Enhancing the Gastrointestinal Stability of Curcumin by Using Sodium Alginate-Based Nanoemulsions Containing Natural Emulsifiers
Abstract
:1. Introduction
2. Results and Discussion
2.1. Initial Systems
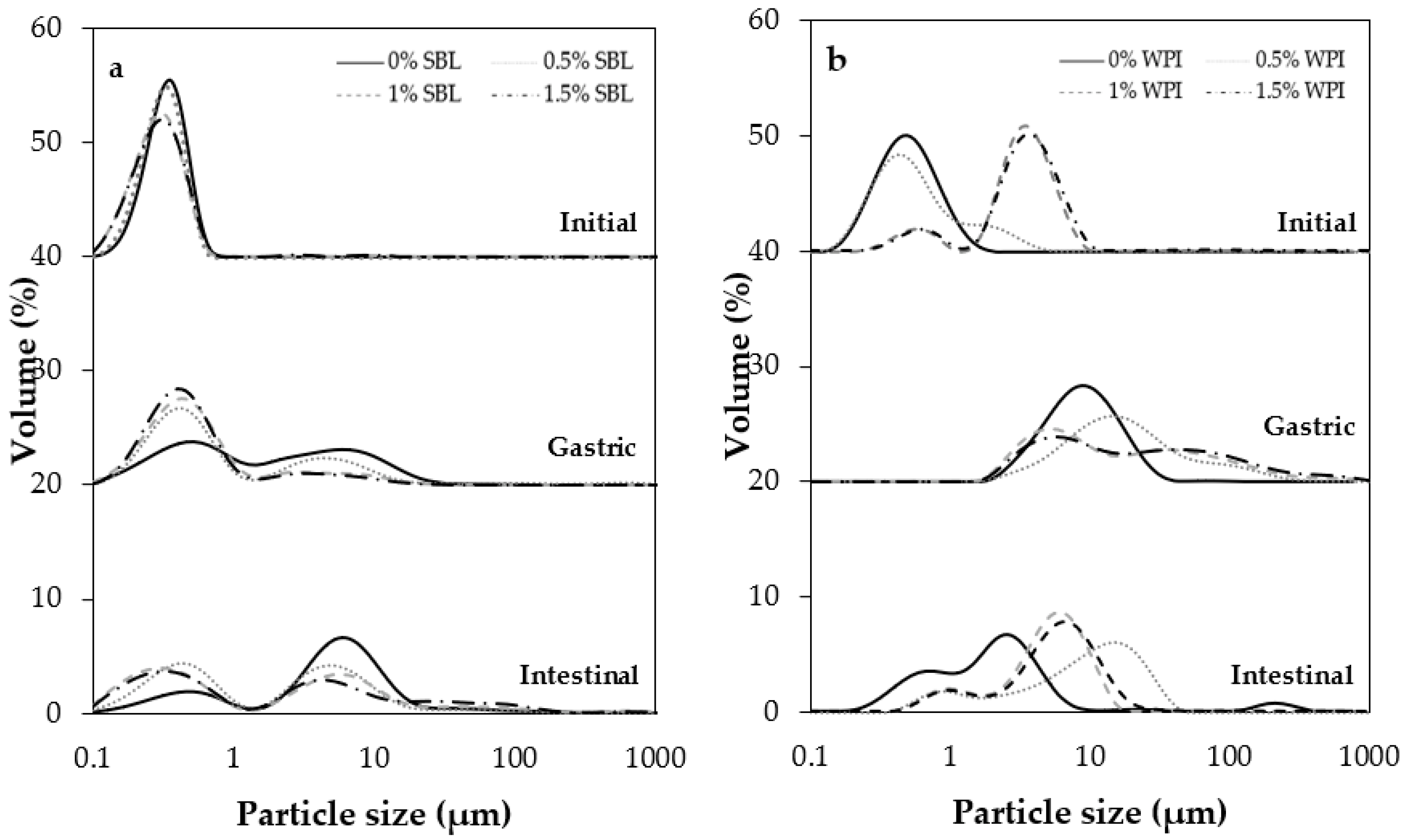
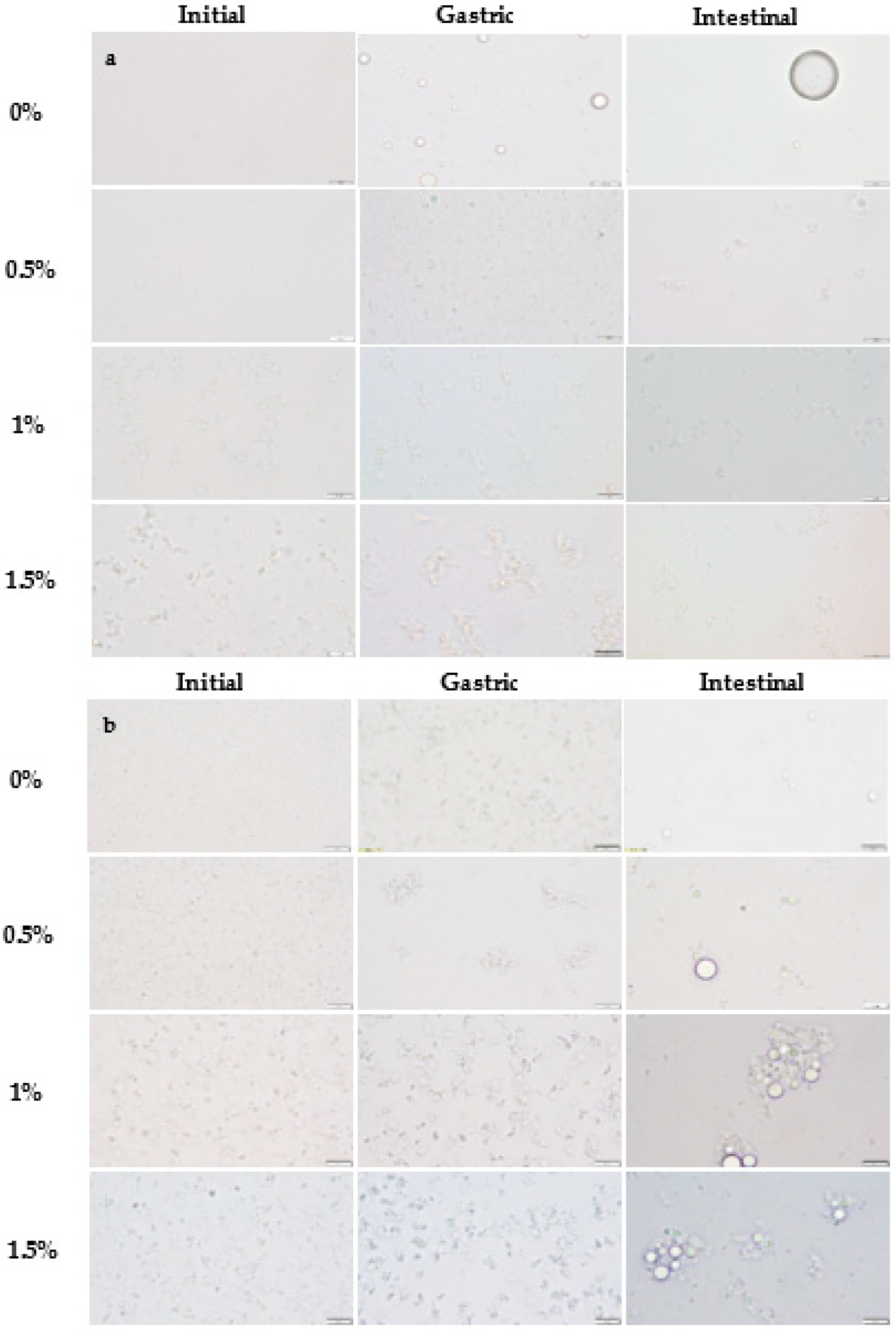
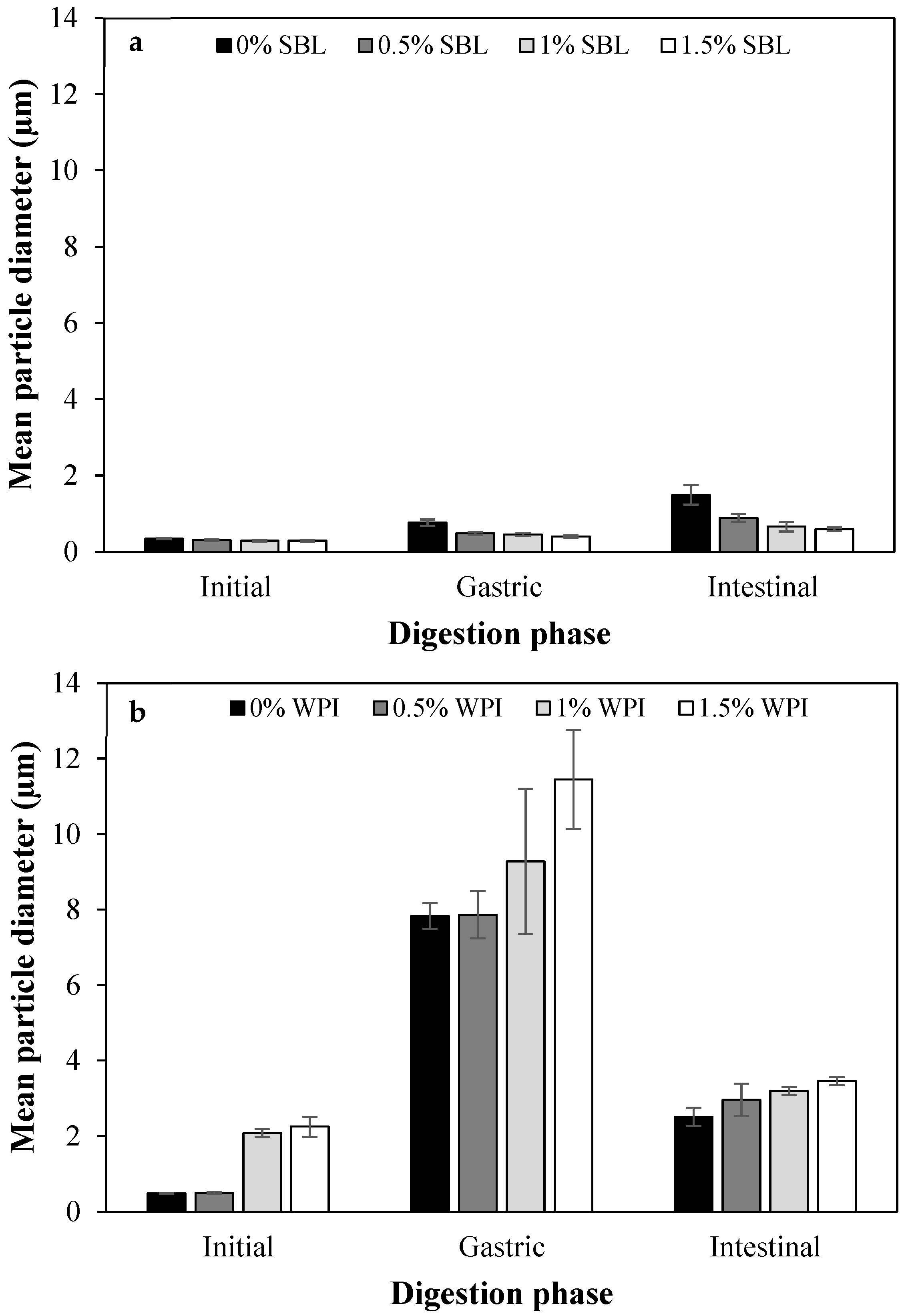
2.1.1. Particle Size and Distribution
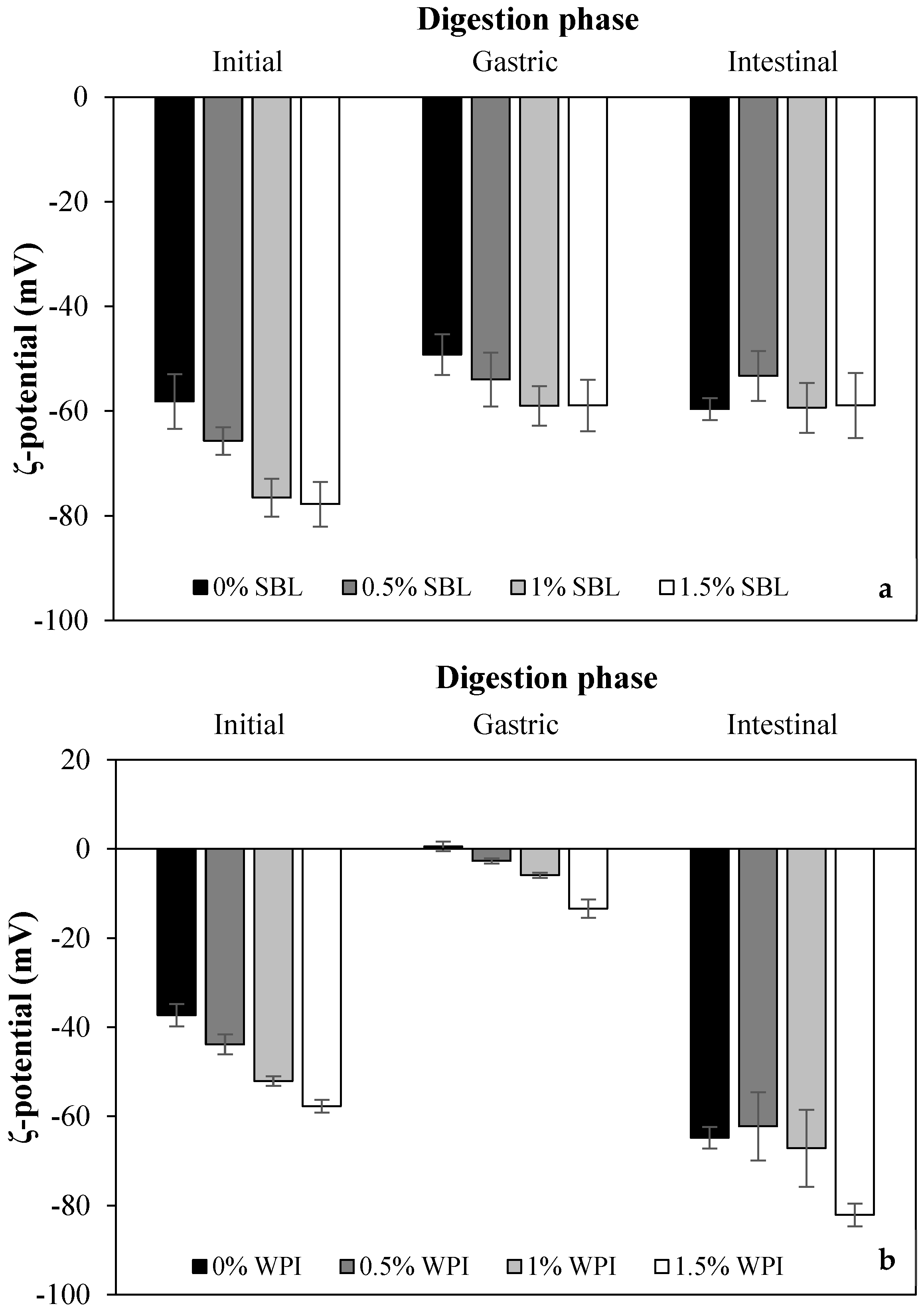
2.1.2. ζ-Potential
2.1.3. Encapsulation Efficiency
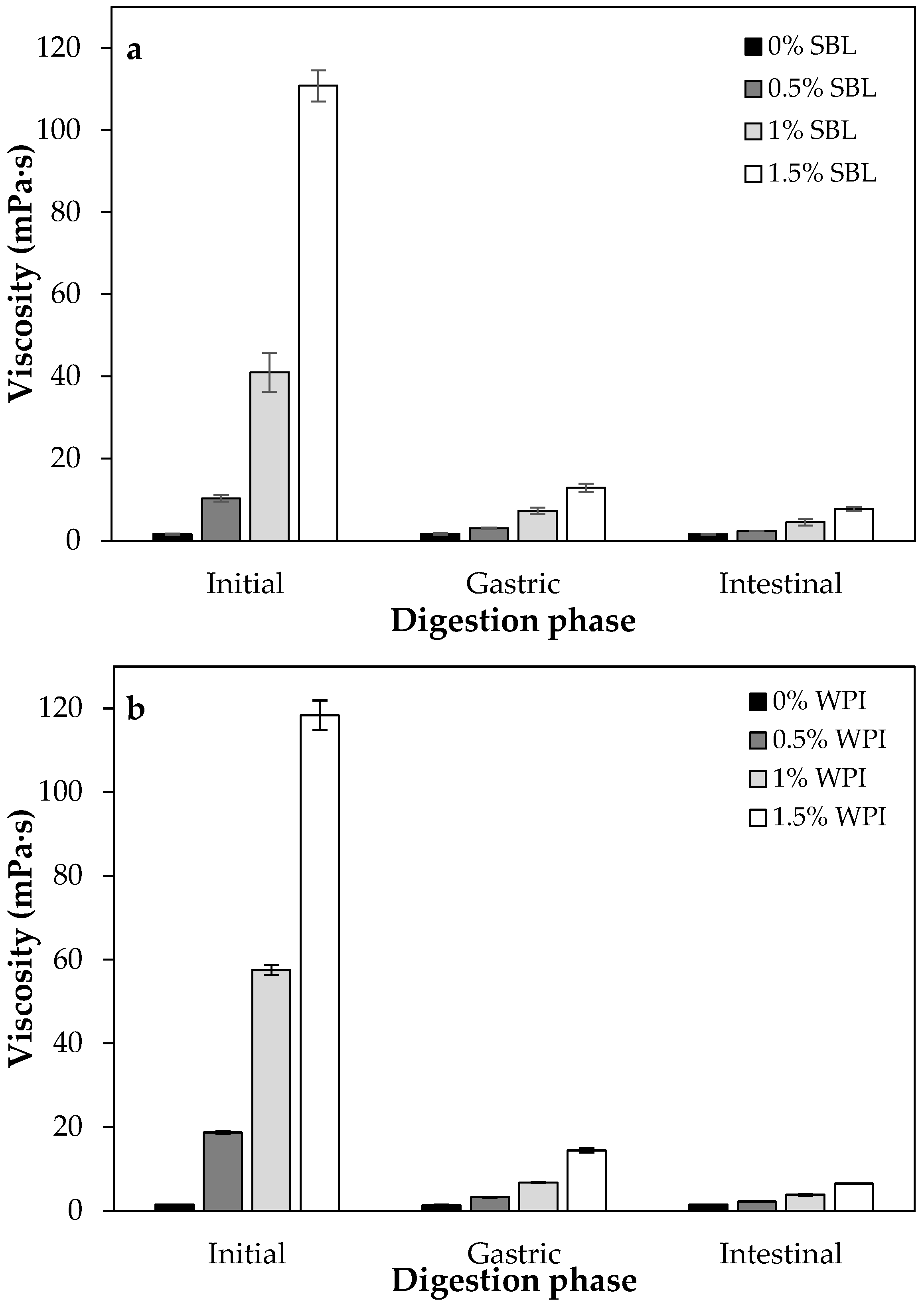
2.1.4. Viscosity
2.1.5. Stability
2.2. Gastrointestinal In Vitro Digestion
2.2.1. Physicochemical Changes during In Vitro Digestion
Gastric Phase
Intestinal Phase
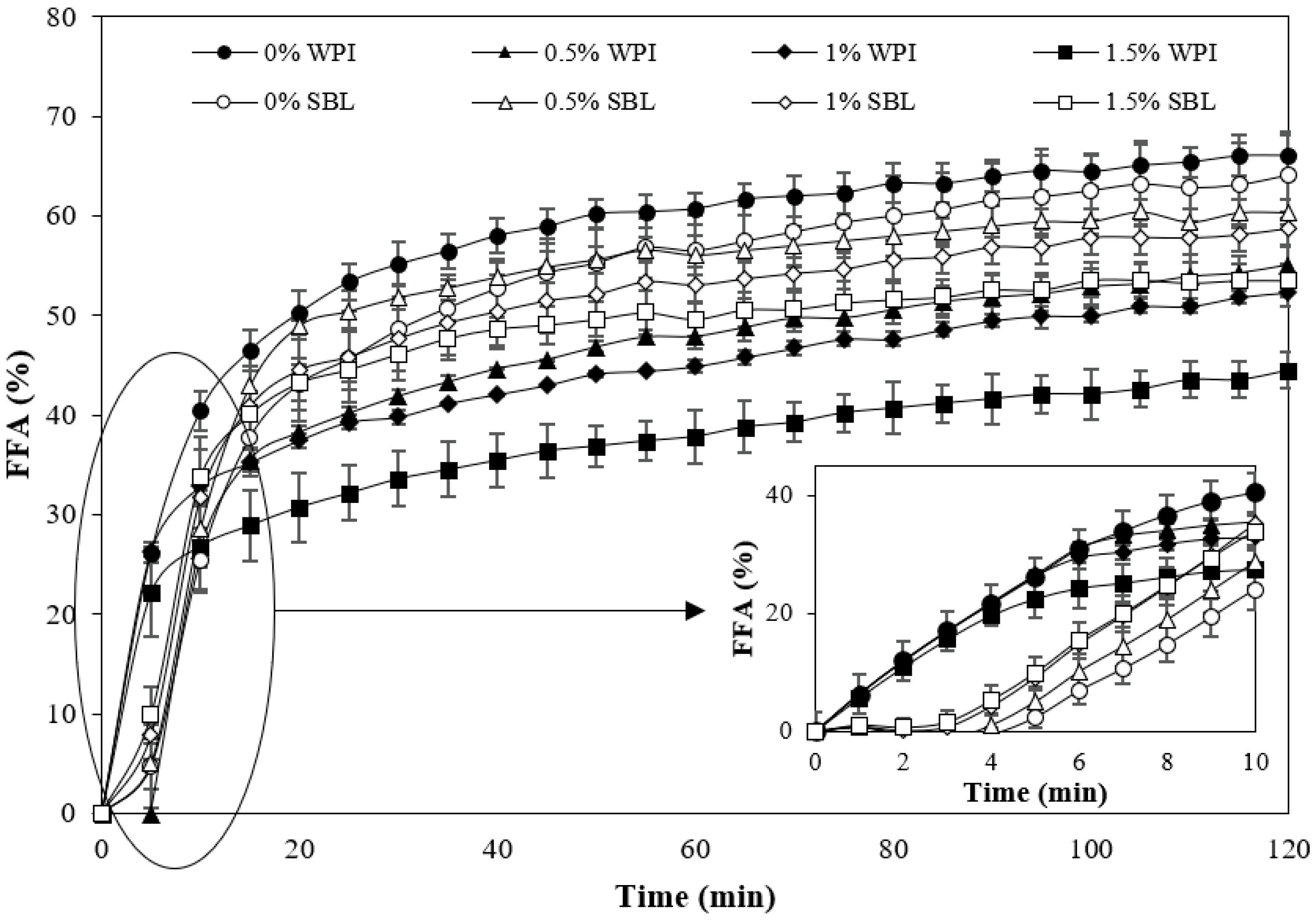
2.2.2. Lipid Digestibility
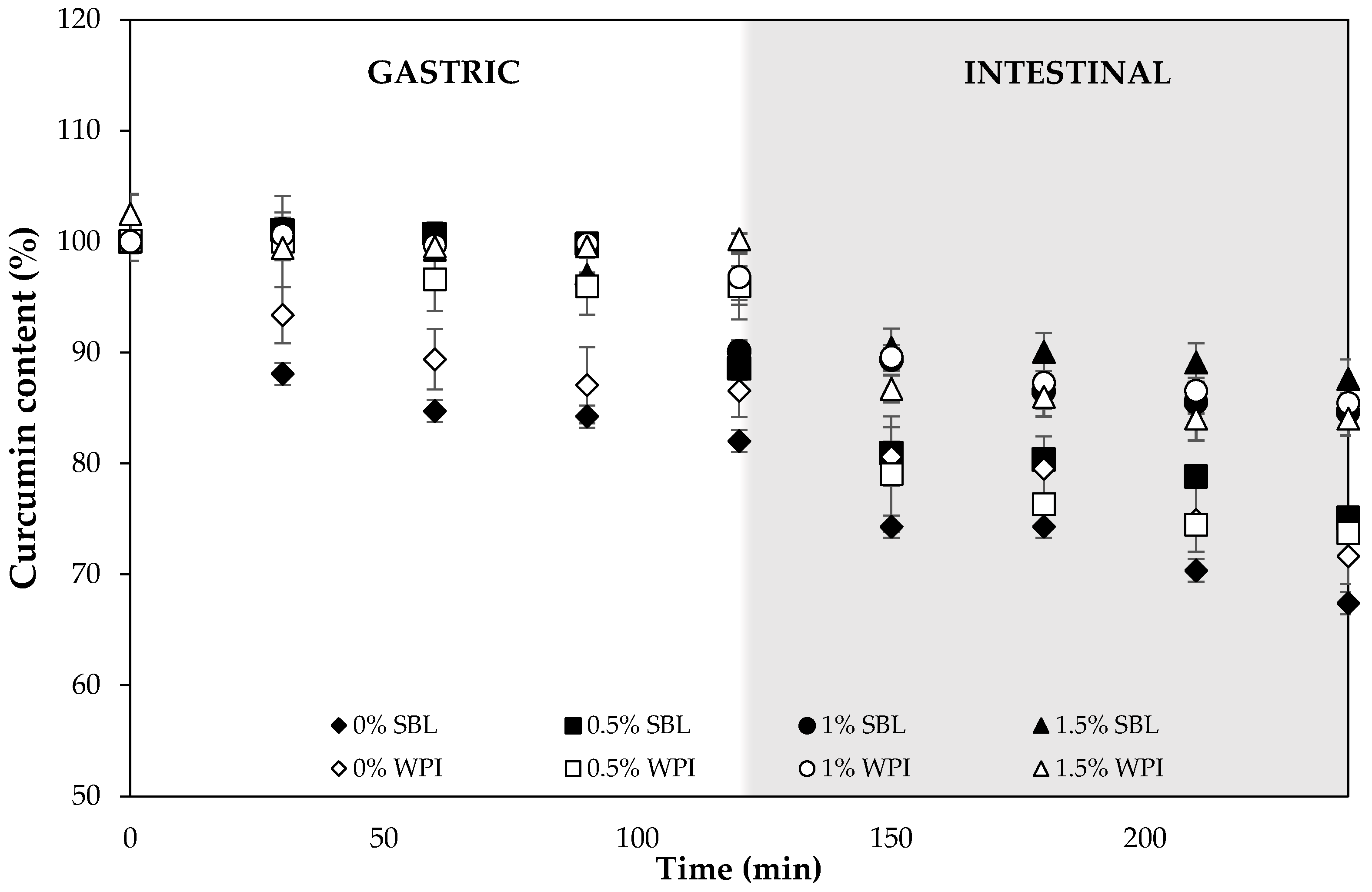
2.2.3. Curcumin Degradation during Digestion
2.2.4. Curcumin Bioaccessibility
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Nanoemulsion Preparation
3.2.2. Physicochemical Characterization
3.2.3. Stability of Nanoemulsions
3.2.4. Curcuminoid Extraction from Nanoemulsions and Quantification
3.2.5. In Vitro Digestion
3.2.6. Curcumin Degradation
3.2.7. Bioaccessibility
3.2.8. Optical Microscopy
3.2.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, Z.; Smart, J.D.; Pannala, A.S. Recent Developments in Formulation Design for Improving Oral Bioavailability of Curcumin: A Review. J. Drug Deliv. Sci. Technol. 2020, 60, 102082. [Google Scholar] [CrossRef]
- Araiza-Calahorra, A.; Akhtar, M.; Sarkar, A. Recent Advances in Emulsion-Based Delivery Approaches for Curcumin: From Encapsulation to Bioaccessibility. Trends Food Sci. Technol. 2018, 71, 155–169. [Google Scholar] [CrossRef]
- Peng, S.; Li, Z.; Zou, L.; Liu, W.; Liu, C.; McClements, D.J. Enhancement of Curcumin Bioavailability by Encapsulation in Sophorolipid-Coated Nanoparticles: An in Vitro and in Vivo Study. J. Agric. Food Chem. 2018, 66, 1488–1497. [Google Scholar] [CrossRef]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of Curcumin: Problems and Promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Hewlings, S.J.; Kalman, D.S. Curcumin: A Review of Its Effects on Human Health. Foods 2017, 6, 92. [Google Scholar] [CrossRef] [Green Version]
- Zheng, B.; Zhang, Z.; Chen, F.; Luo, X.; McClements, D.J. Impact of Delivery System Type on Curcumin Stability: Comparison of Curcumin Degradation in Aqueous Solutions, Emulsions, and Hydrogel Beads. Food Hydrocoll. 2017, 71, 187–197. [Google Scholar] [CrossRef]
- Wang, Y.J.; Pan, M.H.; Cheng, A.L.; Lin, L.I.; Ho, Y.S.; Hsieh, C.Y.; Lin, J.K. Stability of Curcumin in Buffer Solutions and Characterization of Its Degradation Products. J. Pharm. Biomed. Anal. 1997, 15, 1867–1876. [Google Scholar] [CrossRef]
- Ahmed, K.; Li, Y.; McClements, D.J.; Xiao, H. Nanoemulsion- and Emulsion-Based Delivery Systems for Curcumin: Encapsulation and Release Properties. Food Chem. 2012, 132, 799–807. [Google Scholar] [CrossRef]
- Ma, P.; Zeng, Q.; Tai, K.; He, X.; Yao, Y.; Hong, X.; Yuan, F. Preparation of Curcumin-Loaded Emulsion Using High Pressure Homogenization: Impact of Oil Phase and Concentration on Physicochemical Stability. LWT Food Sci. Technol. 2017, 84, 34–46. [Google Scholar] [CrossRef]
- Zhu, X.; Chen, J.; Hu, Y.; Zhang, N.; Fu, Y.; Chen, X. Tuning Complexation of Carboxymethyl Cellulose/Cationic Chitosan to Stabilize Pickering Emulsion for Curcumin Encapsulation. Food Hydrocoll. 2021, 110, 106135. [Google Scholar] [CrossRef]
- Lu, X.; Zhu, J.; Pan, Y.; Huang, Q. Assessment of Dynamic Bioaccessibility of Curcumin Encapsulated in Milled Starch Particle Stabilized Pickering Emulsions Using TNO’s Gastrointestinal Model. Food Funct. 2019, 10, 2583–2594. [Google Scholar] [CrossRef]
- Artiga-Artigas, M.; Lanjari-Pérez, Y.; Martín-Belloso, O. Curcumin-Loaded Nanoemulsions Stability as Affected by the Nature and Concentration of Surfactant. Food Chem. 2018, 266, 466–474. [Google Scholar] [CrossRef]
- Silva, H.D.; Cerqueira, M.Â.; Vicente, A.A. Nanoemulsions for Food Applications: Development and Characterization. Food Bioprocess Technol. 2012, 5, 854–867. [Google Scholar] [CrossRef] [Green Version]
- Zheng, B.; Zhang, X.; Peng, S.; McClements, D.J. Impact of Curcumin Delivery System Format on Bioaccessibility: Nanocrystals, Nanoemulsion Droplets, and Natural Oil Bodies. Food Funct. 2019, 10, 4339–4349. [Google Scholar] [CrossRef]
- Jiang, T.; Liao, W.; Charcosset, C. Recent Advances in Encapsulation of Curcumin in Nanoemulsions: A Review of Encapsulation Technologies, Bioaccessibility and Applications. Food Res. Int. 2020, 132, 109035. [Google Scholar] [CrossRef]
- Pinheiro, A.C.; Lad, M.; Silva, H.D.; Coimbra, M.A.; Boland, M.; Vicente, A.A. Unravelling the Behaviour of Curcumin Nanoemulsions during in Vitro Digestion: Effect of the Surface Charge. Soft. Matter. 2013, 9, 3147–3154. [Google Scholar] [CrossRef] [Green Version]
- Gasa-Falcon, A.; Odriozola-Serrano, I.; Oms-Oliu, G.; Martín-Belloso, O. Impact of Emulsifier Nature and Concentration on the Stability of β-Carotene Enriched Nanoemulsions during: In Vitro Digestion. Food Funct. 2019, 10, 713–722. [Google Scholar] [CrossRef] [Green Version]
- Klang, V.; Valenta, C. Lecithin-Based Nanoemulsions. J. Drug Deliv. Sci. Technol. 2011, 21, 55–76. [Google Scholar] [CrossRef]
- McClements, D.J.; Bai, L.; Chung, C. Recent Advances in the Utilization of Natural Emulsifiers to Form and Stabilize Emulsions. Annu. Rev. Food Sci. Technol. 2017, 8, 205–236. [Google Scholar] [CrossRef]
- Ozturk, B.; Argin, S.; Ozilgen, M.; McClements, D.J. Formation and Stabilization of Nanoemulsion-Based Vitamin e Delivery Systems Using Natural Surfactants: Quillaja Saponin and Lecithin. J. Food Eng. 2014, 142, 57–63. [Google Scholar] [CrossRef]
- Zheng, H.; Li, J.; Ning, F.; Wijaya, W.; Chen, Y.; Xiao, J.; Cao, Y.; Huang, Q. Improving: In Vitro Bioaccessibility and Bioactivity of Carnosic Acid Using a Lecithin-Based Nanoemulsion System. Food Funct. 2021, 12, 1558–1568. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.C.; Lin, H.Y.; Chen, H.C.; Yu, M.W.; Lee, M.H. Stability and Characterisation of Phospholipid-Based Curcumin-Encapsulated Microemulsions. Food Chem. 2009, 116, 923–928. [Google Scholar] [CrossRef]
- Li, J.; Ye, A.; Lee, S.J.; Singh, H. Physicochemical Behaviour of WPI-Stabilized Emulsions in in Vitro Gastric and Intestinal Conditions. Colloids Surf. B Biointerfaces 2013, 111, 80–87. [Google Scholar] [CrossRef]
- Dickinson, E. Milk Protein Interfacial Layers and the Relationship to Emulsion Stability and Rheology. Colloids Surf. B Biointerfaces 2001, 20, 197–210. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Yang, J.; Xu, D.; Yuan, F.; Gao, Y. Effects of Homogenization Models and Emulsifiers on the Physicochemical Properties of β-Carotene Nanoemulsions. J. Dispers. Sci. Technol. 2010, 31, 986–993. [Google Scholar] [CrossRef]
- Jo, Y.J.; Kwon, Y.J. Characterization of β-Carotene Nanoemulsions Prepared by Microfluidization Technique. Food Sci. Biotechnol. 2014, 23, 107–113. [Google Scholar] [CrossRef]
- Bai, L.; Huan, S.; Gu, J.; McClements, D.J. Fabrication of Oil-in-Water Nanoemulsions by Dual-Channel Microfluidization Using Natural Emulsifiers: Saponins, Phospholipids, Proteins, and Polysaccharides. Food Hydrocoll. 2016, 61, 703–711. [Google Scholar] [CrossRef] [Green Version]
- Shah, B.R.; Li, Y.; Jin, W.; An, Y.; He, L.; Li, Z.; Xu, W.; Li, B. Preparation and Optimization of Pickering Emulsion Stabilized by Chitosan-Tripolyphosphate Nanoparticles for Curcumin Encapsulation. Food Hydrocoll. 2016, 52, 369–377. [Google Scholar] [CrossRef]
- Richa, R.; Roy Choudhury, A. Exploration of Polysaccharide Based Nanoemulsions for Stabilization and Entrapment of Curcumin. Int. J. Biol. Macromol. 2020, 156, 1287–1296. [Google Scholar] [CrossRef]
- Artiga-Artigas, M.; Acevedo-Fani, A.; Martín-Belloso, O. Effect of Sodium Alginate Incorporation Procedure on the Physicochemical Properties of Nanoemulsions. Food Hydrocoll. 2017, 70, 191–200. [Google Scholar] [CrossRef]
- Sabet, S.; Seal, C.K.; Swedlund, P.J.; McGillivray, D.J. Depositing Alginate on the Surface of Bilayer Emulsions. Food Hydrocoll. 2020, 100, 105385. [Google Scholar] [CrossRef]
- Chang, Y.; McClements, D.J. Influence of Emulsifier Type on the in Vitro Digestion of Fish Oil-in-Water Emulsions in the Presence of an Anionic Marine Polysaccharide (Fucoidan): Caseinate, Whey Protein, Lecithin, or Tween 80. Food Hydrocoll. 2016, 61, 92–101. [Google Scholar] [CrossRef] [Green Version]
- Verkempinck, S.H.E.; Salvia-Trujillo, L.; Denis, S.; Van Loey, A.M.; Hendrickx, M.E.; Grauwet, T. Pectin Influences the Kinetics of in Vitro Lipid Digestion in Oil-in-Water Emulsions. Food Chem. 2018, 262, 150–161. [Google Scholar] [CrossRef]
- Gasa-Falcon, A.; Odriozola-Serrano, I.; Oms-Oliu, G.; Martín-Belloso, O. Influence of Mandarin Fiber Addition on Physico-Chemical Properties of Nanoemulsions Containing β-Carotene under Simulated Gastrointestinal Digestion Conditions. LWT-Food Sci. Technol. 2017, 84, 331–337. [Google Scholar] [CrossRef]
- Tan, Y.; Li, R.; Liu, C.; Muriel Mundo, J.; Zhou, H.; Liu, J.; McClements, D.J. Chitosan reduces vitamin D bioaccessibility in food emulsions by binding to mixed micelles. Food Funct. 2020, 11, 187–199. [Google Scholar] [CrossRef]
- Wang, H.; Gong, X.; Guo, X.; Liu, C.; Fan, Y.Y.; Zhang, J.; Niu, B.; Li, W. Characterization, Release, and Antioxidant Activity of Curcumin-Loaded Sodium Alginate/ZnO Hydrogel Beads. Int. J. Biol. Macromol. 2019, 121, 1118–1125. [Google Scholar] [CrossRef]
- Pallandre, S.; Decker, E.A.; McClements, D.J. Improvement of Stability of Oil-in-Water Emulsions Containing Caseinate-Coated Droplets by Addition of Sodium Alginate. J. Food Sci. 2007, 72, E518–E524. [Google Scholar] [CrossRef]
- Flores-Andrade, E.; Allende-Baltazar, Z.; Sandoval-González, P.E.; Jiménez-Fernández, M.; Beristain, C.I.; Pascual-Pineda, L.A. Carotenoid Nanoemulsions Stabilized by Natural Emulsifiers: Whey Protein, Gum Arabic, and Soy Lecithin. J. Food Eng. 2021, 290, 110208. [Google Scholar] [CrossRef]
- Dickinson, E.; Golding, M.; Povey, M.J.W. Creaming and Flocculation of Oil-in-Water Emulsions Containing Sodium Caseinate. J. Colloid Interface Sci. 1997, 185, 515–529. [Google Scholar] [CrossRef]
- McClements, D. Food Emulsions: Principles, Practices and Techniques, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Zhang, R.; Zhang, Z.; Zou, L.; Xiao, H.; Zhang, G.; Decker, E.A.; McClements, D.J. Enhancement of Carotenoid Bioaccessibility from Carrots Using Excipient Emulsions: Influence of Particle Size of Digestible Lipid Droplets. Food Funct. 2016, 7, 93–103. [Google Scholar] [CrossRef]
- Kulmyrzaev, A.; Chanamai, R.; McClements, D.J. Influence of PH and CaCl2 on the Stability of Dilute Whey Protein Stabilized Emulsions. Food Res. Int. 2000, 33, 15–20. [Google Scholar] [CrossRef]
- Kumar, M.; Ahuja, M.; Sharma, S.K. Hepatoprotective Study of Curcumin-Soya Lecithin Complex. Sci. Pharm. 2008, 76, 761–774. [Google Scholar] [CrossRef] [Green Version]
- Heckert Bastos, L.P.; Vicente, J.; Corrêa dos Santos, C.H.; Geraldo de Carvalho, M.; Garcia-Rojas, E.E. Encapsulation of Black Pepper (Piper Nigrum L.) Essential Oil with Gelatin and Sodium Alginate by Complex Coacervation. Food Hydrocoll. 2020, 102, 105605. [Google Scholar] [CrossRef]
- Shao, P.; Feng, J.; Sun, P.; Xiang, N.; Lu, B.; Qiu, D. Recent Advances in Improving Stability of Food Emulsion by Plant Polysaccharides. Food Res. Int. 2020, 137, 109376. [Google Scholar] [CrossRef] [PubMed]
- Phillips, G.O.; Williams, P.A. Handbook of Hydrocolloids, 2nd ed.; CRC Press and Woodhead Publishing: Boca Raton, FL, USA, 2009. [Google Scholar]
- Xu, D.; Wang, X.; Jiang, J.; Yuan, F.; Gao, Y. Impact of Whey Protein-Beet Pectin Conjugation on the Physicochemical Stability of β-Carotene Emulsions. Food Hydrocoll. 2012, 28, 258–266. [Google Scholar] [CrossRef]
- Salvia-Trujillo, L.; Decker, E.A.; McClements, D.J. Influence of an Anionic Polysaccharide on the Physical and Oxidative Stability of Omega-3 Nanoemulsions: Antioxidant Effects of Alginate. Food Hydrocoll. 2016, 52, 690–698. [Google Scholar] [CrossRef] [Green Version]
- Mantovani, R.A.; Cavallieri, Â.L.F.; Netto, F.M.; Cunha, R.L. Stability and in Vitro Digestibility of Emulsions Containing Lecithin and Whey Proteins. Food Funct. 2013, 4, 1322–1331. [Google Scholar] [CrossRef]
- Ogawa, S.; Decker, E.A.; McClements, D.J. Production and Characterization of O/W Emulsions Containing Droplets Stabilized by Lecithin-Chitosan-Pectin Multilayered Membranes. J. Agric. Food Chem. 2004, 52, 3595–3600. [Google Scholar] [CrossRef]
- Li, D.; Wei, Z.; Xue, C. Alginate-Based Delivery Systems for Food Bioactive Ingredients: An Overview of Recent Advances and Future Trends. Compr. Rev. Food Sci. Food Saf. 2021, 20, 5345–5370. [Google Scholar] [CrossRef]
- Fioramonti, S.A.; Martinez, M.J.; Pilosof, A.M.R.; Rubiolo, A.C.; Santiago, L.G. Multilayer Emulsions as a Strategy for Linseed Oil Microencapsulation: Effect of PH and Alginate Concentration. Food Hydrocoll. 2015, 43, 8–17. [Google Scholar] [CrossRef]
- Guzey, D.; McClements, D.J. Formation, Stability and Properties of Multilayer Emulsions for Application in the Food Industry. Adv. Colloid Interface Sci. 2006, 128–130, 227–248. [Google Scholar] [CrossRef]
- Gonçalves, R.F.S.; Martins, J.T.; Abrunhosa, L.; Baixinho, J.; Matias, A.A.; Vicente, A.A.; Pinheiro, A.C. Lipid-Based Nanostructures as a Strategy to Enhance Curcumin Bioaccessibility: Behavior under Digestion and Cytotoxicity Assessment. Food Res. Int. 2021, 143, 110278. [Google Scholar] [CrossRef]
- Chen, L.; Yokoyama, W.; Liang, R.; Zhong, F. Enzymatic Degradation and Bioaccessibility of Protein Encapsulated β-Carotene Nano-Emulsions during in Vitro Gastro-Intestinal Digestion. Food Hydrocoll. 2020, 100, 105177. [Google Scholar] [CrossRef]
- Reis, P.; Holmberg, K.; Watzke, H.; Leser, M.E.; Miller, R. Lipases at Interfaces: A Review. Adv. Colloid Interface Sci. 2009, 147–148, 237–250. [Google Scholar] [CrossRef]
- Li, Y.; Hu, M.; McClements, D.J. Factors Affecting Lipase Digestibility of Emulsified Lipids Using an in Vitro Digestion Model: Proposal for a Standardised PH-Stat Method. Food Chem. 2011, 126, 498–505. [Google Scholar] [CrossRef]
- Li, Y.; Hu, M.; Du, Y.; McClements, D.J. Controlling Lipid Nanoemulsion Digestion Using Nanolaminated Biopolymer Coatings. J. Microencapsul. 2011, 28, 166–175. [Google Scholar] [CrossRef]
- Qin, D.; Yang, X.; Gao, S.; Yao, J.; McClements, D.J. Influence of Hydrocolloids (Dietary Fibers) on Lipid Digestion of Protein-Stabilized Emulsions: Comparison of Neutral, Anionic, and Cationic Polysaccharides. J. Food Sci. 2016, 81, C1636–C1645. [Google Scholar] [CrossRef]
- Espinal-Ruiz, M.; Parada-Alfonso, F.; Restrepo-Sánchez, L.P.; Narváez-Cuenca, C.E.; McClements, D.J. Impact of Dietary Fibers [Methyl Cellulose, Chitosan, and Pectin] on Digestion of Lipids under Simulated Gastrointestinal Conditions. Food Funct. 2014, 5, 3083–3095. [Google Scholar] [CrossRef]
- Velderrain-Rodríguez, G.R.; Salvia-Trujillo, L.; Wall-Medrano, A.; González-Aguilar, G.A.; Martín-Belloso, O. In Vitro Digestibility and Release of a Mango Peel Extract Encapsulated within Water-in-Oil-in-Water (W1/O/W2) Emulsions Containing Sodium Carboxymethyl Cellulose. Food Funct. 2019, 10, 6110–6120. [Google Scholar] [CrossRef]
- Dima, C.; Dima, S. Bioaccessibility Study of Calcium and Vitamin D3 Co-Microencapsulated in Water-in-Oil-in-Water Double Emulsions. Food Chem. 2020, 303, 125416. [Google Scholar] [CrossRef]
- Salvia-Trujillo, L.; Qian, C.; Martín-Belloso, O.; McClements, D.J. Influence of Particle Size on Lipid Digestion and β-Carotene Bioaccessibility in Emulsions and Nanoemulsions. Food Chem. 2013, 141, 1472–1480. [Google Scholar] [CrossRef] [PubMed]
- Sneharani, A.H.; Karakkat, J.V.; Singh, S.A.; Rao, A.G.A. Interaction of Curcumin with SS-Lactoglobulin;Stability, Spectroscopic Analysis, and Molecular Modeling of the Complex. J. Agric. Food Chem. 2010, 58, 11130–11139. [Google Scholar] [CrossRef]
- Korhonen, H.; Pihlanto, A. Bioactive Peptides: Production and Functionality. Int. Dairy J. 2006, 16, 945–960. [Google Scholar] [CrossRef]
- Madureira, A.R.; Tavares, T.; Gomes, A.M.P.; Pintado, M.E.; Malcata, F.X. Invited Review: Physiological Properties of Bioactive Peptides Obtained from Whey Proteins. J. Dairy Sci. 2010, 93, 437–455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, L.; Lu, W.; Mata, A.; Nishinari, K.; Fang, Y. Egg-Box Model-Based Gelation of Alginate and Pectin: A Review. Carbohydr. Polym. 2020, 242, 116389. [Google Scholar] [CrossRef] [PubMed]
- Agüero, L.; Zaldivar-Silva, D.; Peña, L.; Dias, M. Alginate Microparticles as Oral Colon Drug Delivery Device: A Review. Carbohydr. Polym. 2017, 168, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Apoorva, A.; Rameshbabu, A.P.; Dasgupta, S.; Dhara, S.; Padmavati, M. Novel PH-Sensitive Alginate Hydrogel Delivery System Reinforced with Gum Tragacanth for Intestinal Targeting of Nutraceuticals. Int. J. Biol. Macromol. 2020, 147, 675–687. [Google Scholar] [CrossRef]
- Zheng, B.; Peng, S.; Zhang, X.; McClements, D.J. Impact of Delivery System Type on Curcumin Bioaccessibility: Comparison of Curcumin-Loaded Nanoemulsions with Commercial Curcumin Supplements. J. Agric. Food Chem. 2018, 66, 10816–10826. [Google Scholar] [CrossRef]
- Zou, L.; Liu, W.; Liu, C.; Xiao, H.; McClements, D.J. Designing Excipient Emulsions to Increase Nutraceutical Bioavailability: Emulsifier Type Influences Curcumin Stability and Bioaccessibility by Altering Gastrointestinal Fate. Food Funct. 2015, 6, 2475–2486. [Google Scholar] [CrossRef]
- Chen, X.; McClements, D.J.; Zhu, Y.; Zou, L.; Li, Z.; Liu, W.; Cheng, C.; Gao, H.; Liu, C. Gastrointestinal Fate of Fluid and Gelled Nutraceutical Emulsions: Impact on Proteolysis, Lipolysis, and Quercetin Bioaccessibility. J. Agric. Food Chem. 2018, 66, 9087–9096. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, R.; Zou, L.; Chen, L.; Ahmed, Y.; Al Bishri, W.; Balamash, K.; McClements, D.J. Encapsulation of Curcumin in Polysaccharide-Based Hydrogel Beads: Impact of Bead Type on Lipid Digestion and Curcumin Bioaccessibility. Food Hydrocoll. 2016, 58, 160–170. [Google Scholar] [CrossRef] [Green Version]
- Xing, F.; Cheng, G.; Yang, B.; Ma, L. Microencapsulation of Capsaicin by the Complex Coacervation of Gelatin, Acacia and Tannins. J. Appl. Polym. Sci. 2004, 91, 2669–2675. [Google Scholar] [CrossRef]
- Setthacheewakul, S.; Mahattanadul, S.; Phadoongsombut, N.; Pichayakorn, W.; Wiwattanapatapee, R. Development and Evaluation of Self-Microemulsifying Liquid and Pellet Formulations of Curcumin, and Absorption Studies in Rats. Eur. J. Pharm. Biopharm. 2010, 76, 475–485. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST Static in Vitro Simulation of Gastrointestinal Food Digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- Li, Y.; Mcclements, D.J. New Mathematical Model for Interpreting Ph-Stat Digestion Profiles: Impact of Lipid Droplet Characteristics on in Vitro Digestibility. J. Agric. Food Chem. 2010, 58, 8085–8092. [Google Scholar] [CrossRef]


| Emulsifier Type | Alginate (%) | d32 (µm) | ζ-Potential (mV) | Encapsulation Efficiency (%) | Viscosity (mPa·s) |
|---|---|---|---|---|---|
| Soybean lecithin | 0 | 0.344 ± 0.01 c | −47.1 ± 3.3 c | 97.0 ± 0.8 ab | 1.59 ± 0.13 a |
| 0.5 | 0.308 ± 0.02 b | −65.7 ± 2.6 b | 97.1 ± 1.0 ab | 10.28 ± 0.79 b | |
| 1 | 0.288 ± 0.02 a | −76.6 ± 3.6 a | 97.5 ± 0.2 b | 41.01 ± 4.76 c | |
| 1.5 | 0.288 ± 0.02 a | −77.8 ± 4.3 a | 96.7 ± 0.3 a | 110.78 ± 3.79 d | |
| Whey protein isolate | 0 | 0.487 ± 0.01 a | −37.3 ± 2.5 d | 95.1 ± 0.2 a | 1.44 ± 0.03 a |
| 0.5 | 0.499 ± 0.03 b | −43.9 ± 2.3 c | 95.2 ± 0.7 a | 18.72 ± 0.30 b | |
| 1 | 2.080 ± 0.11 c | −52.1 ± 1.1 b | 95.6 ± 1.1 a | 57.53 ± 1.16 c | |
| 1.5 | 2.251 ± 0.27 d | −57.7 ± 1.4 a | 95.7 ± 0.2 a | 118.33 ± 3.56 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teixé-Roig, J.; Oms-Oliu, G.; Odriozola-Serrano, I.; Martín-Belloso, O. Enhancing the Gastrointestinal Stability of Curcumin by Using Sodium Alginate-Based Nanoemulsions Containing Natural Emulsifiers. Int. J. Mol. Sci. 2023, 24, 498. https://doi.org/10.3390/ijms24010498
Teixé-Roig J, Oms-Oliu G, Odriozola-Serrano I, Martín-Belloso O. Enhancing the Gastrointestinal Stability of Curcumin by Using Sodium Alginate-Based Nanoemulsions Containing Natural Emulsifiers. International Journal of Molecular Sciences. 2023; 24(1):498. https://doi.org/10.3390/ijms24010498
Chicago/Turabian StyleTeixé-Roig, Júlia, Gemma Oms-Oliu, Isabel Odriozola-Serrano, and Olga Martín-Belloso. 2023. "Enhancing the Gastrointestinal Stability of Curcumin by Using Sodium Alginate-Based Nanoemulsions Containing Natural Emulsifiers" International Journal of Molecular Sciences 24, no. 1: 498. https://doi.org/10.3390/ijms24010498





