Ladinin 1 Shortens Survival via Promoting Proliferation and Enhancing Invasiveness in Lung Adenocarcinoma
Abstract
1. Introduction
2. Results
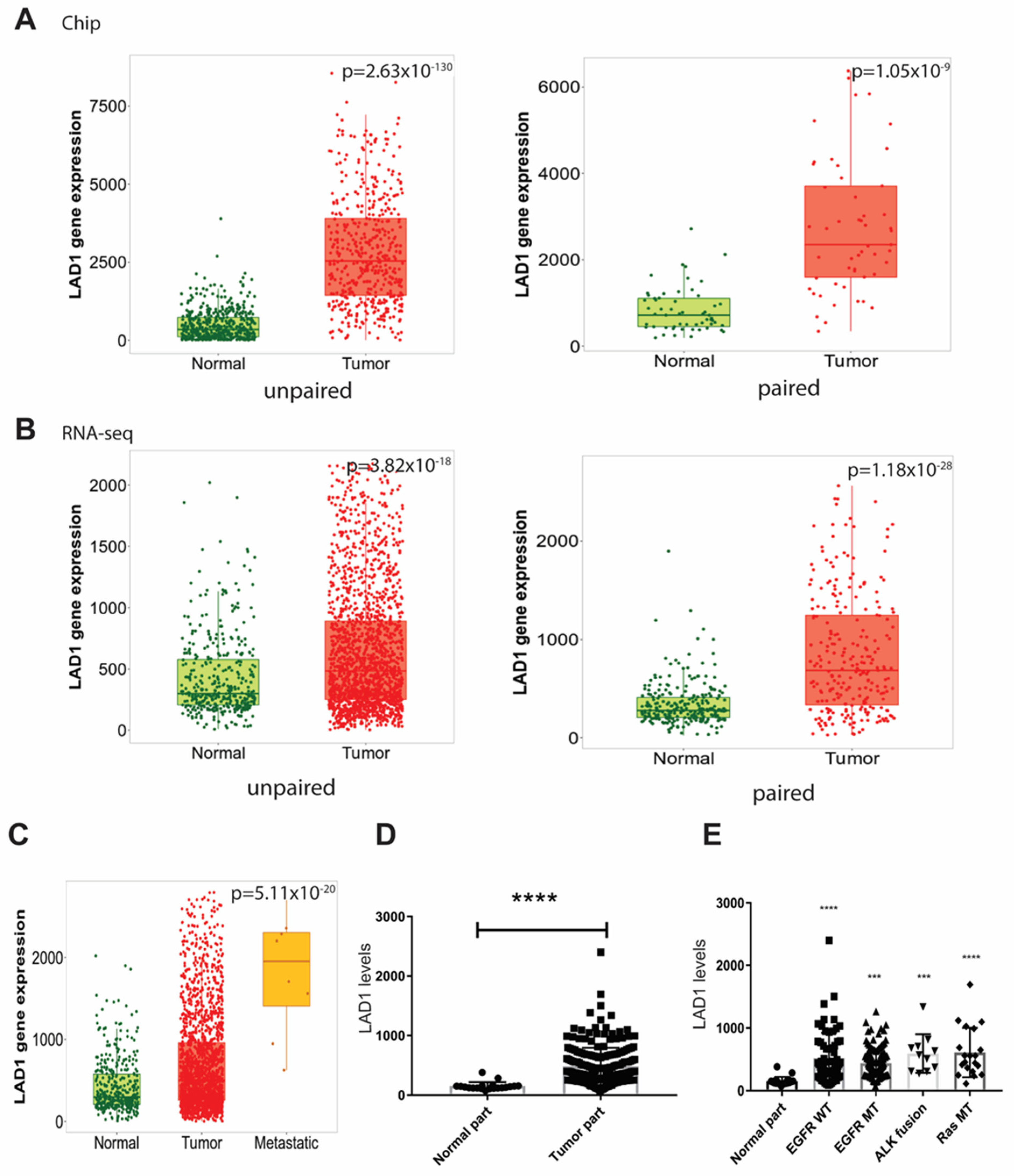
2.1. LAD1 mRNA Expression and Its Association with Mutated Genes in LUAD
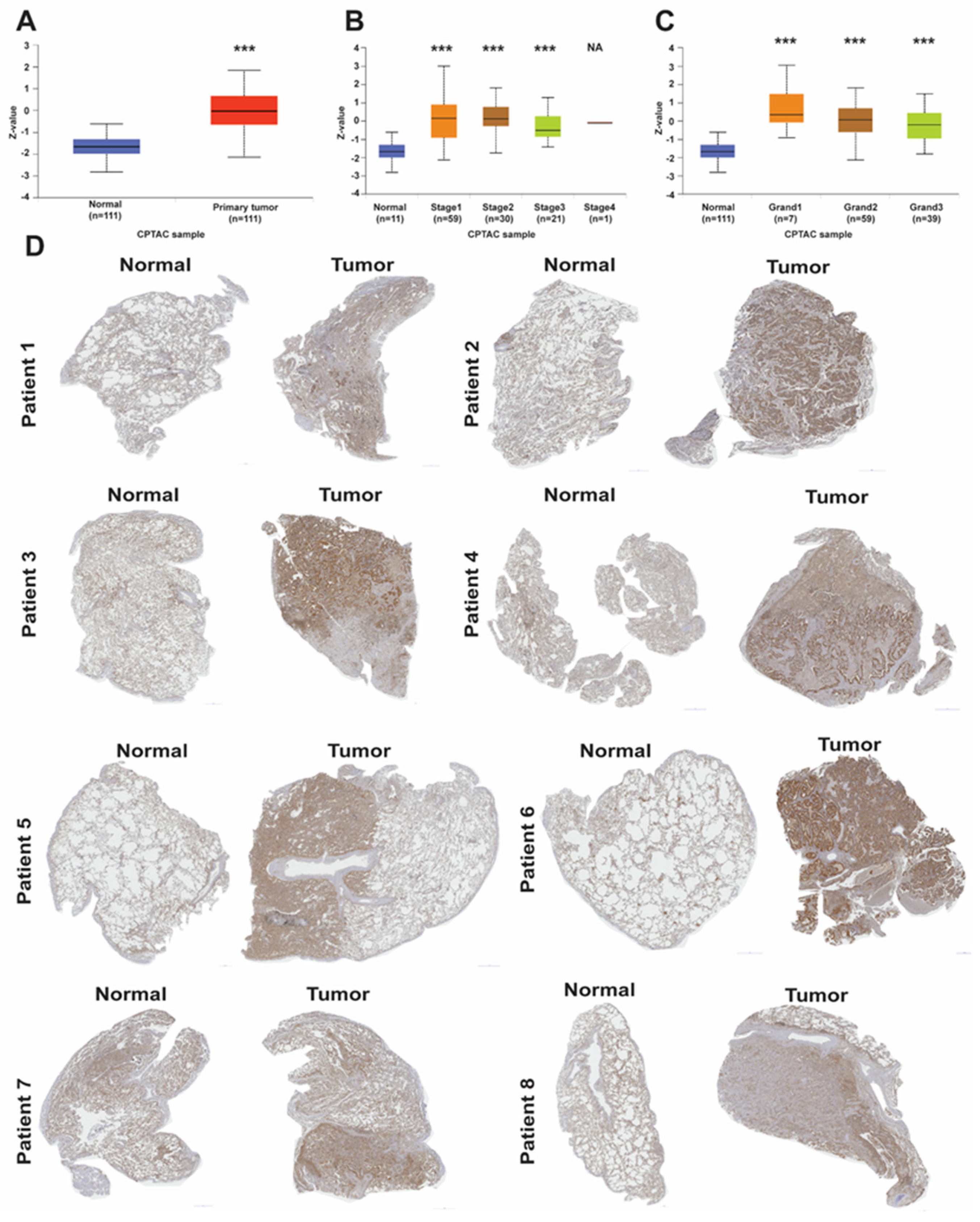
2.2. LAD1 Protein Expression Level in LUAD
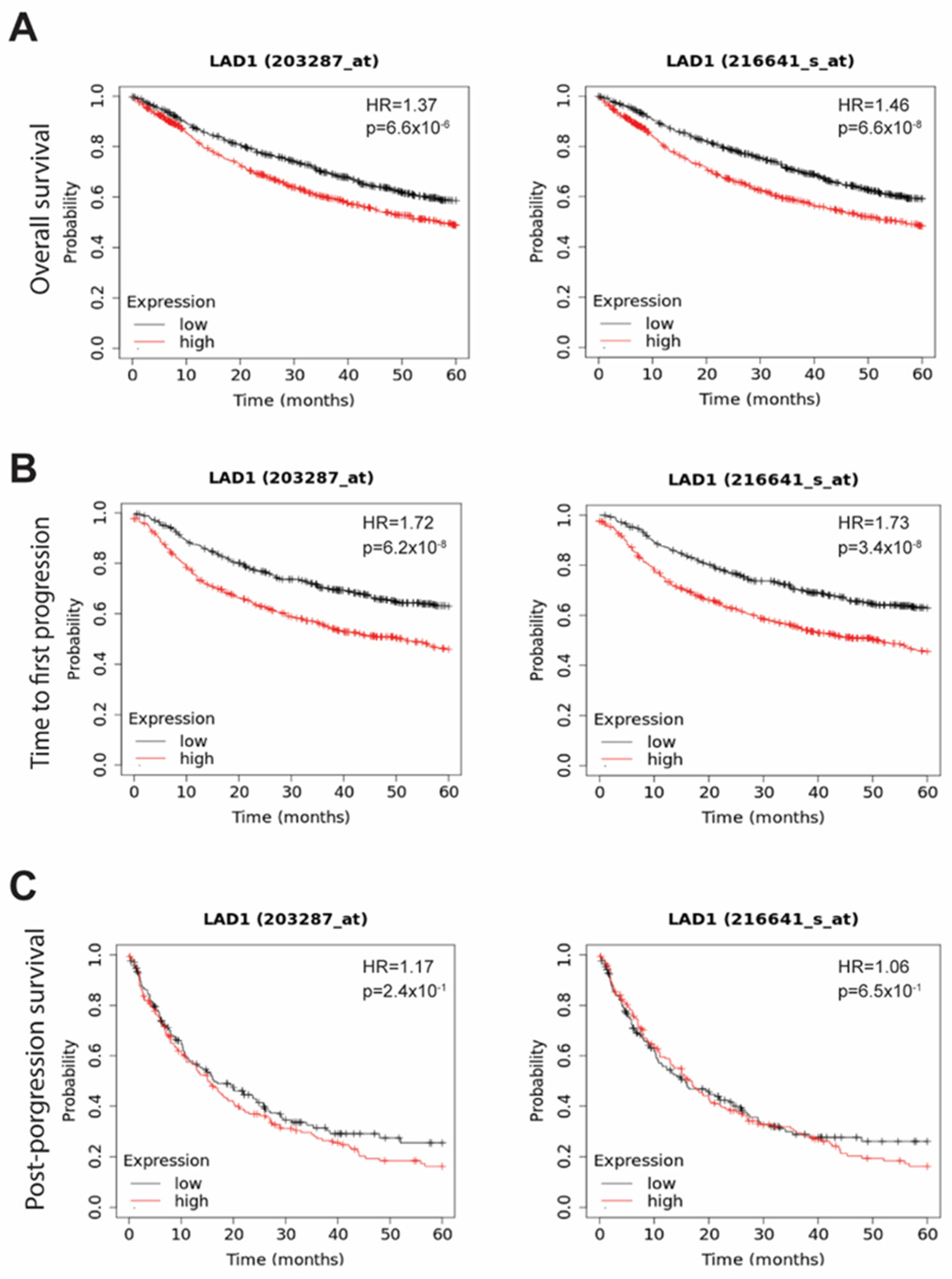
2.3. The Survival Significance of LAD1
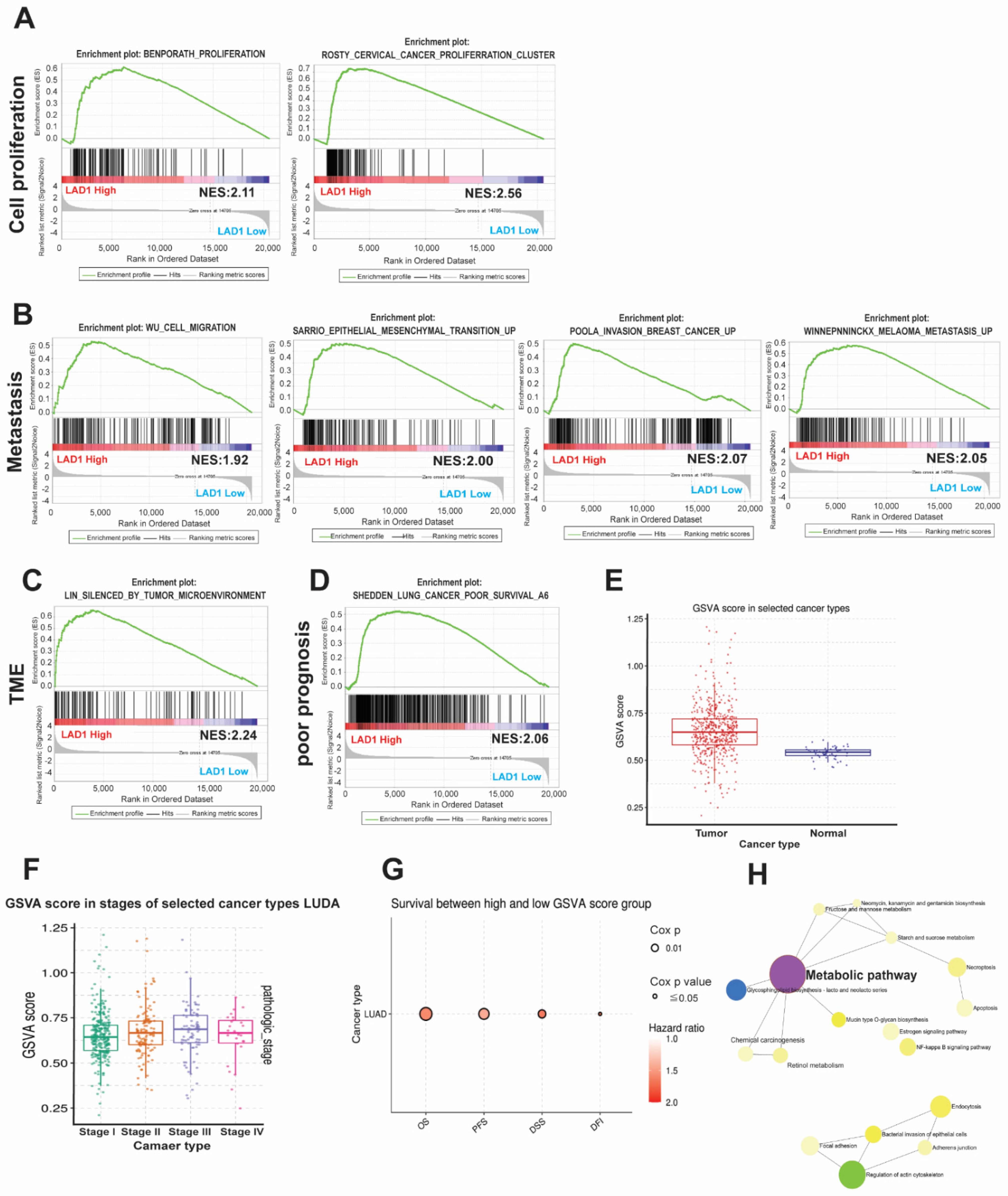
2.4. The Functional Role and Pathways Associated with LAD1
2.5. The Impact of LAD1 Correlated Metabolic Pathway Genes
2.6. The Functional Role of B3GNT3 and Its Association with Targetable Mutated Genes
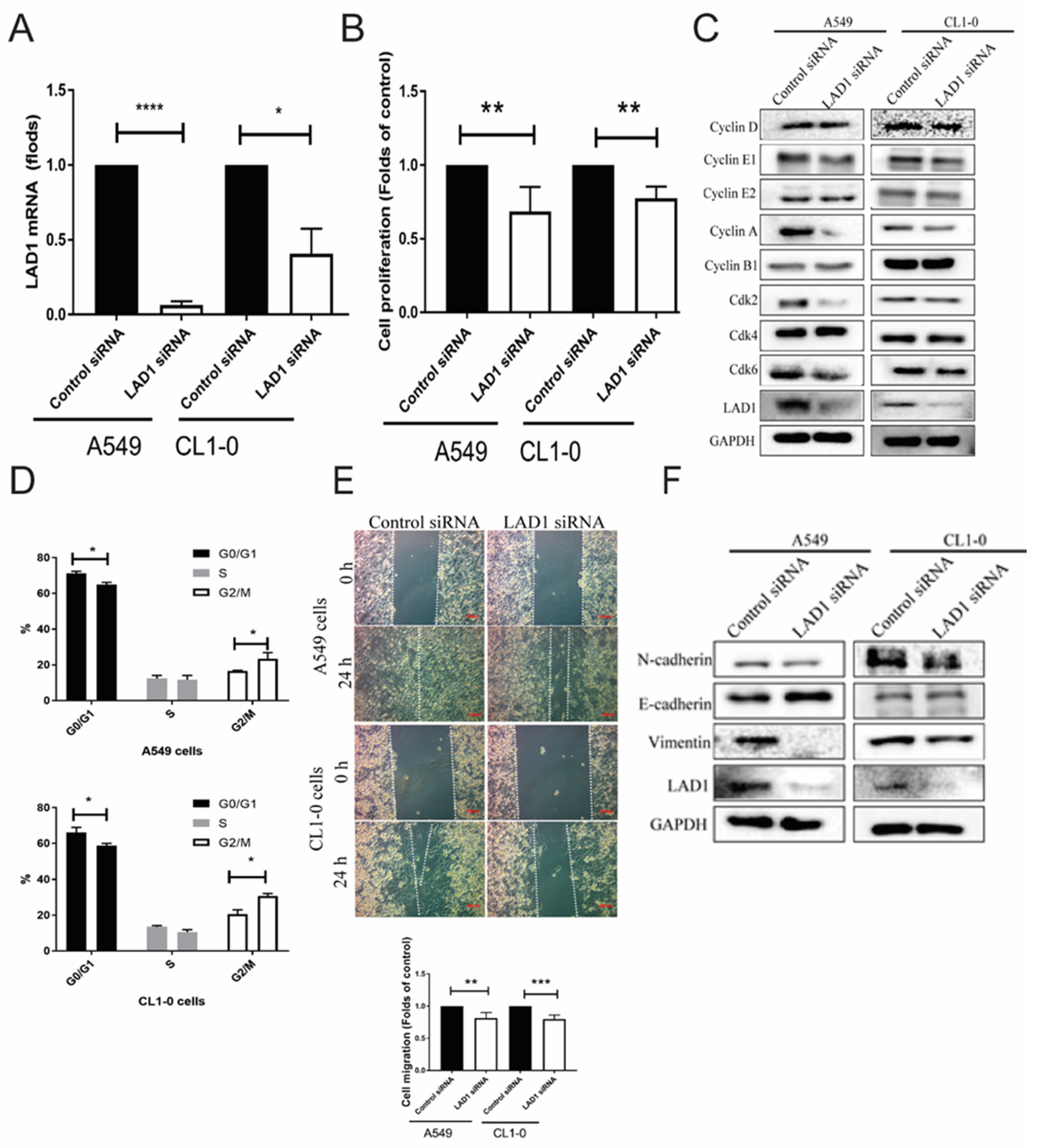
2.7. Functional Analysis of Knockdown LAD1 In Vitro
3. Discussion
4. Materials and Methods
4.1. Bioinformatics
4.2. Survival Analysis
4.3. Immunohistochemistry
4.4. Gene Set Enrichment Analysis (GSEA)
4.5. Gene Set Variation Analysis (GSVA) and Metascape
4.6. Functional Assays
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Lu, H. Adenosquamous carcinoma of the lung. OncoTargets Ther. 2018, 11, 4829–4835. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, A.G.; Tsao, M.S.; Beasley, M.B.; Borczuk, A.C.; Brambilla, E.; Cooper, W.A.; Dacic, S.; Jain, D.; Kerr, K.M.; Lantuejoul, S.; et al. The 2021 WHO Classification of Lung Tumors: Impact of Advances Since 2015. J. Thorac. Oncol. 2022, 17, 362–387. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.-H.; Liang, K.-H.; Huang, H.-C.; Shen, C.-I.; Chiang, C.-L.; Wang, M.-L.; Chiou, S.-H.; Chen, Y.-M. State-of-the-Art Molecular Oncology of Lung Cancer in Taiwan. Int. J. Mol. Sci. 2022, 23, 7037. [Google Scholar] [CrossRef] [PubMed]
- Bonney, A.; Malouf, R.; Marchal, C.; Manners, D.; Fong, K.M.; Marshall, H.M.; Irving, L.B.; Manser, R. Impact of low-dose computed tomography (LDCT) screening on lung cancer-related mortality. Cochrane Database Syst. Rev. 2022, 8, Cd013829. [Google Scholar]
- Deb, D.; Moore, A.C.; Roy, U.B. The 2021 Global Lung Cancer Therapy Landscape. J. Thorac. Oncol. 2022, 17, 931–936. [Google Scholar] [CrossRef]
- Singh, N.; Temin, S.; Baker, S.; Blanchard, E.; Brahmer, J.R.; Celano, P.; Duma, N.; Ellis, P.M.; Elkins, I.B.; Haddad, R.Y.; et al. Therapy for Stage IV Non–Small-Cell Lung Cancer Without Driver Alterations: ASCO Living Guideline. J. Clin. Oncol. 2022, 40, 3323–3343. [Google Scholar] [CrossRef]
- Teixeira, J.C.; de Filippo, C.; Weihmann, A.; Meneu, J.R.; Racimo, F.; Dannemann, M.; Nickel, B.; Fischer, A.; Halbwax, M.; Andre, C.; et al. Long-Term Balancing Selection in LAD1 Maintains a Missense Trans-Species Polymorphism in Humans, Chimpanzees, and Bonobos. Mol. Biol. Evol. 2015, 32, 1186–1196. [Google Scholar] [CrossRef]
- Cozzani, E.; Di Zenzo, G.; Gasparini, G.; Salemme, A.; Agnoletti, A.; Vassallo, C.; Caproni, M.; Antiga, E.; Marzano, A.; Cavalli, R.; et al. Autoantibody Profile of a Cohort of 54 Italian Patients with Linear IgA Bullous Dermatosis: LAD-1 Denoted as a Major Auto-antigen of the Lamina Lucida Subtype. Acta Derm. Venereol. 2020, 100, adv00070. [Google Scholar] [CrossRef]
- Roth, L.; Srivastava, S.; Lindzen, M.; Sas-Chen, A.; Sheffer, M.; Lauriola, M.; Enuka, Y.; Noronha, A.; Mancini, M.; Lavi, S.; et al. SILAC identifies LAD1 as a filamin-binding regulator of actin dynamics in response to EGF and a marker of aggressive breast tumors. Sci. Signal. 2018, 11, eaan0949. [Google Scholar] [CrossRef]
- Moon, B.; Yang, S.J.; Park, S.M.; Lee, S.H.; Song, K.S.; Jeong, E.J.; Park, M.; Kim, J.S.; Yeom, Y.I.; Kim, J.A. LAD1 expression is associated with the metastatic potential of colorectal cancer cells. BMC Cancer 2020, 20, 1180. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, Z.; Tie, C. High expression of ladinin-1 (LAD1) predicts adverse outcomes: A new candidate docetaxel resistance gene for prostatic cancer (PCa). Bioengineered 2021, 12, 5749–5759. [Google Scholar] [CrossRef] [PubMed]
- Codreanu, S.G.; Hoeksema, M.D.; Slebos, R.J.C.; Zimmerman, L.J.; Rahman, S.M.J.; Li, M.; Chen, S.-C.; Chen, H.; Eisenberg, R.; Liebler, D.C.; et al. Identification of Proteomic Features To Distinguish Benign Pulmonary Nodules from Lung Adenocarcinoma. J. Proteome Res. 2017, 16, 3266–3276. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y. circ-ANXA7 facilitates lung adenocarcinoma progression via miR-331/LAD1 axis. Cancer Cell Int. 2021, 21, 85. [Google Scholar] [CrossRef] [PubMed]
- Leng, X.; Wei, S.; Mei, J.; Deng, S.; Yang, Z.; Liu, Z.; Guo, C.; Deng, Y.; Xia, L.; Cheng, J.; et al. Identifying the prognostic significance of B3GNT3 with PD-L1 expression in lung adenocarcinoma. Transl. Lung Cancer Res. 2021, 10, 965–980. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Luo, J.; Li, H.; Huang, Y.; Zhu, Y.; Chen, Q. B3GNT3 as a prognostic biomarker and correlation with immune cell infiltration in lung adenocarcinoma. Ann. Transl. Med. 2022, 10, 295. [Google Scholar] [CrossRef]
- Xu, J.; Guo, Z.; Yuan, S.; Li, H.; Luo, S. Upregulation of B3GNT3 is associated with immune infiltration and activation of NF-κB pathway in gynecologic cancers. J. Reprod. Immunol. 2022, 152, 103658. [Google Scholar] [CrossRef]
- Kong, K.; Zhao, Y.; Xia, L.; Jiang, H.; Xu, M.; Zheng, J. B3GNT3: A prognostic biomarker associated with immune cell infiltration in pancreatic adenocarcinoma. Oncol. Lett. 2021, 21, 159. [Google Scholar] [CrossRef]
- Bartha, A.; Gyorffy, B. TNMplot.com: A Web Tool for the Comparison of Gene Expression in Normal, Tumor and Metastatic Tissues. Int. J. Mol. Sci. 2021, 22, 2622. [Google Scholar] [CrossRef]
- Chandrashekar, D.S.; Karthikeyan, S.K.; Korla, P.K.; Patel, H.; Shovon, A.R.; Athar, M.; Netto, G.J.; Qin, Z.S.; Kumar, S.; Manne, U.; et al. UALCAN: An update to the integrated cancer data analysis platform. Neoplasia 2022, 25, 18–27. [Google Scholar] [CrossRef]
- Okayama, H.; Kohno, T.; Ishii, Y.; Shimada, Y.; Shiraishi, K.; Iwakawa, R.; Furuta, K.; Tsuta, K.; Shibata, T.; Yamamoto, S.; et al. Identification of genes upregulated in ALK-positive and EGFR/KRAS/ALK-negative lung adenocarcinomas. Cancer Res. 2012, 72, 100–111. [Google Scholar] [CrossRef] [PubMed]
- Nagy, A.; Munkacsy, G.; Gyorffy, B. Pancancer survival analysis of cancer hallmark genes. Sci. Rep. 2021, 11, 6047. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.J.; Hu, F.F.; Xia, M.X.; Han, L.; Zhang, Q.; Guo, A.Y. GSCALite: A web server for gene set cancer analysis. Bioinformatics 2018, 34, 3771–3772. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, B.; Pache, L.; Chang, M.; Khodabakhshi, A.H.; Tanaseichuk, O.; Benner, C.; Chanda, S.K. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 2019, 10, 1523. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, C.-Y.; Huang, Y.-C.; Chiang, H.-H.; Wu, Y.-Y.; Wu, K.-L.; Chang, Y.-Y.; Liu, L.-X.; Tsai, Y.-M.; Hsu, Y.-L. Ladinin 1 Shortens Survival via Promoting Proliferation and Enhancing Invasiveness in Lung Adenocarcinoma. Int. J. Mol. Sci. 2023, 24, 431. https://doi.org/10.3390/ijms24010431
Chang C-Y, Huang Y-C, Chiang H-H, Wu Y-Y, Wu K-L, Chang Y-Y, Liu L-X, Tsai Y-M, Hsu Y-L. Ladinin 1 Shortens Survival via Promoting Proliferation and Enhancing Invasiveness in Lung Adenocarcinoma. International Journal of Molecular Sciences. 2023; 24(1):431. https://doi.org/10.3390/ijms24010431
Chicago/Turabian StyleChang, Chao-Yuan, Yung-Chi Huang, Hung-Hsing Chiang, Yu-Yuan Wu, Kuan-Li Wu, Yung-Yun Chang, Lian-Xiu Liu, Ying-Ming Tsai, and Ya-Ling Hsu. 2023. "Ladinin 1 Shortens Survival via Promoting Proliferation and Enhancing Invasiveness in Lung Adenocarcinoma" International Journal of Molecular Sciences 24, no. 1: 431. https://doi.org/10.3390/ijms24010431
APA StyleChang, C.-Y., Huang, Y.-C., Chiang, H.-H., Wu, Y.-Y., Wu, K.-L., Chang, Y.-Y., Liu, L.-X., Tsai, Y.-M., & Hsu, Y.-L. (2023). Ladinin 1 Shortens Survival via Promoting Proliferation and Enhancing Invasiveness in Lung Adenocarcinoma. International Journal of Molecular Sciences, 24(1), 431. https://doi.org/10.3390/ijms24010431





