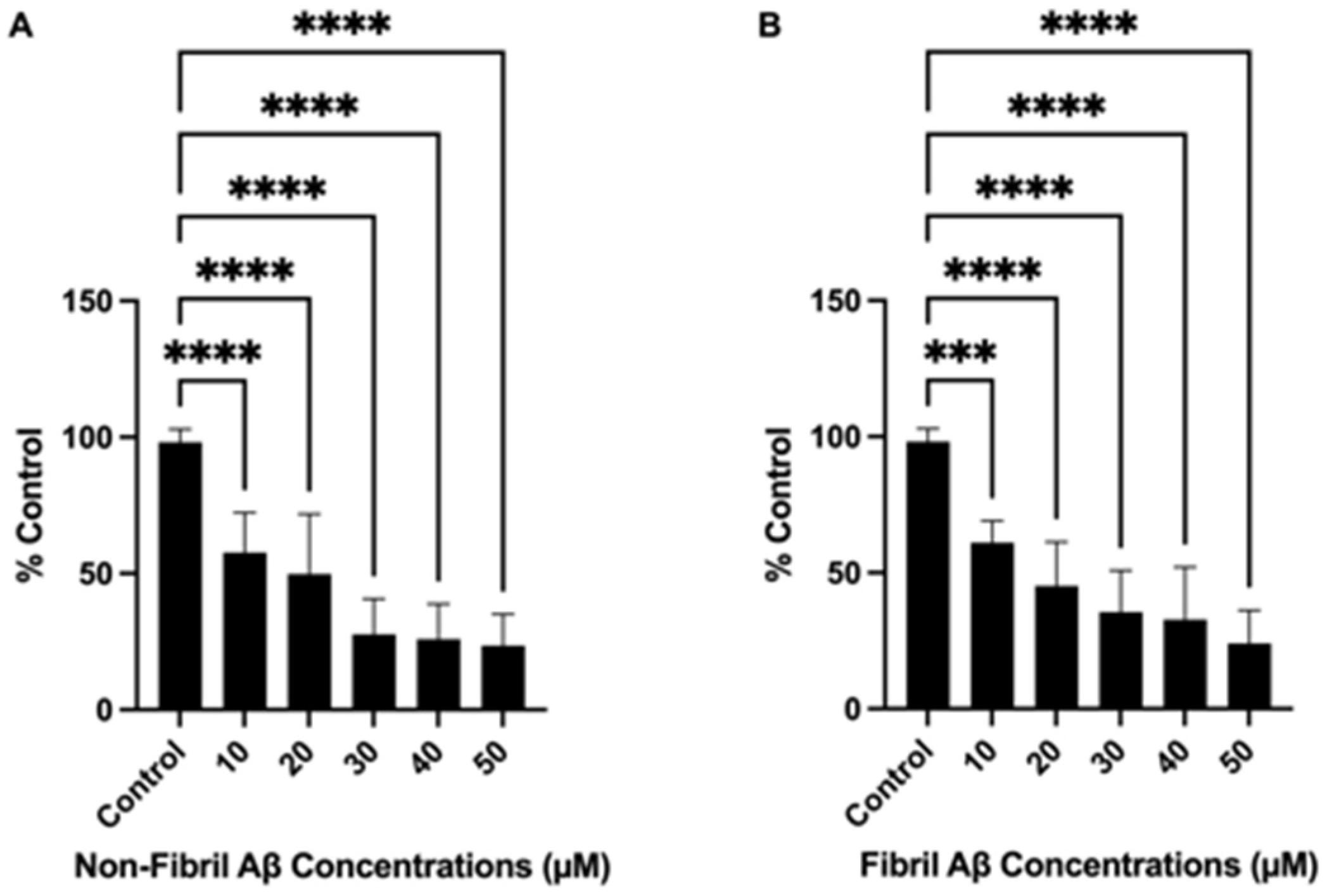
2.1. Non-Fibril and Fibril Aβ25–35 Induced Dose-Dependent Cell Death
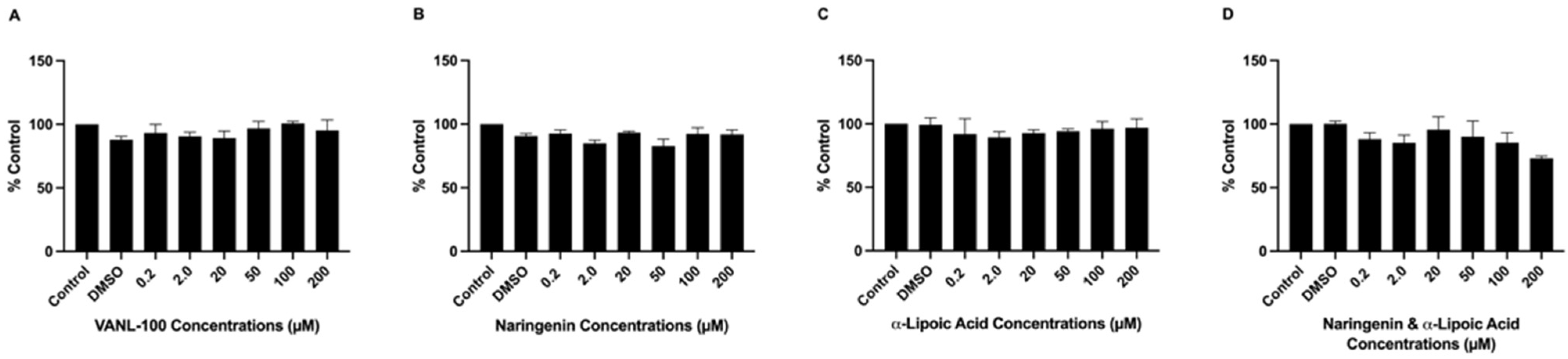
2.2. Antioxidant Compounds Did Not Induce Cell Loss or Reduce Cell Viability
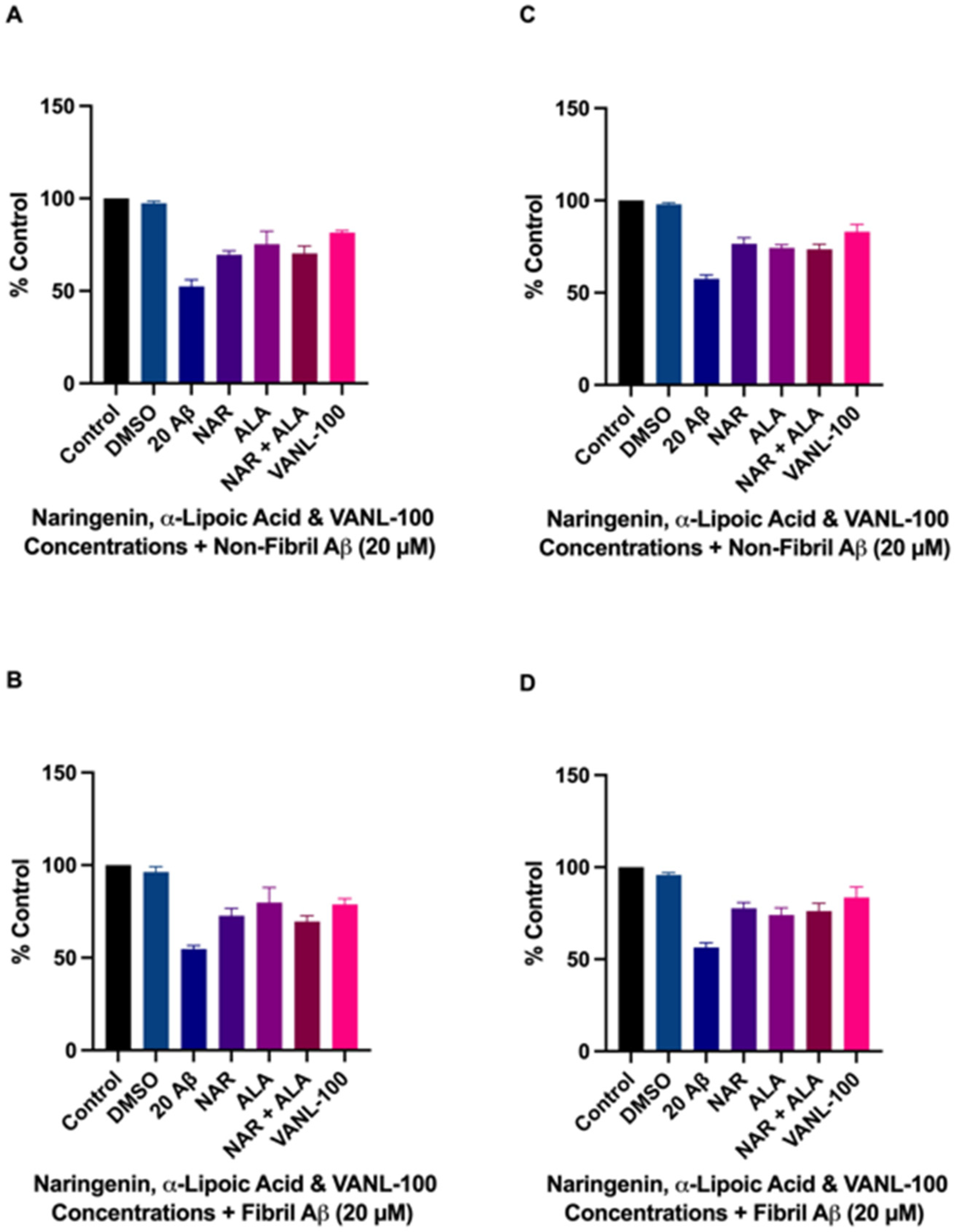
2.3. Effect of Antioxidant Compounds on Aβ-Induced Cell Death
2.4. Cytoprotective Effects of VANL-100 Are Not Significantly Different to Parent Compounds
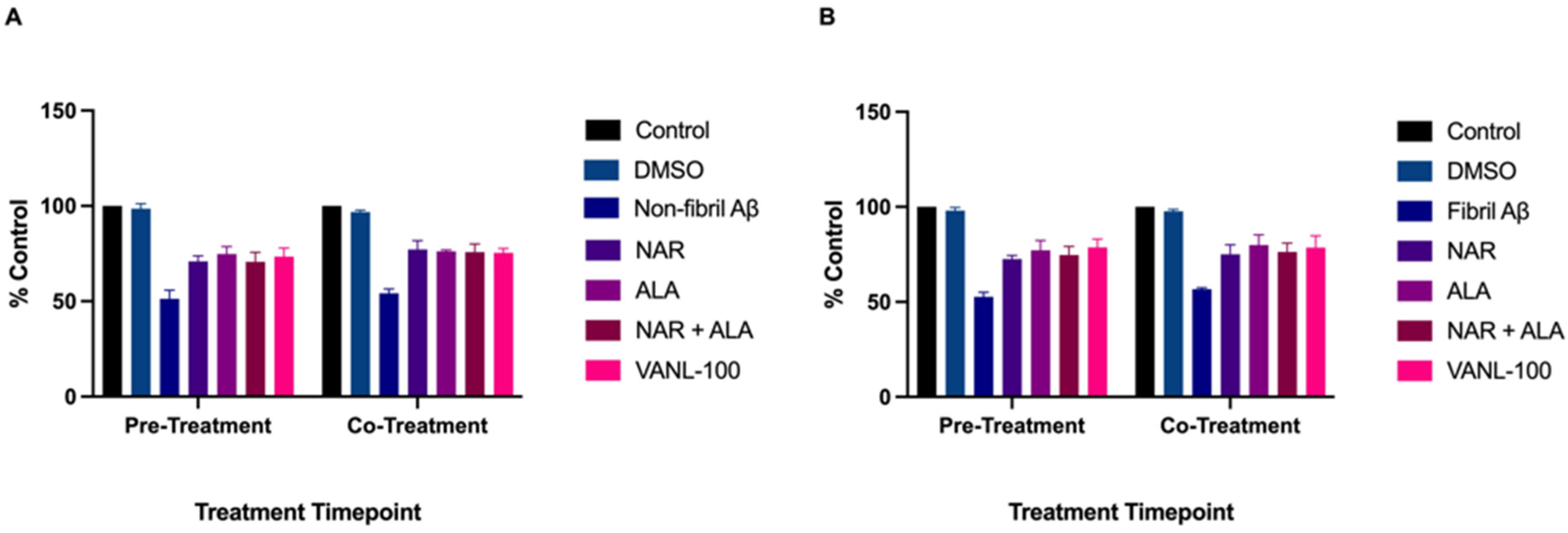
2.5. Effect of Treatment Timepoint on Cell Survival
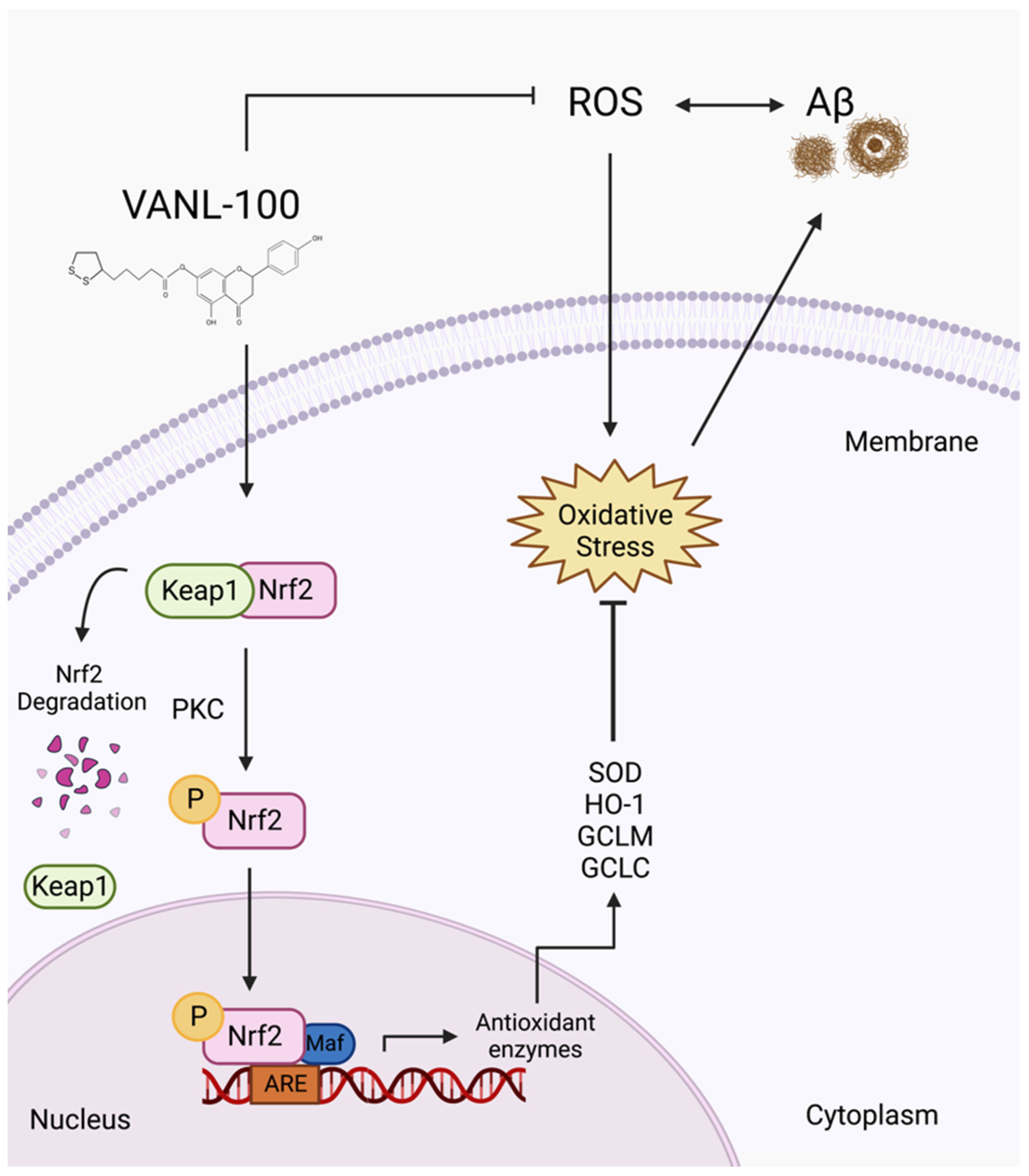
2.6. Effect of VANL-100 on ROS Levels in SH-SY5Y Cells
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture
4.3. Antioxidant Treatments
4.4. Cell Viability and Toxicity Assay
4.5. Reactive Oxygen Species Determination
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Alzheimer Report 2022—Life after Diagnosis: Navigating Treatment, Care and Support. 416. Available online: https://www.alzint.org/u/World-Alzheimer-Report-2022.pdf (accessed on 12 October 2022).
- Mayeux, R.; Stern, Y. Epidemiology of Alzheimer Disease. Cold Spring Harb. Perspect. Med. 2012, 2, a006239. [Google Scholar] [CrossRef] [PubMed]
- Scheltens, P.; Strooper, B.D.; Kivipelto, M.; Holstege, H.; Chételat, G.; Teunissen, C.E.; Cummings, J.; van der Flier, W.M. Alzheimer’s Disease. Lancet 2021, 397, 1577–1590. [Google Scholar] [CrossRef] [PubMed]
- Reitz, C.; Mayeux, R. Alzheimer Disease: Epidemiology, Diagnostic Criteria, Risk Factors and Biomarkers. Biochem. Pharmacol. 2014, 88, 640–651. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Gómez, O.; Palacio-Lacambra, M.E.; Palasí, A.; Ruiz-Laza, A.; Boada-Rovira, M. Prevention of Alzheimer’s Disease: A Global Challenge for next Generation Neuroscientists. J. Alzheimers Dis. 2014, 42 (Suppl. 4), S515–S523. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Ahmad, R.; Khare, S.K. Alzheimer’s Disease and Its Treatment by Different Approaches: A Review. Eur. J. Med. Chem. 2021, 216, 113320. [Google Scholar] [CrossRef]
- Breijyeh, Z.; Karaman, R. Comprehensive Review on Alzheimer’s Disease: Causes and Treatment. Molecules 2020, 25, 5789. [Google Scholar] [CrossRef]
- Yiannopoulou, K.G.; Anastasiou, A.I.; Zachariou, V.; Pelidou, S.-H. Reasons for Failed Trials of Disease-Modifying Treatments for Alzheimer Disease and Their Contribution in Recent Research. Biomedicines 2019, 7, 97. [Google Scholar] [CrossRef]
- Se Thoe, E.; Fauzi, A.; Tang, Y.Q.; Chamyuang, S.; Chia, A.Y.Y. A Review on Advances of Treatment Modalities for Alzheimer’s Disease. Life Sci. 2021, 276, 119129. [Google Scholar] [CrossRef]
- National Institute on Aging (NIA). Evaluation of the Safety, Tolerability and Impact on Biomarkers of Anti-Oxidant Treatment of Mild to Moderate Alzheimer’s Disease; National Institute on Aging: Bethesda, MD, USA, 2009.
- Goktas, Z. The Effect of Black Mulberry (Morus Nigra) Consumption on Cognitive Functions and Antioxidant Capacity in Individuals Diagnosed with Dementia. 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT05406648 (accessed on 12 October 2022).
- Park, S.-Y. Effects of Mitochondrial-Targeted Antioxidant on Carotid Artery Endothelial Function and Brain Blood Flow in Mild Cognitive Impairment (MCI) Patients. 2022. Available online: https://www.clinicaltrials.gov/ct2/show/NCT03514875 (accessed on 12 October 2022).
- Shinto, L. Fish Oil and Alpha Lipoic Acid in Mild Alzheimer’s Disease. 2017. Available online: https://www.clinicaltrials.gov/ct2/show/NCT00090402 (accessed on 12 October 2022).
- US Department of Veterans Affairs. A Single Center, Multi-Site, Randomized, Double-Blind, Placebo-Controlled Trial of Resveratrol With Glucose and Malate (RGM) to Slow the Progression of Alzheimer’s Disease; U.S. Department of Veterans Affairs: Washington, DC, USA, 2012.
- Oregon Health and Science University. Lutein and Oxidative Stress in Alzheimer’s Disease—A Pilot Study; Oregon Health & Science University: Portland, OR, USA, 2019. [Google Scholar]
- National Institute on Aging (NIA). A Randomized, Double-Blind, Placebo-Controlled Trial of Vitamin E and Donepezil HCL (Aricept) to Delay Clinical Progression from Mild Cognitive Impairment (MCI) to Alzheimer’s Disease (AD); National Institute on Aging: Bethesda, MD, USA, 2009.
- Paul, F. Sunphenon EGCg (Epigallocatechin-Gallate) in the Early Stage of Alzheimer’s Disease. 2021. Available online: https://clinicaltrials.gov/ct2/show/NCT00951834 (accessed on 12 October 2022).
- Vina, J. Effect of Activation of the Receptor PPARg/RXR as a Possible Treatment for Alzheimer’s Disease. Role of Genistein. 2021. Available online: https://clinicaltrials.gov/ct2/show/NCT01982578 (accessed on 12 October 2022).
- Taipei City Psychiatric Center, Taiwan. The Effects of Omega-3 Fatty Acids Monotherapy in Alzheimer’s Disease and Mild Cognitive Impairment: A Preliminary Randomized Double-Blind Placebo-Controlled Study; Taipei City Psychiatric Center: Taipei, Taiwan, 2008.
- John Douglas French Foundation. A Phase II, Double-Blind, Placebo-Controlled Study of the Safety and Tolerability of Two Doses of Curcumin C3 Complex Versus Placebo in Patients with Mild to Moderate Alzheimer’s Disease; John Douglas French Foundation: Pasadena, CA, USA, 2009. [Google Scholar]
- Alzheimer’s Disease Cooperative Study (ADCS). Phase II Study to Evaluate the Impact on Biomarkers of Resveratrol Treatment in Patients with Mild to Moderate Alzheimer’s Disease. 2016. Available online: https://scholars.uky.edu/en/projects/phase-ii-study-to-evaluate-the-impact-on-biomarkers-of-resveratro-3 (accessed on 12 October 2022).
- Schmitt, F. Prevention of Alzheimer’s Disease by Vitamin E and Selenium (PREADVISE). 2018. Available online: https://clinicaltrials.gov/ct2/show/NCT00040378 (accessed on 12 October 2022).
- Medical College of Wisconsin. Randomized, Placebo-Controlled Clinical Trial of Resveratrol Supplement Effects on Cognition, Function and Behavior in Patients with Mild-to-Moderate Alzheimer’s Disease; Medical College of Wisconsin: Milwaukee, WI, USA, 2015. [Google Scholar]
- Shinto, L. Lipoic Acid and Omega-3 Fatty Acids in Alzheimer’s Disease. 2017. Available online: https://www.clinicaltrials.gov/ct2/show/NCT01058941 (accessed on 12 October 2022).
- The University of Texas Health Science Center at San Antonio. Pilot Study to Investigate the Safety and Feasibility of Senolytic Therapy to Modulate Progression of Alzheimer’s Disease (SToMP-AD). 2022. Available online: https://www.clinicalconnection.com/clinical-trials-from-other-databases/full-listing-from-other-databases/508404/58195737/pilot-study-to-investigate-the-safety-and-feasibility-of-senolytic-therapy-to-modulate-progression-of-alzheimer-s-disease-stomp-ad (accessed on 12 October 2022).
- US Department of Veterans Affairs. CSP #546—A Randomized, Clinical Trial of Vitamin E and Memantine in Alzheimer’s Disease (TEAM-AD). 2014. Available online: https://www.clinicaltrials.gov/ct2/show/NCT00235716 (accessed on 12 October 2022).
- Life Extension Foundation Inc. Open Label, Crossover, Pilot Study to Assess the Efficacy & Safety of Perispinal Admin.of Etanercept(Enbrel®) in Comb.w/Nutritional Supplements vs. Nutritional Supplements Alone in Subj. w/Mild to Mod. Alzheimer’s Disease Receiving Std. Care. 2016. Available online: https://faseb.onlinelibrary.wiley.com/doi/abs/10.1096/fasebj.30.1_supplement.lb296 (accessed on 12 October 2022).
- Wake Forest University Health Sciences. Phase II Clinical Trial to Evaluate the Safety and Feasibility of Senolytic Therapy in Alzheimer’s Disease. 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT04685590 (accessed on 12 October 2022).
- Kirkland, J.L. ALSENLITE: An Open-Label Pilot Study of Senolytics for Alzheimer’s Disease. 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT04785300 (accessed on 12 October 2022).
- Lipsitz, L. Senolytics to Alleviate Mobility Issues and Neurological Impairment in Aging. 2022. Available online: https://www.marcusinstituteforaging.org/current-studies/senolytics-alleviate-mobility-issues-and-neurological-impairment-aging-stamina-study (accessed on 12 October 2022).
- de la Torre, R. Prevention of Cognitive Decline in ApoE4 Carriers with Subjective Cognitive Decline After EGCG and a Multimodal Intervention. 2020. Available online: https://clinicaltrials.gov/ct2/show/NCT03978052 (accessed on 12 October 2022).
- Shinto, L. Pilot Study: Lipoic Acid and Omega-3 Fatty Acid for Alzheimer’s Disease Prevention. 2017. Available online: https://clinicaltrials.gov/ct2/show/NCT01780974 (accessed on 12 October 2022).
- Markesbery, W.R. Oxidative Stress Hypothesis in Alzheimer’s Disease. Free Radic. Biol. Med. 1997, 23, 134–147. [Google Scholar] [CrossRef]
- Rosini, M.; Simoni, E.; Milelli, A.; Minarini, A.; Melchiorre, C. Oxidative Stress in Alzheimer’s Disease: Are We Connecting the Dots? J. Med. Chem. 2014, 57, 2821–2831. [Google Scholar] [CrossRef]
- Ionescu-Tucker, A.; Cotman, C.W. Emerging Roles of Oxidative Stress in Brain Aging and Alzheimer’s Disease. Neurobiol Aging 2021, 107, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, W.; Li, L.; Perry, G.; Lee, H.; Zhu, X. Oxidative Stress and Mitochondrial Dysfunction in Alzheimer’s Disease. Biochim. Biophys. Acta 2014, 1842, 1240–1247. [Google Scholar] [CrossRef] [PubMed]
- Cioffi, F.; Adam, R.H.I.; Broersen, K. Molecular Mechanisms and Genetics of Oxidative Stress in Alzheimer’s Disease. J. Alzheimers Dis. 2019, 72, 981–1017. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhong, C. Oxidative Stress in Alzheimer’s Disease. Neurosci. Bull. 2014, 30, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative Stress: A Key Modulator in Neurodegenerative Diseases. Molecules 2019, 24, 1583. [Google Scholar] [CrossRef] [PubMed]
- Cenini, G.; Lloret, A.; Cascella, R. Oxidative Stress in Neurodegenerative Diseases: From a Mitochondrial Point of View. Oxid. Med. Cell. Longev. 2019, 2019, 2105607. [Google Scholar] [CrossRef]
- Teleanu, D.M.; Niculescu, A.-G.; Lungu, I.I.; Radu, C.I.; Vladâcenco, O.; Roza, E.; Costăchescu, B.; Grumezescu, A.M.; Teleanu, R.I. An Overview of Oxidative Stress, Neuroinflammation, and Neurodegenerative Diseases. Int. J. Mol. Sci. 2022, 23, 5938. [Google Scholar] [CrossRef]
- Cheignon, C.; Tomas, M.; Bonnefont-Rousselot, D.; Faller, P.; Hureau, C.; Collin, F. Oxidative Stress and the Amyloid Beta Peptide in Alzheimer’s Disease. Redox Biol. 2017, 14, 450–464. [Google Scholar] [CrossRef]
- Hensley, K.; Butterfieldld, D.A.; Hall, N.; Cole, P.; Subramaniam, R.; Mark, R.; Mattson, M.P.; Markesbery, W.R.; Harris, M.E.; Aksenov, M.; et al. Reactive Oxygen Species as Causal Agents in the Neurotoxicity of the Alzheimer’s Disease-Associated Amyloid Beta Peptidea. Ann. N. Y. Acad. Sci. 1996, 786, 120–134. [Google Scholar] [CrossRef]
- Giovanna, C.; Cecchi, C.; Pensalfini, A.; Bonini, S.A.; Ferrari-Toninelli, G.; Liguri, G.; Memo, M.; Uberti, D. Generation of Reactive Oxygen Species by Beta Amyloid Fibrils and Oligomers Involves Different Intra/Extracellular Pathways. Amino Acids 2010, 38, 1101–1106. [Google Scholar] [CrossRef]
- Tamagno, E.; Guglielmotto, M.; Vasciaveo, V.; Tabaton, M. Oxidative Stress and Beta Amyloid in Alzheimer’s Disease. Which Comes First: The Chicken or the Egg? Antioxidants 2021, 10, 1479. [Google Scholar] [CrossRef] [PubMed]
- Guglielmotto, M.; Giliberto, L.; Tamagno, E.; Tabaton, M. Oxidative Stress Mediates the Pathogenic Effect of Different Alzheimer’s Disease Risk Factors. Front. Aging Neurosci. 2010, 2, 3. [Google Scholar] [CrossRef] [PubMed]
- Butterfield, D.A.; Swomley, A.M.; Sultana, R. Amyloid β-Peptide (1–42)-Induced Oxidative Stress in Alzheimer Disease: Importance in Disease Pathogenesis and Progression. Antioxid. Redox Signal. 2013, 19, 823–835. [Google Scholar] [CrossRef]
- Cai, Z.; Zhao, B.; Ratka, A. Oxidative Stress and β-Amyloid Protein in Alzheimer’s Disease. Neuromol. Med. 2011, 13, 223–250. [Google Scholar] [CrossRef] [PubMed]
- Nouri, Z.; Fakhri, S.; El-Senduny, F.F.; Sanadgol, N.; Abd-ElGhani, G.E.; Farzaei, M.H.; Chen, J.-T. On the Neuroprotective Effects of Naringenin: Pharmacological Targets, Signaling Pathways, Molecular Mechanisms, and Clinical Perspective. Biomolecules 2019, 9, 690. [Google Scholar] [CrossRef] [PubMed]
- Goyal, A.; Verma, A.; Dubey, N.; Raghav, J.; Agrawal, A. Naringenin: A Prospective Therapeutic Agent for Alzheimer’s and Parkinson’s Disease. J. Food Biochem. 2022; e14415, Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Heo, H.J.; Kim, D.-O.; Shin, S.C.; Kim, M.J.; Kim, B.G.; Shin, D.-H. Effect of Antioxidant Flavanone, Naringenin, from Citrus junos on Neuroprotection. J. Agric. Food Chem. 2004, 52, 1520–1525. [Google Scholar] [CrossRef]
- Molz, P.; Schröder, N. Potential Therapeutic Effects of Lipoic Acid on Memory Deficits Related to Aging and Neurodegeneration. Front. Pharmacol. 2017, 8, 849. [Google Scholar] [CrossRef]
- Pei, X.; Hu, F.; Luo, F.; Huang, X.; Li, X.; Xing, S.; Long, D. The Neuroprotective Effects of Alpha-Lipoic Acid on an Experimental Model of Alzheimer’s Disease in PC12 Cells. J. Appl. Toxicol. 2022, 42, 285–294. [Google Scholar] [CrossRef]
- Kaur, D.; Behl, T.; Sehgal, A.; Singh, S.; Sharma, N.; Chigurupati, S.; Alhowail, A.; Abdeen, A.; Ibrahim, S.F.; Vargas-De-La-Cruz, C.; et al. Decrypting the Potential Role of α-Lipoic Acid in Alzheimer’s Disease. Life Sci. 2021, 284, 119899. [Google Scholar] [CrossRef]
- Wu, J.; Kou, X.; Ju, H.; Zhang, H.; Yang, A.; Shen, R. Design, Synthesis and Biological Evaluation of Naringenin Carbamate Derivatives as Potential Multifunctional Agents for the Treatment of Alzheimer’s Disease. Bioorg. Med. Chem. Lett. 2021, 49, 128316. [Google Scholar] [CrossRef] [PubMed]
- Ghofrani, S.; Joghataei, M.-T.; Mohseni, S.; Baluchnejadmojarad, T.; Bagheri, M.; Khamse, S.; Roghani, M. Naringenin Improves Learning and Memory in an Alzheimer’s Disease Rat Model: Insights into the Underlying Mechanisms. Eur. J. Pharmacol. 2015, 764, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Lawal, M.; Olotu, F.A.; Soliman, M.E.S. Across the Blood-Brain Barrier: Neurotherapeutic Screening and Characterization of Naringenin as a Novel CRMP-2 Inhibitor in the Treatment of Alzheimer’s Disease Using Bioinformatics and Computational Tools. Comput. Biol. Med. 2018, 98, 168–177. [Google Scholar] [CrossRef]
- Zhou, T.; Liu, L.; Wang, Q.; Gao, Y. Naringenin Alleviates Cognition Deficits in High-Fat Diet-Fed SAMP8 Mice. J. Food Biochem. 2020, 44, e13375. [Google Scholar] [CrossRef] [PubMed]
- Sang, Z.; Wang, K.; Shi, J.; Liu, W.; Cheng, X.; Zhu, G.; Wang, Y.; Zhao, Y.; Qiao, Z.; Wu, A.; et al. The Development of Advanced Structural Framework as Multi-Target-Directed Ligands for the Treatment of Alzheimer’s Disease. Eur. J. Med. Chem. 2020, 192, 112180. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Yang, W.-Q.; Zha, H.; Yu, H.-R. Effect of naringenin on learning and memory ability on model rats with Alzheimer disease. Zhong Yao Cai 2013, 36, 271–276. [Google Scholar]
- Yang, Z.; Kuboyama, T.; Tohda, C. Naringenin Promotes Microglial M2 Polarization and Aβ Degradation Enzyme Expression. Phytother. Res. 2019, 33, 1114–1121. [Google Scholar] [CrossRef] [PubMed]
- Ahsan, A.U.; Sharma, V.L.; Wani, A.; Chopra, M. Naringenin Upregulates AMPK-Mediated Autophagy to Rescue Neuronal Cells From β-Amyloid (1–42) Evoked Neurotoxicity. Mol. Neurobiol. 2020, 57, 3589–3602. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Hu, Z.; Zhang, Z.; Liu, G.; Wang, Y.; Ren, Y.; Wu, X.; Geng, F. Protective Role of Naringenin Against Aβ25–35-Caused Damage via ER and PI3K/Akt-Mediated Pathways. Cell Mol. Neurobiol. 2018, 38, 549–557. [Google Scholar] [CrossRef]
- Md, S.; Gan, S.Y.; Haw, Y.H.; Ho, C.L.; Wong, S.; Choudhury, H. In Vitro Neuroprotective Effects of Naringenin Nanoemulsion against β-Amyloid Toxicity through the Regulation of Amyloidogenesis and Tau Phosphorylation. Int. J. Biol. Macromol. 2018, 118, 1211–1219. [Google Scholar] [CrossRef]
- Shinto, L.; Quinn, J.; Montine, T.; Dodge, H.H.; Woodward, W.; Baldauf-Wagner, S.; Waichunas, D.; Bumgarner, L.; Bourdette, D.; Silbert, L.; et al. A Randomized Placebo-Controlled Pilot Trial of Omega-3 Fatty Acids and Alpha Lipoic Acid in Alzheimer’s Disease. J. Alzheimers Dis. 2014, 38, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-H.; Wang, D.-W.; Xu, S.-F.; Zhang, S.; Fan, Y.-G.; Yang, Y.-Y.; Guo, S.-Q.; Wang, S.; Guo, T.; Wang, Z.-Y.; et al. α-Lipoic Acid Improves Abnormal Behavior by Mitigation of Oxidative Stress, Inflammation, Ferroptosis, and Tauopathy in P301S Tau Transgenic Mice. Redox Biol. 2018, 14, 535–548. [Google Scholar] [CrossRef] [PubMed]
- de Sousa, C.N.S.; da Silva Leite, C.M.G.; da Silva Medeiros, I.; Vasconcelos, L.C.; Cabral, L.M.; Patrocínio, C.F.V.; Patrocínio, M.L.V.; Mouaffak, F.; Kebir, O.; Macedo, D.; et al. Alpha-Lipoic Acid in the Treatment of Psychiatric and Neurological Disorders: A Systematic Review. Metab. Brain Dis. 2019, 34, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Metsla, K.; Kirss, S.; Laks, K.; Sildnik, G.; Palgi, M.; Palumaa, T.; Tõugu, V.; Palumaa, P. α-Lipoic Acid Has the Potential to Normalize Copper Metabolism, Which Is Dysregulated in Alzheimer’s Disease. J. Alzheimers Dis. 2022, 85, 715–728. [Google Scholar] [CrossRef] [PubMed]
- Zarini-Gakiye, E.; Vaezi, G.; Parivar, K.; Sanadgol, N. Age and Dose-Dependent Effects of Alpha-Lipoic Acid on Human Microtubule- Associated Protein Tau-Induced Endoplasmic Reticulum Unfolded Protein Response: Implications for Alzheimer’s Disease. CNS Neurol. Disord. Drug Targets 2021, 20, 451–464. [Google Scholar] [CrossRef]
- Fava, A.; Pirritano, D.; Plastino, M.; Cristiano, D.; Puccio, G.; Colica, C.; Ermio, C.; De Bartolo, M.; Mauro, G.; Bosco, D. The Effect of Lipoic Acid Therapy on Cognitive Functioning in Patients with Alzheimer’s Disease. J. Neurodegener. Dis. 2013, 2013, 454253. [Google Scholar] [CrossRef]
- Memudu, A.E.; Adewumi, A.E. Alpha Lipoic Acid Ameliorates Scopolamine Induced Memory Deficit and Neurodegeneration in the Cerebello-Hippocampal Cortex. Metab. Brain Dis. 2021, 36, 1729–1745. [Google Scholar] [CrossRef]
- Sancheti, H.; Akopian, G.; Yin, F.; Brinton, R.D.; Walsh, J.P.; Cadenas, E. Age-Dependent Modulation of Synaptic Plasticity and Insulin Mimetic Effect of Lipoic Acid on a Mouse Model of Alzheimer’s Disease. PLoS ONE 2013, 8, e69830. [Google Scholar] [CrossRef]
- Sancheti, H.; Kanamori, K.; Patil, I.; Díaz Brinton, R.; Ross, B.D.; Cadenas, E. Reversal of Metabolic Deficits by Lipoic Acid in a Triple Transgenic Mouse Model of Alzheimer’s Disease: A 13C NMR Study. J. Cereb. Blood Flow Metab. 2014, 34, 288–296. [Google Scholar] [CrossRef]
- Erdoğan, M.E.; Aydın, S.; Yanar, K.; Mengi, M.; Kansu, A.D.; Cebe, T.; Belce, A.; Çelikten, M.; Çakatay, U. The Effects of Lipoic Acid on Redox Status in Brain Regions and Systemic Circulation in Streptozotocin-Induced Sporadic Alzheimer’s Disease Model. Metab. Brain Dis. 2017, 32, 1017–1031. [Google Scholar] [CrossRef]
- Park, E.; Gim, J.; Kim, D.K.; Kim, C.-S.; Chun, H.S. Protective Effects of Alpha-Lipoic Acid on Glutamate-Induced Cytotoxicity in C6 Glioma Cells. Biol. Pharm. Bull. 2019, 42, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Dieter, F.; Esselun, C.; Eckert, G.P. Redox Active α-Lipoic Acid Differentially Improves Mitochondrial Dysfunction in a Cellular Model of Alzheimer and Its Control Cells. Int. J. Mol. Sci. 2022, 23, 9186. [Google Scholar] [CrossRef]
- Kamarudin, M.N.A.; Mohd Raflee, N.A.; Hussein, S.S.S.; Lo, J.Y.; Supriady, H.; Abdul Kadir, H. (R)-(+)-α-Lipoic Acid Protected NG108-15 Cells against H₂O₂-Induced Cell Death through PI3K-Akt/GSK-3β Pathway and Suppression of NF-Κβ-Cytokines. Drug Des. Devel. Ther. 2014, 8, 1765–1780. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Staykov, H.; Lazarova, M.; Hassanova, Y.; Stefanova, M.; Tancheva, L.; Nikolov, R. Neuromodulatory Mechanisms of a Memory Loss-Preventive Effect of Alpha-Lipoic Acid in an Experimental Rat Model of Dementia. J. Mol. Neurosci. 2022, 72, 1018–1025. [Google Scholar] [CrossRef] [PubMed]
- Koriyama, Y.; Nakayama, Y.; Matsugo, S.; Kato, S. Protective Effect of Lipoic Acid against Oxidative Stress Is Mediated by Keap1/Nrf2-Dependent Heme Oxygenase-1 Induction in the RGC-5 Cellline. Brain Res. 2013, 1499, 145–157. [Google Scholar] [CrossRef]
- Saleh, T.M.; Saleh, M.C.; Connell, B.J.; Song, Y.-H. A Co-Drug Conjugate of Naringenin and Lipoic Acid Mediates Neuroprotection in a Rat Model of Oxidative Stress. Clin. Exp. Pharmacol. Physiol. 2017, 44, 1008–1016. [Google Scholar] [CrossRef]
- Vander Zanden, C.M.; Wampler, L.; Bowers, I.; Watkins, E.B.; Majewski, J.; Chi, E.Y. Fibrillar and Nonfibrillar Amyloid Beta Structures Drive Two Modes of Membrane-Mediated Toxicity. Langmuir 2019, 35, 16024–16036. [Google Scholar] [CrossRef]
- Kawai, R.; Chiba, S.; Okuwaki, K.; Kanada, R.; Doi, H.; Ono, M.; Mochizuki, Y.; Okuno, Y. Stabilization Mechanism for a Nonfibrillar Amyloid β Oligomer Based on Formation of a Hydrophobic Core Determined by Dissipative Particle Dynamics. ACS Chem. Neurosci. 2020, 11, 385–394. [Google Scholar] [CrossRef]
- Kandel, N.; Matos, J.O.; Tatulian, S.A. Structure of Amyloid Β25–35 in Lipid Environment and Cholesterol-Dependent Membrane Pore Formation. Sci. Rep. 2019, 9, 2689. [Google Scholar] [CrossRef]
- Naldi, M.; Fiori, J.; Pistolozzi, M.; Drake, A.F.; Bertucci, C.; Wu, R.; Mlynarczyk, K.; Filipek, S.; De Simone, A.; Andrisano, V. Amyloid β-Peptide 25–35 Self-Assembly and Its Inhibition: A Model Undecapeptide System to Gain Atomistic and Secondary Structure Details of the Alzheimer’s Disease Process and Treatment. ACS Chem. Neurosci. 2012, 3, 952–962. [Google Scholar] [CrossRef]
- Clementi, M.E.; Marini, S.; Coletta, M.; Orsini, F.; Giardina, B.; Misiti, F. Aβ(31–35) and Aβ(25–35) Fragments of Amyloid Beta-Protein Induce Cellular Death through Apoptotic Signals: Role of the Redox State of Methionine-35. FEBS Lett. 2005, 579, 2913–2918. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.Y.; Jannat, S.; Edraki, N.; Das, S.; Chang, W.K.; Kim, H.C.; Park, S.K.; Chang, M.S. Flavanone Glycosides Inhibit β-Site Amyloid Precursor Protein Cleaving Enzyme 1 and Cholinesterase and Reduce Aβ Aggregation in the Amyloidogenic Pathway. Chem. Biol. Interact. 2019, 309, 108707. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, B.; Dhiman, M.; Mittal, S.; Mantha, A.K. Curcumin Revitalizes Amyloid Beta (25–35)-Induced and Organophosphate Pesticides Pestered Neurotoxicity in SH-SY5Y and IMR-32 Cells via Activation of APE1 and Nrf2. Metab. Brain Dis. 2017, 32, 2045–2061. [Google Scholar] [CrossRef] [PubMed]
- Zhi, Z.; Tang, X.; Wang, Y.; Chen, R.; Ji, H. Sinensetin Attenuates Amyloid Beta25-35-Induced Oxidative Stress, Inflammation, and Apoptosis in SH-SY5Y Cells Through the TLR4/NF-ΚB Signaling Pathway. Neurochem. Res. 2021, 46, 3012–3024. [Google Scholar] [CrossRef]
- Liu, X.-Y.; Zhang, L.-J.; Chen, Z.; Liu, L.-B. The PTEN Inhibitor BpV(Pic) Promotes Neuroprotection against Amyloid β-Peptide (25–35)-Induced Oxidative Stress and Neurotoxicity. Neurol. Res. 2017, 39, 758–765. [Google Scholar] [CrossRef]
- Zheng, X.; Xie, Z.; Zhu, Z.; Liu, Z.; Wang, Y.; Wei, L.; Yang, H.; Yang, H.; Liu, Y.; Bi, J. Methyllycaconitine Alleviates Amyloid-β Peptides-Induced Cytotoxicity in SH-SY5Y Cells. PLoS ONE 2014, 9, e111536. [Google Scholar] [CrossRef]
- Gao, L.; Li, X.; Meng, S.; Ma, T.; Wan, L.; Xu, S. Chlorogenic Acid Alleviates Aβ25-35-Induced Autophagy and Cognitive Impairment via the MTOR/TFEB Signaling Pathway. Drug Des. Devel. Ther. 2020, 14, 1705–1716. [Google Scholar] [CrossRef]
- Adewusi, E.A.; Fouche, G.; Steenkamp, V. Effect of Four Medicinal Plants on Amyloid-β Induced Neurotoxicity in SH-SY5Y Cells. Afr. J. Tradit. Complement. Altern. Med. 2013, 10, 6–11. [Google Scholar]
- How Is Alzheimer’s Disease Treated? Available online: https://www.nia.nih.gov/health/how-alzheimers-disease-treated (accessed on 18 October 2022).
- Amenta, F.; Parnetti, L.; Gallai, V.; Wallin, A. Treatment of Cognitive Dysfunction Associated with Alzheimer’s Disease with Cholinergic Precursors. Ineffective Treatments or Inappropriate Approaches? Mech. Ageing Dev. 2001, 122, 2025–2040. [Google Scholar] [CrossRef]
- Salomone, S.; Caraci, F.; Leggio, G.M.; Fedotova, J.; Drago, F. New Pharmacological Strategies for Treatment of Alzheimer’s Disease: Focus on Disease Modifying Drugs. Br. J. Clin. Pharmacol. 2012, 73, 504–517. [Google Scholar] [CrossRef]
- Pithadia, A.S.; Lim, M.H. Metal-Associated Amyloid-β Species in Alzheimer’s Disease. Curr. Opin. Chem. Biol. 2012, 16, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Greenough, M.A.; Camakaris, J.; Bush, A.I. Metal Dyshomeostasis and Oxidative Stress in Alzheimer’s Disease. Neurochem. Int. 2013, 62, 540–555. [Google Scholar] [CrossRef] [PubMed]
- Das, N.; Raymick, J.; Sarkar, S. Role of Metals in Alzheimer’s Disease. Metab. Brain Dis. 2021, 36, 1627–1639. [Google Scholar] [CrossRef] [PubMed]
- Sadžak, A.; Mravljak, J.; Maltar-Strmečki, N.; Arsov, Z.; Baranović, G.; Erceg, I.; Kriechbaum, M.; Strasser, V.; Přibyl, J.; Šegota, S. The Structural Integrity of the Model Lipid Membrane during Induced Lipid Peroxidation: The Role of Flavonols in the Inhibition of Lipid Peroxidation. Antioxidants 2020, 9, 430. [Google Scholar] [CrossRef] [PubMed]
- Jeynes, B.; Provias, J. Evidence for Altered LRP/RAGE Expression in Alzheimer Lesion Pathogenesis. Curr. Alzheimer Res. 2008, 5, 432–437. [Google Scholar] [CrossRef]
- Reiss, A.B.; Arain, H.A.; Stecker, M.M.; Siegart, N.M.; Kasselman, L.J. Amyloid Toxicity in Alzheimer’s Disease. Rev. Neurosci. 2018, 29, 613–627. [Google Scholar] [CrossRef]
- Marambaud, P.; Dreses-Werringloer, U.; Vingtdeux, V. Calcium Signaling in Neurodegeneration. Mol. Neurodegener. 2009, 4, 20. [Google Scholar] [CrossRef]
- Fernandez-Perez, E.J.; Peters, C.; Aguayo, L.G. Membrane Damage Induced by Amyloid Beta and a Potential Link with Neuroinflammation. Curr. Pharm. Des. 2016, 22, 1295–1304. [Google Scholar] [CrossRef]
- Kommaddi, R.P.; Das, D.; Karunakaran, S.; Nanguneri, S.; Bapat, D.; Ray, A.; Shaw, E.; Bennett, D.A.; Nair, D.; Ravindranath, V. Aβ Mediates F-Actin Disassembly in Dendritic Spines Leading to Cognitive Deficits in Alzheimer’s Disease. J. Neurosci. 2018, 38, 1085–1099. [Google Scholar] [CrossRef]
- Morishima, Y.; Gotoh, Y.; Zieg, J.; Barrett, T.; Takano, H.; Flavell, R.; Davis, R.J.; Shirasaki, Y.; Greenberg, M.E. β-Amyloid Induces Neuronal Apoptosis Via a Mechanism That Involves the c-Jun N-Terminal Kinase Pathway and the Induction of Fas Ligand. J. Neurosci. 2001, 21, 7551–7560. [Google Scholar] [CrossRef]
- Takada, E.; Okubo, K.; Yano, Y.; Iida, K.; Someda, M.; Hirasawa, A.; Yonehara, S.; Matsuzaki, K. Molecular Mechanism of Apoptosis by Amyloid β-Protein Fibrils Formed on Neuronal Cells. ACS Chem. Neurosci. 2020, 11, 796–805. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.; Nguyen, T.-V.V.; Pike, C.J. β-Amyloid-Induced Neuronal Apoptosis Involves c-Jun N-Terminal Kinase-Dependent Downregulation of Bcl-w. J. Neurosci. 2005, 25, 1149–1158. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Xu, T.; Yan, Y.; Zhou, Y.; Jiang, Y.; Melcher, K.; Xu, H.E. Amyloid Beta: Structure, Biology and Structure-Based Therapeutic Development. Acta Pharmacol. Sin. 2017, 38, 1205–1235. [Google Scholar] [CrossRef] [PubMed]
- Tare, M.; Modi, R.M.; Nainaparampil, J.J.; Puli, O.R.; Bedi, S.; Fernandez-Funez, P.; Kango-Singh, M.; Singh, A. Activation of JNK Signaling Mediates Amyloid-ß-Dependent Cell Death. PLoS ONE 2011, 6, e24361. [Google Scholar] [CrossRef]
- Marín, N.; Romero, B.; Bosch-Morell, F.; Llansola, M.; Felipo, V.; Romá, J.; Romero, F.J. β-Amyloid-Induced Activation of Caspase-3 in Primary Cultures of Rat Neurons. Mech. Ageing Dev. 2000, 119, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, R.; Zarifkar, A.; Rastegar, K.; Maghsoudi, N.; Moosavi, M. Repeated Intra-Hippocampal Injection of Beta-Amyloid 25–35 Induces a Reproducible Impairment of Learning and Memory: Considering Caspase-3 and MAPKs Activity. Eur. J. Pharmacol. 2014, 726, 33–40. [Google Scholar] [CrossRef]
- Zhang, G.; Yao, L.; Du, Y.; Zhang, R.; Bu, N.; Liu, J.; Yuan, H.; Wu, H. [Expression of p38MAPK in the hippocampal CA1 region of rats with Abeta25-35-induced Alzheimer disease]. Nan Fang Yi Ke Da Xue Xue Bao 2008, 28, 1176–1179. [Google Scholar]
- Jin, Y.; Wang, H. Naringenin Inhibit the Hydrogen Peroxide-Induced SH-SY5Y Cells Injury Through Nrf2/HO-1 Pathway. Neurotox Res. 2019, 36, 796–805. [Google Scholar] [CrossRef]
- Zeng, H.; Chen, Q.; Zhao, B. Genistein Ameliorates Beta-Amyloid Peptide (25–35)-Induced Hippocampal Neuronal Apoptosis. Free Radic. Biol. Med. 2004, 36, 180–188. [Google Scholar] [CrossRef]
- Lee, S.Y.; Lee, J.W.; Lee, H.; Yoo, H.S.; Yun, Y.P.; Oh, K.W.; Ha, T.Y.; Hong, J.T. Inhibitory Effect of Green Tea Extract on β-Amyloid-Induced PC12 Cell Death by Inhibition of the Activation of NF-ΚB and ERK/P38 MAP Kinase Pathway through Antioxidant Mechanisms. Mol. Brain Res. 2005, 140, 45–54. [Google Scholar] [CrossRef]
- Martorana, F.; Foti, M.; Virtuoso, A.; Gaglio, D.; Aprea, F.; Latronico, T.; Rossano, R.; Riccio, P.; Papa, M.; Alberghina, L.; et al. Differential Modulation of NF-κB in Neurons and Astrocytes Underlies Neuroprotection and Antigliosis Activity of Natural Antioxidant Molecules. Oxidative Med. Cell Longev. 2019, 2019, e8056904. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Chen, Z.; Huang, L.; Meng, B.; Zhou, X.; Wen, X.; Ren, D. Naringenin Reduces Oxidative Stress and Improves Mitochondrial Dysfunction via Activation of the Nrf2/ARE Signaling Pathway in Neurons. Int. J. Mol. Med. 2017, 40, 1582–1590. [Google Scholar] [CrossRef] [PubMed]
- Lou, H.; Jing, X.; Wei, X.; Shi, H.; Ren, D.; Zhang, X. Naringenin Protects against 6-OHDA-Induced Neurotoxicity via Activation of the Nrf2/ARE Signaling Pathway. Neuropharmacology 2014, 79, 380–388. [Google Scholar] [CrossRef]
- de Oliveira, M.R.; Brasil, F.B.; Andrade, C.M.B. Naringenin Attenuates H2O2-Induced Mitochondrial Dysfunction by an Nrf2-Dependent Mechanism in SH-SY5Y Cells. Neurochem. Res. 2017, 42, 3341–3350. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, M.R.; Andrade, C.M.B.; Fürstenau, C.R. Naringenin Exerts Anti-Inflammatory Effects in Paraquat-Treated SH-SY5Y Cells Through a Mechanism Associated with the Nrf2/HO-1 Axis. Neurochem. Res. 2018, 43, 894–903. [Google Scholar] [CrossRef]
- Wang, K.; Chen, Z.; Huang, J.; Huang, L.; Luo, N.; Liang, X.; Liang, M.; Xie, W. Naringenin Prevents Ischaemic Stroke Damage via Anti-Apoptotic and Anti-Oxidant Effects. Clin. Exp. Pharmacol. Physiol. 2017, 44, 862–871. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, B.; Chen, X.; Zhang, N.; Li, G.; Zhang, L.-H.; Tan, L.-Y. Naringenin Ameliorates Behavioral Dysfunction and Neurological Deficits in a D-Galactose-Induced Aging Mouse Model Through Activation of PI3K/Akt/Nrf2 Pathway. Rejuvenation Res. 2017, 20, 462–472. [Google Scholar] [CrossRef]
- Petersen Shay, K.; Moreau, R.F.; Smith, E.J.; Hagen, T.M. Is α-Lipoic Acid a Scavenger of Reactive Oxygen Species in Vivo? Evidence for Its Initiation of Stress Signaling Pathways That Promote Endogenous Antioxidant Capacity. IUBMB Life 2008, 60, 362–367. [Google Scholar] [CrossRef]
- Lee, J.; Jung, S.-Y.; Yang, K.-J.; Kim, Y.; Lee, D.; Lee, M.H.; Kim, D.-K. α-Lipoic Acid Prevents against Cisplatin Cytotoxicity via Activation of the NRF2/HO-1 Antioxidant Pathway. PLoS ONE 2019, 14, e0226769. [Google Scholar] [CrossRef]
- Liu, L.; Yang, S.; Wang, H. α-Lipoic Acid Alleviates Ferroptosis in the MPP+-Induced PC12 Cells via Activating the PI3K/Akt/Nrf2 Pathway. Cell Biol. Int. 2021, 45, 422–431. [Google Scholar] [CrossRef]
- Xia, D.; Zhai, X.; Wang, H.; Chen, Z.; Fu, C.; Zhu, M. Alpha Lipoic Acid Inhibits Oxidative Stress-induced Apoptosis by Modulating of Nrf2 Signalling Pathway after Traumatic Brain Injury. J. Cell. Mol. Med. 2019, 23, 4088–4096. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Guo, X.; Wang, Z.; Wang, P.; Zhang, Z.; Dong, J.; Zhuang, R.; Zhou, Y.; Ma, G.; Cai, W. Alphalipoic Acid Prevents Oxidative Stress and Peripheral Neuropathy in Nab-Paclitaxel-Treated Rats through the Nrf2 Signalling Pathway. Oxidative Med. Cell Longev. 2019, 2019, 3142732. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhou, X.; Wu, W.; Wang, J.; Xie, H.; Wu, Z. Regeneration of Glutathione by α-Lipoic Acid via Nrf2/ARE Signaling Pathway Alleviates Cadmium-Induced HepG2 Cell Toxicity. Environ. Toxicol. Pharmacol. 2017, 51, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Zgorzynska, E.; Dziedzic, B.; Walczewska, A. An Overview of the Nrf2/ARE Pathway and Its Role in Neurodegenerative Diseases. Int. J. Mol. Sci. 2021, 22, 9592. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q. Role of Nrf2 in Oxidative Stress and Toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Davies, K.J.A.; Forman, H.J. Oxidative Stress Response and Nrf2 Signaling in Aging. Free Radic. Biol. Med. 2015, 88, 314–336. [Google Scholar] [CrossRef]
- Huang, Y.; Li, W.; Su, Z.; Kong, A.-N.T. The Complexity of the Nrf2 Pathway: Beyond the Antioxidant Response. J. Nutr. Biochem. 2015, 26, 1401–1413. [Google Scholar] [CrossRef]
- Wang, H.; Ma, J.; Tan, Y.; Wang, Z.; Sheng, C.; Chen, S.; Ding, J. Amyloid-β 1–42 Induces Reactive Oxygen Species-Mediated Autophagic Cell Death in U87 and SH-SY5Y Cells. J. Alzheimer’s Dis. 2010, 21, 597–610. [Google Scholar] [CrossRef]
- Qu, M.; Zhou, Z.; Xu, S.; Chen, C.; Yu, Z.; Wang, D. Mortalin Overexpression Attenuates Beta-Amyloid-Induced Neurotoxicity in SH-SY5Y Cells. Brain Res. 2011, 1368, 336–345. [Google Scholar] [CrossRef]
- Shi, C.; Zhao, L.; Zhu, B.; Li, Q.; Yew, D.T.; Yao, Z.; Xu, J. Protective Effects of Ginkgo Biloba Extract (EGb761) and Its Constituents Quercetin and Ginkgolide B against β-Amyloid Peptide-Induced Toxicity in SH-SY5Y Cells. Chem.-Biol. Interact. 2009, 181, 115–123. [Google Scholar] [CrossRef]
- Zhang, L.; Yu, H.; Zhao, X.; Lin, X.; Tan, C.; Cao, G.; Wang, Z. Neuroprotective Effects of Salidroside against Beta-Amyloid-Induced Oxidative Stress in SH-SY5Y Human Neuroblastoma Cells. Neurochem. Int. 2010, 57, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Sheehan, J.P.; Swerdlow, R.H.; Miller, S.W.; Davis, R.E.; Parks, J.K.; Parker, W.D.; Tuttle, J.B. Calcium Homeostasis and Reactive Oxygen Species Production in Cells Transformed by Mitochondria from Individuals with Sporadic Alzheimer’s Disease. J. Neurosci. 1997, 17, 4612–4622. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-T.; Kuo, Y.-Y.; Lin, G.-B.; Lu, C.-H.; Hsu, H.-P.; Sun, Y.-K.; Chao, C.-Y. Thermal Cycling Protects SH-SY5Y Cells against Hydrogen Peroxide and β-Amyloid-Induced Cell Injury through Stress Response Mechanisms Involving Akt Pathway. PLoS ONE 2020, 15, e0240022. [Google Scholar] [CrossRef] [PubMed]
- Wiatrak, B.; Jawień, P.; Matuszewska, A.; Szeląg, A.; Kubis-Kubiak, A. Effect of Amyloid-β on the Redox System Activity in SH-SY5Y Cells Preincubated with Lipopolysaccharide or Co-Cultured with Microglia Cells. Biomed. Pharmacother. 2022, 149, 112880. [Google Scholar] [CrossRef]
- Wang, H.; Xu, Y.; Yan, J.; Zhao, X.; Sun, X.; Zhang, Y.; Guo, J.; Zhu, C. Acteoside Protects Human Neuroblastoma SH-SY5Y Cells against β-Amyloid-Induced Cell Injury. Brain Res. 2009, 1283, 139–147. [Google Scholar] [CrossRef]
- Mani, S.; Sekar, S.; Chidambaram, S.B.; Sevanan, M. Naringenin Protects against 1-Methyl-4-Phenylpyridinium- Induced Neuroinflammation and Resulting Reactive Oxygen Species Production in SH-SY5Y Cell Line: An in Vitro Model of Parkinson’s Disease. Pharmacogn. Mag. 2018, 14, 458. [Google Scholar] [CrossRef]
- Cores, Á.; Michalska, P.; Pérez, J.M.; Crisman, E.; Gómez, C.; Villacampa, M.; Menéndez, J.C.; León, R. Enantioselective Synthesis and Pharmacological Evaluation of Aza-CGP37157–Lipoic Acid Hybrids for the Treatment of Alzheimer’s Disease. Antioxidants 2022, 11, 112. [Google Scholar] [CrossRef]
- Korecka, J.A.; van Kesteren, R.E.; Blaas, E.; Spitzer, S.O.; Kamstra, J.H.; Smit, A.B.; Swaab, D.F.; Verhaagen, J.; Bossers, K. Phenotypic Characterization of Retinoic Acid Differentiated SH-SY5Y Cells by Transcriptional Profiling. PLoS ONE 2013, 8, e63862. [Google Scholar] [CrossRef]
- Encinas, M.; Iglesias, M.; Liu, Y.; Wang, H.; Muhaisen, A.; Ceña, V.; Gallego, C.; Comella, J.X. Sequential Treatment of SH-SY5Y Cells with Retinoic Acid and Brain-Derived Neurotrophic Factor Gives Rise to Fully Differentiated, Neurotrophic Factor-Dependent, Human Neuron-like Cells. J. Neurochem. 2000, 75, 991–1003. [Google Scholar] [CrossRef]
- Sasaguri, H.; Nilsson, P.; Hashimoto, S.; Nagata, K.; Saito, T.; De Strooper, B.; Hardy, J.; Vassar, R.; Winblad, B.; Saido, T.C. APP Mouse Models for Alzheimer’s Disease Preclinical Studies. EMBO J. 2017, 36, 2473–2487. [Google Scholar] [CrossRef]
- Haider, S.; Liaquat, L.; Ahmad, S.; Batool, Z.; Siddiqui, R.A.; Tabassum, S.; Shahzad, S.; Rafiq, S.; Naz, N. Naringenin Protects AlCl3/D-Galactose Induced Neurotoxicity in Rat Model of AD via Attenuation of Acetylcholinesterase Levels and Inhibition of Oxidative Stress. PLoS ONE 2020, 15, e0227631. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.B.; Khan, M.M.; Khan, A.; Ahmed, M.E.; Ishrat, T.; Tabassum, R.; Vaibhav, K.; Ahmad, A.; Islam, F. Naringenin Ameliorates Alzheimer’s Disease (AD)-Type Neurodegeneration with Cognitive Impairment (AD-TNDCI) Caused by the Intracerebroventricular-Streptozotocin in Rat Model. Neurochem. Int. 2012, 61, 1081–1093. [Google Scholar] [CrossRef] [PubMed]
- Farr, S.A.; Price, T.O.; Banks, W.A.; Ercal, N.; Morley, J.E. Effect of Alpha-Lipoic Acid on Memory, Oxidation, and Lifespan in SAMP8 Mice. J. Alzheimers Dis. 2012, 32, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Sharman, M.J.; Gyengesi, E.; Liang, H.; Chatterjee, P.; Karl, T.; Li, Q.-X.; Wenk, M.R.; Halliwell, B.; Martins, R.N.; Münch, G. Assessment of Diets Containing Curcumin, Epigallocatechin-3-Gallate, Docosahexaenoic Acid and α-Lipoic Acid on Amyloid Load and Inflammation in a Male Transgenic Mouse Model of Alzheimer’s Disease: Are Combinations More Effective? Neurobiol. Dis. 2019, 124, 505–519. [Google Scholar] [CrossRef]
- Garcia, N.; Santafé, M.M.; Tomàs, M.; Lanuza, M.A.; Tomàs, J. Short-Term Effects of β-Amyloid25-35 Peptide Aggregates on Transmitter Release in Neuromuscular Synapses. J. Neuropathol. Exp. Neurol. 2008, 67, 250–259. [Google Scholar] [CrossRef]
- Ng, N.S.; Ooi, L. A Simple Microplate Assay for Reactive Oxygen Species Generation and Rapid Cellular Protein Normalization. Bio Protoc. 2021, 11, e3877. [Google Scholar] [CrossRef]




| Timepoint | Antioxidant Compound(s) | Treatment | Result (p-Value) | Figure |
|---|---|---|---|---|
| 24-h pre-treatment | VANL-100 | 0.2 μM + 20 μM NF Aβ 0.2 μM + 20 μM F Aβ | p > 0.05 p > 0.05 | Figure 4A,B |
| 2.0 μM + 20 μM NF Aβ 2.0 μM + 20 μM F Aβ | p > 0.05 p = 0.0051 | |||
| 20 μM + 20 μM NF Aβ 20 μM + 20 μM F Aβ | p < 0.0001 p = 0.0001 | |||
| 50 μM + 20 μM NF Aβ 50 μM + 20 μM F Aβ | p < 0.0001 p = 0.0013 | |||
| 100 μM + 20 μM NF Aβ 100 μM + 20 μM F Aβ | p < 0.0001 p = 0.0022 | |||
| 200 μM + 20 μM NF Aβ 200 μM + 20 μM F Aβ | p = 0.0066 p = 0.0041 | |||
| NAR | 0.2 μM + 20 μM NF Aβ 0.2 μM + 20 μM F Aβ | p > 0.05 p > 0.05 | Figure 4C,D | |
| 2.0 μM + 20 μM NF Aβ 2.0 μM + 20 μM F Aβ | p = 0.0008 p = 0.0139 | |||
| 20 μM + 20 μM NF Aβ 20 μM + 20 μM F Aβ | p < 0.0001 p < 0.0001 | |||
| 50 μM + 20 μM NF Aβ 50 μM + 20 μM F Aβ | p = 0.017 p = 0.0018 | |||
| 100 μM + 20 μM NF Aβ 100 μM + 20 μM F Aβ | p = 0.0004 p < 0.0001 | |||
| 200 μM + 20 μM NF Aβ 200 μM + 20 μM F Aβ | p = 0.0104 p = 0.0061 | |||
| ALA | 0.2 μM + 20 μM NF Aβ 0.2 μM + 20 μM F Aβ | p > 0.05 p > 0.05 | Figure 4E,F | |
| 2.0 μM + 20 μM NF Aβ 2.0 μM + 20 μM F Aβ | p > 0.05 p > 0.05 | |||
| 20 μM + 20 μM NF Aβ 20 μM + 20 μM F Aβ | p = 0.0070 p = 0.0025 | |||
| 50 μM + 20 μM NF Aβ 50 μM + 20 μM F Aβ | p = 0.0451 p = 0.0230 | |||
| 100 μM + 20 μM NF Aβ 100 μM + 20 μM F Aβ | p = 0.0058 p = 0.0045 | |||
| 200 μM + 20 μM NF Aβ 200 μM + 20 μM F Aβ | p = 0.0063 p = 0.0059 | |||
| NAR + ALA | 0.2 μM + 20 μM NF Aβ 0.2 μM + 20 μM F Aβ | p > 0.05 p > 0.05 | Figure 4G,H | |
| 2.0 μM + 20 μM NF Aβ 2.0 μM + 20 μM F Aβ | p = 0.0018 p > 0.05 | |||
| 20 μM + 20 μM NF Aβ 20 μM + 20 μM F Aβ | p = 0.0002 p = 0.0008 | |||
| 50 μM + 20 μM NF Aβ 50 μM + 20 μM F Aβ | p = 0.0020 p = 0.0129 | |||
| 100 μM + 20 μM NF Aβ 100 μM + 20 μM F Aβ | p = 0.0001 p = 0.0005 | |||
| 200 μM + 20 μM NF Aβ 200 μM + 20 μM F Aβ | p = 0.0423 p = 0.0044 | |||
| Co-treatment | VANL-100 | 0.2 μM + 20 μM NF Aβ 0.2 μM + 20 μM F Aβ | p > 0.05 p > 0.05 | Figure 5A,B |
| 2.0 μM + 20 μM NF Aβ 2.0 μM + 20 μM F Aβ | p > 0.05 p > 0.05 | |||
| 20 μM + 20 μM NF Aβ 20 μM + 20 μM F Aβ | p = 0.0017 p = 0.0019 | |||
| 50 μM + 20 μM NF Aβ 50 μM + 20 μM F Aβ | p > 0.05 p > 0.05 | |||
| 100 μM + 20 μM NF Aβ 100 μM + 20 μM F Aβ | p > 0.05 p > 0.05 | |||
| 200 μM + 20 μM NF Aβ 200 μM + 20 μM F Aβ | p > 0.05 p > 0.05 | |||
| NAR | 0.2 μM + 20 μM NF Aβ 0.2 μM + 20 μM F Aβ | p > 0.05 p > 0.05 | Figure 5C,D | |
| 2.0 μM + 20 μM NF Aβ 2.0 μM + 20 μM F Aβ | p > 0.05 p = 0.0115 | |||
| 20 μM + 20 μM NF Aβ 20 μM + 20 μM F Aβ | p = 0.0014 p = 0.0001 | |||
| 50 μM + 20 μM NF Aβ 50 μM + 20 μM F Aβ | p = 0.0038 p = 0.0005 | |||
| 100 μM + 20 μM NF Aβ 100 μM + 20 μM F Aβ | p < 0.0001 p = 0.0001 | |||
| 200 μM + 20 μM NF Aβ 200 μM + 20 μM F Aβ | p = 0.0161 p = 0.0023 | |||
| ALA | 0.2 μM + 20 μM NF Aβ 0.2 μM + 20 μM F Aβ | p > 0.05 p > 0.05 | Figure 5E,F | |
| 2.0 μM + 20 μM NF Aβ 2.0 μM + 20 μM F Aβ | p > 0.05 p > 0.05 | |||
| 20 μM + 20 μM NF Aβ 20 μM + 20 μM F Aβ | p = 0.0003 p < 0.0001 | |||
| 50 μM + 20 μM NF Aβ 50 μM + 20 μM F Aβ | p = 0.0011 p = 0.0011 | |||
| 100 μM + 20 μM NF Aβ 100 μM + 20 μM F Aβ | p = 0.0010 p = 0.0031 | |||
| 200 μM + 20 μM NF Aβ 200 μM + 20 μM F Aβ | p = 0.0207 p = 0.0005 | |||
| NAR + ALA | 0.2 μM + 20 μM NF Aβ 0.2 μM + 20 μM F Aβ | p > 0.05 p > 0.05 | Figure 5G,H | |
| 2.0 μM + 20 μM NF Aβ 2.0 μM + 20 μM F Aβ | p > 0.05 p > 0.05 | |||
| 20 μM + 20 μM NF Aβ 20 μM + 20 μM F Aβ | p = 0.034 p = 0.0036 | |||
| 50 μM + 20 μM NF Aβ 50 μM + 20 μM F Aβ | p = 0.0026 p = 0.0338 | |||
| 100 μM + 20 μM NF Aβ 100 μM + 20 μM F Aβ | p = 0.0002 p = 0.0066 | |||
| 200 μM + 20 μM NF Aβ 200 μM + 20 μM F Aβ | p = 0.0012 p > 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Collins, A.E.; Saleh, T.M.; Kalisch, B.E. VANL-100 Attenuates Beta-Amyloid-Induced Toxicity in SH-SY5Y Cells. Int. J. Mol. Sci. 2023, 24, 442. https://doi.org/10.3390/ijms24010442
Collins AE, Saleh TM, Kalisch BE. VANL-100 Attenuates Beta-Amyloid-Induced Toxicity in SH-SY5Y Cells. International Journal of Molecular Sciences. 2023; 24(1):442. https://doi.org/10.3390/ijms24010442
Chicago/Turabian StyleCollins, Andrila E., Tarek M. Saleh, and Bettina E. Kalisch. 2023. "VANL-100 Attenuates Beta-Amyloid-Induced Toxicity in SH-SY5Y Cells" International Journal of Molecular Sciences 24, no. 1: 442. https://doi.org/10.3390/ijms24010442
APA StyleCollins, A. E., Saleh, T. M., & Kalisch, B. E. (2023). VANL-100 Attenuates Beta-Amyloid-Induced Toxicity in SH-SY5Y Cells. International Journal of Molecular Sciences, 24(1), 442. https://doi.org/10.3390/ijms24010442






