Protective Effects of Currants (Vitis vinifera) on Corticolimbic Serotoninergic Alterations and Anxiety-like Comorbidity in a Rat Model of Parkinson’s Disease
Abstract
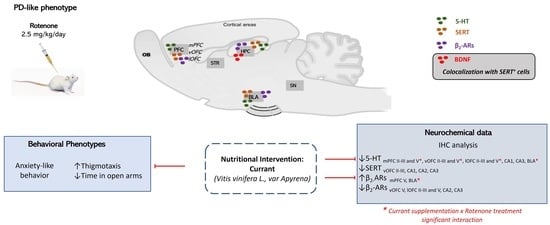
1. Introduction
2. Results
2.1. Effects of Rotenone Treatment and Currant Consumption on Thigmotaxic Behavior Using the Open Field Test (OFT)
2.2. Effects of Rotenone Treatment and Currant Consumption on Behavioral Parameters in the Elevated Plus-Maze (EPM)
2.3. Prefrontal Cortex, Hippocampus and Basolateral Amygdala 5-HT Immunoreactivity Following Rotenone Treatment and Complementary Diet with Currants
2.4. SERT Expression Pattern Following Rotenone Treatment and Complementary Diet with Currants
2.5. β2-AR Expression Pattern Following Rotenone Treatment and Complementary Diet with Currants
2.6. Anxiety-like Behavior Is Correlated with Altered 5-HT and SERT Immunodensity Levels
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. Animals
4.3. Experimental Design
4.4. Behavioral Testing
4.4.1. Open Field
4.4.2. Elevated Plus Maze
4.5. Immunohistochemistry: Immunofluorescence
4.6. Quantifications and Photomicrograph Production
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Dorsey, E.R.; Constantinescu, R.; Thompson, J.P.; Biglan, K.M.; Holloway, R.G.; Kieburtz, K.; Marshall, F.J.; Ravina, B.M.; Schifitto, G.; Siderowf, A.; et al. Projected Number of People with Parkinson Disease in the Most Populous Nations, 2005 through 2030. Neurology 2007, 68, 384–386. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, D.A.; Schrag, A. Psychosis, Apathy, Depression and Anxiety in Parkinson’s Disease. Neurobiol. Dis. 2012, 46, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Ryu, Y.H.; Cho, W.G.; Kang, Y.W.; Lee, S.J.; Jeon, T.J.; Lyoo, C.H.; Kim, C.H.; Kim, D.G.; Lee, K.; et al. Relationship between Dopamine Deficit and the Expression of Depressive Behavior Resulted from Alteration of Serotonin System. Synapse 2015, 69, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Pavese, N.; Rivero-Bosch, M.; Lewis, S.J.; Whone, A.L.; Brooks, D.J. Progression of Monoaminergic Dysfunction in Parkinson’s Disease: A Longitudinal 18F-Dopa PET Study. Neuroimage 2011, 56, 1463–1468. [Google Scholar] [CrossRef] [PubMed]
- Castrioto, A.; Thobois, S.; Carnicella, S.; Maillet, A.; Krack, P. Emotional Manifestations of PD: Neurobiological Basis. Mov. Disord. 2016, 31, 1103–1113. [Google Scholar] [CrossRef] [PubMed]
- Paredes-Rodriguez, E.; Vegas-Suarez, S.; Morera-Herreras, T.; de Deurwaerdere, P.; Miguelez, C. The Noradrenergic System in Parkinson’s Disease. Front. Pharmacol. 2020, 11, 435. [Google Scholar] [CrossRef]
- Kinnerup, M.B.; Sommerauer, M.; Damholdt, M.F.; Schaldemose, J.L.; Ismail, R.; Terkelsen, A.J.; Stær, K.; Hansen, A.; Fedorova, T.D.; Knudsen, K.; et al. Preserved Noradrenergic Function in Parkinson’s Disease Patients with Rest Tremor. Neurobiol. Dis. 2021, 152, 105295. [Google Scholar] [CrossRef]
- McCall, J.G.; Siuda, E.R.; Bhatti, D.L.; Lawson, L.A.; McElligott, Z.A.; Stuber, G.D.; Bruchas, M.R. Locus Coeruleus to Basolateral Amygdala Noradrenergic Projections Promote Anxiety-like Behavior. Elife 2017, 6, e18247. [Google Scholar] [CrossRef]
- Carey, G.; Görmezoğlu, M.; de Jong, J.J.A.; Hofman, P.A.M.; Backes, W.H.; Dujardin, K.; Leentjens, A.F.G. Neuroimaging of Anxiety in Parkinson’s Disease: A Systematic Review. Mov. Disord. 2021, 36, 327–339. [Google Scholar] [CrossRef]
- Schwarz, L.A.; Luo, L. Organization of the Locus Coeruleus-Norepinephrine System. Curr. Biol. 2015, 25, R1051–R1056. [Google Scholar] [CrossRef]
- Karuppagounder, S.S.; Madathil, S.K.; Pandey, M.; Haobam, R.; Rajamma, U.; Mohanakumar, K.P. Quercetin Up-Regulates Mitochondrial Complex-I Activity to Protect against Programmed Cell Death in Rotenone Model of Parkinson’s Disease in Rats. Neuroscience 2013, 236, 136–148. [Google Scholar] [CrossRef]
- Lacerda, D.C.; Urquiza-Martínez, M.V.; Manhaes-de-Castro, R.; Visco, D.B.; Derosier, C.; Mercado-Camargo, R.; Torner, L.; Toscano, A.E.; Guzmán-Quevedo, O. Metabolic and Neurological Consequences of the Treatment with Polyphenols: A Systematic Review in Rodent Models of Noncommunicable Diseases. Nutr. Neurosci. 2021, 25, 1–17. [Google Scholar] [CrossRef]
- Chen, T.Y.; Kritchevsky, J.; Hargett, K.; Feller, K.; Klobusnik, R.; Song, B.J.; Cooper, B.; Jouni, Z.; Ferruzzi, M.G.; Janle, E.M. Plasma Bioavailability and Regional Brain Distribution of Polyphenols from Apple/Grape Seed and Bilberry Extracts in a Young Swine Model. Mol. Nutr. Food Res. 2015, 59, 2432–2447. [Google Scholar] [CrossRef]
- Lin, L.C.; Wang, M.N.; Tseng, T.Y.; Sung, J.S.; Tsai, T.H. Pharmacokinetics of (-)-Epigallocatechin-3-Gallate in Conscious and Freely Moving Rats and Its Brain Regional Distribution. J. Agric. Food Chem. 2007, 55, 1517–1524. [Google Scholar] [CrossRef]
- Vasilakopoulou, P.B.; Fanarioti, Ε.; Tsarouchi, M.; Kokotou, M.G.; Dermon, C.R.; Karathanos, V.T.; Chiou, A. Polar Phenol Detection in Rat Brain: Development and Validation of a Versatile UHPLC-MS Method and Application on the Brain Tissues of Corinthian Currant (Vitis vinifera L., var. Apyrena) Fed Rats. Food Chem. 2022, 390, 133131. [Google Scholar] [CrossRef]
- Knight, E.; Geetha, T.; Burnett, D.; Babu, J.R. The Role of Diet and Dietary Patterns in Parkinson’s Disease. Nutrients 2022, 14, 4472. [Google Scholar] [CrossRef]
- Tapias, V.; Cannon, J.R.; Greenamyre, J.T. Pomegranate Juice Exacerbates Oxidative Stress and Nigrostriatal Degeneration in Parkinson’s Disease. Neurobiol. Aging 2014, 35, 1162–1176. [Google Scholar] [CrossRef]
- Gravandi, M.M.; Fakhri, S.; Zarneshan, S.N.; Yarmohammadi, A.; Khan, H. Flavonoids Modulate AMPK/PGC-1α and Interconnected Pathways toward Potential Neuroprotective Activities. Metab. Brain Dis. 2021, 36, 1501–1521. [Google Scholar] [CrossRef]
- Ramassamy, C. Emerging Role of Polyphenolic Compounds in the Treatment of Neurodegenerative Diseases: A Review of Their Intracellular Targets. Eur. J. Pharmacol. 2006, 545, 51–64. [Google Scholar] [CrossRef]
- Simunkova, M.; Barbierikova, Z.; Jomova, K.; Hudecova, L.; Lauro, P.; Alwasel, S.H.; Alhazza, I.; Rhodes, C.J.; Valko, M. Antioxidant vs. Prooxidant Properties of the Flavonoid, Kaempferol, in the Presence of Cu(II) Ions: A Ros-Scavenging Activity, Fenton Reaction and DNA Damage Study. Int. J. Mol. Sci. 2021, 22, 1619. [Google Scholar] [CrossRef]
- Fanarioti, E.; Tsarouchi, M.; Vasilakopoulou, P.B.; Chiou, A.; Karvelas, M.; Karathanos, V.T.; Dermon, C.R. Brain Polar Phenol Content, Behavioural and Neurochemical Effects of Corinthian Currant in a Rotenone Rat Model of Parkinson’s Disease. Nutr. Neurosci. 2022, 3, 1–15. [Google Scholar] [CrossRef]
- Betarbet, R.; Sherer, T.B.; MacKenzie, G.; Garcia-Osuna, M.; Panov, A.V.; Greenamyre, J.T. Chronic Systemic Pesticide Exposure Reproduces Features of Parkinson’s Disease. Nat. Neurosci. 2000, 3, 1301–1306. [Google Scholar] [CrossRef] [PubMed]
- Santiago, R.M.; Barbieiro, J.; Lima, M.M.S.; Dombrowski, P.A.; Andreatini, R.; Vital, M.A.B.F. Depressive-like Behaviors Alterations Induced by Intranigral MPTP, 6-OHDA, LPS and Rotenone Models of Parkinson’s Disease Are Predominantly Associated with Serotonin and Dopamine. Prog. Neuro. Psychopharmacol. Biol. Psychiatry 2010, 34, 1104–1114. [Google Scholar] [CrossRef] [PubMed]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 6th ed.; Academic Press: Cambridge, MA, USA, 2006. [Google Scholar]
- Cannon, J.R.; Tapias, V.; Na, H.M.; Honick, A.S.; Drolet, R.E.; Greenamyre, J.T. A Highly Reproducible Rotenone Model of Parkinson’s Disease. Neurobiol. Dis. 2009, 34, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Bonito-Oliva, A.; Masini, D.; Fisone, G. A Mouse Model of Non-Motor Symptoms in Parkinson’s Disease: Focus on Pharmacological Interventions Targeting Affective Dysfunctions. Front. Behav. Neurosci. 2014, 8, 290. [Google Scholar] [CrossRef]
- Tadaiesky, M.T.; Dombrowski, P.A.; Figueiredo, C.P.; Cargnin-Ferreira, E.; da Cunha, C.; Takahashi, R.N. Emotional, Cognitive and Neurochemical Alterations in a Premotor Stage Model of Parkinson’s Disease. Neuroscience 2008, 156, 830–840. [Google Scholar] [CrossRef]
- Prediger, R.D.S.; Matheus, F.C.; Schwarzbold, M.L.; Lima, M.M.S.; Vital, M.A.B.F. Anxiety in Parkinson’s Disease: A Critical Review of Experimental and Clinical Studies. Neuropharmacology 2012, 62, 115–124. [Google Scholar] [CrossRef]
- Menza, M.A.; Robertson-Hoffman, D.E.; Bonapace, A.S. Parkinson’s Disease and Anxiety: Comorbidity with Depression. Biol. Psychiatry 1993, 34, 465–470. [Google Scholar] [CrossRef]
- Goto, Y.; Grace, A.A. Dopaminergic Modulation of Limbic and Cortical Drive of Nucleus Accumbens in Goal-Directed Behavior. Nat. Neurosci. 2005, 8, 805–812. [Google Scholar] [CrossRef]
- Jin, J.; Maren, S. Prefrontal-Hippocampal Interactions in Memory and Emotion. Front. Syst. Neurosci. 2015, 9, 170. [Google Scholar] [CrossRef]
- Ressler, K.J.; Nemeroff, C.B. Role of Serotonergic and Noradrenergic Systems in the Pathophysiology of Depression and Anxiety Disorders. Depress. Anxiety 2000, 12 (Suppl. 1), 2–19. [Google Scholar] [CrossRef]
- Sun, Y.N.; Wang, T.; Wang, Y.; Han, L.N.; Li, L.B.; Zhang, Y.M.; Liu, J. Activation of 5-HT1A Receptors in the Medial Subdivision of the Central Nucleus of the Amygdala Produces Anxiolytic Effects in a Rat Model of Parkinson’s Disease. Neuropharmacology 2015, 95, 181–191. [Google Scholar] [CrossRef]
- Scatton, B.; Javoy-Agid, F.; Rouquier, L.; Dubois, B.; Agid, Y. Reduction of Cortical Dopamine, Noradrenaline, Serotonin and Their Metabolites in Parkinson’s Disease. Brain Res. 1983, 275, 321–328. [Google Scholar] [CrossRef]
- Weintraub, D.; Burn, D.J. Parkinson’s disease: The quintessential neuropsychiatric disorder. Mov. Disord. 2011, 26, 1022–1031. [Google Scholar] [CrossRef]
- Taylor, T.N.; Caudle, W.M.; Shepherd, K.R.; Noorian, A.R.; Jackson, C.R.; Iuvone, P.M.; Weinshenker, D.; Greene, J.G.; Miller, G.W. Nonmotor Symptoms of Parkinson’s Disease Revealed in an Animal Model with Reduced Monoamine Storage Capacity. J. Neurosci. 2009, 29, 8103–8113. [Google Scholar] [CrossRef]
- Van de Werd, H.J.J.M.; Uylings, H.B.M. The Rat Orbital and Agranular Insular Prefrontal Cortical Areas: A Cytoarchitectonic and Chemoarchitectonic Study. Brain Struct. Funct. 2008, 212, 387–401. [Google Scholar] [CrossRef]
- Santana, N.; Artigas, F. Laminar and Cellular Distribution of Monoamine Receptors in Rat Medial Prefrontal Cortex. Front. Neuroanat. 2017, 11, 87. [Google Scholar] [CrossRef]
- Santana, N.; Mengod, G.; Artigas, F. Expression of A1-Adrenergic Receptors in Rat Prefrontal Cortex: Cellular Co-Localization with 5-HT2A Receptors. Int. J. Neuropsychopharmacol. 2013, 16, 1139–1151. [Google Scholar] [CrossRef]
- Gagnon, D.; Gregoire, L.; di Paolo, T.; Parent, M. Serotonin Hyperinnervation of the Striatum with High Synaptic Incidence in Parkinsonian Monkeys. Brain Struct. Funct. 2016, 221, 3675–3691. [Google Scholar] [CrossRef]
- D’Amato, R.J.; Zweig, R.M.; Whitehouse, P.J.; Wenk, G.L.; Singer, H.S.; Mayeux, R.; Price, D.L.; Snyder, S.H. Aminergic Systems in Alzheimer’s Disease and Parkinson’s Disease. Ann. Neurol. 1987, 22, 229–236. [Google Scholar] [CrossRef]
- Guttman, M.; Boileau, I.; Warsh, J.; Saint-Cyr, J.A.; Ginovart, N.; McCluskey, T.; Houle, S.; Wilson, A.; Mundo, E.; Rusjan, P.; et al. Brain Serotonin Transporter Binding in Non-Depressed Patients with Parkinson’s Disease. Eur. J. Neurol. 2007, 14, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Haapaniemi, T.H.; Ahonen, A.; Torniainen, P.; Sotaniemi, K.A.; Myllylä, V.V. [123I] β-CIT SPECT demonstrates decreased brain dopamine and serotonin transporter levels in untreated parkinsonian patients. Mov. Disord. Off. J. Mov. Disord. Soc. 2001, 16, 124–130. [Google Scholar] [CrossRef]
- Martinowich, K.; Manji, H.; Lu, B. New Insights into BDNF Function in Depression and Anxiety. Nat. Neurosci. 2007, 10, 1089–1093. [Google Scholar] [CrossRef] [PubMed]
- Mamounas, L.A.; Altar, C.A.; Blue, M.E.; Kaplan, D.R.; Tessarollo, L.; Lyons, W.E. BDNF promotes the regenerative sprouting, but not survival, of injured serotonergic axons in the adult rat brain. J. Neurosci. Off. J. Soc. Neurosci. 2000, 20, 771–782. [Google Scholar] [CrossRef] [PubMed]
- Flügge, G.; Ahrens, O.; Fuchs, E. Beta-Adrenoceptors in the Tree Shrew Brain. II. Time-Dependent Effects of Chronic Psychosocial Stress on [125I] Iodocyanopindolol Bindings Sites. Cell. Mol. Neurobiol. 1997, 17, 417–432. [Google Scholar] [CrossRef]
- Engeln, M.; de Deurwaerdère, P.; Li, Q.; Bezard, E.; Fernagut, P.O. Widespread Monoaminergic Dysregulation of Both Motor and Non-Motor Circuits in Parkinsonism and Dyskinesia. Cereb. Cortex 2015, 25, 2783–2792. [Google Scholar] [CrossRef]
- Tohgi, H.; Abe, T.; Takahashi, S. The Effects of L-Threo-3,4-Dihydroxyphenylserine on the Total Norepinephrine and Dopamine Concentrations in the Cerebrospinal Fluid and Freezing Gait in Parkinsonian Patients. J. Neural Transm. Park. Dis. Dement. Sect. 1993, 5, 27–34. [Google Scholar] [CrossRef]
- Giuliano, C.; Cerri, S.; Blandini, F. Potential Therapeutic Effects of Polyphenols in Parkinson’s Disease: In Vivo and in Vitro Pre-Clinical Studies. Neural Regen. Res. 2021, 16, 234–241. [Google Scholar] [CrossRef]
- Kujawska, M.; Jodynis-Liebert, J. Polyphenols in Parkinson’s Disease: A Systematic Review of In Vivo Studies. Nutrients 2018, 10, 642. [Google Scholar] [CrossRef]
- Singh, S.; Kumar, P. Piperine in Combination with Quercetin Halt 6-OHDA Induced Neurodegeneration in Experimental Rats: Biochemical and Neurochemical Evidences. Neurosci. Res. 2018, 133, 38–47. [Google Scholar] [CrossRef]
- Liu, D.; Xie, K.; Yang, X.; Gu, J.; Ge, L.; Wang, X.; Wang, Z. Resveratrol Reverses the Effects of Chronic Unpredictable Mild Stress on Behavior, Serum Corticosterone Levels and BDNF Expression in Rats. Behav. Brain Res. 2014, 264, 9–16. [Google Scholar] [CrossRef]
- Machado, D.G.; Bettio, L.E.B.; Cunha, M.P.; Santos, A.R.S.; Pizzolatti, M.G.; Brighente, I.M.C.; Rodrigues, A.L.S. Antidepressant-like Effect of Rutin Isolated from the Ethanolic Extract from Schinus Molle L. in Mice: Evidence for the Involvement of the Serotonergic and Noradrenergic Systems. Eur. J. Pharmacol. 2008, 587, 163–168. [Google Scholar] [CrossRef]
- Sarbishegi, M.; Charkhat Gorgich, E.A.; Khajavi, O.; Komeili, G.; Salimi, S. The Neuroprotective Effects of Hydro-Alcoholic Extract of Olive (Olea europaea L.) Leaf on Rotenone-Induced Parkinson’s Disease in Rat. Metab. Brain Dis. 2018, 33, 79–88. [Google Scholar] [CrossRef]
- Lister, R.G. The Use of a Plus-Maze to Measure Anxiety in the Mouse. Psychopharmacology 1987, 92, 180–185. [Google Scholar] [CrossRef]
- Cohen, H.; Liu, T.; Kozlovsky, N.; Kaplan, Z.; Zohar, J.; Mathé, A.A. The neuropeptide Y (NPY)-ergic system is associated with behavioral resilience to stress exposure in an animal model of post-traumatic stress disorder. Neuropsychopharmacology 2012, 37, 350–363. [Google Scholar] [CrossRef]
- Ampatzis, K.; Dermon, C.R. Regional Distribution and Cellular Localization of Β2-Adrenoceptors in the Adult Zebrafish Brain (Danio Rerio). J. Comp. Neurol. 2010, 518, 1418–1441. [Google Scholar] [CrossRef]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsarouchi, M.; Fanarioti, E.; Karathanos, V.T.; Dermon, C.R. Protective Effects of Currants (Vitis vinifera) on Corticolimbic Serotoninergic Alterations and Anxiety-like Comorbidity in a Rat Model of Parkinson’s Disease. Int. J. Mol. Sci. 2023, 24, 462. https://doi.org/10.3390/ijms24010462
Tsarouchi M, Fanarioti E, Karathanos VT, Dermon CR. Protective Effects of Currants (Vitis vinifera) on Corticolimbic Serotoninergic Alterations and Anxiety-like Comorbidity in a Rat Model of Parkinson’s Disease. International Journal of Molecular Sciences. 2023; 24(1):462. https://doi.org/10.3390/ijms24010462
Chicago/Turabian StyleTsarouchi, Martha, Eleni Fanarioti, Vaios T. Karathanos, and Catherine R. Dermon. 2023. "Protective Effects of Currants (Vitis vinifera) on Corticolimbic Serotoninergic Alterations and Anxiety-like Comorbidity in a Rat Model of Parkinson’s Disease" International Journal of Molecular Sciences 24, no. 1: 462. https://doi.org/10.3390/ijms24010462
APA StyleTsarouchi, M., Fanarioti, E., Karathanos, V. T., & Dermon, C. R. (2023). Protective Effects of Currants (Vitis vinifera) on Corticolimbic Serotoninergic Alterations and Anxiety-like Comorbidity in a Rat Model of Parkinson’s Disease. International Journal of Molecular Sciences, 24(1), 462. https://doi.org/10.3390/ijms24010462