Brassinosteroid Promotes Grape Berry Quality-Focus on Physicochemical Qualities and Their Coordination with Enzymatic and Molecular Processes: A Review
Abstract
1. Introduction
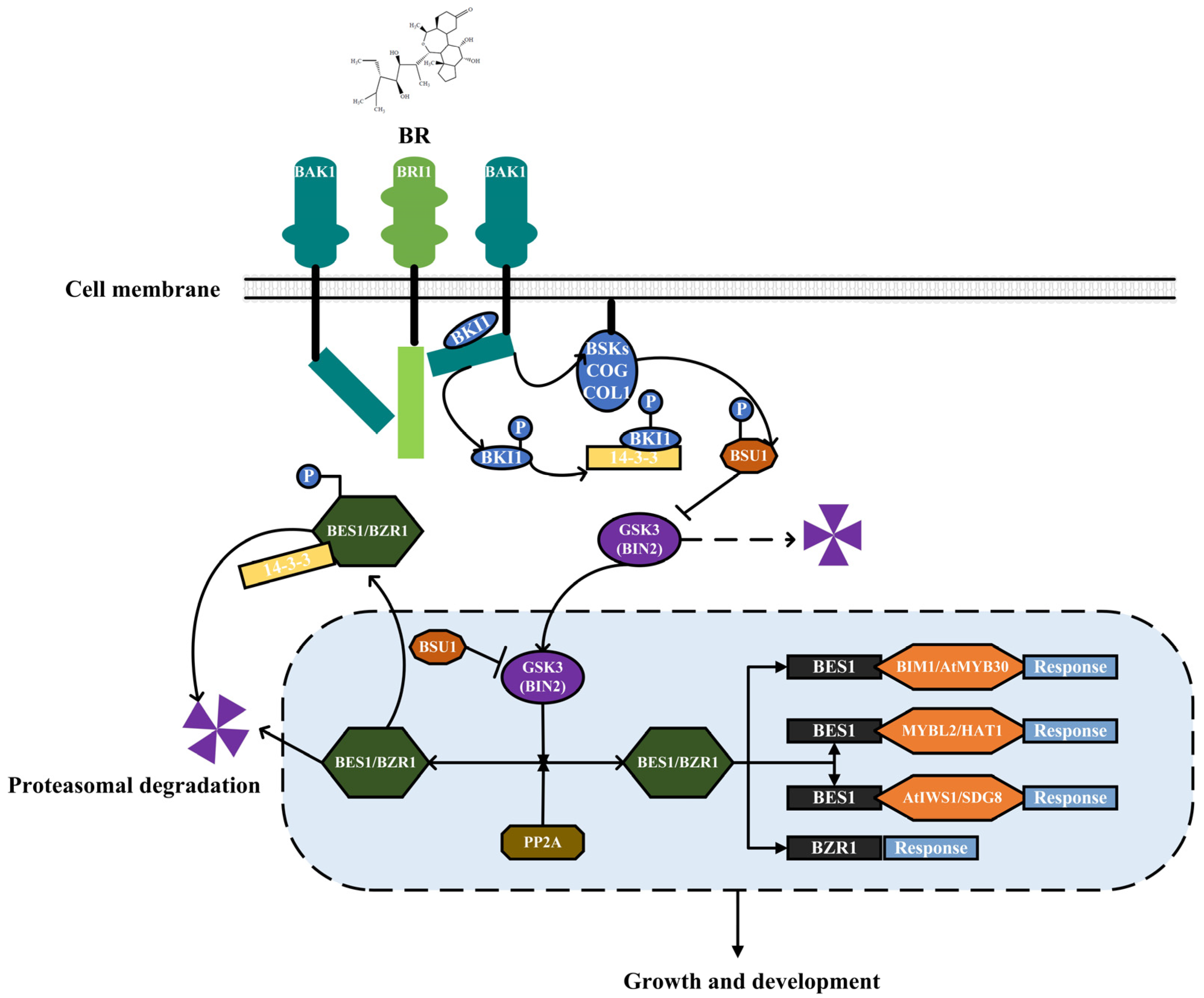
2. Regulation of BR Biosynthesis and Signal Transduction
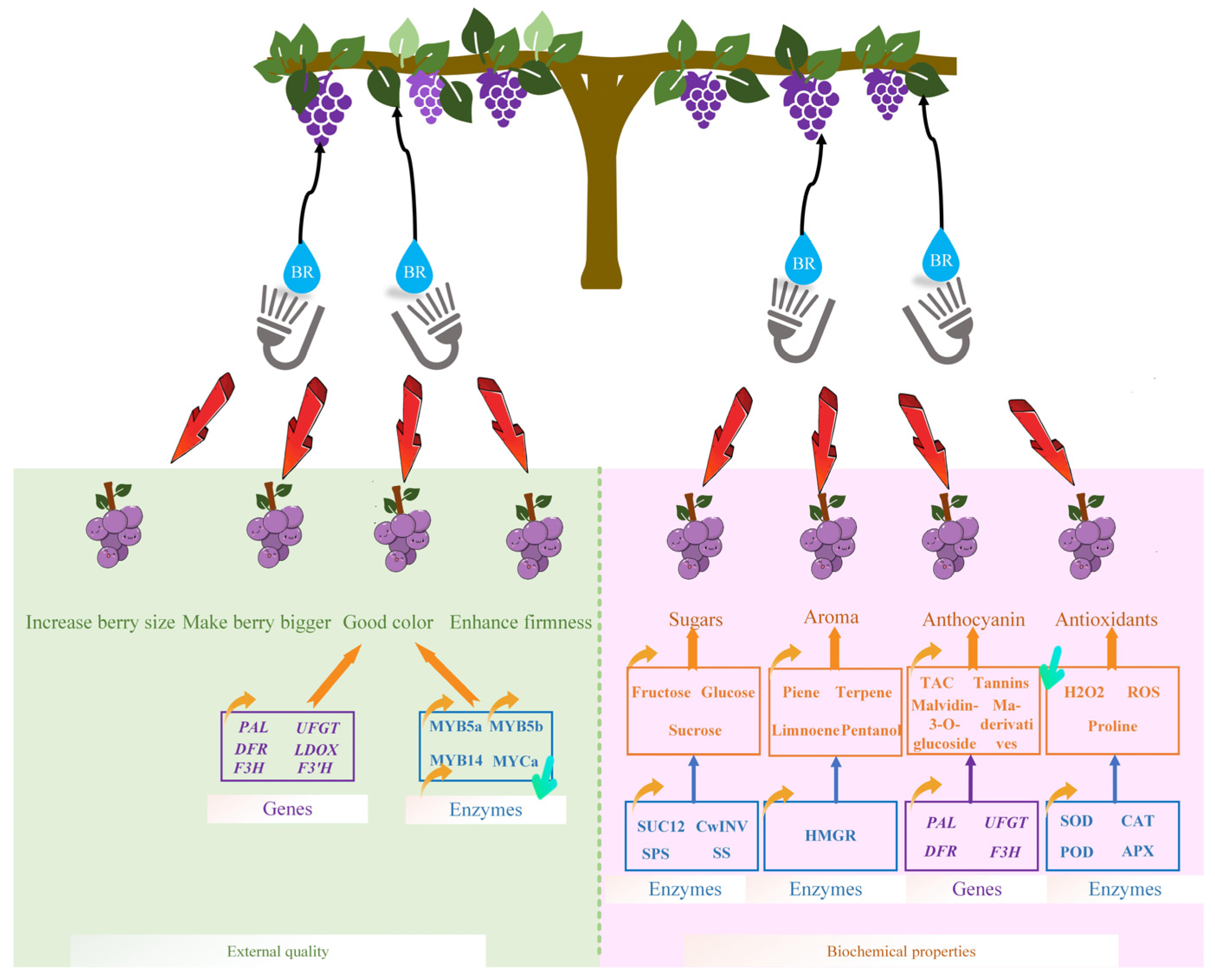
3. Effect of BR on Grape Berry Development and Quality Improvement
3.1. Effect of BR on Table Grape External Quality Development
3.2. Effect of BR on Grape Biochemical Properties
3.2.1. Role of Biochemical Properties on Fruit Flavor
3.2.2. Effect of BR on Sugars Accumulation in Grape Berries
3.2.3. Effect of BR on Organic Acids Formation in Grape Berries
3.2.4. Effect of BR on Astringency Reduction in Grape Berries
3.3. Effect of BR on Grape Aroma Enrichment
3.3.1. Aroma Components Existed in Grape Berries
3.3.2. The Effect of BR on Aroma Components in Grape Berries
3.3.3. Regulating the Effect of BR on Key Rate-Limiting Enzyme HMGR
3.4. Effect of BR on Grape Pericarp Coloration
3.5. Effect of BR on Grape Antioxidant Metabolites Accumulation
4. The Interaction of Endogenous Phytohormones Regulated Plant Growth and Development
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, T.W.; Hwang, J.Y.; Kim, Y.S.; Joo, S.H.; Chang, S.C.; Lee, J.S.; Kim, S.K. Arabidopsis CYP85A2, a cytochrome P450, mediates the Baeyer-Villiger oxidation of castasterone to brassinolide in brassinosteroid biosynthesis. Plant Cell 2005, 17, 2397–2412. [Google Scholar] [CrossRef]
- Zhang, F.; Lu, K.; Gu, Y.; Zhang, L.; Li, W.; Li, Z. Effects of low-temperature stress and brassinolide application on the photosynthesis and leaf structure of tung tree seedlings. Front. Plant Sci. 2020, 10, 1767. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wu, Y.; Liao, W.; Hu, L.; Dawuda, M.M.; Jin, X.; Yu, J. Nitric oxide is involved in the brassinolide-induced adventitious root development in cucumber. BMC Plant Biol. 2020, 20, 102. [Google Scholar] [CrossRef] [PubMed]
- An, L.; Ma, J.; Wang, H.; Li, F.; Qin, D.; Wu, J.; Wu, X. NMR-based global metabolomics approach to decipher the metabolic effects of three plant growth regulators on strawberry maturation. Food Chem. 2018, 269, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Tanveer, M.; Shahzad, B.; Sharma, A.; Biju, S.; Bhardwaj, R. 24-Epibrassinolide; an active brassinolide and its role in salt stress tolerance in plants: A review. Plant Physiol. Biochem. 2018, 130, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.A.; Ezz, T.M.; El-Megeed, A.; Ismail, M. Foliar application of Kelpak, Brassinolide and Boron in relation to fruit set, drop, yield and fruit quality of’Canino’apricot trees. J. Adv. Agric. Res. 2019, 24, 194–211. [Google Scholar] [CrossRef]
- Hu, S.; Liu, L.; Li, S.; Shao, Z.; Meng, F.; Liu, H.; Wang, Q. Regulation of fruit ripening by the brassinosteroid biosynthetic gene SlCYP90B3 via an ethylene-dependent pathway in tomato. Hortic. Res. 2020, 7, 163. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, H.; Xiang, J.; Zhang, Y.; Wang, Z.; Zhu, D.; Wang, Y. Rice spikelet formation inhibition caused by decreased sugar utilization under high temperature is associated with brassinolide decomposition. Environ. Exp. Bot. 2021, 190, 104585. [Google Scholar] [CrossRef]
- Wang, Y.; Fu, X.; He, W.; Chen, Q.; Wang, X. Effect of spraying brassinolide on fruit quality of Citrus grandis cv.‘Huangjinmiyou’and ‘Hongroumiyou’. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2019; p. 022029. Available online: https://iopscience.iop.org/article/10.1088/1755-1315/358/2/022029/meta (accessed on 31 December 2019).
- Wang, M.; Cai, C.; Lin, J.; Tao, H.; Zeng, W.; Zhang, F.; Wang, Q. Combined treatment of epi-brassinolide and NaCl enhances the main phytochemicals in Chinese kale sprouts. Food Chem. 2020, 315, 126275. [Google Scholar] [CrossRef]
- Al-Shammari, M.Z.F. A study of the effect of NPK nanofertilizer and traditional NPK fertilizers and brassinolide spray on the ratio of some amino acids in the seeds of Fenugreek Trigonella Foenum-graecum L. EurAsian J. BioSci. 2020, 14, 7759–7766. Available online: https://www.proquest.com/scholarly-journals/study-effect-npk-nanofertilizer-traditional/docview/2568034891/se-2?accountid=1381 (accessed on 31 December 2020).
- Zheng, T.; Dong, T.; Haider, M.S.; Jin, H.; Jia, H.; Fang, J. Brassinosteroid regulates 3-hydroxy-3-methylglutaryl CoA reductase to promote grape fruit development. J. Agric. Food Chem. 2020, 68, 11987–11996. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Dong, T.; Zhang, Y.; Ku, Y.; Zheng, T.; Jia, H.; Fang, J. Metabolomic profiling of brassinolide and abscisic acid in response to high-temperature stress. Plant Cell Rep. 2022, 41, 935–946. [Google Scholar] [CrossRef]
- Li, B.; Zhang, C.; Cao, B.; Qin, G.; Wang, W.; Tian, S. Brassinolide enhances cold stress tolerance of fruit by regulating plasma membrane proteins and lipids. Amino Acids 2012, 43, 2469–2480. [Google Scholar] [CrossRef]
- Aghdam, M.S.; Mohammadkhani, N. Enhancement of chilling stress tolerance of tomato fruit by postharvest brassinolide treatment. Food Bioprocess Technol. 2014, 7, 909–914. [Google Scholar] [CrossRef]
- Durbak, A.; Yao, H.; McSteen, P. Hormone signaling in plant development. Curr. Opin. Plant Biol. 2012, 15, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Ramos, Â.P.; Zanardi, A.M.; Amarante, C.V.T.D.; Steffens, C.A.; Pereira-Netto, A.B. Effects of an Auxin and a brassinosteroid on physical, chemical and biochemical attributes of ‘Galaxy’apples. Ciênc. Rural. 2019, 49, e20180311. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, Z.; Yang, Y.; Li, M.; Xu, B. Abscisic acid and brassinolide combined application synergistically enhances drought tolerance and photosynthesis of tall fescue under water stress. Sci. Hortic. 2018, 228, 1–9. [Google Scholar] [CrossRef]
- Zhang, X.C.; Zhu, Y.Q.; Wang, Y.N.; Luo, C.; Wang, X. Effects of different plant growth regulators on blueberry fruit quality. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2017; p. 012038. Available online: https://iopscience.iop.org/article/10.1088/1755-1315/81/1/012038/meta (accessed on 31 August 2017).
- Sheng, J.; Li, X.; Zhang, D. Gibberellins, brassinolide, and ethylene signaling were involved in flower differentiation and development in Nelumbo nucifera. Hortic. Plant J. 2022, 8, 243–250. [Google Scholar] [CrossRef]
- Zheng, L.; Gao, C.; Zhao, C.; Zhang, L.; Han, M.; An, N.; Ren, X. Effects of brassinosteroid associated with auxin and gibberellin on apple tree growth and gene expression patterns. Hortic. Plant J. 2019, 5, 93–108. [Google Scholar] [CrossRef]
- Ghassemi-Golezani, K.; Hassanzadeh, N.; Shakiba, M.R.; Esmaeilpour, B. Exogenous salicylic acid and 24-epi-brassinolide improve antioxidant capacity and secondary metabolites of Brassica nigra. Biocatal. Agric. Biotechnol. 2020, 26, 101636. [Google Scholar] [CrossRef]
- Li, N.; Han, X.; Feng, D.; Yuan, D.; Huang, L.J. Signaling crosstalk between salicylic acid and ethylene/jasmonate in plant defense: Do we understand what they are whispering? Int. J. Mol. Sci. 2019, 20, 671. [Google Scholar] [CrossRef] [PubMed]
- Chavan, C.S.; Chavan, S.S.; Velhal, R.S.; Patil, A.G.; Deshmukh, L.P.; Bapat, V.A. Effect of Brassinolide on Invitro growth of sugarcane Co 86032 (Saccharum spp.). In AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2018; p. 030004. [Google Scholar] [CrossRef]
- Han, X.; Xue, T.; Liu, X.; Wang, Z.; Zhang, L.; Wang, Y.; Yao, F.; Wang, H.; Li, H. A sustainable viticulture method adapted to the cold climate zone in China. Horticulturae 2021, 7, 150. [Google Scholar] [CrossRef]
- Fujioka, S.; Yokota, T. Biosynthesis and metabolism of brassinosteroids. Annu. Rev. Plant Biol. 2003, 54, 137–164. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Y.; Seto, H.; Fujioka, S.; Yoshida, S.; Chory, J. BRI1 is a critical component of a plasma-membrane receptor for plant steroids. Nature 2001, 410, 380–383. [Google Scholar] [CrossRef]
- Li, J.; Wen, J.; Lease, K.A.; Doke, J.T.; Tax, F.E.; Walker, J.C. BAK1, an Arabidopsis LRR receptor-like protein kinase, interacts with BRI1 and modulates brassinosteroid signaling. Cell 2002, 110, 213–222. [Google Scholar] [CrossRef]
- Kim, T.W.; Guan, S.; Sun, Y.; Deng, Z.; Tang, W.; Shang, J.X.; Wang, Z.Y. Brassinosteroid signal transduction from cell-surface receptor kinases to nuclear transcription factors. Nat. Cell Biol. 2009, 11, 1254–1260. [Google Scholar] [CrossRef]
- Peng, P.; Yan, Z.; Zhu, Y.; Li, J. Regulation of the Arabidopsis GSK3-like kinase BRASSINOSTEROID-INSENSITIVE 2 through proteasome-mediated protein degradation. Mol. Plant 2008, 1, 338–346. [Google Scholar] [CrossRef]
- Wang, X.; Chory, J. Brassinosteroids regulate dissociation of BKI1, a negative regulator of BRI1 signaling, from the plasma membrane. Science 2006, 313, 1118–1122. [Google Scholar] [CrossRef]
- He, J.X.; Gendron, J.M.; Yang, Y.; Li, J.; Wang, Z.Y. The GSK3-like kinase BIN2 phosphorylates and destabilizes BZR1, a positive regulator of the brassinosteroid signaling pathway in Arabidopsis. Proc. Natl. Acad. Sci. USA 2002, 99, 10185–10190. [Google Scholar] [CrossRef]
- Boca, G.D. Factors influencing consumer behavior in sustainable fruit and vegetable consumption in maramures county, Romania. Sustainability 2021, 13, 1812. [Google Scholar] [CrossRef]
- Kupe, M.; Ercisli, S.; Baron, M.; Sochor, J. Sustainable Viticulture on Traditional ‘Baran’Training System in Eastern Turkey. Sustainability 2021, 13, 10236. [Google Scholar] [CrossRef]
- Sharma, S.K. Brassinosteroids Application Responses in Fruit Crops-A Review. Int. J. Agric. Environ. Biotechnol. 2021, 14, 123–140. [Google Scholar] [CrossRef]
- Babalık, Z.; Demirci, T.; Aşcı, Ö.A.; Baydar, N.G. Brassinosteroids modify yield, quality, and antioxidant components in Grapes (Vitis vinifera cv. Alphonse lavallée). J. Plant Growth Regul. 2020, 39, 147–156. [Google Scholar] [CrossRef]
- Pakkish, Z.; Ghorbani, B.; Najafzadeh, R. Fruit quality and shelf life improvement of grape cv. Rish Baba using Brassinosteroid during cold storage. J. Food Meas. Charact. 2019, 13, 967–975. [Google Scholar] [CrossRef]
- Champa, W.H.; Gill, M.I.S.; Mahajan, B.V.C.; Aror, N.K.; Bedi, S. Brassinosteroids improve quality of table grapes (Vitis vinifera L.) cv. flame seedless. Trop. Agric. Res. 2015, 26, 368. Available online: http://192.248.43.153/bitstream/1/2985/2/PGIATAR_26_2_368.pdf (accessed on 31 December 2015). [CrossRef]
- IŞÇI, B. Yield and quality of Sultani grapes (Vitis vinifera L.) treated with 28-homobrassinolide and gibberellic acid. Appl. Ecol. Environ. Res. 2019, 17, 12441–12450. Available online: https://www.epa.hu/02500/02583/00061/pdf/EPA02583_applied_ecology_2019_5_1244112450.pdf (accessed on 31 July 2019). [CrossRef]
- Liu, Q.; Xi, Z.; Gao, J.; Meng, Y.; Lin, S.; Zhang, Z. Effects of exogenous 24-epibrassinolide to control grey mould and maintain postharvest quality of table grapes. Int. J. Food Sci. Technol. 2016, 51, 1236–1243. [Google Scholar] [CrossRef]
- Vergara, A.E.; Díaz, K.; Carvajal, R.; Espinoza, L.; Alcalde, J.A.; Pérez-Donoso, A.G. Exogenous applications of brassinosteroids improve color of red table grape (Vitis vinifera L. Cv.“Redglobe”) berries. Front. Plant Sci. 2018, 9, 363. [Google Scholar] [CrossRef]
- Tadayon, M.S.; Moafpourian, G. Effects of Exogenous epi-brassinolid, zinc and boron foliar nutrition on fruit development and ripening of grape (Vitis vinifera L. clv.‘Khalili’). Sci. Hortic. 2019, 244, 94–101. [Google Scholar] [CrossRef]
- Zhou, Y.; Yuan, C.; Ruan, S.; Zhang, Z.; Meng, J.; Xi, Z. Exogenous 24-epibrassinolide interacts with light to regulate anthocyanin and proanthocyanidin biosynthesis in cabernet sauvignon (Vitis vinifera L.). Molecules 2018, 23, 93. [Google Scholar] [CrossRef]
- Xu, F.; Gao, X.; Xi, Z.M.; Zhang, H.; Peng, X.Q.; Wang, Z.Z.; Meng, Y. Application of exogenous 24-epibrassinolide enhances proanthocyanidin biosynthesis in Vitis vinifera ‘Cabernet Sauvignon’berry skin. Plant Growth Regul. 2015, 75, 741–750. [Google Scholar] [CrossRef]
- Belal, B.E. Improvement of physical and chemical properties of Thompson Seedless grapes (H4 Strain) by application of Brassinolide and Gibberellic acid. Egypt. J. Hortic. 2019, 46, 251–262. [Google Scholar] [CrossRef]
- Xi, Z.M.; Zhang, Z.W.; Huo, S.S.; Luan, L.Y.; Gao, X.; Ma, L.N.; Fang, Y.L. Regulating the secondary metabolism in grape berry using exogenous 24-epibrassinolide for enhanced phenolics content and antioxidant capacity. Food Chem. 2013, 141, 3056–3065. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.T.; Chen, Z.Y.; Jiang, Y.; Duan, B.B.; Xi, Z.M. Involvement of ABA and antioxidant system in brassinosteroid-induced water stress tolerance of grapevine (Vitis vinifera L.). Sci. Hortic. 2019, 256, 108596. [Google Scholar] [CrossRef]
- Zareei, E.; Zaare-Nahandi, F.; Oustan, S.; Hajilou, J. Effects of magnetic solutions on some biochemical properties and production of some phenolic compounds in grapevine (Vitis vinifera L.). Sci. Hortic. 2019, 253, 217–226. [Google Scholar] [CrossRef]
- Li, J.; Li, X.; Wang, L.; Wang, S. The Regulation of Brassinolide Biosynthesis and Physiological Effects in Grape Berries. Mol. Plant Breed. 2021, 12, 1–10. Available online: https://genbreedpublisher.com/index.php/mpb/article/view/3854 (accessed on 31 October 2021). [CrossRef]
- Yan, H.E.; Yanli, S.U.N.; Fangfang, Z.H.A.O.; Hongjun, D.A.I. Effect of Exogenous Brassinolides Treatment on Sugar Metabolism of Merlot Grape Berries. Acta Hortic. Sin. 2022, 49, 117. [Google Scholar] [CrossRef]
- Asghari, M.; Rezaei-Rad, R. 24-Epibrassinolide enhanced the quality parameters and phytochemical contents of table grape. J. Appl. Bot. Food Qual. 2018, 91, 226–231. [Google Scholar] [CrossRef]
- Chen, Z.Y.; Wang, Y.T.; Pan, X.B.; Xi, Z.M. Amelioration of cold-induced oxidative stress by exogenous 24-epibrassinolide treatment in grapevine seedlings: Toward regulating the ascorbate–glutathione cycle. Sci. Hortic. 2019, 244, 379–387. [Google Scholar] [CrossRef]
- Babalik, Z. Increasing of Phenolic Compounds by Brassinosteroid Applications in Immobilized Cell Suspension Cultures of Vitis vinifera L. cv. Cinsault. J. Agric. Sci. 2021, 27, 298–303. [Google Scholar] [CrossRef]
- Luan, L.Y.; Zhang, Z.W.; Xi, Z.M.; Huo, S.S.; Ma, L.N. Brassinosteroids regulate anthocyanin biosynthesis in the ripening of grape berries. S. Afr. J. Enol. Vitic. 2013, 34, 196–203. [Google Scholar] [CrossRef]
- Vergara, A.; Torrealba, M.; Alcalde, J.A.; Pérez-Donoso, A.G. Commercial brassinosteroid increases the concentration of anthocyanin in red tablegrape cultivars (Vitis vinifera L.). Aust. J. Grape Wine Res. 2020, 26, 427–433. [Google Scholar] [CrossRef]
- Wang, Z.; Jiang, Y.; Peng, X.; Xu, S.; Zhang, H.; Gao, J.; Xi, Z. Exogenous 24-epibrassinolide regulates antioxidant and pesticide detoxification systems in grapevine after chlorothalonil treatment. Plant Growth Regul. 2017, 81, 455–466. [Google Scholar] [CrossRef]
- Campbell, J.; Sarkhosh, A.; Habibi, F.; Gajjar, P.; Ismail, A.; Tsolova, V.; El-Sharkawy, I. Evaluation of biochemical juice attributes and color-related traits in muscadine grape population. Foods 2021, 10, 1101. [Google Scholar] [CrossRef]
- Agasse, A.; Vignault, C.; Kappel, C.; Conde, C.; Gerós, H.; Delrot, S. Sugar transport & sugar sensing in grape. In Grapevine Molecular Physiology & Biotechnology; Springer: Dordrecht, The Netherlands, 2009; pp. 105–139. [Google Scholar] [CrossRef]
- Zeng, J.; Haider, M.S.; Huang, J.; Xu, Y.; Pervaiz, T.; Feng, J.; Tao, J. Functional Characterization of VvSK Gene Family in Grapevine Revealing Their Role in Berry Ripening. Int. J. Mol. Sci. 2020, 21, 4336. [Google Scholar] [CrossRef]
- Etienne, A.; Génard, M.; Lobit, P.; Mbeguié-A-Mbéguié, D.; Bugaud, C. What controls fleshy fruit acidity? A review of malate and citrate accumulation in fruit cells. J. Exp. Bot. 2013, 64, 1451–1469. [Google Scholar] [CrossRef]
- Wu, W.; Zhu, Q.G.; Wang, W.Q.; Grierson, D.; Yin, X.R. Molecular basis of the formation and removal of fruit astringency. Food Chem. 2022, 372, 131234. [Google Scholar] [CrossRef]
- Liu, M.Y.; Song, C.Z.; Chi, M.; Wang, T.M.; Zuo, L.L.; Li, X.L.; Xi, Z.M. The effects of light and ethylene and their interaction on the regulation of proanthocyanidin and anthocyanin synthesis in the skins of Vitis vinifera berries. Plant Growth Regul. 2016, 79, 377–390. [Google Scholar] [CrossRef]
- Ma, W.; Guo, A.; Zhang, Y.; Wang, H.; Liu, Y.; Li, H. A review on astringency and bitterness perception of tannins in wine. Trends Food Sci. Technol. 2014, 40, 6–19. [Google Scholar] [CrossRef]
- Lin, J.; Massonnet, M.; Cantu, D. The genetic basis of grape and wine aroma. Hortic. Res. 2019, 6, 81. [Google Scholar] [CrossRef]
- El Hadi, M.A.M.; Zhang, F.J.; Wu, F.F.; Zhou, C.H.; Tao, J. Advances in fruit aroma volatile research. Molecules 2013, 18, 8200–8229. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, Y.; Lin, K.; Wang, B.; Shi, X.; Cheng, W. June Comparison of sugars, organic acids and aroma components of five table grapes in Xinjiang. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2021; p. 012029. Available online: https://iopscience.iop.org/article/10.1088/1755-1315/792/1/012029/meta (accessed on 30 May 2021).
- Yang, Y.; Jin, G.J.; Wang, X.J.; Kong, C.L.; Liu, J.; Tao, Y.S. Chemical profiles and aroma contribution of terpene compounds in Meili (Vitis vinifera L.) grape and wine. Food Chem. 2019, 284, 155–161. [Google Scholar] [CrossRef]
- Ferreira, V.; Lopez, R. The actual and potential aroma of winemaking grapes. Biomolecules 2019, 9, 818. [Google Scholar] [CrossRef]
- Chappell, J.; Wolf, F.; Proulx, J.; Cuellar, R.; Saunders, C. Is the reaction catalyzed by 3-hydroxy-3-methylglutaryl coenzyme A reductase a rate-limiting step for isoprenoid biosynthesis in plants? Plant Physiol. 1995, 109, 1337–1343. [Google Scholar] [CrossRef]
- Wu, L.; Chen, Y.; Gao, M.; Zhao, Y.; Wang, Y. Sir2 family proteins regulate terpenoid synthesis by deacetylation of 3-hydroxy-3-methylglutaryl-CoA synthase. Ind. Crops Prod. 2021, 170, 113770. [Google Scholar] [CrossRef]
- Zheng, T.; Guan, L.; Yu, K.; Haider, M.S.; Nasim, M.; Liu, Z.; Fang, J. Expressional diversity of grapevine 3-Hydroxy-3-methylglutaryl-CoA reductase (VvHMGR) in different grapes genotypes. BMC Plant Biol. 2021, 21, 279. [Google Scholar] [CrossRef]
- Suzuki, M.; Kamide, Y.; Nagata, N.; Seki, H.; Ohyama, K.; Kato, H.; Muranaka, T. Loss of function of 3-hydroxy-3-methylglutaryl coenzyme A reductase 1 (HMG1) in Arabidopsis leads to dwarfing, early senescence and male sterility, and reduced sterol levels. Plant J. 2004, 37, 750–761. [Google Scholar] [CrossRef]
- Kyraleou, M.; Kallithraka, S.; Gkanidi, E.; Koundouras, S.; Mannion, D.T.; Kilcawley, K.N. Discrimination of five Greek red grape varieties according to the anthocyanin and proanthocyanidin profiles of their skins and seeds. J. Food Compos. Anal. 2020, 92, 103547. [Google Scholar] [CrossRef]
- Yue, X.; Zhao, Y.; Ma, X.; Jiao, X.; Fang, Y.; Zhang, Z.; Ju, Y. Effects of leaf removal on the accumulation of anthocyanins and the expression of anthocyanin biosynthetic genes in Cabernet Sauvignon (Vitis vinifera L.) grapes. J. Sci. Food Agric. 2021, 101, 3214–3224. [Google Scholar] [CrossRef]
- de Rosas, I.; Ponce, M.T.; Malovini, E.; Deis, L.; Cavagnaro, B.; Cavagnaro, P. Loss of anthocyanins and modification of the anthocyanin profiles in grape berries of Malbec and Bonarda grown under high temperature conditions. Plant Sci. 2017, 258, 137–145. [Google Scholar] [CrossRef]
- Sun, L.; Li, S.; Tang, X.; Fan, X.; Zhang, Y.; Jiang, J.; Liu, C. Transcriptome analysis reveal the putative genes involved in light-induced anthocyanin accumulation in grape ‘Red Globe’ (V. vinifera L.). Gene 2020, 728, 144284. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; He, S.; Liu, Y.; Liu, B.; Ju, Y.; Kang, D.; Fang, Y. Transcriptomics integrated with metabolomics reveals the effect of regulated deficit irrigation on anthocyanin biosynthesis in Cabernet Sauvignon grape berries. Food Chem. 2020, 314, 126170. [Google Scholar] [CrossRef] [PubMed]
- Panić, M.; Gunjević, V.; Cravotto, G.; Redovniković, I.R. Enabling technologies for the extraction of grape-pomace anthocyanins using natural deep eutectic solvents in up-to-half-litre batches extraction of grape-pomace anthocyanins using NADES. Food Chem. 2019, 300, 125185. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Xi, Z.; Huo, S.; Luan, L.; Gao, X.; Zhao, X. Studies on the regulation of anthocyanin biosynthesis in grape berry by brassinosteroid and abscisic acid. J. Fruit Sci. 2012, 29, 830–836. Available online: https://www.cabdirect.org/cabdirect/abstract/20133014426 (accessed on 30 November 2016).
- Yang, N.; Zhou, Y.; Wang, Z.; Zhang, Z.; Xi, Z.; Wang, X. Emerging roles of brassinosteroids and light in anthocyanin biosynthesis and ripeness of climacteric and non-climacteric fruits. Crit. Rev. Food Sci. Nutr. 2021, 2004579, 1–13. [Google Scholar] [CrossRef]
- Sachdev, S.; Ansari, S.A.; Ansari, M.I.; Fujita, M.; Hasanuzzaman, M. Abiotic stress and reactive oxygen species: Generation, signaling, and defense mechanisms. Antioxidants 2021, 10, 277. [Google Scholar] [CrossRef]
- Savoi, S.; Wong, D.C.; Arapitsas, P.; Miculan, M.; Bucchetti, B.; Peterlunger, E.; Castellarin, S.D. Transcriptome and metabolite profiling reveals that prolonged drought modulates the phenylpropanoid and terpenoid pathway in white grapes (Vitis vinifera L.). BMC Plant Biol. 2016, 16, 67. [Google Scholar] [CrossRef]
- Abdulkadhim, S.J.; Hadi, A.A.A. Effectiveness of Brassinolide and Dry Yeast Extract Spraying on Growth Parameters and the Chemical Content of the Grape Seedlings. Available online: https://www.researchgate.net/publication/342924750 (accessed on 31 October 2019).
- Iqbal, N.; Khan, N.A.; Ferrante, A.; Trivellini, A.; Francini, A.; Khan, M.I.R. Ethylene role in plant growth, development and senescence: Interaction with other phytohormones. Front. Plant Sci. 2017, 8, 475. [Google Scholar] [CrossRef]
- Symons, G.M.; Davies, C.; Shavrukov, Y.; Dry, I.B.; Reid, J.B.; Thomas, M.R. Grapes on steroids. Brassinosteroids are involved in grape berry ripening. Plant Physiol. 2006, 140, 150–158. [Google Scholar] [CrossRef]
- Li, N.; Euring, D.; Cha, J.Y.; Lin, Z.; Lu, M.; Huang, L.J.; Kim, W.Y. Plant hormone-mediated regulation of heat tolerance in response to global climate change. Front. Plant Sci. 2021, 11, 627969. [Google Scholar] [CrossRef]
- Hu, Y.; Yu, D. BRASSINOSTEROID INSENSITIVE2 interacts with ABSCISIC ACID INSENSITIVE5 to mediate the antagonism of brassinosteroids to abscisic acid during seed germination in Arabidopsis. Plant Cell 2014, 26, 4394–4408. [Google Scholar] [CrossRef]
- Divi, U.K.; Rahman, T.; Krishna, P. Brassinosteroid-mediated stress tolerance in Arabidopsis shows interactions with abscisic acid, ethylene and salicylic acid pathways. BMC Plant Biol. 2010, 10, 151. [Google Scholar] [CrossRef] [PubMed]
- Nemhauser, J.L.; Mockler, T.C.; Chory, J.; Dangl, J. Interdependency of brassinosteroid and auxin signaling in Arabidopsis. PLoS Biol. 2004, 2, e258. [Google Scholar] [CrossRef]
- Wang, D.; Yang, Z.; Wu, M.; Wang, W.; Wang, Y.; Nie, S. Enhanced brassinosteroid signaling via the overexpression of SlBRI1 positively regulates the chilling stress tolerance of tomato. Plant Sci. 2022, 320, 111281. [Google Scholar] [CrossRef] [PubMed]
- De Vleesschauwer, D.; Van Buyten, E.; Satoh, K.; Balidion, J.; Mauleon, R.; Choi, I.R.; Höfte, M. Brassinosteroids antagonize gibberellin-and salicylate-mediated root immunity in rice. Plant Physiol. 2012, 158, 1833–1846. [Google Scholar] [CrossRef] [PubMed]
- Saud, S.; Shi, Z.; Xiong, L.; Danish, S.; Datta, R.; Ahmad, I.; Banout, J. Recognizing the basics of phytochrome-interacting factors in plants for abiotic stress tolerance. Plant Stress 2022, 3, 100050. [Google Scholar] [CrossRef]



| S. No | Compounds | Best Concentration (From Literature Sources) | Best Concentration (Concerted to Molar) | Variety | Age of Grapevine | Key Findings |
|---|---|---|---|---|---|---|
| 1 | Brassinosteroid | 100 μmol L−1 | 100 μmol L−1 | Kyoho | 5-year | BR treatment improved grape pericarp coloring at various phases of fruit development, which had most noticeable effect occurring at the start of veraison [12]. |
| 2 | Brassinosteroid | 0.2 mg L−1 | 0.42 μmol L−1 | Alphonse Lavallée | 9-year | Responsible for the maximum berry weight (7.65 g and 7.87 g), cluster weight (374.98 g and 418.75 g), and yield production (26.25 vine kg−1 and 30.15 vine kg−1) in both production years, respectively [36]. |
| 3 | Brassinosteroid | 1.5 ppm | 4.58 μmol L−1 | Rish Baba | Not mentioned | 1.5 ppm BR treatment was effective and responsible for reducing single fruit weight loss from 29.75% to 29.48% under cold storage for 5 weeks [37]. |
| 4 | Brassinosteroid | 0.5 ppm and 1 ppm | 1.53 μmol L−1 and 3.05 μmol L−1 | Flame Seedless | 12-year | 0.5 ppm and 1 ppm of BR significantly increased berry weight (2.55 and 2.57 g), berry length (1.84 and 1.89 cm), and berry breadth (1.73 and 1.74 cm) in comparison to the control group (2.38, 1.71, and 1.59 cm, respectively) [38]. |
| 5 | Brassinosteroid | 0.5 ppm and 1 ppm | 1.53 μmol L−1 and 3.05 μmol L−1 | Flame Seedless | 12-year | The application of BRs on the grape cluster effectively delayed the deviation rates of L*, A*, and B* and was responsible for color changing from relatively pure green to yellow and subsequently to red [38]. |
| 6 | 24-Epibrassinosteroid | 0.4 mg L−1 | 0.83 μmol L−1 | Khalili | 8-year | 0.4 mg L−1 EBR significantly increased the cluster weight (223.77 g), which was higher than that of 0.2 mg L−1 EBR treatment (208.30 g) and the control group (185.69 g). As well, the cluster length of 0.4 mg L−1 (25.26 cm) was higher than that of 0.2 mg L−1 EBR treatment (23.16 cm) and the control group (19.29 cm) [42]. |
| 7 | 24-Epibrassinolide | 0.4 mg L−1 | 0.83 μmol L−1 | Cabernet Sauvignon | 7-year | Exogenous EBL promoted the total weight of 100-berry of Cabernet Sauvignon (~140 g) as compared to the control group (~135 g) after 46 days of treatment [43]. |
| 8 | 24-Epibrassinolide | 0.4 mg L−1 | 0.83 μmol L−1 | Cabernet Sauvignon | 5-year | The EBL treatment (twice at fruit setting), and EBL application (once at fruit set stage), were effective for the development of cluster weights (190 g and 170 g, respectively) in 116 DAA, in comparison with the control group (140 g) [44]. |
| 9 | Brassinolide | 0.5 ppm | 1.53 μmol L−1 | Thompson seedless grapes | 4-year | BL could significantly improve the cluster weight and yield of grape berries, which was 1–2 times higher than that of the control group [45]. |
| 10 | 24-Epibrassinolide | 0.4 mg L−1 | 0.83 μmol L−1 | Cabernet Sauvignon | Not mentioned | The single berry weight (~1.3 g) of grapes sprayed with EBL was significantly higher than that of the control group (~1.2 g) [46]. |
| 11 | 24-Epibrassinolide | 0.4 mg L−1 | 0.83 μmol L−1 | Yan 73 | Not mentioned | The single berry weight (~1.5 g) of grapes sprayed with EBL was significantly higher than that of the control group (~1.6 g) [46]. |
| 12 | ABA, Brassinosteroid, ABA + Brassinosteroid | 10 μmol L−1 | 10 μmol L−1 | Shine Muscat | 8-year | At 12, 24, 36, and 48 h, BR treatment gained the weight (69.39, 69.18, 68.99, and 68.89, respectively), followed by BR + ABA (65.87, 65.65, 65.46, and 65.39, respectively), control (65.77, 65.55, 65.30, and 65.23, respectively), and ABA (65.74, 65.53, 65.33, and 65.26, respectively) [13]. |
| 13 | 28-homobrassinolide | 8 ppm | 16.19 μmol L−1 | Sultani | 12-year | Exogenous application of 28-homobrassinolide (8 ppm) improved fruit firmness (7.11 N) more in grape berries than the control group (6.19 N) [39]. |
| 14 | 24-Epibrassinolide | 0.8 mg L−1 | 1.66 μmol L−1 | Red globe | 5-year | The fruit firmness was decreased after 15 days (5.25 N), 30 days (5.15 N), and 60 days (4.98 N) of storage under 0.8 mg L−1 EBL treatment [40]. |
| 15 | 3α-hydroxy-20-RB-homo-7-oxa-5α-cholestan-6-one | 0.4 mg L−1 | 0.83 μmol L−1 | Red globe | 16-year | Exogenous BR significantly contributed to the CIRG (the color parameter of red grape variety, which was evaluated according to CIELAB parameters L* (brightness), H (tone angle), and C (chroma) of sixteen-year-old self-rooted “Redglobe” grapevine [41]. |
| 16 | 24-Epibrassinosteroid | 0.1 μmol L−1 | 0.1 μmol L−1 | Cabernet Sauvignon grape seedlings | Not mentioned | EBR treatment was more effective in alleviating the damage of grapevine phenotypes under water stress. Compared with the control group, EBR had less damage (moderate dehydration, drooping and curling of leaves) [47]. |
| S. No | Compounds | Best Concentration (From Literature Sources) | Best Concentration (Concerted to Molar) | Variety | Age of Grapevine | Key Findings |
|---|---|---|---|---|---|---|
| 1 | Brassinosteroid | 100 μmol L−1 | 100 μmol L−1 | Kyoho | 5-year | BRs applied at the commencement of veraison on grapes had little influence on the content of organic acids. After 20 DAT (days after treatments), tartaric acid concentrations dropped from 80 mg g−1 to 70 mg g−1 and remained low during postorbital storage (60 mg g−1). Meanwhile, the content of organic acids in grape pericarp was higher (an average of 90 mg g−1) than in berry flesh (an average of 70 mg g−1), and tartaric acid accounted for a leading organic acid (more than 50%) [12]. |
| 2 | Brassinosteroid | 100 μmol L−1 | 100 μmol L−1 | Kyoho | 5-year | At 30 days after BR treatment, anthocyanin content was 0.0998 mg g−1 in the BR treatment group, the value was higher than in control groups of the initiation of the veraison, half veraison stage (0.0560 mg g−1), and the full veraison stage (0.0281 mg g−1). During postharvest storage, the anthocyanin content of BR-treated grapes was higher than that of control-treated grapes [12]. |
| 3 | Brassinosteroid | 0.6 mg L−1 | 1.25 μmol L−1 | Alphonse Lavallée | 9-year | In both growing years, compared with the control group (42.82 mg 100 g−1, 49.53 mg 100 g−1) and other BR concentrations, the application of 0.6 mg L−1 of BR to vines three times (7 days after berry set + veraison + 30 days after veraison) furnished the maximum anthocyanin content (75.89 mg 100 g−1 and 86.90 mg 100 g−1) [36]. |
| 4 | 24-Epibrassinosteroid | 0.4 mg L−1 | 0.83 μmol L−1 | Khalili | 8-year | EBR significantly increased the total soluble solid content (22.26 °Bx), which was higher than that of 0.2 mg L−1 EBR treatment (21.33 °Bx) and the control group (18.94 °Bx) [42]. |
| 5 | 24-Epibrassinolide | 0.4 mg L−1 | 0.83 μmol L−1 | Cabernet Sauvignon | 7-year | The highest total anthocyanin contents were observed in 0.4 mg L−1 EBL + light condition treatment (0.862 mg g−1), following 0.4 mg L−1 EBL with dark condition (0.024 mg g−1) and control group (dark condition; 0.0089 mg g−1). The 0.4 mg L−1 EBL with light condition treatment (11.55 mg g−1) significantly increased the content of malvidin-3-O-glucoside [43]. |
| 6 | 24-Epibrassinolide | 0.4 mg L−1 | 0.83 μmol L−1 | Cabernet Sauvignon | 5-year | The EBL treatments (twice at the fruit set stage) were more effective for the production of total tannin (95 mg g−1) in 60 DAA, followed by EBL application (once at fruit set stage) and the control group (70 mg g−1) [44]. |
| 7 | 24-Epibrassinolide | 0.40 mg L−1 | 0.83 μmol L−1 | Cabernet Sauvignon and Yan 73 | Not mentioned | The total soluble solids (19 °Bx and 18 °Bx, respectively) or reducing sugar content (167 g L−1 and 165 g L−1, respectively) of grapes sprayed with EBL were significantly higher than that of the control group (Total soluble solids: 17 °Bx and 15 °Bx, respectively; reducing sugar: 160 g L−1 and 150 g L−1, respectively) [46]. |
| 8 | 24-Epibrassinolide | 0.4 mg L−1 | 0.83 μmol L−1 | Yan 73 and Cabernet Sauvignon | Not mentioned | EBL treatment could promote the production of DPPH, ABTS, and HRSA (secondary metabolites and antioxidant parameters) in these two grape varieties, as well as an increase of anthocyanin monomers such as TAC, TFOC, TPC, and TTC by 16.0%, 8.2%, 40.0%, and 18.2% respectively, in “Cabernet Sauvignon” grape berry and 28.0%, 9.4%, 19.4%, and 21.9%, respectively, in “Yan 73” grape berry [46]. |
| 9 | ABA, Brassinosteroid, ABA + Brassinosteroid | 10 μmol L−1 | 10 μmol L−1 | Shine Muscat | 8-year | The application of BR on the grapevine after 12 and 48 h were significantly increased the tartaric acid content (0.5 mg g−1 and 0.5 mg g−1, respectively) and total organic acid content (0.9 mg g−1 and 0.8 mg g−1, respectively) as compared to control group (tartaric acid content: 0.4 mg g−1 and 0.4 mg g−1, respectively; total organic acid content: 0.8 mg g−1 and 0.8 mg g−1, respectively) during the fruit maturity stage [13]. |
| 10 | 28-homobrassinolide | 0.4 mg L−1 | 1.22 μmol L−1 | Redglobe | 16-year | The content of cyanidin-3-glucoside and peonidin-3-glucosidein (anthocyanin compounds) in the grape pericarp was ~1.5 times more than the control group under BL treatment [39]. |
| 11 | 24-Epibrassinolide | 0.6 mg L−1 | 1.25 μmol L−1 | Merlot | 10-year | EBL treatment significantly enhanced the accumulation of monosaccharides in grape berries, including an increase in glucose and fructose content (16.95% and 39.31%, respectively) at the maturity stage, compared to the control [50]. |
| 12 | 24-Epibrassinolide | 3 μmol L−1 and 6 μmol L−1 | 3 μmol L−1 and 6 μmol L−1 | Thompson Seedless | 12-year | 3 and 6 μmol L−1 EBL significantly increased TSS levels (22 °Bx, 22.5 °Bx, respectively) as compared to the control group (18.5 °Bx) [51]. |
| 13 | 24-Epibrassinolide | 3 μmol L−1 and 6 mol L−1 | 3 μmol L−1 and 6 μmol L−1 | Thompson Seedless | 12-year | 3 and 6 μM EBL significantly increased TSS levels (22 °Bx, 22.5 °Bx, respectively) as compared to the control group (18.5 °Bx) [51]. |
| 14 | 24-Epibrassinolide | 3 μmol L−1 | 3 μmol L−1 | Thompson seedless | 12-year | EBL treatments could significantly increase total organic acid content with the value of 0.675 g 100 g−1 and 0.670 g 100 g−1, respectively [51]. |
| 15 | 24-Epibrassinolide | 0.1 mg L−1 | 0.31 μmol L−1 | Cabernet Sauvignon | 1-year | The application of exogenous EBL significantly increased ascorbic acid (AsA) after 24 h treatment (~190 mg 100 g−1) and 48 h treatment (~200 mg 100 g−1) compared with the control group (~140 mg 100 g−1, ~150 mg 100 g−1, respectively) [52]. |
| 16 | 24-Epibrassinolide | 0.1 mg L−1 | 0.21 μmol L−1 | Cabernet Sauvignon | 1-year | Exogenous application of EBL improved the ability of one-year-old “Cabernet Sauvignon” grape seedlings to resist low-temperature stress, as evidenced by a significant decrease in H2O2 (9 mol g−1) and ROS (14 g g−1) after EBL 6 h treatment compared to the control group [52]. |
| 17 | 24-Epibrassinosteroid | 0.5 mg L−1 and 0.75 mg L−1 | 1.04 μmol L−1 and 1.56 μmol L−1 | Cinsaul | Not mentioned | 0.5 mg L−1 and 0.75 mg L−1 of EBR significantly increased total tannins content (37.54 mg g−1 and 46.66 mg g−1, respectively) compared to the control group (29.76 mg g−1) [53]. |
| 18 | 24-Epibrassinosteroid | 0.4 mg L−1 | 0.83 μmol L−1 | Cabernet Sauvignon | 6-year | Exogenous EBR was the most effective treatment for increasing total anthocyanin content and significant color development was observed 7 days earlier than in the control group [54]. |
| 19 | 24-Epibrassinosteroid | 0.4 mg L−1 | 0.83 μmol L−1 | Cabernet Sauvignon | 6-year | EBR was responsible for increasing total anthocyanins production in grape pericarp at late fruit ripening stage (3.913 mg g−1) compared to the control group (3.369 mg g−1) [54]. |
| 20 | BR, BR treated twice after the first BRs | 0.06 mg L−1 | 0.18 μmol L−1 | Red Globe and Crimson Seedless | 6-year | They found that BR had significantly greater anthocyanin concentrations in Redglobe (~1.45 mg berry−1) and Crimson Seedless (~4.5 mg berry−1) grape berries, in comparison to control (~1.2 and ~3 mg berry−1, respectively) [55]. |
| 21 | 24-Epibrassinolide | 0.42 μmol L−1 and 0.21 μmol L−1 | 0.42 μmol L−1 and 0.21 μmol L−1 | Cabernet Sauvignon | 1-year | On the 3rd–5th day after EBL treatment, the total protein content of EBL1 (0.21 μM) and EBL2 (0.42 μM) treatment increased by 12% and 13%, respectively, compared with the control [56]. |
| S. No | Compounds | Best Concentration (From Literature Sources) | Best Concentration (Concerted to Molar) | Variety | Age of Grapevine | Key Findings |
|---|---|---|---|---|---|---|
| 1 | Brassinosteroid | 100 μmol L−1 | 100 μmol L−1 | Kyoho | 5-year | Exogenous BRs could change the aroma composition of grapes and increase the proportion of terpenes compounds such as α-pinene (0.03%), d-limonene (0.07%), and γ-terpene (63.67%) compared with the control group (0.01%, 0.04%, and 62.96%, respectively) [12]. |
| 2 | Brassinosteroid | 10 μmol L−1 | 10 μmol L−1 | Shine Muscat | 8-year | Exogenous BR plays an essential role in boosting the concentration of alcohols and aldehydes in eight-year-old “Shine Muscat” grape berries, as evidenced by a two-fold rise in 1-pentanol and sinapyl alcohol proportions than the control group after 48 h treatment. Additionally, the alcoholic compounds such as 1-hexanol and acetoin proportion (4.68% and 10.35%, respectively) were significantly more in content than the control group (2.92%, 5.73%, respectively). In the case of aldehydes, the proportions of methyl glyoxal (12.12%), pentanal (2.31%), and hexanal (22.96%) were found in abundance when compared with the control group (0%, 2.08%, and 15.27%, respectively) [13]. |
| S. No | Compounds | Best Concentration (From Literature Sources) | Best Concentration (Concerted to Molar) | Variety | Age of Grapevine | Key Findings |
|---|---|---|---|---|---|---|
| 1 | 24-Epibrassinolide | 0.4 mg L−1 | 0.83 μmol L−1 | Cabernet Sauvignon | 7-year | Exogenous EBL promoted the accumulation of total anthocyanins (~5 mg g−1) in grape berries compared to the control group (~2.5 mg L−1) and developed its pericarp color after 46 days treatment by up-regulating the expressions of genes VvCHI1, VvCHS3, VvF3′5′H, VvDFR, and VvUFGT (about 1.2–3 times increment) [43]. |
| 2 | 24-Epibrassinolide | 0.4 mg L−1 | 0.83 μmol L−1 | Cabernet Sauvignon | 7-year | MYB5a, MYB5b, and MYBPA1, which interacted with anthocyanin biosynthesis structural genes. As seen in the late stage of grape berry ripening (120 days after anthesis), the EBL treatment (0.4 mg L−1) dramatically boosted the relative expressions of MYB5a (~0.5), MYB5b (~0.25), and MYBPA1 (~7) at 46 days after treatment as compared to control (~0.2, ~0.15, ~2, respectively) [43]. |
| 3 | 24-Epibrassinolide | 0.4 mg L−1 | 0.83 μmol L−1 | Cabernet Sauvignon | 5-year | EBL treatment on grape increased the transcription levels of VvHT3, VvHT4, VvHT5, and VvHT6 (monosaccharide-coding genes) at various stages of berry development, including half veraison stage and maturity stage, but had little effect on VvHT1 and VvHT2 expressions. The transcription level of VvSUC27 (genes encoding disaccharide) was also considerably greater in grape berries after EBL treatment [44]. |
| 4 | ABA, Brassinosteroid, ABA + Brassinoster-oid | 10 μmol L−1 | 10 μmol L−1 | Shine Muscat | 8-year | The expression level of VvHsfA2, VvGols1 and VvHSP17.9 (stress resistance related genes) were all higher in BR-treated groups than that of in the control group (~1–1.5 times higher) [13]. |
| 5 | 24-Epibrassinolide | 0.6 mg L−1 | 1.25 μmol L−1 | Merlot | 10-year | EBL significantly increased the expression of VvSS (Sucrose biosynthesis related gene) compared to the control group (around 2.75 times higher) [50]. |
| 6 | 24-Epibrassinosteroid | 1.5 μmol L−1 | 1.5 μmol L−1 | Shine Muscat | 5-year | The application of EBR and 1.5 μmol L−1 on grape berries significantly promoted soluble solids accumulation by inhibition of VvSKs’ (glycogen biosynthesis related genes) expression [59]. |
| 7 | 24-Epibrassinolide | 0.1 μmol L−1 | 0.1 μmol L−1 | Cabernet Sauvignon | 1-year | The expression of GST (pesticide degradation related gene) under EBL treatment was fourfold higher than that of the control group at third day after treatment and increased twofold at fifth day after treatment [56]. |
| 8 | 24-Epibrassinolide | 0.1 μmol L−1 | 0.1 μmol L−1 | Cabernet Sauvignon | 1-year | The expression of MRP (pesticide degradation related gene) increased slightly in EBL treatment compared with the control group (~1.5 times higher). The transcription level of P450 (pesticide degradation related gene) increased sharply to 4–6 times that of the control group after the application of EBL [56]. |
| S. No | Compounds | Best Concentration (From Literature Sources) | Best Concentration (Concerted to Molar) | Variety | Age of Grapevine | Key Findings |
|---|---|---|---|---|---|---|
| 1 | Brassinosteroid | 100 μmol L−1 | 100 μmol L−1 | Kyoho | 5-year | The HMGR activity under exogenous BR treatment was significantly higher (10 times up-regulation) than the control group at the beginning and half veraison stages of grapevine [12]. |
| 2 | Brassinosteroid | 0.75 ppm | 2.29 μmol L−1 | Rish Baba | Not mentioned | Superoxide dismutase (SOD, ~50 U mg−1) activities of exogenous BR treatments were significantly higher than those of control group (~20 U mg−1, ~25 U mg−1, respectively) [37]. |
| 3 | Brassinosteroid | 1.5 ppm | 4.58 μmol L−1 | Rish Baba | Not mentioned | Exogenous BR grapes has significantly increased the levels of catalase (CAT, ~55 U mg−1) and peroxidase (POD, ~40 U mg−1) while 0.75 ppm treatment responsible to enhance the activity of ascorbate peroxidase (APX, ~35 U mg−1) than the control group [37]. |
| 4 | 24-Epibrassinolide | 0.4 mg L−1 | 0.83 μmol L−1 | Cabernet Sauvignon | 5-year | EBL treatment in grape pericarp from days after application (DAA) 85 to 100, the glucose and fructose conversion enzymes “acidic invertase (INV) and neutral invertase (SuSyn)” significantly up-regulate their activity, while the 1.31 mg L−1 Brz (BR signaling inhibitor) application significantly reduced the acidic invertase (INV) activity and the INV activity at 60 DAA and 66 DAA, respectively [44]. |
| 5 | 24-Epibrassinolide | 0.4 mg L−1 | 1.22 μmol L−1 | Cabernet Sauvignon | 5-year | Exogenous EBL significantly up-regulated the activities of key rate-limiting enzymes such as UDP-glucose: flavonoid 3-O-glucosyl transferase (UFGT: ~9 mmol g−1) and phenylalanine ammonia-lyase (PAL, ~240 U g−1) in comparison with the control group (~5 mmol g−1 and ~210 U g−1, respectively) [46]. |
| 6 | 24-Epibrassinolide | 0.4 mg L−1 | 1.22 μmol L−1 | Yan 73 | 5-year | Exogenous EBL treatment had higher UFGT (~23 mmol g−1) and PAL (~170 U g−1) activity than the control group (~18 mmol g−1 and ~150 U g−1, respectively) [46]. |
| 7 | 24-Epibrassinolide | 0.4 mg L−1 | 0.83 μmol L−1 | Redglobe | Not mentioned | The POD activity of grape berries treated with EBL (~30 U g−1, ~40 U g−1, ~36 U g−1, respectively) was significantly higher than that of control group at the 2nd, 4th, and 6th day (~8 U g−1, ~12 U g−1, ~28 U g−1, respectively) [40]. |
| 8 | 24-Epibrassinolide | 0.8 mg L−1 | 1.66 μmol L−1 | Redglobe | Not mentioned | The SOD activity of grape berries treated with EBL (~50 U g−1, ~58 U g−1, respectively) was significantly higher than that of control group at the 2nd and 6th day (~35 U g−1, ~44 U g−1, respectively) [40]. |
| 9 | 24-Epibrassinosteroid | 0.1 μmol L−1 | 0.1 μmol L−1 | Cabernet Sauvignon grape seedlings | Not mentioned | EBR on grape seedlings under drought stress significantly enhanced the level of APX activity after 12 h (~1.1 U g−1) and 24 h (~1.15 U g−1) of treatment as compared to control (stressed and unstressed). The EBR application was also responsible for enhancing the considerable activity of glutathione reductase (GR) at 48 h and 72 h treatment (~0.045 U g−1 and ~0.04 U g−1, respectively) compared with the drought stress treatment group (~0.03 U g−1 and ~0.035 U g−1, respectively) [47]. |
| 10 | 24-Epibrassinolide | 0.6 mg L−1 | 1.25 μmol L−1 | Merlot | Ten-year-old | Exogenous EBL enhanced the activity of sucrose phosphate synthase (SPS), significantly up-regulated activities of cell wall acid invertase (VvcwINV), sucrose transporter (VvSUC12), and sucrose synthase (VvSS) during veraison to ripening stage [50]. |
| 11 | 24-Epibrassinolide | 3 μmol L−1 and 6 μmol L−1 | 3 μmol L−1 and 6 μmol L−1 | Thompson Seedless | 12-year | Both EBL applications (3 μmol L−1 and 6 μmol L−1) significantly promote the activities of polyphenol oxidase (PPO) with the value of ~12,000 U mg−1 and ~13,000 U mg−1, respectively). Interestingly, the content of total antioxidant activity (TAA) was significantly increased under 3 μmol L−1 EBL treatment (~4000 mmol 100 g−1) compared with the control group (~2000 mmol 100 g−1) [51]. |
| 12 | 24-Epibrassinolide | 0.1 mg L−1 | 0.21 μmol L−1 | Cabernet Sauvignon | 1-year | After 12 h of EBL treatment, there was a substantial increase in superoxide dismutase (SOD, 13 U g−1 min−1) compared to the control group (9 U g−1 min−1) [52]. |
| 13 | 24-Epibrassinolide | 0.1 μmol L−1 | 0.1 μmol L−1 | Cabernet Sauvignon | 1-year | EBL treatment (61.97 U mg−1 min−1) showed higher APX activity than the control group (37.33 U mg−1 min−1), and the average maximum increase of SOD activity was 25% higher than the control group [56]. |
| 14 | Brassinosteroid | 0.8 mg L−1 | 1.66 μmol L−1 | Yan 73 | 5-year | The activity of PAL gradually rose, whereas the activity of UFGT increased and subsequently dropped. When compared to the control group, a concentration of exogenous BR significantly improved the activity of PAL (130 U g−1) and UFGT (28 U g−1) during the late stages of fruit ripening [79]. |
| 15 | 24-Epibrassinolide | 0.8 mg L−1 | 2.44 μmol L−1 | Summer Roya | Not mentioned | The EBL application was also responsible for enhancing the considerable activity of photosynthesis enzymes (35.16 SPAD) compared with the control group and the 0.4 mg L−1 EBL treatment group (28.12 SPAD, 33.53 SPAD, respectively) [83]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Quan, Y.; Wang, L.; Wang, S. Brassinosteroid Promotes Grape Berry Quality-Focus on Physicochemical Qualities and Their Coordination with Enzymatic and Molecular Processes: A Review. Int. J. Mol. Sci. 2023, 24, 445. https://doi.org/10.3390/ijms24010445
Li J, Quan Y, Wang L, Wang S. Brassinosteroid Promotes Grape Berry Quality-Focus on Physicochemical Qualities and Their Coordination with Enzymatic and Molecular Processes: A Review. International Journal of Molecular Sciences. 2023; 24(1):445. https://doi.org/10.3390/ijms24010445
Chicago/Turabian StyleLi, Jiajia, Yi Quan, Lei Wang, and Shiping Wang. 2023. "Brassinosteroid Promotes Grape Berry Quality-Focus on Physicochemical Qualities and Their Coordination with Enzymatic and Molecular Processes: A Review" International Journal of Molecular Sciences 24, no. 1: 445. https://doi.org/10.3390/ijms24010445
APA StyleLi, J., Quan, Y., Wang, L., & Wang, S. (2023). Brassinosteroid Promotes Grape Berry Quality-Focus on Physicochemical Qualities and Their Coordination with Enzymatic and Molecular Processes: A Review. International Journal of Molecular Sciences, 24(1), 445. https://doi.org/10.3390/ijms24010445






