JMJD8 Functions as a Novel AKT1 Lysine Demethylase
Abstract
1. Introduction
2. Results
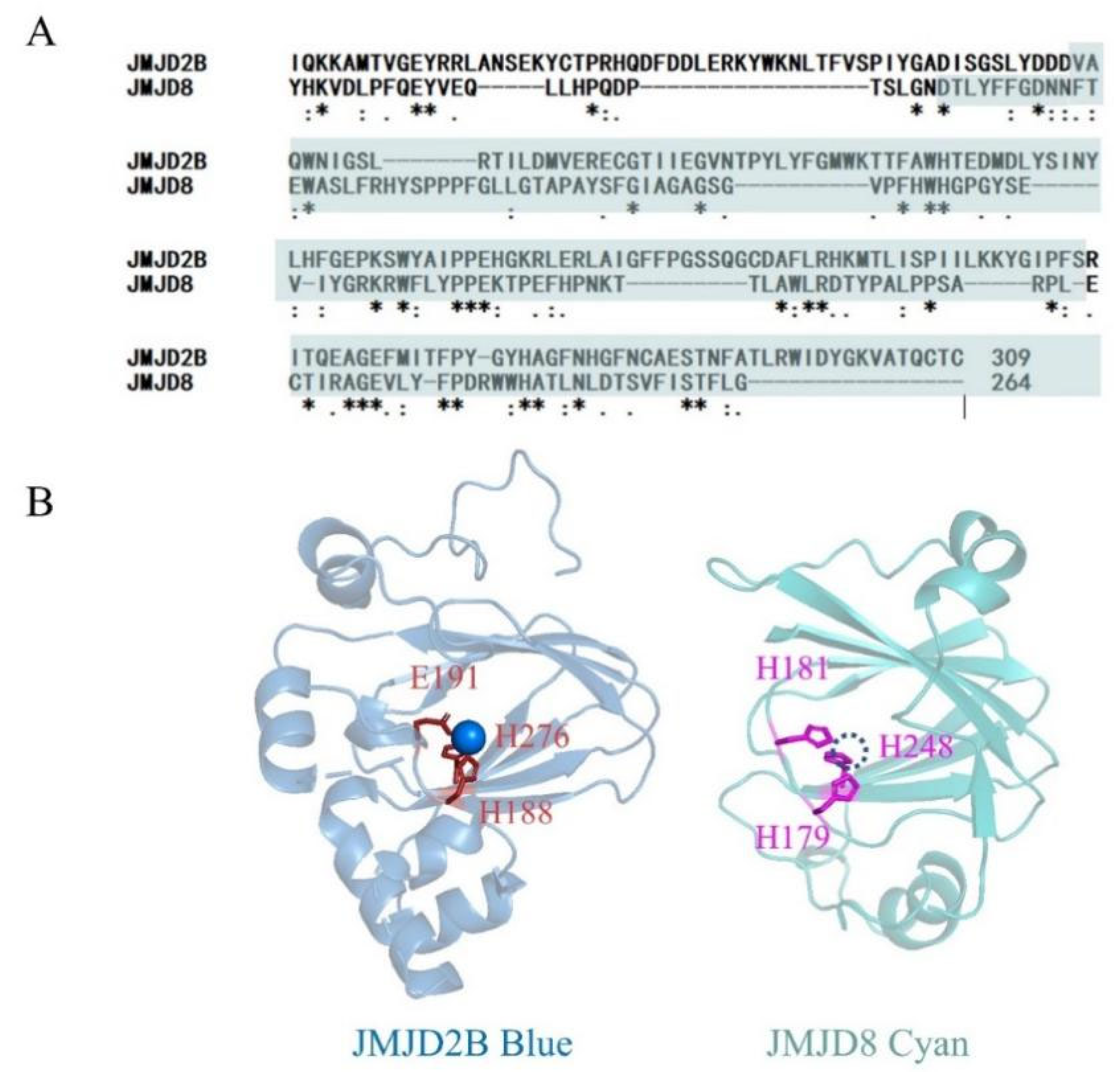
2.1. JMJD8 Has KDM Activity
2.2. NMR Analysis of KDM Activity
2.3. Demethylation of Methylated Full-Length AKT1
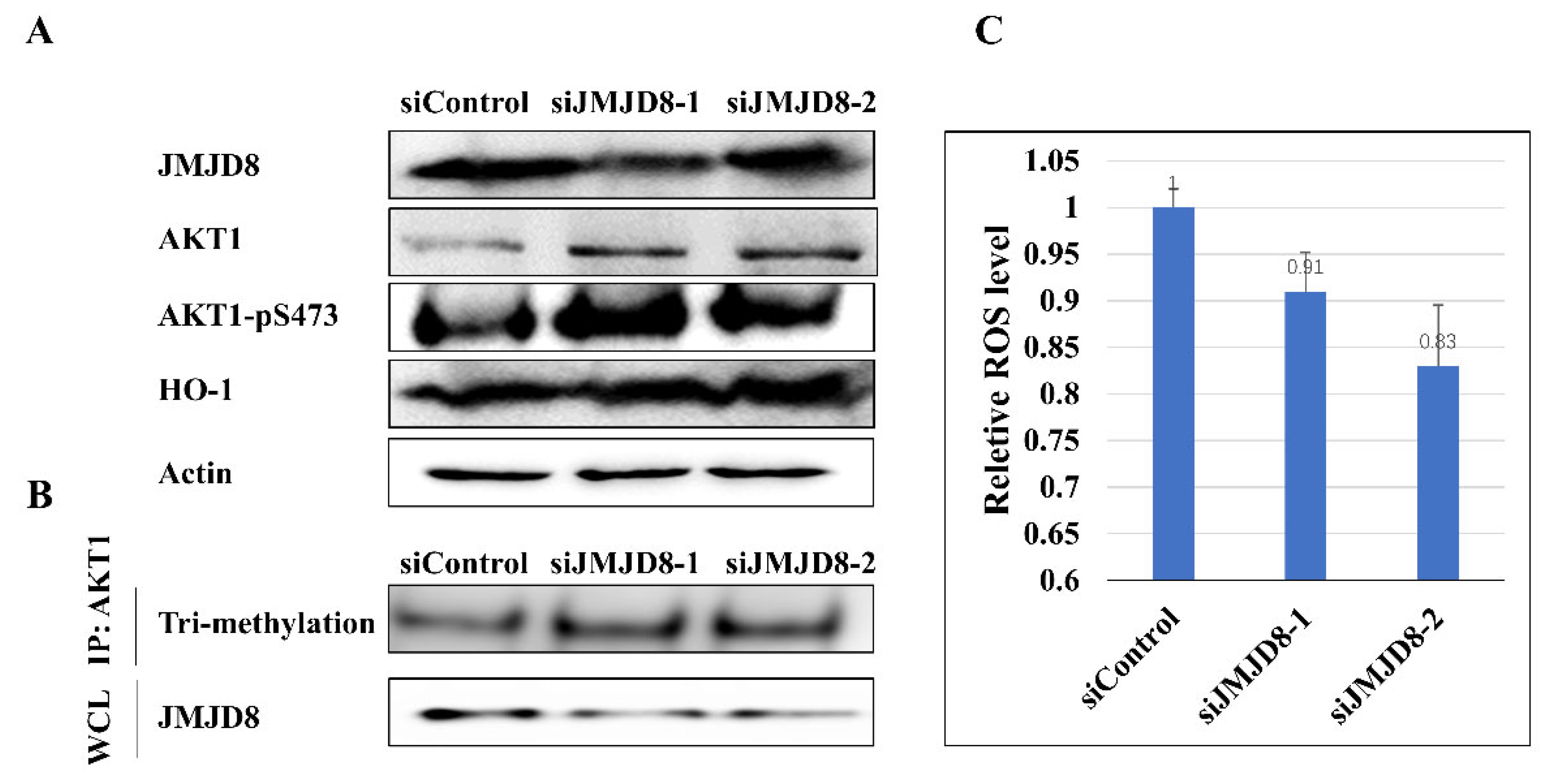
2.4. JMJD8 Modulates the Activity of AKT1 in H1299 Cells
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Plasmid and Expression Vectors
4.3. Reagents and Antibodies
4.4. SiRNA Transfection
4.5. Activity Assays
4.5.1. LC-MS Activity Assays
4.5.2. NMR Assays
4.5.3. WB Assay
4.6. Western Blotting Analysis and Immunoprecipitation
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tsukada, Y.; Fang, J.; Erdjument-Bromage, H.; Warren, M.E.; Borchers, C.H.; Tempst, P.; Zhang, Y. Histone Demethylation by a Family of JmjC Domain-Containing Proteins. Nature 2006, 439, 811–816. [Google Scholar] [CrossRef]
- Krishnan, S.; Trievel, R.C. Purification, Biochemical Analysis, and Structure Determination of JmjC Lysine Demethylases. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 2016; Volume 573, pp. 279–301. ISBN 978-0-12-805365-2. [Google Scholar]
- Zhang, X.; Liu, L.; Yuan, X.; Wei, Y.; Wei, X. JMJD3 in the Regulation of Human Diseases. Protein Cell 2019, 10, 864–882. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.S.; Kavanagh, K.L.; McDonough, M.A.; Butler, D.; Pilka, E.S.; Lienard, B.M.R.; Bray, J.E.; Savitsky, P.; Gileadi, O.; von Delft, F.; et al. Crystal Structures of Histone Demethylase JMJD2A Reveal Basis for Substrate Specificity. Nature 2007, 448, 87–91. [Google Scholar] [CrossRef]
- Guo, J.; Dai, X.; Laurent, B.; Zheng, N.; Gan, W.; Zhang, J.; Guo, A.; Yuan, M.; Liu, P.; Asara, J.M.; et al. AKT Methylation by SETDB1 Promotes AKT Kinase Activity and Oncogenic Functions. Nat. Cell Biol. 2019, 21, 226–237. [Google Scholar] [CrossRef]
- Wang, G.; Long, J.; Gao, Y.; Zhang, W.; Han, F.; Xu, C.; Sun, L.; Yang, S.-C.; Lan, J.; Hou, Z.; et al. SETDB1-Mediated Methylation of Akt Promotes Its K63-Linked Ubiquitination and Activation Leading to Tumorigenesis. Nat. Cell Biol. 2019, 21, 214–225. [Google Scholar] [CrossRef] [PubMed]
- Luciano, A.K.; Guertin, D.A. Oncogenic AKTivation by Methylation. Nat. Cell Biol. 2019, 21, 114–115. [Google Scholar] [CrossRef]
- Walport, L.J.; Hopkinson, R.J.; Chowdhury, R.; Schiller, R.; Ge, W.; Kawamura, A.; Schofield, C.J. Arginine Demethylation Is Catalysed by a Subset of JmjC Histone Lysine Demethylases. Nat. Commun. 2016, 7, 11974. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; Shin, S.; Janknecht, R. The Small Members of the JMJD Protein Family. Biochim. Biophys. Acta BBA—Rev. Cancer 2019, 1871, 406–418. [Google Scholar] [CrossRef]
- Markolovic, S.; Zhuang, Q.; Wilkins, S.E.; Eaton, C.D.; Abboud, M.I.; Katz, M.J.; McNeil, H.E.; Leśniak, R.K.; Hall, C.; Struwe, W.B.; et al. The Jumonji-C Oxygenase JMJD7 Catalyzes (3S)-Lysyl Hydroxylation of TRAFAC GTPases. Nat. Chem. Biol. 2018, 14, 688–695. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, S.E.; Islam, M.S.; Gannon, J.M.; Markolovic, S.; Hopkinson, R.J.; Ge, W.; Schofield, C.J.; Chowdhury, R. JMJD5 Is a Human Arginyl C-3 Hydroxylase. Nat. Commun. 2018, 9, 1180. [Google Scholar] [CrossRef]
- Han, G.; Li, J.; Wang, Y.; Li, X.; Mao, H.; Liu, Y.; Chen, C.D. The Hydroxylation Activity of Jmjd6 Is Required for Its Homo-Oligomerization. J. Cell. Biochem. 2011, 113, 1663–1670. [Google Scholar] [CrossRef]
- Böttger, A.; Islam, M.S.; Chowdhury, R.; Schofield, C.J.; Wolf, A. The Oxygenase Jmjd6—A Case Study in Conflicting Assignments. Biochem. J. 2015, 468, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; McDonough, M.A.; Chowdhury, R.; Gault, J.; Khan, A.; Pires, E.; Schofield, C.J. Biochemical and Structural Investigations Clarify the Substrate Selectivity of the 2-Oxoglutarate Oxygenase JMJD6. J. Biol. Chem. 2019, 294, 11637–11652. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, S.S.; Jaber Sathik Rifayee, S.B.; Waheed, S.O.; Wildey, J.; Warner, C.; Schofield, C.J.; Karabencheva-Christova, T.G.; Christov, C.Z. Can Second Coordination Sphere and Long-Range Interactions Modulate Hydrogen Atom Transfer in a Non-Heme Fe(II)-Dependent Histone Demethylase? JACS Au 2022, 2, 2169–2186. [Google Scholar] [CrossRef]
- Chaturvedi, S.S.; Ramanan, R.; Lehnert, N.; Schofield, C.J.; Karabencheva-Christova, T.G.; Christov, C.Z. Catalysis by the Non-Heme Iron(II) Histone Demethylase PHF8 Involves Iron Center Rearrangement and Conformational Modulation of Substrate Orientation. ACS Catal. 2020, 10, 1195–1209. [Google Scholar] [CrossRef]
- Ramanan, R.; Chaturvedi, S.S.; Lehnert, N.; Schofield, C.J.; Karabencheva-Christova, T.G.; Christov, C.Z. Catalysis by the JmjC Histone Demethylase KDM4A Integrates Substrate Dynamics, Correlated Motions and Molecular Orbital Control. Chem. Sci. 2020, 11, 9950–9961. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, C.; Lee, S.; Deng, Y.; Wither, M.; Oh, S.; Ning, F.; Dege, C.; Zhang, Q.; Liu, X.; et al. Clipping of Arginine-Methylated Histone Tails by JMJD5 and JMJD7. Proc. Natl. Acad. Sci. USA 2017, 114, E7717–E7726. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Xiang, X.; Chen, L.; Wang, H.; Wu, L.; Sun, Y.; Ma, L.; Gu, X.; Liu, H.; Wang, L.; et al. JMJD5 Cleaves Monomethylated Histone H3 N-tail under DNA Damaging Stress. EMBO Rep. 2017, 18, 2131–2143. [Google Scholar] [CrossRef]
- Lee, S.; Liu, H.; Hill, R.; Chen, C.; Hong, X.; Crawford, F.; Kingsley, M.; Zhang, Q.; Liu, X.; Chen, Z.; et al. JMJD6 Cleaves MePCE to Release Positive Transcription Elongation Factor b (P-TEFb) in Higher Eukaryotes. eLife 2020, 9, e53930. [Google Scholar] [CrossRef] [PubMed]
- Yeo, K.S.; Tan, M.C.; Lim, Y.-Y.; Ea, C.-K. JMJD8 Is a Novel Endoplasmic Reticulum Protein with a JmjC Domain. Sci. Rep. 2017, 7, 15407. [Google Scholar] [CrossRef]
- Yeo, K.S.; Tan, M.C.; Wong, W.Y.; Loh, S.W.; Lam, Y.L.; Tan, C.L.; Lim, Y.-Y.; Ea, C.-K. JMJD8 Is a Positive Regulator of TNF-Induced NF-ΚB Signaling. Sci. Rep. 2016, 6, 34125. [Google Scholar] [CrossRef] [PubMed]
- Boeckel, J.-N.; Derlet, A.; Glaser, S.F.; Luczak, A.; Lucas, T.; Heumüller, A.W.; Krüger, M.; Zehendner, C.M.; Kaluza, D.; Doddaballapur, A.; et al. JMJD8 Regulates Angiogenic Sprouting and Cellular Metabolism by Interacting with Pyruvate Kinase M2 in Endothelial Cells. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 1425–1433. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Xu, Y.; Song, M.; Chen, G.; Wang, H.; Zhao, Y.; Wang, Z.; Li, F. PRDM16 Is Associated with Evasion of Apoptosis by Prostatic Cancer Cells According to RNA Interference Screening. Mol. Med. Rep. 2016, 14, 3357–3361. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Pan, H.; Li, J.; Zhong, Q.; Chen, X.; Dry, S.M.; Wang, C.-Y. Epigenetic Activation of AP1 Promotes Squamous Cell Carcinoma Metastasis. Sci. Signal. 2013, 6, ra28. [Google Scholar] [CrossRef] [PubMed]
- You, D.; Jung, B.C.; Villivalam, S.D.; Lim, H.-W.; Kang, S. JMJD8 Is a Novel Molecular Nexus Between Adipocyte-Intrinsic Inflammation and Insulin Resistance. Diabetes 2022, 71, 43–59. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Wang, J. JmjC Domain-Containing Protein 8 (JMJD8) Represses Ku70/Ku80 Expression via Attenuating AKT/NF-ΚB/COX-2 Signaling. Biochim. Biophys. Acta BBA—Mol. Cell Res. 2019, 1866, 118541. [Google Scholar] [CrossRef]
- Su, Y.; Wang, X.; Guo, Z.; Wang, J. Aberrant JmjC Domain-Containing Protein 8 (JMJD8) Expression Promotes Activation of AKT and Tumor Epithelial–Mesenchymal Transition. Oncogene 2020, 39, 6451–6467. [Google Scholar] [CrossRef]
- Hopkinson, R.J.; Hamed, R.B.; Rose, N.R.; Claridge, T.D.W.; Schofield, C.J. Monitoring the Activity of 2-Oxoglutarate Dependent Histone Demethylases by NMR Spectroscopy: Direct Observation of Formaldehyde. ChemBioChem 2010, 11, 506–510. [Google Scholar] [CrossRef]
- Chu, C.-H.; Wang, L.-Y.; Hsu, K.-C.; Chen, C.-C.; Cheng, H.-H.; Wang, S.-M.; Wu, C.-M.; Chen, T.-J.; Li, L.-T.; Liu, R.; et al. KDM4B as a Target for Prostate Cancer: Structural Analysis and Selective Inhibition by a Novel Inhibitor. J. Med. Chem. 2014, 57, 5975–5985. [Google Scholar] [CrossRef]
- Hillringhaus, L.; Yue, W.W.; Rose, N.R.; Ng, S.S.; Gileadi, C.; Loenarz, C.; Bello, S.H.; Bray, J.E.; Schofield, C.J.; Oppermann, U. Structural and Evolutionary Basis for the Dual Substrate Selectivity of Human KDM4 Histone Demethylase Family. J. Biol. Chem. 2011, 286, 41616–41625. [Google Scholar] [CrossRef]
- Carlson, S.M.; Gozani, O. Nonhistone Lysine Methylation in the Regulation of Cancer Pathways. Cold Spring Harb. Perspect. Med. 2016, 6, a026435. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Wei, W. Fine-Tuning AKT Kinase Activity through Direct Lysine Methylation. Cell Cycle 2019, 18, 917–922. [Google Scholar] [CrossRef] [PubMed]
- Whetstine, J.R.; Nottke, A.; Lan, F.; Huarte, M.; Smolikov, S.; Chen, Z.; Spooner, E.; Li, E.; Zhang, G.; Colaiacovo, M.; et al. Reversal of Histone Lysine Trimethylation by the JMJD2 Family of Histone Demethylases. Cell 2006, 125, 467–481. [Google Scholar] [CrossRef] [PubMed]
- Nigi, I.; Fairall, L.; Schwabe, J.W.R. Expression and Purification of Protein Complexes Suitable for Structural Studies Using Mammalian HEK 293F Cells. Curr. Protoc. Protein Sci. 2017, 90, 5–28. [Google Scholar] [CrossRef]




| siRNA | Sequences (5′-3′) |
|---|---|
| Negative control siRNA | UUCUCCGAACGUGUCACGU |
| si-JMJD8-1 | GACUUGCCCUUCCAGGAGU |
| si-JMJD8-2 | GUCGUAAGCGCUGGUUCCU |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Zhang, Y.; Li, Z.; Wang, J. JMJD8 Functions as a Novel AKT1 Lysine Demethylase. Int. J. Mol. Sci. 2023, 24, 460. https://doi.org/10.3390/ijms24010460
Wang Y, Zhang Y, Li Z, Wang J. JMJD8 Functions as a Novel AKT1 Lysine Demethylase. International Journal of Molecular Sciences. 2023; 24(1):460. https://doi.org/10.3390/ijms24010460
Chicago/Turabian StyleWang, Yujuan, Yaoyao Zhang, Zehua Li, and Junfeng Wang. 2023. "JMJD8 Functions as a Novel AKT1 Lysine Demethylase" International Journal of Molecular Sciences 24, no. 1: 460. https://doi.org/10.3390/ijms24010460
APA StyleWang, Y., Zhang, Y., Li, Z., & Wang, J. (2023). JMJD8 Functions as a Novel AKT1 Lysine Demethylase. International Journal of Molecular Sciences, 24(1), 460. https://doi.org/10.3390/ijms24010460





