Geraniol-Mediated Suppression of Endoplasmic Reticulum Stress Protects against Cerebral Ischemia–Reperfusion Injury via the PERK-ATF4-CHOP Pathway
Abstract
:1. Introduction
2. Results
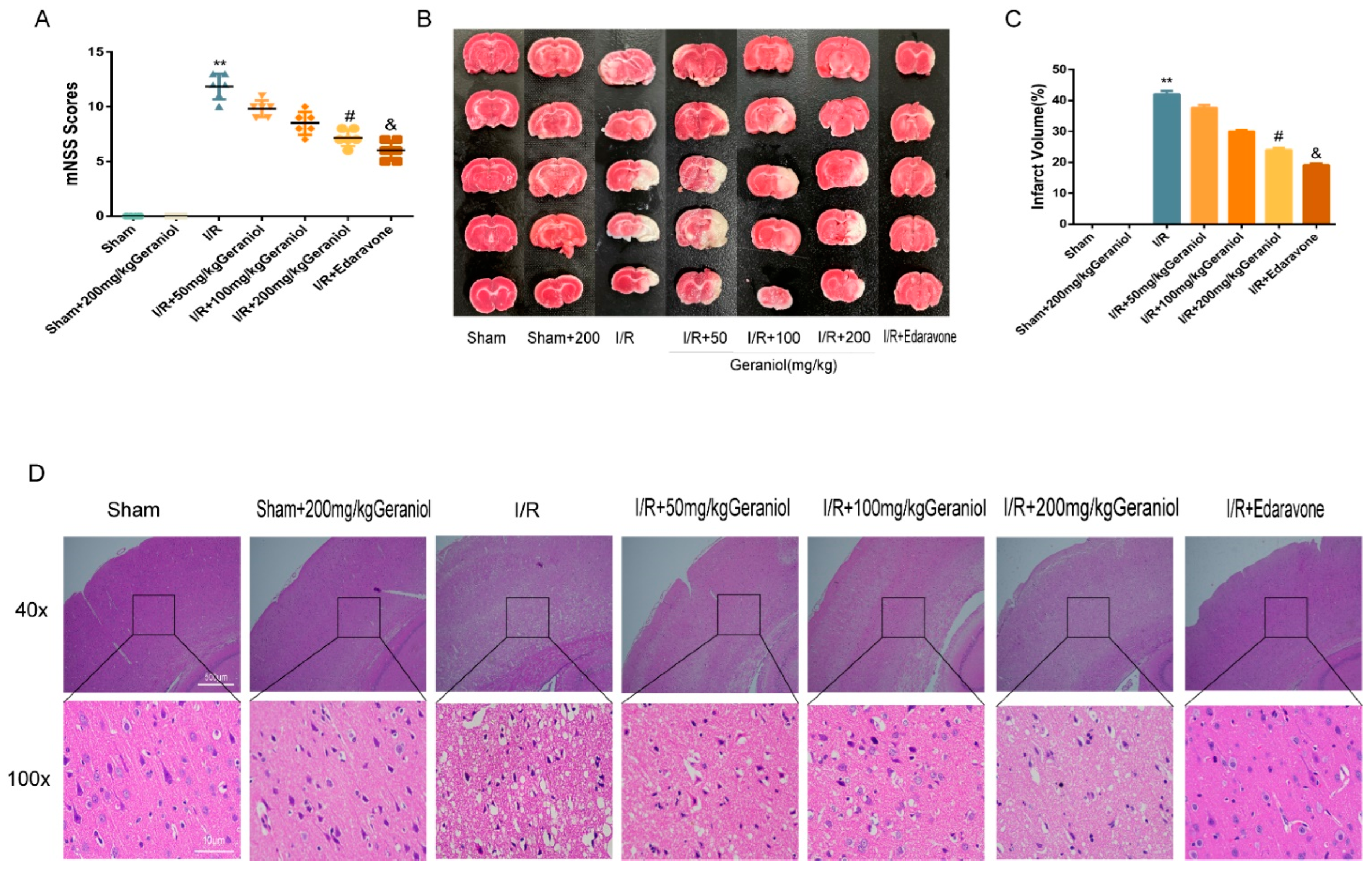
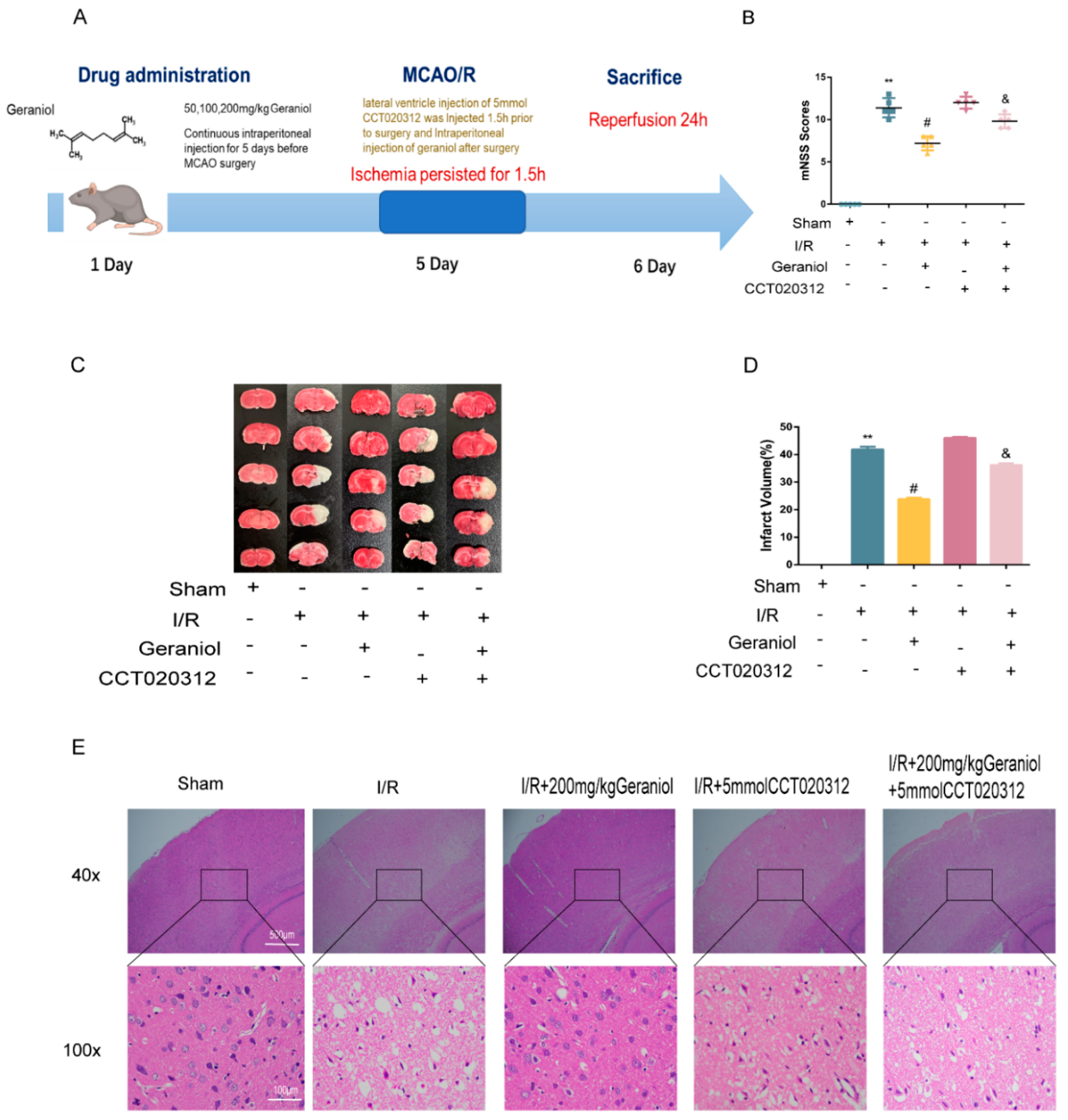
2.1. Protective Effect of Geraniol against I/R-Induced Brain Damage
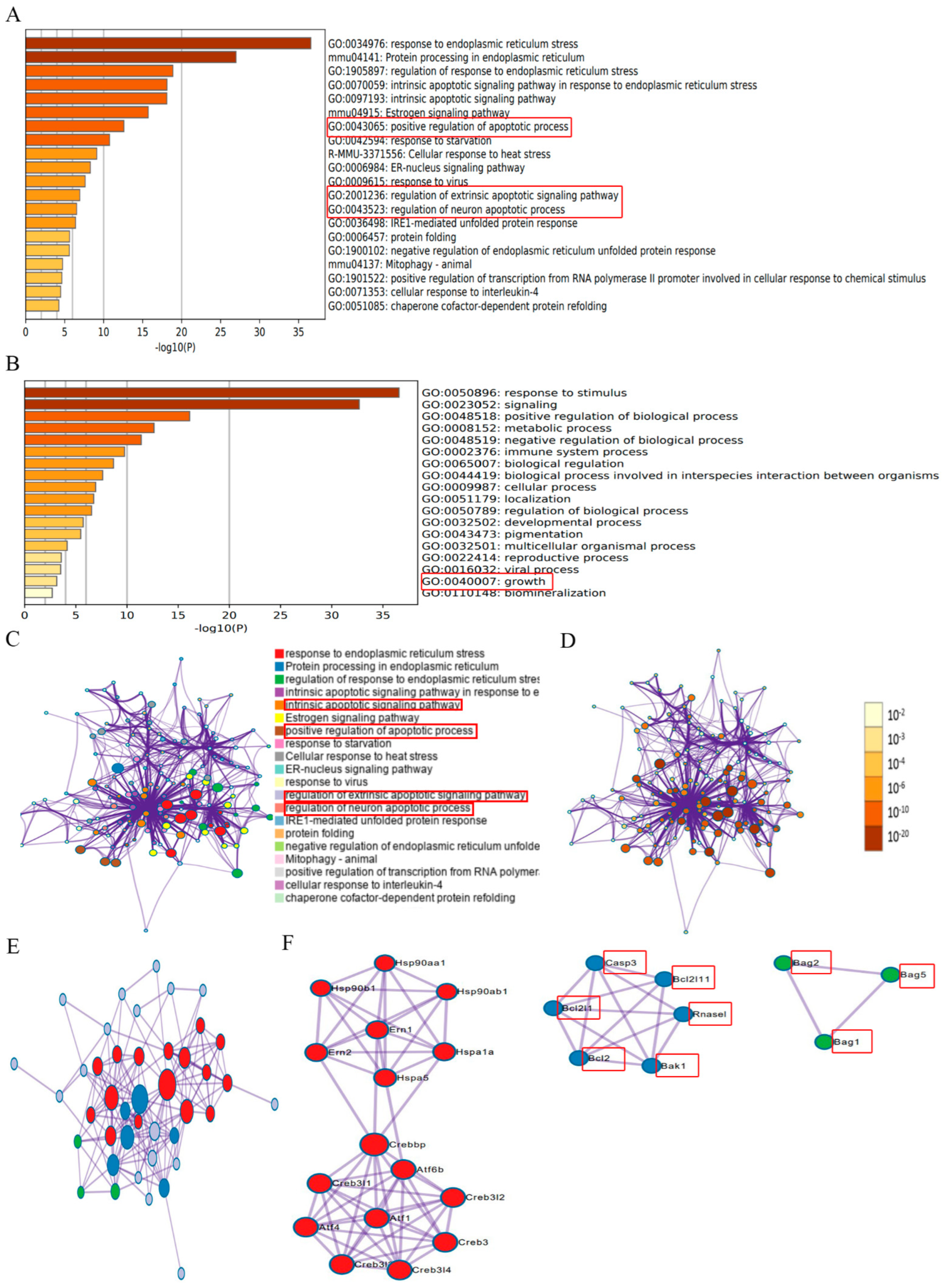
2.2. Identification of ER Stress Regulators via Metascape Online Analysis
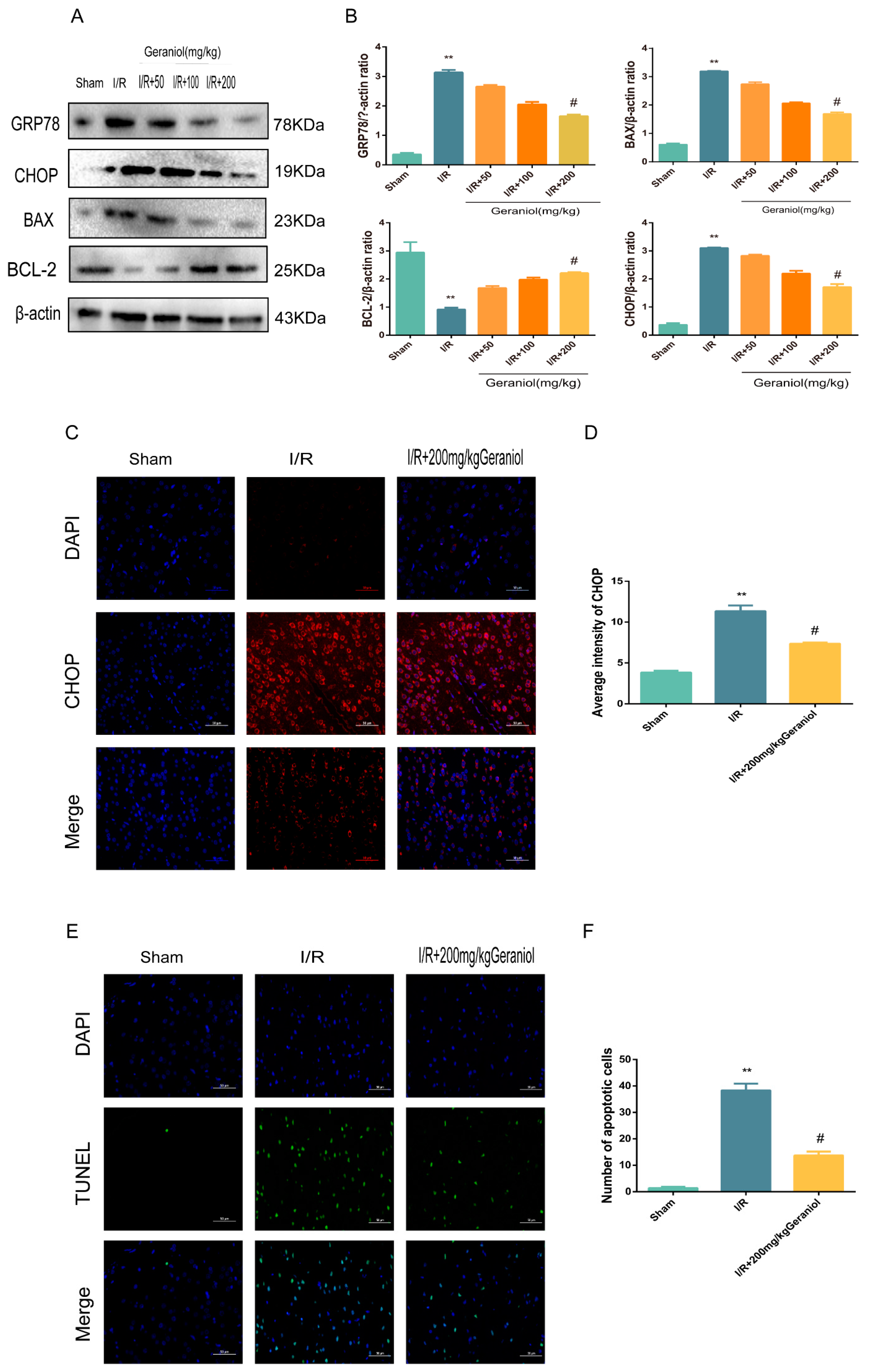
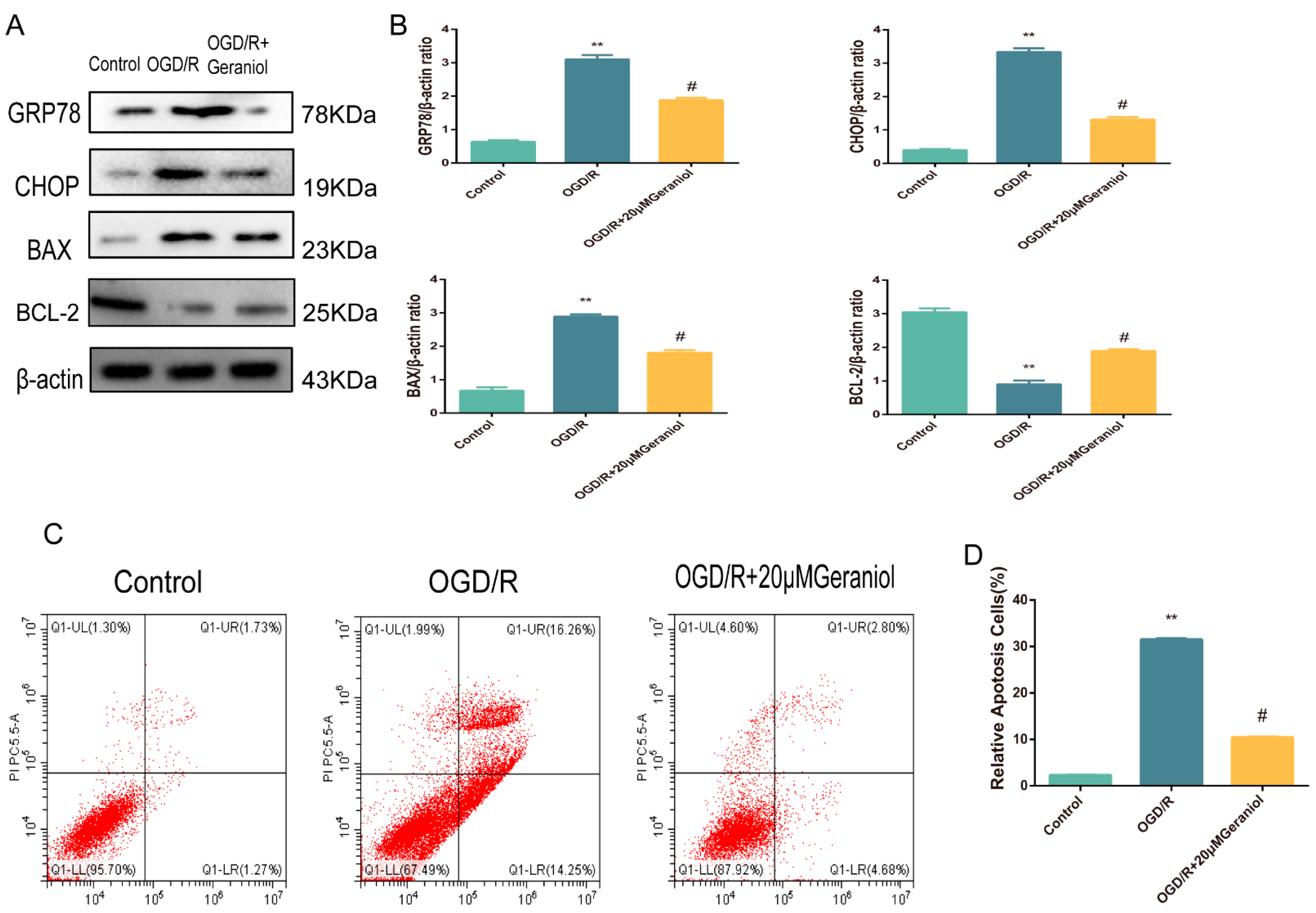
2.3. Geraniol Reduces I/R-Induced ER Stress and Cell Apoptosis
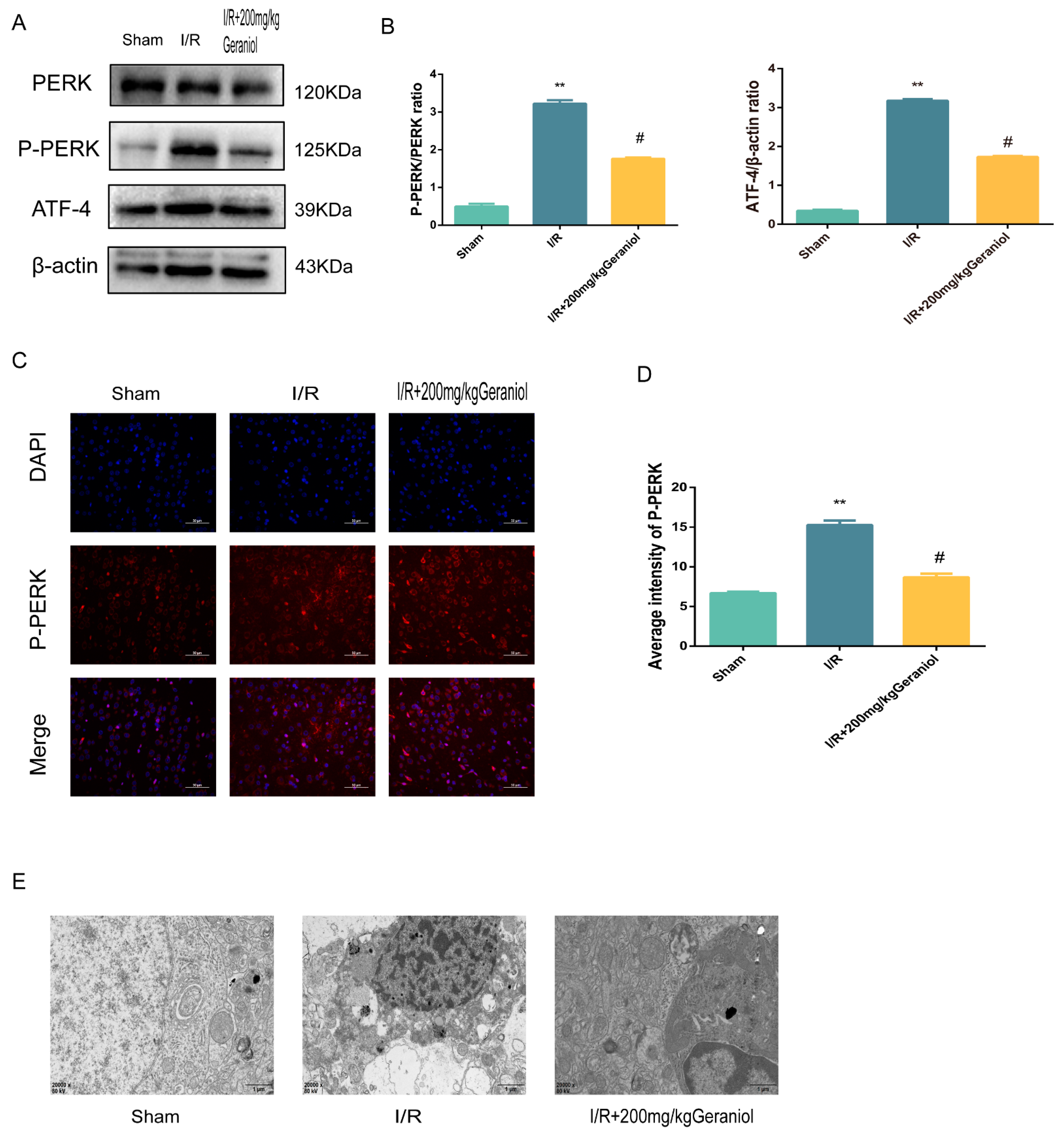
2.4. Geraniol Regulates the ER-Stress-Mediated PERK-ATF4 Pathway in the Cerebral Cortex after I/R
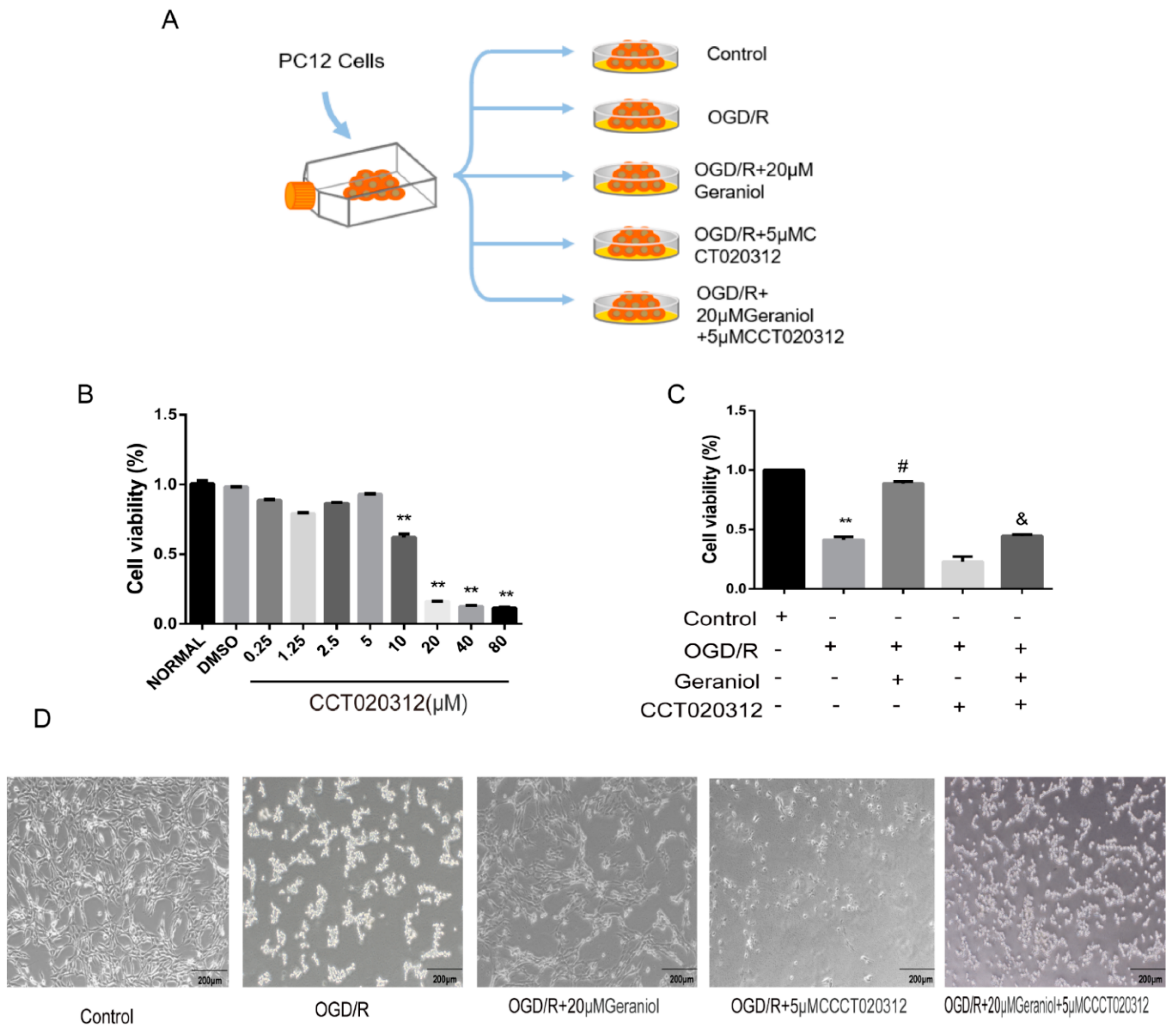
2.5. Inhibition of ER Stress via the PERK-ATF4-CHOP Pathway Is Required for the Geraniol-Mediated Protective Effect against I/R in the Cerebral Cortex
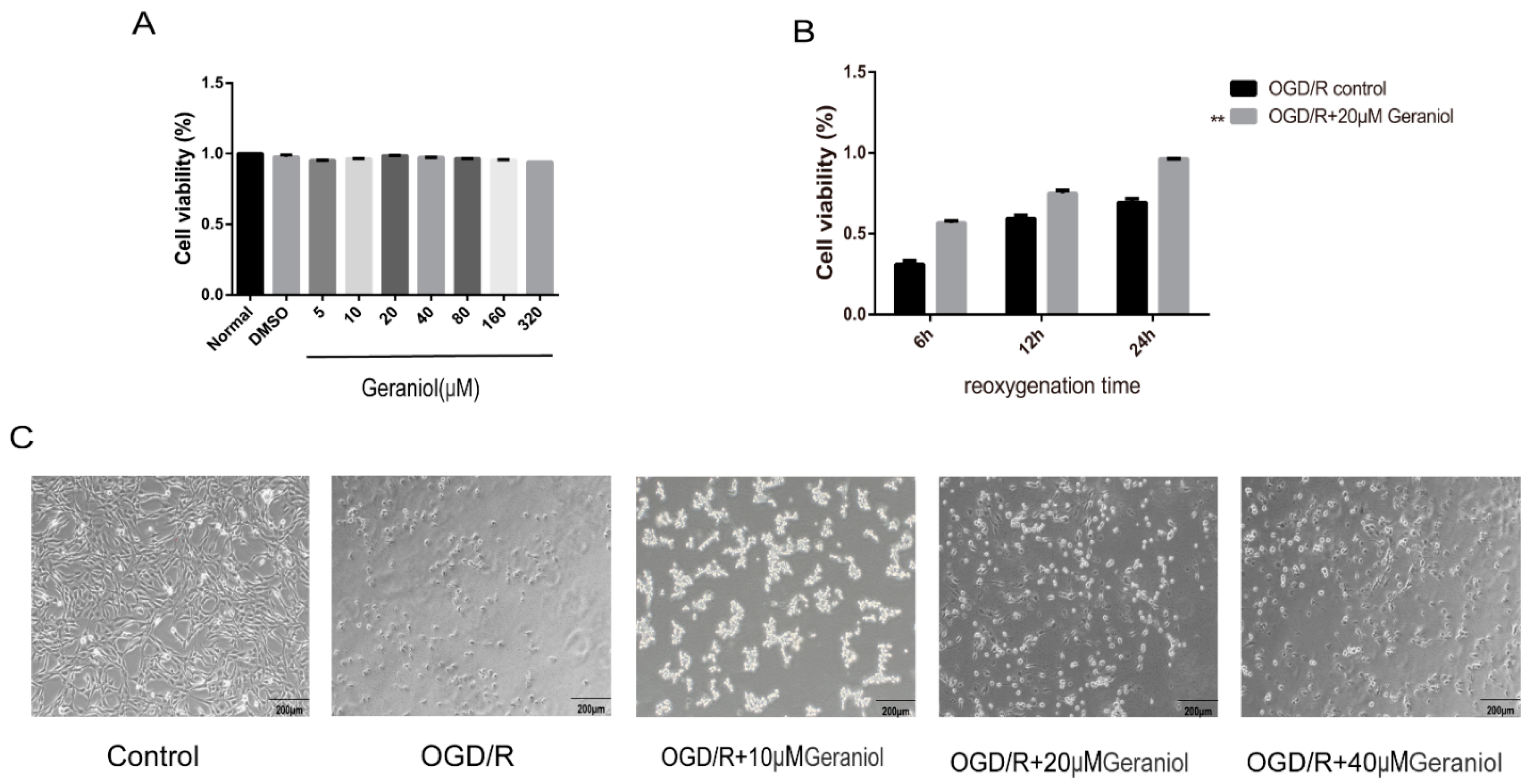
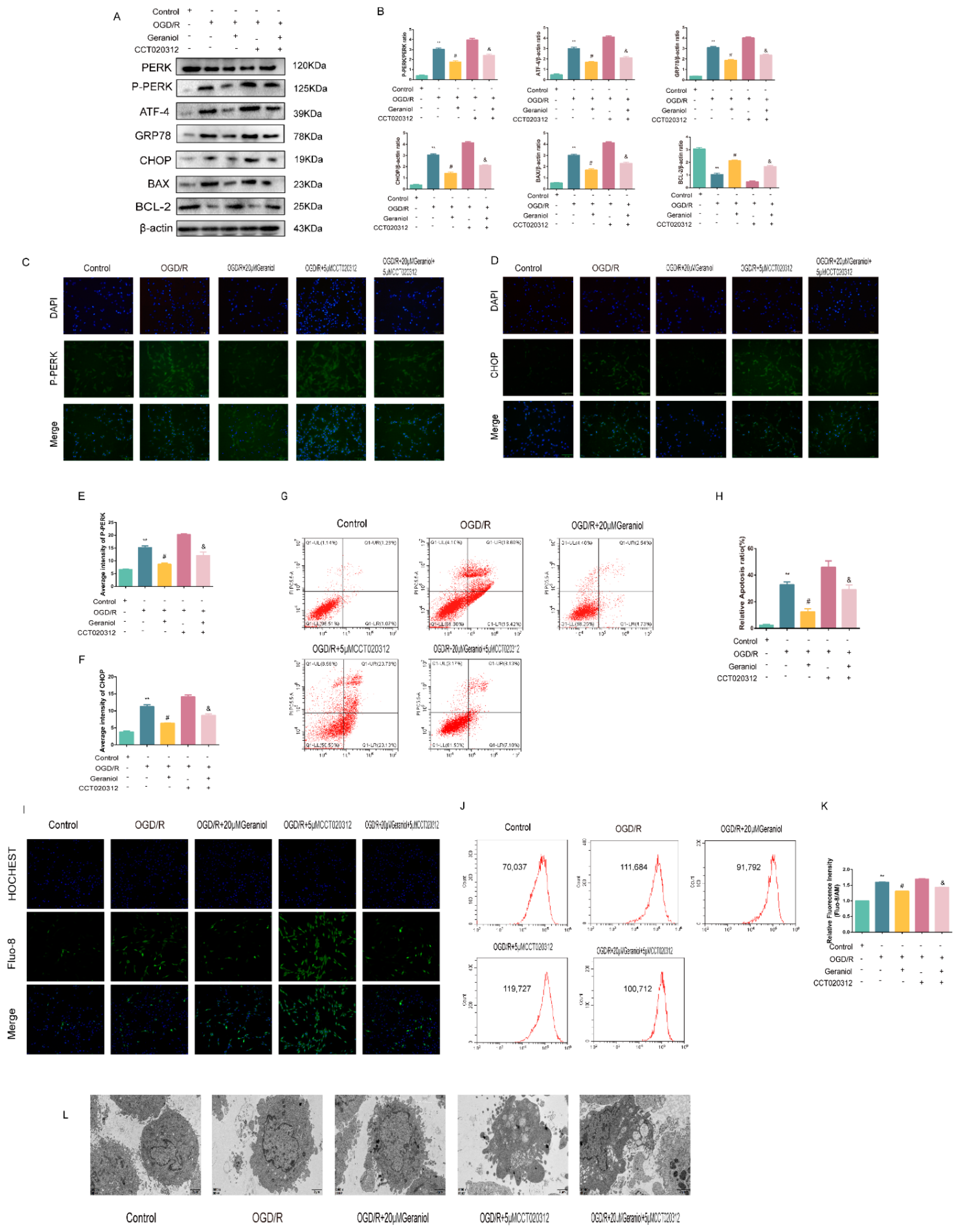
2.6. Geraniol Increases Cell Viability of PC12 Cells Induced by OGD/R
2.7. Geraniol Reduces ER Stress and Cell Apoptosis in OGD/R-Induced PC12 Cells
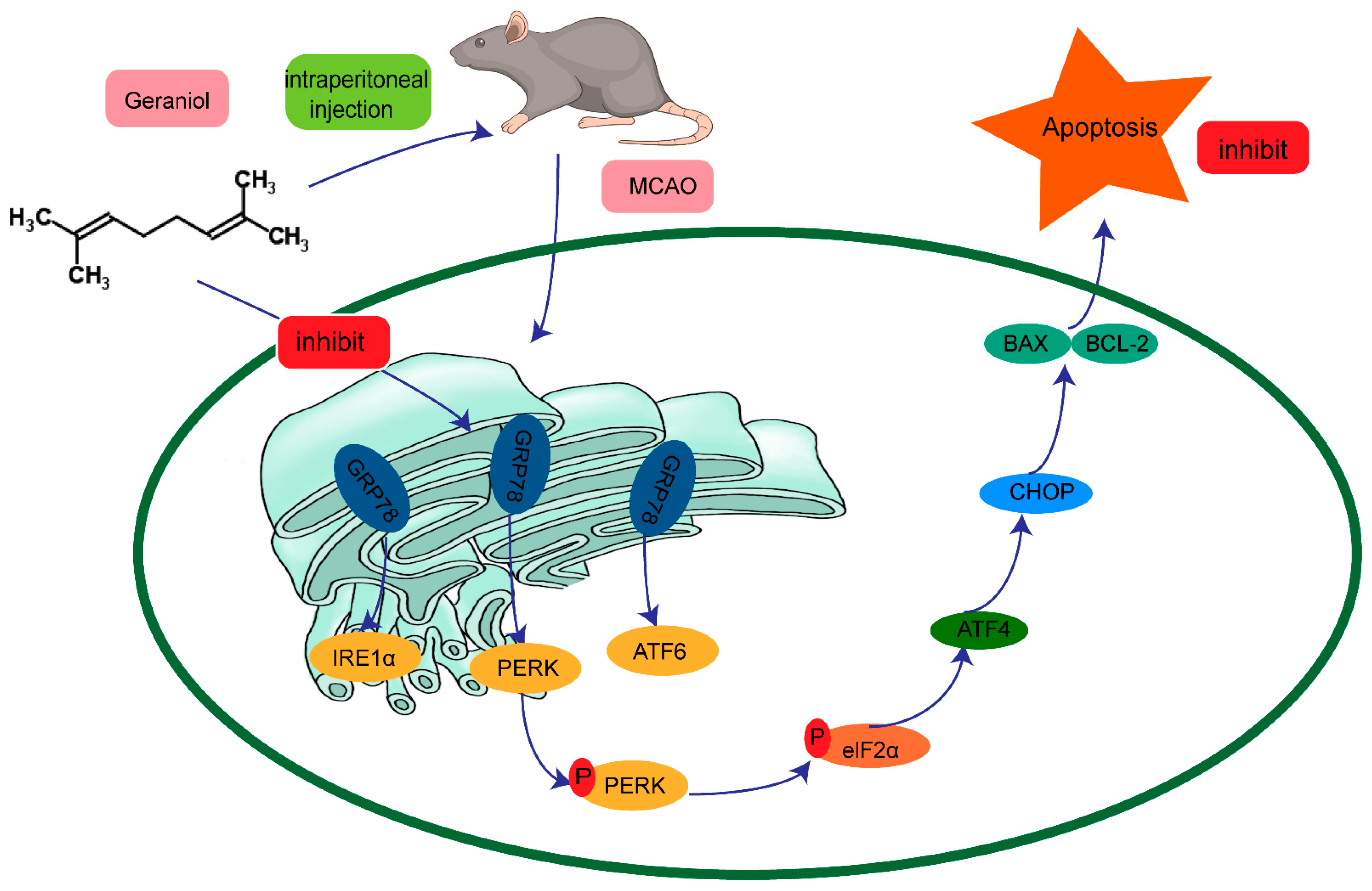
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Grouping and Drug Treatment
4.3. MCAO Model
4.4. Neurological Function Evaluation
4.5. 2,3,5-Triphenyltetrazolium Chloride (TTC) Staining of Brain Infarct Volume
4.6. Hematoxylin and Eosin (HE) Staining
4.7. TUNEL Staining
4.8. Cell Culture
4.9. Oxygen–Glucose Deprivation/Reoxygenation (OGD/R)
4.10. Immunofluorescence Staining
4.11. Transmission Electron Microscopy
4.12. Western Blotting
4.13. Cell Viability Assay
4.14. Flow Cytometric Apoptosis Assays
4.15. Intracellular Calcium Ion Measurement
4.16. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Emam, A.M.; Saad, M.A.; Ahmed, N.A.; Zaki, H.F. Vortioxetine mitigates neuronal damage by restricting PERK/eIF2α/ATF4/CHOP signaling pathway in rats subjected to focal cerebral ischemia-reperfusion. Life Sci. 2021, 283, 119865. [Google Scholar] [CrossRef]
- Moskowitz, M.A.; Lo, E.H.; Iadecola, C. The science of stroke: Mechanisms in search of treatments. Neuron 2010, 67, 181–198. [Google Scholar] [CrossRef] [Green Version]
- Gauberti, M.; Lapergue, B.; de Lizarrondo, S.M.; Vivien, D.; Richard, S.; Bracard, S.; Piotin, M.; Gory, B. Ischemia-reperfusion injury after endovascular thrombectomy for ischemic stroke. Stroke 2018, 49, 3071–3074. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Deng, H.; Xu, S.; Zhang, J. MicroRNAs regulate mitochondrial function in cerebral ischemia-reperfusion injury. Int. J. Mol. Sci. 2015, 16, 24895–24917. [Google Scholar] [CrossRef] [Green Version]
- Lim, S.; Kim, T.J.; Kim, Y.J.; Kim, C.; Ko, S.B.; Kim, B.S. Senolytic therapy for cerebral ischemia-reperfusion injury. Int. J. Mol. Sci. 2021, 22, 11967. [Google Scholar] [CrossRef]
- Yang, Z.; Weian, C.; Susu, H.; Hanmin, W. Protective effects of mangiferin on cerebral ischemia-reperfusion injury and its mechanisms. Eur. J. Pharm. 2016, 771, 145–151. [Google Scholar] [CrossRef]
- Gong, L.; Tang, Y.; An, R.; Lin, M.; Chen, L.; Du, J. RTN1-C mediates cerebral ischemia/reperfusion injury via ER stress and mitochondria-associated apoptosis pathways. Cell Death Dis. 2017, 8, e3080. [Google Scholar] [CrossRef] [Green Version]
- Estaquier, J.; Vallette, F.; Vayssiere, J.L.; Mignotte, B. The mitochondrial pathways of apoptosis. Adv. Exp. Med. Biol. 2012, 942, 157–183. [Google Scholar]
- Araki, K.; Nagata, K. Protein folding and quality control in the ER. Cold Spring Harb Perspect Biol. 2011, 3, a007526. [Google Scholar] [CrossRef] [Green Version]
- Kaufman, R.J.; Scheuner, D.; Schroder, M.; Shen, X.; Lee, K.; Liu, C.Y.; Arnold, S.M. The unfolded protein response in nutrient sensing and differentiation. Nat. Rev. Mol. Cell Biol. 2002, 3, 411–421. [Google Scholar] [CrossRef]
- Martins, A.S.; Alves, I.; Helguero, L.; Domingues, M.R.; Neves, B.M. The unfolded protein response in homeostasis and modulation of mammalian immune cells. Int. Rev. Immunol. 2016, 35, 457–476. [Google Scholar] [CrossRef]
- Cubillos-Ruiz, J.R.; Bettigole, S.E.; Glimcher, L.H. Tumorigenic and immunosuppressive effects of endoplasmic reticulum stress in cancer. Cell 2017, 168, 692–706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, A.; Song, N.J.; Riesenberg, B.P.; Li, Z. The emerging roles of endoplasmic reticulum stress in balancing immunity and tolerance in health and diseases: Mechanisms and opportunities. Front. Immunol. 2019, 10, 3154. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, H. ER stress and diseases. FEBS J. 2007, 274, 630–658. [Google Scholar] [CrossRef]
- Liu, C.; Fu, Q.; Mu, R.; Wang, F.; Zhou, C.; Zhang, L.; Yu, B.; Zhang, Y.; Fang, T.; Tian, F. Dexmedetomidine alleviates cerebral ischemia-reperfusion injury by inhibiting endoplasmic reticulum stress dependent apoptosis through the PERK-CHOP-Caspase-11 pathway. Brain Res. 2018, 1701, 246–254. [Google Scholar] [CrossRef]
- Li, H.; Zhang, X.; Qi, X.; Zhu, X.; Cheng, L. Icariin inhibits endoplasmic reticulum stress-induced neuronal apoptosis after spinal cord injury through modulating the PI3K/AKT signaling pathway. Int. J. Biol. Sci. 2019, 15, 277–286. [Google Scholar] [CrossRef] [Green Version]
- Sano, R.; Reed, J.C. ER stress-induced cell death mechanisms. Biochim. Biophys. Acta 2013, 1833, 3460–3470. [Google Scholar] [CrossRef] [Green Version]
- Fei, H.; Xiang, P.; Luo, W.; Tan, X.; Gu, C.; Liu, M.; Chen, M.; Wang, Q.; Yang, J. CTRP1 attenuates cerebral ischemia/reperfusion injury via the PERK signaling pathway. Front. Cell Dev. Biol. 2021, 9, 700854. [Google Scholar] [CrossRef]
- Luo, S.; Baumeister, P.; Yang, S.; Abcouwer, S.F.; Lee, A.S. Induction of Grp78/BiP by translational block: Activation of the Grp78 promoter by ATF4 through and upstream ATF/CRE site independent of the endoplasmic reticulum stress elements. J. Biol. Chem. 2003, 278, 37375–37385. [Google Scholar] [CrossRef] [Green Version]
- Verfaillie, T.; Rubio, N.; Garg, A.D.; Bultynck, G.; Rizzuto, R.; Decuypere, J.P.; Piette, J.; Linehan, C.; Gupta, S.; Samali, A.; et al. PERK is required at the ER-mitochondrial contact sites to convey apoptosis after ROS-based ER stress. Cell Death Differ. 2012, 19, 1880–1891. [Google Scholar] [CrossRef] [Green Version]
- Cao, G.; Zhou, H.; Jiang, N.; Han, Y.; Hu, Y.; Zhang, Y.; Qi, J.; Kou, J.; Yu, B. YiQiFuMai powder injection ameliorates cerebral ischemia by inhibiting endoplasmic reticulum stress-mediated neuronal apoptosis. Oxid. Med. Cell. Longev. 2016, 2016, 5493279. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Fu, H.; Huang, H.; Lu, Q.; Qin, H.; Wu, Y.; Huang, H.; Mao, G.; Wei, Z.; et al. Hes1 knockdown exacerbates ischemic stroke following tMCAO by increasing ER stress-dependent apoptosis via the PERK/eIF2alpha/ATF4/CHOP signaling pathway. Neurosci Bull 2020, 36, 134–142. [Google Scholar] [CrossRef]
- So, J.S. Roles of endoplasmic reticulum stress in immune responses. Mol. Cells 2018, 41, 705–716. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Wang, H.; Fang, S.; Xu, C. Roles of endoplasmic reticulum stress and autophagy on H2O2 induced oxidative stress injury in HepG2 cells. Mol. Med. Rep. 2018, 18, 4163–4174. [Google Scholar] [CrossRef] [Green Version]
- Xu, F.; Zhang, G.; Yin, J.; Zhang, Q.; Ge, M.Y.; Peng, L.; Wang, S.; Li, Y. Fluoxetine mitigating late-stage cognition and neurobehavior impairment induced by cerebral ischemia reperfusion injury through inhibiting ERS-mediated neurons apoptosis in the hippocampus. Behav. Brain Res. 2019, 370, 111952. [Google Scholar] [CrossRef]
- Khan, A.Q.; Khan, R.; Qamar, W.; Lateef, A.; Rehman, M.U.; Tahir, M.; Ali, F.; Hamiza, O.O.; Hasan, S.K.; Sultana, S. Geraniol attenuates 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced oxidative stress and inflammation in mouse skin: Possible role of p38 MAP Kinase and NF-kappaB. Exp. Mol. Pathol. 2013, 94, 419–429. [Google Scholar] [CrossRef]
- Tiwari, M.; Kakkar, P. Plant derived antioxidants—Geraniol and camphene protect rat alveolar macrophages against t-BHP induced oxidative stress. Toxicol. Vitr. 2009, 23, 295–301. [Google Scholar] [CrossRef]
- Hasan, S.K.; Sultana, S. Geraniol attenuates 2-acetylaminofluorene induced oxidative stress, inflammation and apoptosis in the liver of wistar rats. Toxicol Mech. Methods 2015, 25, 559–573. [Google Scholar]
- Kim, S.H.; Bae, H.C.; Park, E.J.; Lee, C.R.; Kim, B.J.; Lee, S.; Park, H.H.; Kim, S.J.; So, I.; Kim, T.W.; et al. Geraniol inhibits prostate cancer growth by targeting cell cycle and apoptosis pathways. Biochem. Biophys. Res. Commun. 2011, 407, 129–134. [Google Scholar] [CrossRef]
- Deng, Y.; Zou, W.; Chen, G.; Shangguan, S.; Zhou, F.; Jiang, W.; Li, X. Comparative studies on the effects of different doses of atorvastatin combined with aspirin on inflammatory cytokines and carotid plaques in patients with ischemic cerebrovascular disease. Int. J. Neurosci. 2019, 129, 1133–1138. [Google Scholar] [CrossRef]
- Feng, S.Q.; Zong, S.Y.; Liu, J.X.; Chen, Y.; Xu, R.; Yin, X.; Zhao, R.; Li, Y.; Luo, T.T. VEGF antagonism attenuates cerebral ischemia/reperfusion-induced injury via inhibiting endoplasmic reticulum stress-mediated apoptosis. Biol. Pharm. Bull. 2019, 42, 692–702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soliman, S.M.; Sheta, N.M.; Ibrahim, B.M.M.; El-Shawwa, M.M.; El-Halim, S.M.A. Novel intranasal drug delivery: Geraniol charged polymeric mixed micelles for targeting cerebral insult as a result of ischaemia/reperfusion. Pharmaceutics 2020, 12, 76. [Google Scholar] [CrossRef] [PubMed]
- Xin, Q.; Ji, B.; Cheng, B.; Wang, C.; Liu, H.; Chen, X.; Chen, J.; Bai, B. Endoplasmic reticulum stress in cerebral ischemia. Neurochem. Int. 2014, 68, 18–27. [Google Scholar] [CrossRef]
- Yang, W.; Paschen, W. Unfolded protein response in brain ischemia: A timely update. J. Cereb. Blood Flow Metab. 2016, 36, 2044–2050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urban, P.; Pavlikova, M.; Sivonova, M.; Kaplan, P.; Tatarkova, Z.; Kaminska, B.; Lehotsky, J. Molecular analysis of endoplasmic reticulum stress response after global forebrain ischemia/reperfusion in rats: Effect of neuroprotectant simvastatin. Cell. Mol. Neurobiol. 2009, 29, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Li, F. Endoplasmic reticulum stress in brain ischemia. Int. J. Neurosci. 2016, 126, 681–691. [Google Scholar] [CrossRef]
- Han, Z.W.; Chang, Y.C.; Zhou, Y.; Zhang, H.; Chen, L.; Zhang, Y.; Si, J.Q.; Li, L. GPER agonist G1 suppresses neuronal apoptosis mediated by endoplasmic reticulum stress after cerebral ischemia/reperfusion injury. Neural Regen. Res. 2019, 14, 1221–1229. [Google Scholar] [PubMed]
- Zhong, C.J.; Chen, M.M.; Lu, M.; Ding, J.H.; Du, R.H.; Hu, G. Astrocyte-specific deletion of Kir6.1/K-ATP channel aggravates cerebral ischemia/reperfusion injury through endoplasmic reticulum stress in mice. Exp. Neurol. 2019, 311, 225–233. [Google Scholar] [CrossRef]
- Li, H.Q.; Xia, S.N.; Xu, S.Y.; Liu, P.Y.; Gu, Y.; Bao, X.Y.; Xu, Y.; Cao, X. γ-Glutamylcysteine alleviates ischemic stroke-induced neuronal apoptosis by inhibiting ROS-mediated endoplasmic reticulum stress. Oxid. Med. Cell. Longev. 2021, 2021, 2961079. [Google Scholar] [CrossRef]
- Gao, Y.; Li, L.; Yu, J.; Zhang, Z. Rosuvastatin protects PC12 cells from hypoxia/reoxygenation-induced injury by inhibiting endoplasmic reticulum stress-induced apoptosis. Exp. Ther. Med. 2021, 22, 1189. [Google Scholar] [CrossRef]
- Chen, Y.; Brandizzi, F. IRE1: ER stress sensor and cell fate executor. Trends Cell Biol. 2013, 23, 547–555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, J.; Back, S.H.; Hur, J.; Lin, Y.-H.; Gildersleeve, R.; Shan, J.; Yuan, C.L.; Krokowski, D.; Wang, S.; Hatzoglou, M.; et al. ER-stress-induced transcriptional regulation increases protein synthesis leading to cell death. Nat. Cell Biol. 2013, 15, 481–490. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Zhu, X.; Fang, F.; Jiang, D.; Tang, L. Down-regulation of GRP78 enhances apoptosis via CHOP pathway in retinal ischemia-reperfusion injury. Neurosci. Lett. 2014, 575, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Szegezdi, E.; Logue, S.E.; Gorman, A.M.; Samali, A. Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep. 2006, 7, 880–885. [Google Scholar] [CrossRef] [Green Version]
- Marciniak, S.J.; Garcia-Bonilla, L.; Hu, J.; Harding, H.P.; Ron, D. Activation-dependent substrate recruitment by the eukaryotic translation initiation factor 2 kinase PERK. J. Cell Biol. 2006, 172, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.S.; Luo, K.L.; Shi, L. Endoplasmic reticulum stress interacts with inflammation in human diseases. J. Cell Physiol. 2016, 231, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.H.; Li, H.; Zhang, Y.; Ron, D.; Walter, P. Divergent effects of PERK and IRE1 signaling on cell viability. PLoS ONE 2009, 4, e4170. [Google Scholar] [CrossRef] [PubMed]
- Darling, N.J.; Cook, S.J. The role of MAPK signalling pathways in the response to endoplasmic reticulum stress. Biochim. Biophys. Acta 2014, 1843, 2150–2163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, B.M.; Li, Z.W.; Wang, Z.Y. PERK activator CCT020312 prevents inflammation-mediated osteoporosis in the ovariectomized rats. Gynecol. Endocrinol. 2021, 37, 342–348. [Google Scholar] [CrossRef]
- Li, X.; Yu, X.; Zhou, D.; Chen, B.; Li, W.; Zheng, X.; Zeng, H.; Long, L.; Zhou, W. CCT020312 inhibits triple-negative breast cancer through PERK pathway-mediated G1 phase cell cycle arrest and apoptosis. Front. Pharmacol. 2020, 11, 737. [Google Scholar] [CrossRef]
- Lei, Y.; He, L.; Yan, C.; Wang, Y.; Lv, G. PERK activation by CCT020312 chemosensitizes colorectal cancer through inducing apoptosis regulated by ER stress. Biochem. Biophys. Res. Commun. 2021, 557, 316–322. [Google Scholar] [CrossRef]
- Liu, S.; Liu, J.; Wang, Y.; Deng, L.; Chen, S.; Wang, X.; Zuo, T.; Hu, Q.; Rao, J.; Wang, Q.; et al. Differentially expressed genes induced by β-caryophyllene in a rat model of cerebral ischemia-reperfusion injury. Life Sci. 2021, 273, 119293. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Fan, X.; Chen, S.; Deng, L.; Jiang, L.; Yang, S.; Dong, Z. Geraniol-Mediated Suppression of Endoplasmic Reticulum Stress Protects against Cerebral Ischemia–Reperfusion Injury via the PERK-ATF4-CHOP Pathway. Int. J. Mol. Sci. 2023, 24, 544. https://doi.org/10.3390/ijms24010544
Wu Y, Fan X, Chen S, Deng L, Jiang L, Yang S, Dong Z. Geraniol-Mediated Suppression of Endoplasmic Reticulum Stress Protects against Cerebral Ischemia–Reperfusion Injury via the PERK-ATF4-CHOP Pathway. International Journal of Molecular Sciences. 2023; 24(1):544. https://doi.org/10.3390/ijms24010544
Chicago/Turabian StyleWu, Yu, Xiaomei Fan, Sha Chen, Ling Deng, Lu Jiang, Shaonan Yang, and Zhi Dong. 2023. "Geraniol-Mediated Suppression of Endoplasmic Reticulum Stress Protects against Cerebral Ischemia–Reperfusion Injury via the PERK-ATF4-CHOP Pathway" International Journal of Molecular Sciences 24, no. 1: 544. https://doi.org/10.3390/ijms24010544



