Next-Generation Sequencing in the Assessment of the Transcriptomic Landscape of DNA Damage Repair Genes in Abdominal Aortic Aneurysm, Chronic Venous Disease and Lower Extremity Artery Disease
Abstract
1. Introduction
1.1. Cardiovascular Diseases
1.2. Reactive Oxygen Species and DNA Repair in Cardiovascular Diseases
2. Results
2.1. Study Group Attributes
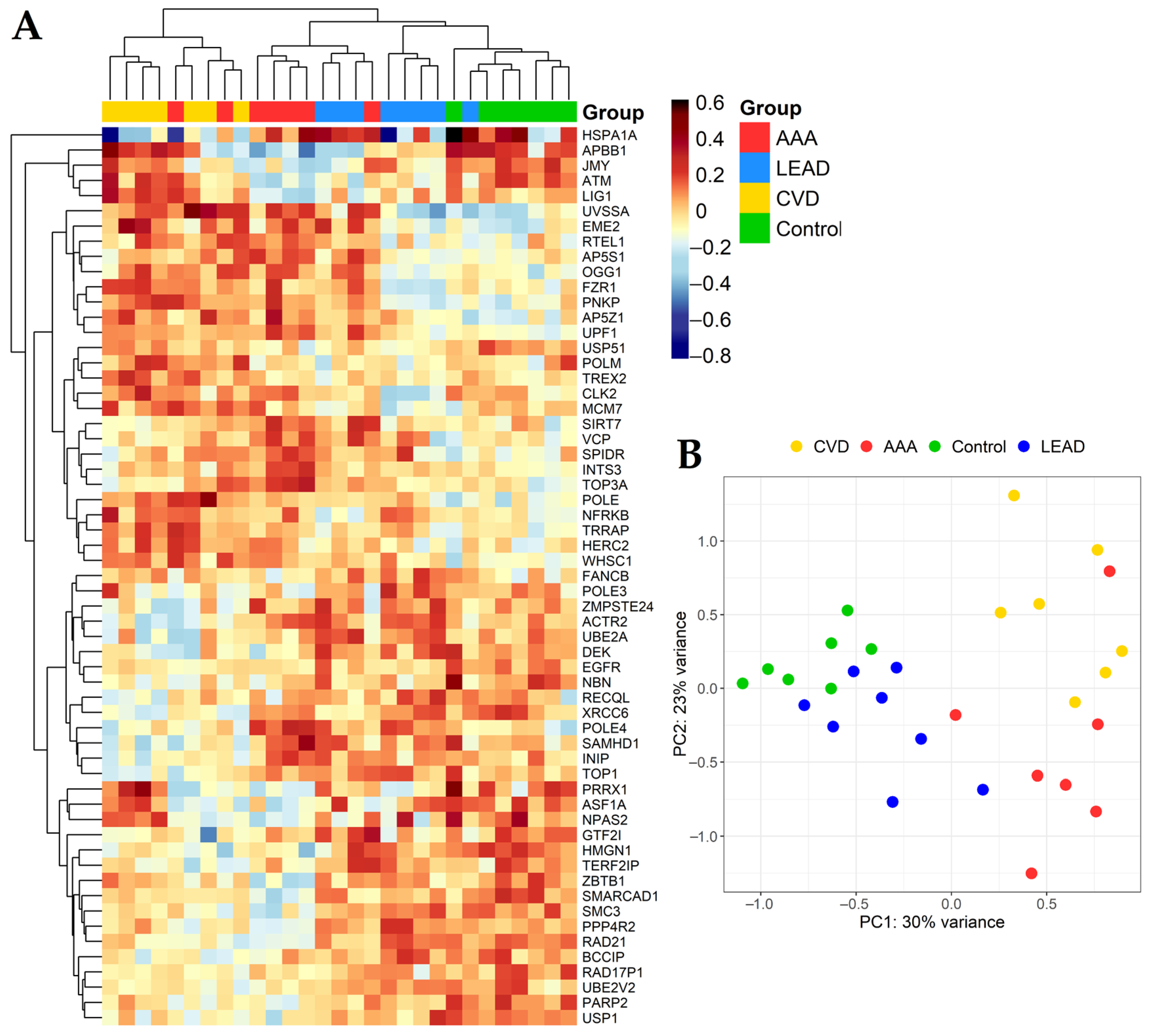
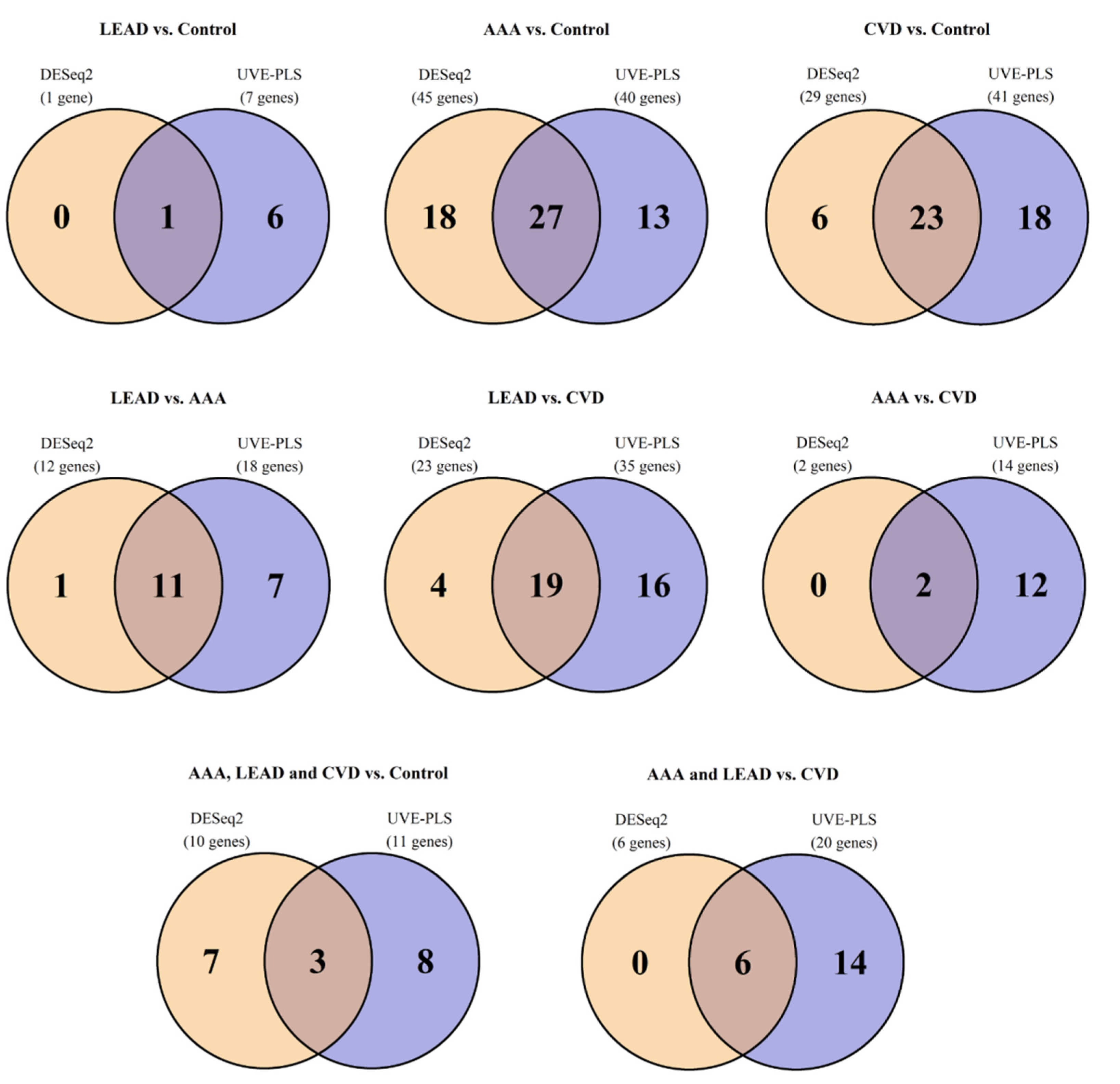
2.2. The Differential Expression Analysis of DNA Damage Metabolism Genes in AAA, CVD, LEAD and Control Subjects
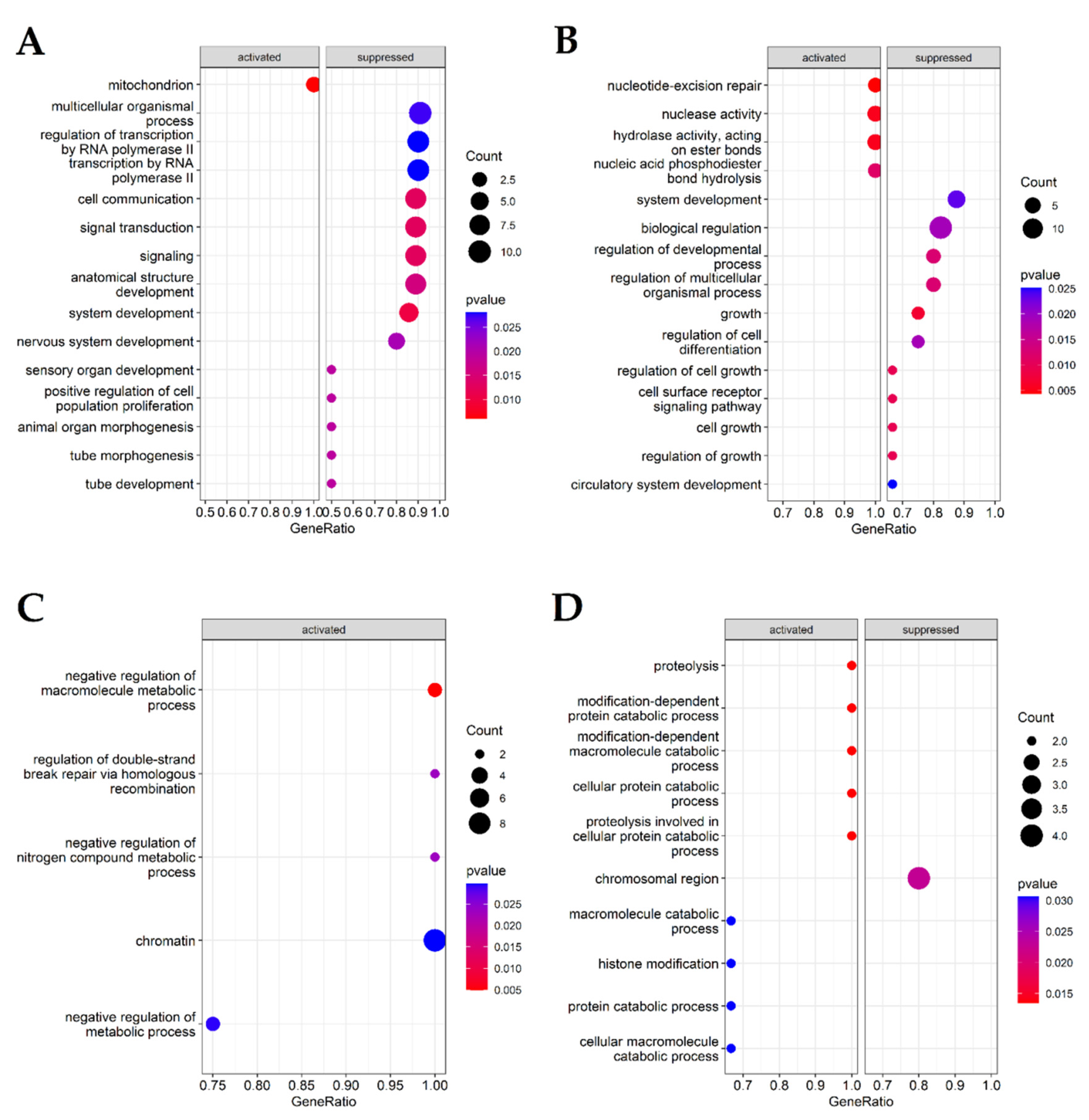
2.3. Functional Analysis of Selected Genes
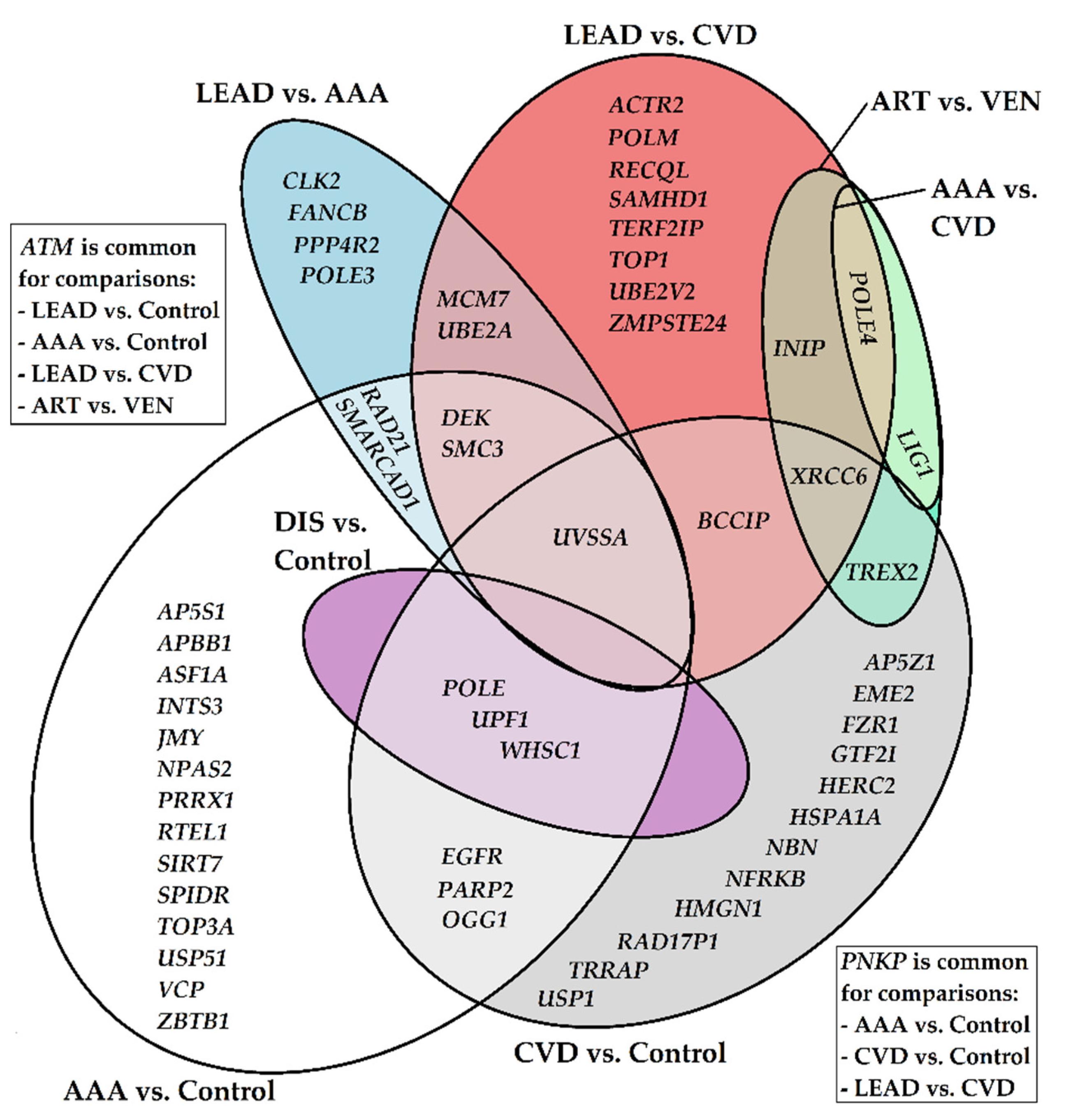
2.4. Relationships between Gene Expression Patterns and Characteristics of the Study Groups of Patients from Performed Comparisons
3. Discussion
3.1. Gene Ontology (GO) and Gene Set Enrichment Analysis (GSEA) Reveals Terms and Categories Associated with Biological Process Regulation, DNA Repair Regulation, Double-Strand Break Repair and Homologous Recombination, Differentiating AAA, CVD and LEAD
3.2. Expression of DNA Double Strand Break Repair Genes Is Altered in Vascular Diseases
3.3. Specific Gene Expression Changes May Be Indicative for Altered Oxidative DNA Damage Responses in AAA, CVD and LEAD
3.4. Analysis Reveals Known Genes Being Involved in Vascular Diseases’ Initiation and Progression
- AAA vs. control group: NPAS2 (MOP4), PRRX1, RTEL1, SIRT7, USP10, VCP (p97)
- LEAD vs. CVD group: ACTR2 (ARP2), SAMHD1, TERF2IP (RAP1)
- CVD vs. control group: HSPA1A (HSP70), TRRAP
3.5. Chromatin Remodelling Could Be the Process Shaping and Regulating DNA Damage Responses in Vascular Diseases
3.6. Ubiquitination and Deubiquitination Could Be a Prominent Mechanism Regulating Gene Activities in Vascular Diseases
3.7. Limitations of the Study and Accompanying Issues
4. Materials and Methods
4.1. Study Groups Participants Characteristics
4.2. Gene Expression Datasets Generation
4.3. Data Analysis Methods and Tools
4.4. Advantages of Peripheral Blood Mononuclear Cells’ (PBMCs) Utilization for DNA Damage Metabolism and Vascular Conditions Assessment
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 8–oxoG | 8–oxo–7:8–dihydroguanine |
| AAA | Abdominal aortic aneurysm |
| BAF200 | BAF Nuclear Assembly Factor 1 |
| BER | Base Excision Repair |
| BMI | Body mass index |
| CVD | Chronic Venous Disease |
| DSBs | Double-strand breaks |
| FDR | False Discovery Rate |
| HUGO | Human Genome Organization |
| LEAD | Lower Extremities Arterial Disease |
| MA plot | Bland–Altman plot for visual representation of genomic data |
| NER | Nucleotide excision repair |
| NGS | Next-Generation Sequencing |
| NHEJ | Non–Homologous End Joining |
| p53 | Tumor Protein P53 |
| PAD | Peripheral Arterial Disease |
| PBMCs | Peripheral blood mononuclear cells |
| ROC | Receiver Operating Characteristics |
| ROS | Reactive oxygen species |
| Swi/Snf complex | SWItch/Sucrose Non–Fermentable complex |
| UV | Ultraviolet |
| UVE–PLS | Uninformative Variable Elimination by Partial Least Squares |
| VEGFA | Vascular Endothelial Growth Factor A |
| VSMCs | Vascular Smooth Muscle Cells |
Appendix A
| Comparison | DESeq2 | UVE–PLS | Number of Genes Common for Sets of Genes Selected from DESeq2 (p < 0.05) and from UVE–PLS as Informative | |
|---|---|---|---|---|
| Number of Differentially Expressed Genes with p < 0.05 and Fold Change > 1.2 (for Upregulated Genes) or Fold Change < 0.8 (for Downregulated Genes) | Number of PLS Components/Iterations | Number of Informative Genes (Default Cutoff Threshold of Reliability Score) | ||
| LEAD vs. control | 1 | 4/1000 | 7 | 1 |
| AAA vs. control | 45 | 2/1000 | 40 | 27 |
| CVD vs. control | 29 | 3/1000 | 41 | 23 |
| LEAD vs. AAA | 12 | 3/1000 | 18 | 11 |
| LEAD vs. CVD | 23 | 2/1000 | 35 | 19 |
| AAA vs. CVD | 2 | 2/1000 | 14 | 2 |
| LEAD, AAA and CVD vs. control | 10 | 3/1000 | 11 | 3 |
| LEAD and AAA vs. CVD | 6 | 4/1000 | 20 | 6 |
References
- Fuster, V.; Kelly, B.B. Promoting Cardiovascular Health in the Developing World: A Critical Challenge to Acheive Global Health; The National Academies Press: Washington, DC, USA, 2010. [Google Scholar]
- Joseph, P.; Leong, D.; McKee, M.; Anand, S.S.; Schwalm, J.D.; Teo, K.; Mente, A.; Yusuf, S. Reducing the global burden of cardiovascular disease, part 1: The epidemiology and risk factors. Circ. Res. 2017, 121, 677–694. [Google Scholar] [CrossRef] [PubMed]
- Kaptoge, S.; Pennells, L.; De Bacquer, D.; Cooney, M.T.; Kavousi, M.; Stevens, G.; Riley, L.M.; Savin, S.; Khan, T.; Altay, S.; et al. World Health Organization cardiovascular disease risk charts: Revised models to estimate risk in 21 global regions. Lancet Glob. Health. 2019, 7, e1332–e1345. [Google Scholar] [CrossRef] [PubMed]
- Criqui, M.H.; Matsushita, K.; Aboyans, V.; Hess, C.N.; Hicks, C.W.; Kwan, T.W.; McDermott, M.M.; Misra, S.; Ujueta, F. American Heart Association Council on Epidemiology and Prevention; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular Radiology and Intervention; Council on Lifestyle and Cardiometabolic Health; Council on Peripheral Vascular Disease; and Stroke Council Lower Extremity Peripheral Artery Disease: Contemporary Epidemiology, Management Gaps, and Future Directions: A Scientific Statement From the American Heart Association. Circulation 2021, 144, e171–e191. [Google Scholar] [CrossRef] [PubMed]
- Aboyans, V.; Ricco, J.B.; Bartelink, M.L.E.L.; Björck, M.; Brodmann, M.; Cohnert, T.; Collet, J.P.; Czerny, M.; De Carlo, M.; Debusa, S.; et al. ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS). Eur. Heart J. 2017, 39, 763–816. [Google Scholar] [CrossRef]
- Song, P.; Rudan, D.; Zhu, Y.; Fowkes, F.J.I.; Rahimi, K.; Fowkes, F.G.R.; Rudan, I. Global, regional, and national prevalence and risk factors for peripheral artery disease in 2015: An updated systematic review and analysis. Lancet Glob. Health 2019, 7, e1020–e1030. [Google Scholar] [CrossRef]
- Ullery, B.W.; Hallett, R.L.; Fleischmann, D. Epidemiology and contemporary management of abdominal aortic aneurysms. Abdom. Radiol. 2018, 43, 1032–1043. [Google Scholar] [CrossRef]
- Lattanzi, S. Abdominal aortic aneurysms: Pathophysiology and clinical issues. J. Intern. Med. 2020, 288, 376–378. [Google Scholar] [CrossRef]
- Eberhardt, R.T.; Raffetto, J.D. Chronic venous insufficiency. Circulation 2014, 130, 333–346. [Google Scholar] [CrossRef]
- Ligi, D.; Croce, L.; Mannello, F. Chronic venous disorders: The dangerous, the good, and the diverse. Int. J. Mol. Sci. 2018, 19, 2544. [Google Scholar] [CrossRef]
- Mansilha, A.; Sousa, J. Pathophysiological mechanisms of chronic venous disease and implications for venoactive drug therapy. Int. J. Mol. Sci. 2018, 19, 1669. [Google Scholar] [CrossRef]
- Lurie, F.; Passman, M.; Meisner, M.; Dalsing, M.; Masuda, E.; Welch, H.; Bush, R.L.; Blebea, J.; Carpentier, P.H.; De Maeseneer, M.; et al. The 2020 update of the CEAP classification system and reporting standards. J. Vasc. Surg. Venous Lymphat. Disord. 2020, 8, 342–352. [Google Scholar] [CrossRef] [PubMed]
- Cervelli, T.; Borghini, A.; Galli, A.; Andreassi, M.G. DNA damage and repair in atherosclerosis: Current insights and future perspectives. Int. J. Mol. Sci. 2012, 13, 16929–16944. [Google Scholar] [CrossRef] [PubMed]
- Martinet, W.; Knaapen, M.W.; De Meyer, G.R.; Herman, A.G.; Kockx, M.M. Elevated levels of oxidative DNA damage and DNA repair enzymes in human atherosclerotic plaques. Circulation 2002, 106, 927–932. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, M.; Mercer, J.; Bennett, M. DNA damage and repair in atherosclerosis. Cardiovasc. Res. 2006, 71, 259–268. [Google Scholar] [CrossRef]
- Shah, A.; Gray, K.; Figg, N.; Finigan, A.; Starks, L.; Bennett, M. Defective Base Excision Repair of Oxidative DNA Damage in Vascular Smooth Muscle Cells Promotes Atherosclerosis. Circulation 2018, 138, 1446–1462. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.; Meira, L.B.; Elliott, R.M.; Hoole, S.P.; West, N.E.; Brown, A.J.; Bennett, M.R.; Garcia-Garcia, H.M.; Kuku, K.O.; Dan, K.; et al. DNA Damage and Repair in Patients with Coronary Artery Disease: Correlation with Plaque Morphology Using Optical Coherence Tomography (DECODE Study). Cardiovasc. Revasc. Med. 2019, 20, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Skarpengland, T.; Dahl, T.B.; Skjelland, M.; Scheffler, K.; de Sousa, M.; Gregersen, I.; Kuśnierczyk, A.; Sharma, A.; Slupphaug, G.; Eide, L.; et al. Enhanced base excision repair capacity in carotid atherosclerosis may protect nuclear DNA but not mitochondrial DNA. Free Radic. Biol. Med. 2016, 97, 386–397. [Google Scholar] [CrossRef]
- Hu, L.; Wang, Z.; Carmone, C.; Keijer, J.; Zhang, D. Role of Oxidative DNA Damage and Repair in Atrial Fibrillation and Ischemic Heart Disease. Int. J. Mol. Sci. 2021, 22, 3838. [Google Scholar] [CrossRef]
- Simon, R.; Meller, R.; Yang, T.; Pearson, A.; Wilson, G. Enhancing Base Excision Repair of Mitochondrial DNA to Reduce Ischemic Injury Following Reperfusion. Transl. Stroke Res. 2019, 10, 664–671. [Google Scholar] [CrossRef]
- Marín-García, J. Mitochondrial DNA repair: A novel therapeutic target for heart failure. Heart Fail. Rev. 2016, 21, 475–487. [Google Scholar] [CrossRef]
- Bogucka-Kocka, A.; Zalewski, D.P.; Ruszel, K.P.; Stępniewski, A.; Gałkowski, D.; Bogucki, J.; Komsta, Ł.; Kołodziej, P.; Zubilewicz, T.; Feldo, M.; et al. Dysregulation of MicroRNA Regulatory Network in Lower Extremities Arterial Disease. Front. Genet. 2019, 10, 1200. [Google Scholar] [CrossRef] [PubMed]
- Zalewski, D.P.; Ruszel, K.P.; Stępniewski, A.; Gałkowski, D.; Bogucki, J.; Komsta, Ł.; Kołodziej, P.; Chmiel, P.; Zubilewicz, T.; Feldo, M.; et al. Dysregulations of MicroRNA and Gene Expression in Chronic Venous Disease. J. Clin. Med. 2020, 9, 1251. [Google Scholar] [CrossRef] [PubMed]
- Zalewski, D.P.; Ruszel, K.P.; Stępniewski, A.; Gałkowski, D.; Bogucki, J.; Komsta, Ł.; Kołodziej, P.; Chmiel, P.; Zubilewicz, T.; Feldo, M.; et al. Dysregulation of microRNA Modulatory Network in Abdominal Aortic Aneurysm. J. Clin. Med. 2020, 9, 1974. [Google Scholar] [CrossRef] [PubMed]
- Zalewski, D.P.; Ruszel, K.P.; Stępniewski, A.; Gałkowski, D.; Bogucki, J.; Kołodziej, P.; Szymańska, J.; Płachno, B.J.; Zubilewicz, T.; Feldo, M.; et al. Identification of Transcriptomic Differences between Lower Extremities Arterial Disease, Abdominal Aortic Aneurysm and Chronic Venous Disease in Peripheral Blood Mononuclear Cells Specimens. Int. J. Mol. Sci. 2021, 22, 3200. [Google Scholar] [CrossRef] [PubMed]
- Ougland, R.; Rognes, T.; Klungland, A.; Larsen, E. Non—Homologous functions of the AlkB homologs. J. Mol. Cell Biol. 2015, 7, 494–504. [Google Scholar] [CrossRef]
- Sherman, B.T.; Hao, M.; Qiu, J.; Jiao, X.; Baseler, M.W.; Lane, H.C.; Imamichi, T.; Chang, W. DAVID: A web server for functional enrichment analysis and functional annotation of gene lists (2021 update). Nucleic Acids Res. 2022, 50, W216–W221. [Google Scholar] [CrossRef]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID Bioinformatics Resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef]
- Wu, T.; Hu, E.; Xu, S.; Chen, M.; Guo, P.; Dai, Z.; Feng, T.; Zhou, L.; Tang, W.; Zhan, L.; et al. clusterProfiler 4.0: A universal enrichment tool for interpreting omics data. Innovation 2021, 2, 100141. [Google Scholar] [CrossRef]
- Yu, G.; Wang, L.G.; Han, Y.; He, Q.Y. clusterProfiler: An R package for comparing biological themes among gene clusters. OMICS J. Integr. Biol. 2012, 16, 284–287. [Google Scholar] [CrossRef]
- Matsumoto, T.; Mugishima, H. Signal transduction via vascular endothelial growth factor (VEGF) receptors and their roles in atherogenesis. J. Atheroscler. Thromb. 2006, 13, 130–135. [Google Scholar] [CrossRef]
- Yue, L.; Wan, R.; Luan, S.; Zeng, W.; Cheung, T.H. Dek Modulates Global Intron Retention during Muscle Stem Cells Quiescence exit. Dev. Cell 2020, 53, 661–676.e6. [Google Scholar] [CrossRef]
- Zhang, R.; Ji, Z.; Yao, Y.; Zuo, W.; Yang, M.; Qu, Y.; Su, Y.; Ma, G.; Li, Y. Identification of hub genes in unstable atherosclerotic plaque by conjoint analysis of bioinformatics. Life Sci. 2020, 262, 118517. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.M.; Meng, L.B.; Yu, S.J.; Ma, D.X. Identification of potential crucial genes in monocytes for atherosclerosis using bioinformatics analysis. Int. J. Med. Res. 2020, 48, 300060520909277. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Chen, K.; Wang, H.; Chen, Z.; Xi, Y.; Yin, H.; Lai, K.; Liu, Y. SIRT7 Regulates the Vascular Smooth Muscle Cells Proliferation and Migration via Wnt/β—Catenin Signaling Pathway. Biomed. Res. Int. 2018, 2018, 4769596. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Hu, T.; He, J.; Xu, Q.; Yu, C.; Liu, X.; Shao, Z.; Liao, Y.; Huang, H.; Liu, N. USP10 deletion inhibits macrophage—Derived foam cell formation and cellular—Oxidized low density lipoprotein uptake by promoting the degradation of CD36. Aging 2020, 12, 22892–22905. [Google Scholar] [CrossRef]
- Abbey, D.; Conlon, D.; Rainville, C.; Elwyn, S.; Quiroz-Figueroa, K.; Billheimer, J.; Schultz, D.C.; Hand, N.J.; Cherry, S.; Rader, D.J. Lipid droplet screen in human hepatocytes identifies TRRAP as a regulator of cellular triglyceride metabolism. Clin. Transl. Sci. 2021, 14, 1369–1379. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, A.E.; Zheng, T.; Ba, Y.; Zhu, Y. The circadian gene NPAS2, a putative tumor suppressor, is involved in DNA damage response. Mol. Cancer Res. MCR 2008, 6, 1461–1468. [Google Scholar] [CrossRef]
- Shrivastav, M.; De Haro, L.P.; Nickoloff, J.A. Regulation of DNA double-strand break repair pathway choice. Cell Res. 2008, 18, 134–147. [Google Scholar] [CrossRef]
- Marini, F.; Rawal, C.C.; Liberi, G.; Pellicioli, A. Regulation of DNA Double Strand Breaks Processing: Focus on Barriers. Front. Mol. Biosci. 2019, 6, 55. [Google Scholar] [CrossRef]
- Jilani, A.; Ramotar, D.; Slack, C.; Ong, C.; Yang, X.M.; Scherer, S.W.; Lasko, D.D. Molecular cloning of the human gene, PNKP, encoding a polynucleotide kinase 3’-phosphatase and evidence for its role in repair of DNA strand breaks caused by oxidative damage. J. Biol. Chem. 1999, 274, 24176–24186. [Google Scholar] [CrossRef]
- Casari, E.; Gobbini, E.; Gnugnoli, M.; Mangiagalli, M.; Clerici, M.; Longhese, M.P. Dpb4 promotes resection of DNA double—Strand breaks and checkpoint activation by acting in two different protein complexes. Nat. Commun. 2021, 12, 4750. [Google Scholar] [CrossRef] [PubMed]
- Pursell, Z.F.; Isoz, I.; Lundström, E.B.; Johansson, E.; Kunkel, T.A. Yeast DNA polymerase epsilon participates in leading—Strand DNA replication. Science 2007, 317, 127–130. [Google Scholar] [CrossRef] [PubMed]
- Ngo, G.; Grimstead, J.W.; Baird, D.M. UPF1 promotes the formation of R loops to stimulate DNA double—Strand break repair. Nat. Commun. 2021, 12, 3849. [Google Scholar] [CrossRef] [PubMed]
- Benedict, B.; van Bueren, M.A.; van Gemert, F.P.; Lieftink, C.; Guerrero Llobet, S.; van Vugt, M.A.; Beijersbergen, R.L.; Te Riele, H. The RECQL helicase prevents replication fork collapse during replication stress. Life Sci. Alliance 2020, 3, e202000668. [Google Scholar] [CrossRef]
- Debnath, S.; Sharma, S. RECQ1 Helicase in Genomic Stability and Cancer. Genes 2020, 11, 622. [Google Scholar] [CrossRef]
- Li, J.; Ma, X.; Banerjee, S.; Baruah, S.; Schnicker, N.J.; Roh, E.; Ma, W.; Liu, K.; Bode, A.M.; Dong, Z. Structural basis for multifunctional roles of human Ints3 C—Terminal domain. J. Biol. Chem. 2021, 296, 100112. [Google Scholar] [CrossRef]
- Jia, Y.; Cheng, Z.; Bharath, S.R.; Sun, Q.; Su, N.; Huang, J.; Song, H. Crystal structure of the INTS3/INTS6 complex reveals the functional importance of INTS3 dimerization in DSB repair. Cell Discov. 2021, 7, 66. [Google Scholar] [CrossRef]
- Vazquez, B.N.; Thackray, J.K.; Simonet, N.G.; Kane-Goldsmith, N.; Martinez-Redondo, P.; Nguyen, T.; Bunting, S.; Vaquero, A.; Tischfield, J.A.; Serrano, L. SIRT7 promotes genome integrity and modulates non-homologous end joining DNA repair. EMBO J. 2016, 35, 1488–1503. [Google Scholar] [CrossRef]
- Prakash, R.; Sandoval, T.; Morati, F.; Zagelbaum, J.A.; Lim, P.X.; White, T.; Taylor, B.; Wang, R.; Desclos, E.; Sullivan, M.R.; et al. Distinct pathways of homologous recombination controlled by the SWS1—SWSAP1—SPIDR complex. Nat. Commun. 2021, 12, 4255. [Google Scholar] [CrossRef]
- Martino, J.; Brunette, G.J.; Barroso-González, J.; Moiseeva, T.N.; Smith, C.M.; Bakkenist, C.J.; O’Sullivan, R.J.; Bernstein, K.A. The human Shu complex functions with PDS5B and SPIDR to promote homologous recombination. Nucleic Acids Res. 2019, 47, 10151–10165. [Google Scholar] [CrossRef]
- Daddacha, W.; Koyen, A.E.; Bastien, A.J.; Head, P.E.; Dhere, V.R.; Nabeta, G.N.; Connolly, E.C.; Werner, E.; Madden, M.Z.; Daly, M.B.; et al. SAMHD1 Promotes DNA End Resection to Facilitate DNA Repair by Homologous Recombination. Cell Rep. 2017, 20, 1921–1935. [Google Scholar] [CrossRef] [PubMed]
- Rice, G.I.; Bond, J.; Asipu, A.; Brunette, R.L.; Manfield, I.W.; Carr, I.M.; Fuller, J.C.; Jackson, R.M.; Lamb, T.; Briggs, T.A.; et al. Mutations involved in Aicardi-Goutières syndrome implicate SAMHD1 as regulator of the innate immune response. Nat. Genet. 2009, 41, 829–832. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Guo, X.; Meng, X.; Liu, J.; Allen, C.; Wray, J.; Nickoloff, J.A.; Shen, Z. The BRCA2—Interacting protein BCCIP functions in RAD51 and BRCA2 focus formation and homologous recombinational repair. Mol. Cell. Biol. 2005, 25, 1949–1957. [Google Scholar] [CrossRef] [PubMed]
- Jurkiw, T.J.; Tumbale, P.P.; Schellenberg, M.J.; Cunningham-Rundles, C.; Williams, R.S.; O’Brien, P.J. LIG1 syndrome mutations remodel a cooperative network of ligand binding interactions to compromise ligation efficiency. Nucleic Acids Res. 2021, 49, 1619–1630. [Google Scholar] [CrossRef]
- Bellelli, R.; Youds, J.; Borel, V.; Svendsen, J.; Pavicic-Kaltenbrunner, V.; Boulton, S.J. Synthetic Lethality between DNA Polymerase Epsilon and RTEL1 in Metazoan DNA Replication. Cell Rep. 2020, 31, 107675. [Google Scholar] [CrossRef]
- Björkman, A.; Johansen, S.L.; Lin, L.; Schertzer, M.; Kanellis, D.C.; Katsori, A.M.; Christensen, S.T.; Luo, Y.; Andersen, J.S.; Elsässer, S.J.; et al. Human RTEL1 associates with Poldip3 to facilitate responses to replication stress and R—Loop resolution. Genes Dev. 2020, 34, 1065–1074. [Google Scholar] [CrossRef]
- Wyatt, H.D.; Sarbajna, S.; Matos, J.; West, S.C. Coordinated actions of SLX1-SLX4 and MUS81-EME1 for Holliday junction resolution in human cells. Mol. Cell 2013, 52, 234–247. [Google Scholar] [CrossRef]
- Amangyeld, T.; Shin, Y.K.; Lee, M.; Kwon, B.; Seo, Y.S. Human MUS81-EME2 can cleave a variety of DNA structures including intact Holliday junction and nicked duplex. Nucleic Acids Res. 2014, 42, 5846–5862. [Google Scholar] [CrossRef]
- Falquet, B.; Rass, U. Structure-Specific Endonucleases and the Resolution of Chromosome Underreplication. Genes 2019, 10, 232. [Google Scholar] [CrossRef]
- Nomura, Y.; Adachi, N.; Koyama, H. Human Mus81 and FANCB independently contribute to repair of DNA damage during replication. Genes Cells 2007, 12, 1111–1122. [Google Scholar] [CrossRef]
- Ghosh, D.; Raghavan, S.C. 20 years of DNA Polymerase μ, the polymerase that still surprises. FEBS J. 2021, 288, 7230–7242. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Im, J.S.; Shibata, E.; Dutta, A. ASF1a Promotes Non—Homologous End Joining Repair by Facilitating Phosphorylation of MDC1 by ATM at Double-Strand Breaks. Mol. Cell 2017, 68, 61–75.e5. [Google Scholar] [CrossRef]
- Francica, P.; Mutlu, M.; Blomen, V.A.; Oliveira, C.; Nowicka, Z.; Trenner, A.; Gerhards, N.M.; Bouwman, P.; Stickel, E.; Hekkelman, M.L.; et al. Functional Radiogenetic Profiling Implicates ERCC6L2 in Non-homologous End Joining. Cell Rep. 2020, 32, 108068. [Google Scholar] [CrossRef] [PubMed]
- Hang, Q.; Zeng, L.; Wang, L.; Nie, L.; Yao, F.; Teng, H.; Deng, Y.; Yap, S.; Sun, Y.; Frank, S.J.; et al. Non-canonical function of DGCR8 in DNA double-strand break repair signaling and tumor radioresistance. Nat. Commun. 2021, 12, 4033. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, H.; Liu, J.; Cheruiyot, A.; Lee, J.H.; Ordog, T.; Lou, Z.; You, Z.; Zhang, Z. USP51 deubiquitylates H2AK13,15ub and regulates DNA damage response. Genes Dev. 2016, 30, 946–959. [Google Scholar] [CrossRef]
- Aquila, L.; Atanassov, B.S. Regulation of Histone Ubiquitination in Response to DNA Double Strand Breaks. Cells 2020, 9, 1699. [Google Scholar] [CrossRef] [PubMed]
- Keyamura, K.; Arai, K.; Hishida, T. Srs2 and Mus81-Mms4 Prevent Accumulation of Toxic Inter-Homolog Recombination Intermediates. PLoS Genet. 2016, 12, e1006136. [Google Scholar] [CrossRef] [PubMed]
- Cervantes Gracia, K.; Llanas-Cornejo, D.; Husi, H. CVD and Oxidative Stress. J. Clin. Med. 2017, 6, 22. [Google Scholar] [CrossRef]
- Bomfim, M.M.; Andrade, G.M.; Del Collado, M.; Sangalli, J.R.; Fontes, P.K.; Nogueira, M.; Meirelles, F.V.; da Silveira, J.C.; Perecin, F. Antioxidant responses and deregulation of epigenetic writers and erasers link oxidative stress and DNA methylation in bovine blastocysts. Mol. Reprod. Dev. 2017, 84, 1296–1305. [Google Scholar] [CrossRef]
- Friedberg, E.C.; Wood, R.D. New insights into the combined Cockayne/xeroderma pigmentosum complex: Human XPG protein can function in transcription factor stability. Mol. Cell 2007, 26, 162–164. [Google Scholar] [CrossRef]
- Ferri, D.; Orioli, D.; Botta, E. Heterogeneity and overlaps in nucleotide excision repair disorders. Clin. Genet. 2020, 97, 12–24. [Google Scholar] [CrossRef]
- Schwartz, S.M.; Schwartz, H.T.; Horvath, S.; Schadt, E.; Lee, S.I. A systematic approach to multifactorial cardiovascular disease: Causal analysis. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 2821–2835. [Google Scholar] [CrossRef] [PubMed]
- Manils, J.; Gómez, D.; Salla-Martret, M.; Fischer, H.; Fye, J.M.; Marzo, E.; Marruecos, L.; Serrano, I.; Salgado, R.; Rodrigo, J.P.; et al. Multifaceted role of TREX2 in the skin defense against UV—Induced skin carcinogenesis. Oncotarget 2015, 6, 22375–22396. [Google Scholar] [CrossRef] [PubMed]
- Aguilera-Aguirre, L.; Hosoki, K.; Bacsi, A.; Radák, Z.; Wood, T.G.; Widen, S.G.; Sur, S.; Ameredes, B.T.; Saavedra-Molina, A.; Brasier, A.R.; et al. Whole transcriptome analysis reveals an 8-oxoguanine DNA glycosylase-1—Driven DNA repair—Dependent gene expression linked to essential biological processes. Free Radic. Biol. Med. 2015, 81, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Brasier, A.R.; Boldogh, I. Targeting inducible epigenetic reprogramming pathways in chronic airway remodeling. Drugs Context 2019, 8, 2019-8-3. [Google Scholar] [CrossRef] [PubMed]
- Hao, W.; Wang, J.; Zhang, Y.; Wang, C.; Xia, L.; Zhang, W.; Zafar, M.; Kang, J.Y.; Wang, R.; Ali Bohio, A.; et al. Enzymatically inactive OGG1 binds to DNA and steers base excision repair toward gene transcription. FASEB J. 2020, 34, 7427–7441. [Google Scholar] [CrossRef] [PubMed]
- Tumurkhuu, G.; Shimada, K.; Dagvadorj, J.; Crother, T.R.; Zhang, W.; Luthringer, D.; Gottlieb, R.A.; Chen, S.; Arditi, M. Ogg1 –Dependent DNA Repair Regulates NLRP3 Inflammasome and Prevents Atherosclerosis. Circ. Res. 2016, 119, e76–e90. [Google Scholar] [CrossRef] [PubMed]
- McNamara, P.; Seo, S.B.; Rudic, R.D.; Sehgal, A.; Chakravarti, D.; FitzGerald, G.A. Regulation of CLOCK and MOP4 by nuclear hormone receptors in the vasculature: A humoral mechanism to reset a peripheral clock. Cell 2001, 105, 877–889. [Google Scholar] [CrossRef]
- Kang, T.H.; Lindsey-Boltz, L.A.; Reardon, J.T.; Sancar, A. Circadian control of XPA and excision repair of cisplatin—DNA damage by cryptochrome and HERC2 ubiquitin ligase. Proc. Natl. Acad. Sci USA 2010, 107, 4890–4895. [Google Scholar] [CrossRef]
- Collis, S.J.; Barber, L.J.; Clark, A.J.; Martin, J.S.; Ward, J.D.; Boulton, S.J. HCLK2 is essential for the mammalian S-phase checkpoint and impacts on Chk1 stability. Nat. Cell Biol. 2007, 9, 391–401. [Google Scholar] [CrossRef]
- Higuchi, M.; Kato, T.; Yoshida, S.; Ueharu, H.; Nishimura, N.; Kato, Y. PRRX1—And PRRX2—Positive mesenchymal stem/progenitor cells are involved in vasculogenesis during rat embryonic pituitary development. Cell Tissue Res. 2015, 361, 557–565. [Google Scholar] [CrossRef]
- Mancini, M.; Petretto, E.; Kleinert, C.; Scavone, A.; De, T.; Cook, S.; Silhavy, J.; Zidek, V.; Pravenec, M.; d’Amati, G.; et al. Mapping genetic determinants of coronary microvascular remodeling in the spontaneously hypertensive rat. Basic Res. Cardiol. 2013, 108, 316. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.; Izumiya, Y.; Araki, S.; Yamamura, S.; Hanatani, S.; Onoue, Y.; Ishida, T.; Arima, Y.; Nakamura, T.; Yamamoto, E.; et al. Sirt7 Deficiency Attenuates Neointimal Formation Following Vascular Injury by Modulating Vascular Smooth Muscle Cell Proliferation. Circ. J. 2021, 85, CJ-20. [Google Scholar] [CrossRef] [PubMed]
- Frösen, J.; Tulamo, R.; Heikura, T.; Sammalkorpi, S.; Niemelä, M.; Hernesniemi, J.; Levonen, A.L.; Hörkkö, S.; Ylä-Herttuala, S. Lipid accumulation, lipid oxidation, and low plasma levels of acquired antibodies against oxidized lipids associate with degeneration and rupture of the intracranial aneurysm wall. Acta Neuropathol. Commun. 2013, 1, 71. [Google Scholar] [CrossRef] [PubMed]
- Ollikainen, E.; Tulamo, R.; Lehti, S.; Lee-Rueckert, M.; Hernesniemi, J.; Niemelä, M.; Ylä-Herttuala, S.; Kovanen, P.T.; Frösen, J. Smooth Muscle Cell Foam Cell Formation, Apolipoproteins, and ABCA1 in Intracranial Aneurysms: Implications for Lipid Accumulation as a Promoter of Aneurysm Wall Rupture. J. Neuropathol. Exp. Neurol. 2016, 75, 689–699. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, U.; Ishida, M.; Berk, B.C. Angiotensin II stimulates tyrosine phosphorylation of phospholipase C-gamma-associated proteins. Characterization of a c-Src-dependent 97-kD protein in vascular smooth muscle cells. Circ. Res. 1997, 81, 550–557. [Google Scholar] [CrossRef]
- Benigni, A.; Cassis, P.; Remuzzi, G. Angiotensin II revisited: New roles in inflammation, immunology and aging. EMBO Mol. Med. 2010, 2, 247–257. [Google Scholar] [CrossRef]
- Lv, P.; Zhang, F.; Yin, Y.J.; Wang, Y.C.; Gao, M.; Xie, X.L.; Zhao, L.L.; Dong, L.H.; Lin, Y.L.; Shu, Y.N.; et al. SM22α inhibits lamellipodium formation and migration via Ras—Arp2/3 signaling in synthetic VSMCs. Am. J. Physiol. Cell Physiol. 2016, 311, C758–C767. [Google Scholar] [CrossRef]
- Li, W.; Xin, B.; Yan, J.; Wu, Y.; Hu, B.; Liu, L.; Wang, Y.; Ahn, J.; Skowronski, J.; Zhang, Z.; et al. SAMHD1 Gene Mutations Are Associated with Cerebral Large-Artery Atherosclerosis. Biomed. Res. Int. 2015, 2015, 739586. [Google Scholar] [CrossRef]
- Wu, C.C.; Peng, S.S.; Lee, W.T. Intracerebral large artery disease in Aicardi-Goutières syndrome with TREX1 mutation: A case report. Neurol. Sci. 2020, 41, 3353–3356. [Google Scholar] [CrossRef]
- Xin, B.; Jones, S.; Puffenberger, E.G.; Hinze, C.; Bright, A.; Tan, H.; Zhou, A.; Wu, G.; Vargus-Adams, J.; Agamanolis, D.; et al. Homozygous mutation in SAMHD1 gene causes cerebral vasculopathy and early onset stroke. Proc. Natl. Acad. Sci. USA 2011, 108, 5372–5377. [Google Scholar] [CrossRef]
- Kotla, S.; Vu, H.T.; Ko, K.A.; Wang, Y.; Imanishi, M.; Heo, K.S.; Fujii, Y.; Thomas, T.N.; Gi, Y.J.; Mazhar, H.; et al. Endothelial senescence is induced by phosphorylation and nuclear export of telomeric repeat binding factor 2-interacting protein. JCI Insight 2019, 4, e124867. [Google Scholar] [CrossRef] [PubMed]
- Dulin, E.; García-Barreno, P.; Guisasola, M.C. Extracellular heat shock protein 70 (HSPA1A) and classical vascular risk factors in a general population. Cell Stress Chaperones 2010, 15, 929–937. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.D.; Song, Y.; Kong, P.; Xu, X.; Gao, Y.K.; Dou, Y.Q.; Weng, L.; Wang, X.W.; Lin, Y.L.; Zhang, F.; et al. Smooth muscle 22 alpha protein inhibits VSMC foam cell formation by supporting normal LXRα signaling, ameliorating atherosclerosis. Cell Death Dis. 2021, 12, 982. [Google Scholar] [CrossRef] [PubMed]
- Berger, N.D.; Stanley, F.; Moore, S.; Goodarzi, A.A. ATM—Dependent pathways of chromatin remodelling and oxidative DNA damage responses. Philos. Trans. R. Soc. B Biol. Sci. 2017, 372, 20160283. [Google Scholar] [CrossRef]
- Bellelli, R.; Belan, O.; Pye, V.E.; Clement, C.; Maslen, S.L.; Skehel, J.M.; Cherepanov, P.; Almouzni, G.; Boulton, S.J. POLE3-POLE4 Is a Histone H3-H4 Chaperone that Maintains Chromatin Integrity during DNA Replication. Mol. Cell 2018, 72, 112–126.e5. [Google Scholar] [CrossRef]
- Murphy, K.J.; Cutter, A.R.; Fang, H.; Postnikov, Y.V.; Bustin, M.; Hayes, J.J. HMGN1 and 2 remodel core and linker histone tail domains within chromatin. Nucleic Acids Res. 2017, 45, 9917–9930. [Google Scholar] [CrossRef]
- Subramanian, M.; Gonzalez, R.W.; Patil, H.; Ueda, T.; Lim, J.H.; Kraemer, K.H.; Bustin, M.; Bergel, M. The nucleosome—Binding protein HMGN2 modulates global genome repair. FEBS J. 2009, 276, 6646–6657. [Google Scholar] [CrossRef]
- Gerlitz, G. HMGNs, DNA repair and cancer. Biochim. Biophys. Acta 2010, 1799, 80–85. [Google Scholar] [CrossRef]
- De Castro, R.O.; Previato, L.; Goitea, V.; Felberg, A.; Guiraldelli, M.F.; Filiberti, A.; Pezza, R.J. The chromatin—Remodeling subunit Baf200 promotes homology—Directed DNA repair and regulates distinct chromatin—Remodeling complexes. J. Biol. Chem. 2017, 292, 8459–8471. [Google Scholar] [CrossRef]
- Caridi, C.P.; D’Agostino, C.; Ryu, T.; Zapotoczny, G.; Delabaere, L.; Li, X.; Khodaverdian, V.Y.; Amaral, N.; Lin, E.; Rau, A.R.; et al. Nuclear F-actin and myosins drive relocalization of heterochromatic breaks. Nature 2018, 559, 54–60. [Google Scholar] [CrossRef]
- Hurst, V.; Shimada, K.; Gasser, S.M. Nuclear Actin and Actin—Binding Proteins in DNA Repair. Trends Cell Biol. 2019, 29, 462–476. [Google Scholar] [CrossRef] [PubMed]
- Yao, T.; Song, L.; Jin, J.; Cai, Y.; Takahashi, H.; Swanson, S.K.; Washburn, M.P.; Florens, L.; Conaway, R.C.; Cohen, R.E.; et al. Distinct modes of regulation of the Uch37 deubiquitinating enzyme in the proteasome and in the Ino80 chromatin-remodeling complex. Mol. Cell 2008, 31, 909–917. [Google Scholar] [CrossRef] [PubMed]
- Masuda, Y.; Piao, J.; Kamiya, K. DNA replication-coupled PCNA mono-ubiquitination and polymerase switching in a human in vitro system. J. Mol. Biol. 2010, 396, 487–500. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wang, D.; Wu, J.; Keller, J.; Ma, T.; Yu, X. RNF168 forms a functional complex with RAD6 during the DNA damage response. J. Cell Sci. 2013, 126, 2042–2051. [Google Scholar] [CrossRef]
- Zhang, M.; Hu, C.; Tong, D.; Xiang, S.; Williams, K.; Bai, W.; Li, G.M.; Bepler, G.; Zhang, X. Ubiquitin—Specific Peptidase 10 (USP10) Deubiquitinates and Stabilizes MutS Homolog 2 (MSH2) to Regulate Cellular Sensitivity to DNA Damage. J. Biol. Chem. 2016, 291, 10783–10791. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Dejsuphong, D.; Adelmant, G.; Ceccaldi, R.; Yang, K.; Marto, J.A.; D’Andrea, A.D. Transcriptional repressor ZBTB1 promotes chromatin remodeling and translesion DNA synthesis. Mol. Cell 2014, 54, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Sato, K.; Koike, A.; Nishikawa, H.; Koizumi, H.; Venkitaraman, A.R.; Ohta, T. HERC2 is an E3 ligase that targets BRCA1 for degradation. Cancer Res. 2010, 70, 6384–6392. [Google Scholar] [CrossRef]
- Laurent, G.; Shtokalo, D.; Tackett, M.R.; Yang, Z.; Vyatkin, Y.; Milos, P.M.; Seilheimer, B.; McCaffrey, T.A.; Kapranov, P. On the importance of small changes in RNA expression. Methods. 2013, 63, 18–24. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Centner, V.; Massart, D.-L.; de Noord, O.E.; de Jong, S.; Vandeginste, B.M.; Sterna, C. Elimination of Uninformative Variables for Multivariate Calibration. Anal. Chem. 1996, 68, 3851–3858. [Google Scholar] [CrossRef]
- Andries, J.P.M.; Vander Heyden, Y.; Buydens, L.M.C. Improved variable reduction in partial least squares modelling by Global-Minimum Error Uninformative-Variable Elimination. Anal. Chim Acta. 2017, 982, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Mehmood, T.; Liland, K.H.; Snipen, L.; Sæbø, S. A Review of Variable Selection Methods in Partial Least Squares Regression. Chemom. Intell. Lab. Syst. 2012, 118, 62–69. [Google Scholar] [CrossRef]
- Robin, X.; Turck, N.; Hainard, A.; Tiberti, N.; Lisacek, F.; Sanchez, J.-C.; Müller, M. PROC: An Open-Source Package for R and S+ to Analyze and Compare ROC Curves. BMC Bioinform. 2011, 12, 77. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, R.; Maqbool, M.; Mohamed, A.L.; Noah, R.M. Elevated neutrophil respiratory burst activity in essential hypertensive patients. Cell. Immunol. 2010, 263, 230–234. [Google Scholar] [CrossRef]
- Hand, W.L.; Hand, D.L.; Vasquez, Y. Increased polymorphonuclear leukocyte respiratory burst function in type 2 diabetes. Diabetes Res. Clin. Pract. 2007, 76, 44–50. [Google Scholar] [CrossRef]
- Mazor, R.; Shurtz-Swirski, R.; Farah, R.; Kristal, B.; Shapiro, G.; Dorlechter, F.; Cohen-Mazor, M.; Meilin, E.; Tamara, S.; Sela, S. Primed polymorphonuclear leukocytes constitute a possible link between inflammation and oxidative stress in hyperlipidemic patients. Atherosclerosis 2008, 197, 937–943. [Google Scholar] [CrossRef]
- Leclercq, A.; Houard, X.; Philippe, M.; Ollivier, V.; Sebbag, U.; Meilhac, O.; Michel, J.B. Involvement of intraplaque hemorrhage in atherothrombosis evolution via neutrophil protease enrichment. J. Leukoc. Biol. 2007, 82, 1420–1429. [Google Scholar] [CrossRef]
- Dulin, E.; García-Barreno, P.; Guisasola, M.C. Genetic variations of HSPA1A, the heat shock protein levels, and risk of atherosclerosis. Cell Stress Chaperones 2012, 17, 507–516. [Google Scholar] [CrossRef]
- Botto, N.; Rizza, A.; Colombo, M.G.; Mazzone, A.M.; Manfredi, S.; Masetti, S.; Clerico, A.; Biagini, A.; Andreassi, M.G. Evidence for DNA damage in patients with coronary artery disease. Mutation Res. 2001, 493, 23–30. [Google Scholar] [CrossRef]
| Characteristic | AAA (n = 7) | CVD (n = 7) | LEAD (n = 8) | Control (n = 7) | p1 |
|---|---|---|---|---|---|
| Age | 66.3 ± 4.03 2 59–71 3 | 41.3 ± 4.03 2 35–47 3 | 62 ± 7.82 2 48–71 3 | 33.6 ± 9.64 2 24–54 3 | 6.290 × 10−5 4 |
| Body mass index (BMI) | 27.23 ± 2.76 2 23.66–30.85 3 | 23.36 ± 1.94 2 20.94–25.83 3 | 28.25 ± 2.07 2 25.5–31.2 3 | 21.73 ± 3.30 2 19.33–29.00 3 | 0.448 5 |
| Gender: males/females | 6 (85.7%)/ 1 (14.3%) | 3 (42.9%)/ 4 (57.1%) | 6 (75%)/ 2 (25%) | 5 (71.4%)/ 2 (28.6%) | 1.564 × 10−3 4 |
| Smoking: never and former/current | 5 (71.4%)/ 2 (28.6%) | 5 (71.4%)/ 2 (28.6%) | 6 (75%)/ 2 (25%) | 7 (100%)/ 0 (0%) | 0.535 5 |
| No. | Gene Symbol 1 | Gene Name 1 | p | Fold Change | PLS Coefficient | ROC–AUC |
|---|---|---|---|---|---|---|
| LEAD vs. Control group | ||||||
| 1. | ATM | ATM serine/threonine kinase | 3.969 × 10−2 | 0.762 | −5.042 × 10−1 | 1.000 |
| AAA vs. Control group | ||||||
| 1. | RAD21 | RAD21 cohesin complex component | 4.926 × 10−7 | 0.757 | −7.573 × 10−2 | 1.000 |
| 2. | UPF1 | UPF1 RNA helicase and ATPase | 7.004 × 10−5 | 1.286 | 7.567 × 10−2 | 1.000 |
| 3. | SMARCAD1 | SWI/SNF-related, matrix-associated actin-dependent regulator of chromatin, subfamily a, containing DEAD/H box 1 | 2.260 × 10−4 | 0.725 | −8.092 × 10−2 | 0.959 |
| 4. | WHSC1 | Nuclear receptor-binding SET Domain protein 2 | 4.903 × 10−4 | 1.300 | 7.990 × 10−2 | 1.000 |
| 5. | TOP3A | DNA topoisomerase III alpha | 2.659 × 10−3 | 1.320 | 6.685 × 10−2 | 0.918 |
| 6. | INTS3 | Integrator complex subunit 3 | 3.802 × 10−3 | 1.227 | 5.369 × 10−2 | 0.918 |
| 7. | PNKP | Polynucleotide kinase 3’-phosphatase | 3.955 × 10−3 | 1.311 | 8.177 × 10−2 | 0.959 |
| 8. | PRRX1 | Paired related homeobox 1 | 3.955 × 10−3 | 0.208 | −1.010 × 10−1 | 0.918 |
| 9. | UVSSA | UV stimulated scaffold protein A | 3.955 × 10−3 | 1.482 | 1.246 × 10−1 | 0.980 |
| 10. | ZBTB1 | Zinc finger and BTB domain containing 1 | 3.955 × 10−3 | 0.778 | −5.801 × 10−2 | 0.918 |
| 11. | PARP2 | Poly(ADP-ribose) polymerase 2 | 4.303 × 10−3 | 0.692 | −5.700 × 10−2 | 0.918 |
| 12. | SMC3 | Structural maintenance of chromosomes 3 | 4.303 × 10−3 | 0.795 | −5.300 × 10−2 | 0.898 |
| 13. | OGG1 | 8-oxoguanine DNA glycosylase | 8.026 × 10−3 | 1.314 | 7.994 × 10−2 | 0.918 |
| 14. | ATM | ATM serine/threonine kinase | 8.031× 10−3 | 0.761 | −6.130 × 10−2 | 0.918 |
| 15. | EGFR | Epidermal growth factor receptor | 8.031× 10−3 | 0.250 | −4.767 × 10−2 | 0.959 |
| 16. | ASF1A | Anti-silencing function 1A histone chaperone | 8.587 × 10−3 | 0.681 | −1.029 × 10−1 | 0.959 |
| 17. | RTEL1 | Regulator of telomere elongation helicase 1 | 9.642 × 10−3 | 1.805 | 6.994 × 10−2 | 0.939 |
| 18. | APBB1 | Amyloid beta precursor protein-binding family B member 1 | 1.132 × 10−2 | 0.638 | −9.918 × 10−2 | 0.878 |
| 19. | NPAS2 | Neuronal PAS domain protein 2 | 1.145 × 10−2 | 0.384 | −6.987 × 10−2 | 1.000 |
| 20. | DEK | DEK proto-oncogene | 1.163 × 10−2 | 0.794 | −6.876 × 10−2 | 0.980 |
| 21. | POLE | DNA polymerase epsilon, catalytic subunit | 1.656 × 10−2 | 1.235 | 6.854× 10−2 | 0.959 |
| 22. | SIRT7 | Sirtuin 7 | 1.656 × 10−2 | 1.236 | 6.185× 10−2 | 0.918 |
| 23. | USP51 | Ubiquitin specific peptidase 51 | 1.656 × 10−2 | 0.541 | −5.382 × 10−2 | 0.959 |
| 24. | AP5S1 | Adaptor-related protein complex 5 subunit sigma 1 | 2.422 × 10−2 | 1.350 | 5.630 × 10−2 | 0.959 |
| 25. | SPIDR | Scaffold protein involved in DNA repair | 2.655 × 10−2 | 1.237 | 4.797 × 10−2 | 0.898 |
| 26. | JMY | Junction-mediating and regulatory protein, p53 cofactor | 2.874 × 10−2 | 0.767 | −5.160 × 10−2 | 0.898 |
| 27. | VCP | Valosin-containing protein | 3.095 × 10−2 | 1.209 | 5.048 × 10−2 | 0.898 |
| CVD vs. Control group | ||||||
| 1. | POLE | DNA polymerase epsilon, catalytic subunit | 2.750 × 10−4 | 1.389 | −1.007 × 10−1 | 1.000 |
| 2. | UVSSA | UV stimulated scaffold protein A | 5.268 × 10−4 | 1.608 | −1.571 × 10−1 | 1.000 |
| 3. | PNKP | Polynucleotide kinase 3’–phosphatase | 3.628 × 10−3 | 1.324 | −9.409 × 10−2 | 0.980 |
| 4. | RAD17P1 | RAD17 pseudogene 1 | 3.628 × 10−3 | 0.241 | 6.482 × 10−2 | 0.980 |
| 5. | WHSC1 | Nuclear receptor-binding SET domain protein 2 | 3.628 × 10−3 | 1.267 | −7.529 × 10−2 | 0.980 |
| 6. | XRCC6 | X-ray repair cross complementing 6 | 3.894 × 10−3 | 0.773 | 7.724 × 10−2 | 1.000 |
| 7. | TRRAP | Transformation/transcription domain-associated protein | 3.937 × 10−3 | 1.247 | −8.331 × 10−2 | 1.000 |
| 8. | AP5Z1 | Adaptor-related protein complex 5 subunit zeta 1 | 4.601 × 10−3 | 1.319 | −1.079 × 10−1 | 0.980 |
| 9. | EME2 | Essential meiotic structure–specific endonuclease subunit 2 | 4.601 × 10−3 | 1.445 | −1.024 × 10−1 | 0.939 |
| 10. | UPF1 | UPF1 RNA helicase and ATPase | 4.601 × 10−3 | 1.211 | −8.287 × 10−2 | 1.000 |
| 11. | NBN | Nibrin | 7.122 × 10−3 | 0.767 | 6.012 × 10−2 | 0.980 |
| 12. | USP1 | Ubiquitin-specific peptidase 1 | 8.767 × 10−3 | 0.786 | 6.183 × 10−2 | 1.000 |
| 13. | GTF2I | General transcription factor IIi | 1.106 × 10−2 | 0.748 | 1.004 × 10−1 | 0.980 |
| 14. | HERC2 | HECT and RLD domain containing E3 ubiquitin protein ligase 2 | 1.291 × 10−2 | 1.229 | −5.896 × 10−2 | 0.959 |
| 15. | TREX2 | Three prime repair exonuclease 2 | 1.291 × 10−2 | 2.039 | −5.716 × 10−2 | 0.959 |
| 16. | HMGN1 | High-mobility group nucleosome-binding domain 1 | 1.303 × 10−2 | 0.760 | 1.011 × 10−1 | 1.000 |
| 17. | EGFR | Epidermal growth factor receptor | 1.396 × 10−2 | 0.244 | 4.115 × 10−2 | 0.918 |
| 18. | OGG1 | 8-oxoguanine DNA glycosylase | 2.368 × 10−2 | 1.282 | −7.221 × 10−2 | 0.898 |
| 19. | PARP2 | Poly(ADP-ribose) polymerase 2 | 2.490 × 10−2 | 0.725 | 5.284 × 10−2 | 0.918 |
| 20. | NFRKB | Nuclear factor related to kappaB-binding protein | 2.618 × 10−2 | 1.249 | −9.573 × 10−2 | 0.980 |
| 21. | BCCIP | BRCA2 and CDKN1A interacting protein | 3.014 × 10−2 | 0.788 | 6.583 × 10−2 | 0.939 |
| 22. | FZR1 | Fizzy and cell division cycle 20 related 1 | 3.418 × 10−2 | 1.250 | −9.201 × 10−2 | 0.980 |
| 23. | HSPA1A | Heat shock protein family A (Hsp70) member 1A | 3.418 × 10−2 | 0.533 | 1.376 × 10−1 | 0.898 |
| LEAD vs. AAA | ||||||
| 1. | PPP4R2 | Protein phosphatase 4 regulatory subunit 2 | 2.732 × 10−6 | 1.335 | 1.229 × 10−1 | 1.000 |
| 2. | RAD21 | RAD21 cohesin complex component | 9.034 × 10−6 | 1.272 | 1.049× 10−1 | 1.000 |
| 3. | SMC3 | Structural maintenance of chromosomes 3 | 7.431 × 10−4 | 1.294 | 1.195 × 10−1 | 0.964 |
| 4. | POLE3 | DNA polymerase epsilon 3, accessory subunit | 5.547 × 10−3 | 1.326 | 1.340 × 10−1 | 0.946 |
| 5. | MCM7 | Minichromosome maintenance complex component 7 | 9.869 × 10−3 | 0.786 | −1.065 × 10−1 | 0.911 |
| 6. | CLK2 | CDC like kinase 2 | 1.350 × 10−2 | 0.774 | −1.063 × 10−1 | 0.946 |
| 7. | DEK | DEK proto–oncogene | 1.350 × 10−2 | 1.274 | 1.133 × 10−1 | 0.929 |
| 8. | SMARCAD1 | SWI/SNF–related, matrix–associated actin–dependent regulator of chromatin, subfamily a, containing DEAD/H box 1 | 1.350 × 10−2 | 1.265 | 1.110 × 10−1 | 0.964 |
| 9. | UVSSA | UV stimulated scaffold protein A | 1.350 × 10−2 | 0.703 | −1.890× 10−1 | 0.875 |
| 10. | FANCB | FA complementation group B | 2.563 × 10−2 | 1.598 | 9.226 × 10−2 | 0.929 |
| 11. | UBE2A | Ubiquitin conjugating enzyme E2 A | 3.975 × 10−2 | 1.291 | 1.428 × 10−1 | 0.911 |
| LEAD vs. CVD | ||||||
| 1. | UVSSA | UV-stimulated scaffold protein A | 2.430 × 10−3 | 0.648 | −1.885 × 10−1 | 0.893 |
| 2. | MCM7 | Minichromosome maintenance complex component 7 | 5.487 × 10−3 | 0.771 | −9.060 × 10−2 | 0.964 |
| 3. | ACTR2 | Actin-related protein 2 | 7.278 × 10−3 | 1.290 | 6.713 × 10−2 | 0.946 |
| 4. | POLM | DNA polymerase mu | 7.278 × 10−3 | 0.774 | −8.552 × 10−2 | 0.946 |
| 5. | PNKP | Polynucleotide kinase 3′–phosphatase | 1.780 × 10−2 | 0.793 | −6.935 × 10−2 | 0.946 |
| 6. | UBE2A | Ubiquitin conjugating enzyme E2 A | 1.780 × 10−2 | 1.327 | 6.779 × 10−2 | 0.893 |
| 7. | DEK | DEK proto–oncogene | 1.795 × 10−2 | 1.256 | 5.554 × 10−2 | 0.875 |
| 8. | INIP | INTS3 and NABP interacting protein | 1.795 × 10−2 | 1.283 | 6674 × 10−2 | 0.929 |
| 9. | XRCC6 | X–ray repair cross complementing 6 | 1.795 × 10−2 | 1.239 | 7.014 × 10−2 | 0.911 |
| 10. | UBE2V2 | Ubiquitin conjugating enzyme E2 V2 | 1.820 × 10−2 | 1.250 | 7.772 × 10−2 | 1.000 |
| 11. | BCCIP | BRCA2 and CDKN1A interacting protein | 1.992 × 10−2 | 1.271 | 8.439 × 10−2 | 0.964 |
| 12. | ATM | ATM serine/threonine kinase | 2.223 × 10−2 | 0.785 | −6.956 × 10−2 | 0.911 |
| 13. | SMC3 | Structural maintenance of chromosomes 3 | 2.226 × 10−2 | 1.204 | 7.135 × 10−2 | 0.982 |
| 14. | RECQL | RecQ like helicase | 2.592 × 10−2 | 1.246 | 5.847 × 10−2 | 0.857 |
| 15. | TOP1 | DNA topoisomerase I | 2.677 × 10−2 | 1.219 | 6.918 × 10−2 | 0.893 |
| 16. | ZMPSTE24 | Zinc metallopeptidase STE24 | 2.677 × 10−2 | 1.314 | 5.716 × 10−2 | 0.929 |
| 17. | POLE4 | DNA polymerase epsilon 4, accessory subunit | 3.143 × 10−2 | 1.297 | 6.662 × 10−2 | 0.929 |
| 18. | TERF2IP | TERF2 interacting protein | 4.007 × 10−2 | 1.231 | 8.871 × 10−2 | 0.946 |
| 19. | SAMHD1 | SAM and HD domain containing deoxynucleoside triphosphate triphosphohydrolase 1 | 4.624 × 10−2 | 1.270 | 6.332 × 10−2 | 0.893 |
| AAA vs. CVD | ||||||
| 1. | LIG1 | DNA ligase 1 | 1.405 × 10−2 | 0.734 | −2.215 × 10−1 | 0.878 |
| 2. | POLE4 | DNA polymerase epsilon 4. accessory subunit | 1.405 × 10−2 | 1.381 | 2.107 × 10−1 | 0.918 |
| LEAD, AAA and CVD vs. Control group | ||||||
| 3. | WHSC1 | Nuclear receptor binding SET domain protein 2 | 2.248 × 10−3 | 1.245 | 1.269 × 10−1 | 0.981 |
| 4. | UPF1 | UPF1 RNA helicase and ATPase | 3.682 × 10−3 | 1.204 | 9.414 × 10−2 | 0.955 |
| 5. | POLE | DNA polymerase epsilon, catalytic subunit | 3.016 × 10−2 | 1.242 | 9.073 × 10−2 | 0.909 |
| LEAD and AAA vs. CVD | ||||||
| 1. | ATM | ATM serine/threonine kinase | 2.116 × 10−2 | 0.791 | −1.108 × 10−1 | 0.867 |
| 2. | INIP | INTS3 and NABP interacting protein | 2.116 × 10−2 | 1.282 | 1.131 × 10−1 | 0.914 |
| 3. | LIG1 | DNA ligase 1 | 2.116 × 10−2 | 0.764 | −1.165 × 10−1 | 0.895 |
| 4. | POLE4 | DNA polymerase epsilon, 4 accessory subunit | 2.116 × 10−2 | 1.334 | 1.197 × 10−1 | 0.924 |
| 5. | TREX2 | Three prime repair exonuclease 2 | 2.116 × 10−2 | 0.569 | −1.122 × 10−1 | 0.914 |
| 6. | XRCC6 | X–ray repair cross complementing 6 | 2.116 × 10−2 | 1.222 | 8.096 × 10−2 | 0.914 |
| Comparison | Gene Symbol | R | p |
| Age | |||
| AAA vs. control | APBB1 | −0.590 | 7.116 × 10−3 |
| NPAS2 | −0.456 | 4.393 × 10−2 | |
| PRRX1 | −0.510 | 2.300 × 10−2 | |
| SIRT7 | 0.499 | 2.630 × 10−2 | |
| TOP3A | 0.579 | 8.596 × 10−3 | |
| USP51 | −0.672 | 1.536 × 10−3 | |
| VCP | 0.619 | 4.216 × 10−3 | |
| JMY | −0.490 | 2.959 × 10−2 | |
| LEAD vs. control, AAA vs. control, LEAD vs. CVD, ART vs. CVD | ATM | −0.653 | 2.262 × 10−3 |
| AAA vs. CVD, ART vs. CVD | LIG1 | −0.461 | 4.177 × 10−2 |
| LEAD vs. CVD, AAA vs. CVD, ART vs. CVD | POLE4 | 0.580 | 8.546 × 10−3 |
| AAA vs. control, CVD vs. control, Disease vs. control | UPF1 | 0.558 | 1.200 × 10−2 |
| WHSC1 | 0.479 | 3.370 × 10−2 | |
| BMI | |||
| AAA vs. control | PRRX1 | −0.519 | 2.061 × 10−2 |
| USP51 | −0.533 | 1.726 × 10−2 | |
| VCP | 0.467 | 3.826 × 10−2 | |
| LEAD vs. CVD | RECQL | 0.450 | 4.744 × 10−2 |
| LEAD vs. CVD | POLM | −0.469 | 3.729 × 10−2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruszel, K.P.; Zalewski, D.P.; Stępniewski, A.; Gałkowski, D.; Bogucki, J.; Feldo, M.; Płachno, B.J.; Kocki, J.; Bogucka-Kocka, A. Next-Generation Sequencing in the Assessment of the Transcriptomic Landscape of DNA Damage Repair Genes in Abdominal Aortic Aneurysm, Chronic Venous Disease and Lower Extremity Artery Disease. Int. J. Mol. Sci. 2023, 24, 551. https://doi.org/10.3390/ijms24010551
Ruszel KP, Zalewski DP, Stępniewski A, Gałkowski D, Bogucki J, Feldo M, Płachno BJ, Kocki J, Bogucka-Kocka A. Next-Generation Sequencing in the Assessment of the Transcriptomic Landscape of DNA Damage Repair Genes in Abdominal Aortic Aneurysm, Chronic Venous Disease and Lower Extremity Artery Disease. International Journal of Molecular Sciences. 2023; 24(1):551. https://doi.org/10.3390/ijms24010551
Chicago/Turabian StyleRuszel, Karol P., Daniel P. Zalewski, Andrzej Stępniewski, Dariusz Gałkowski, Jacek Bogucki, Marcin Feldo, Bartosz J. Płachno, Janusz Kocki, and Anna Bogucka-Kocka. 2023. "Next-Generation Sequencing in the Assessment of the Transcriptomic Landscape of DNA Damage Repair Genes in Abdominal Aortic Aneurysm, Chronic Venous Disease and Lower Extremity Artery Disease" International Journal of Molecular Sciences 24, no. 1: 551. https://doi.org/10.3390/ijms24010551
APA StyleRuszel, K. P., Zalewski, D. P., Stępniewski, A., Gałkowski, D., Bogucki, J., Feldo, M., Płachno, B. J., Kocki, J., & Bogucka-Kocka, A. (2023). Next-Generation Sequencing in the Assessment of the Transcriptomic Landscape of DNA Damage Repair Genes in Abdominal Aortic Aneurysm, Chronic Venous Disease and Lower Extremity Artery Disease. International Journal of Molecular Sciences, 24(1), 551. https://doi.org/10.3390/ijms24010551








