Agronomic Trait Analysis and Genetic Mapping of a New Wheat Semidwarf Gene Rht-SN33d
Abstract
1. Introduction
2. Results
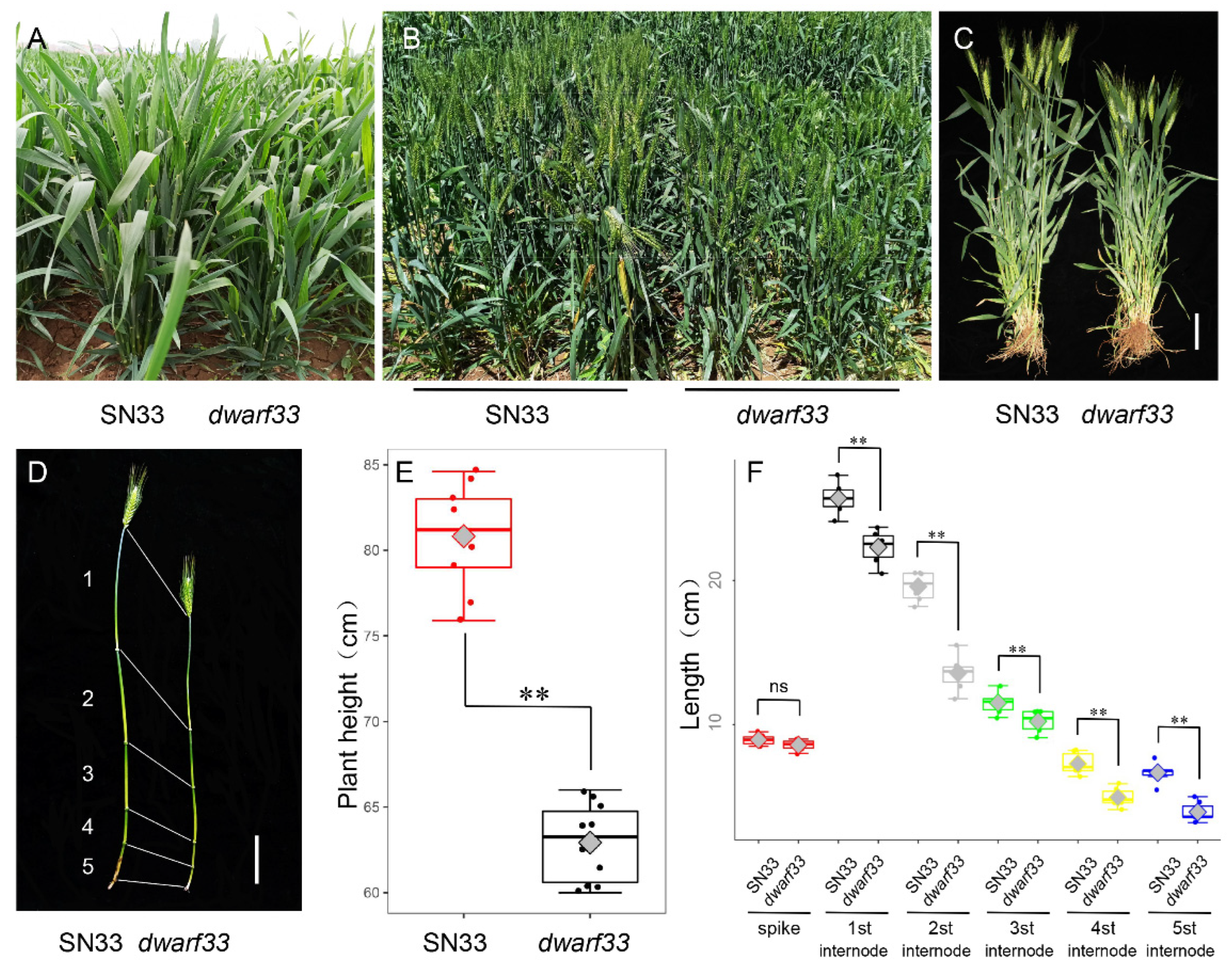
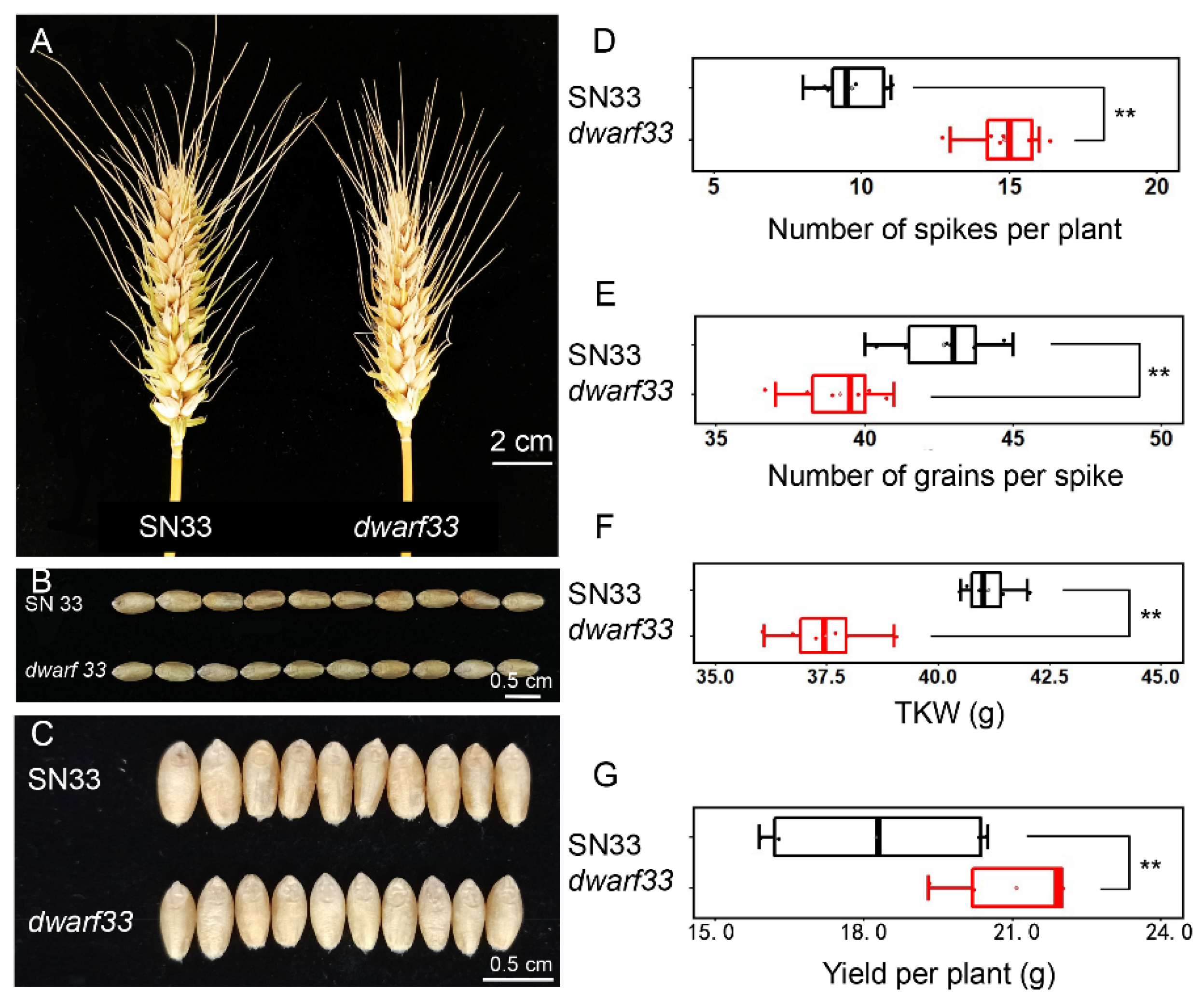
2.1. dwarf33 Shows Decreased Plant Height
2.2. Cytological Observation of Internode Cells
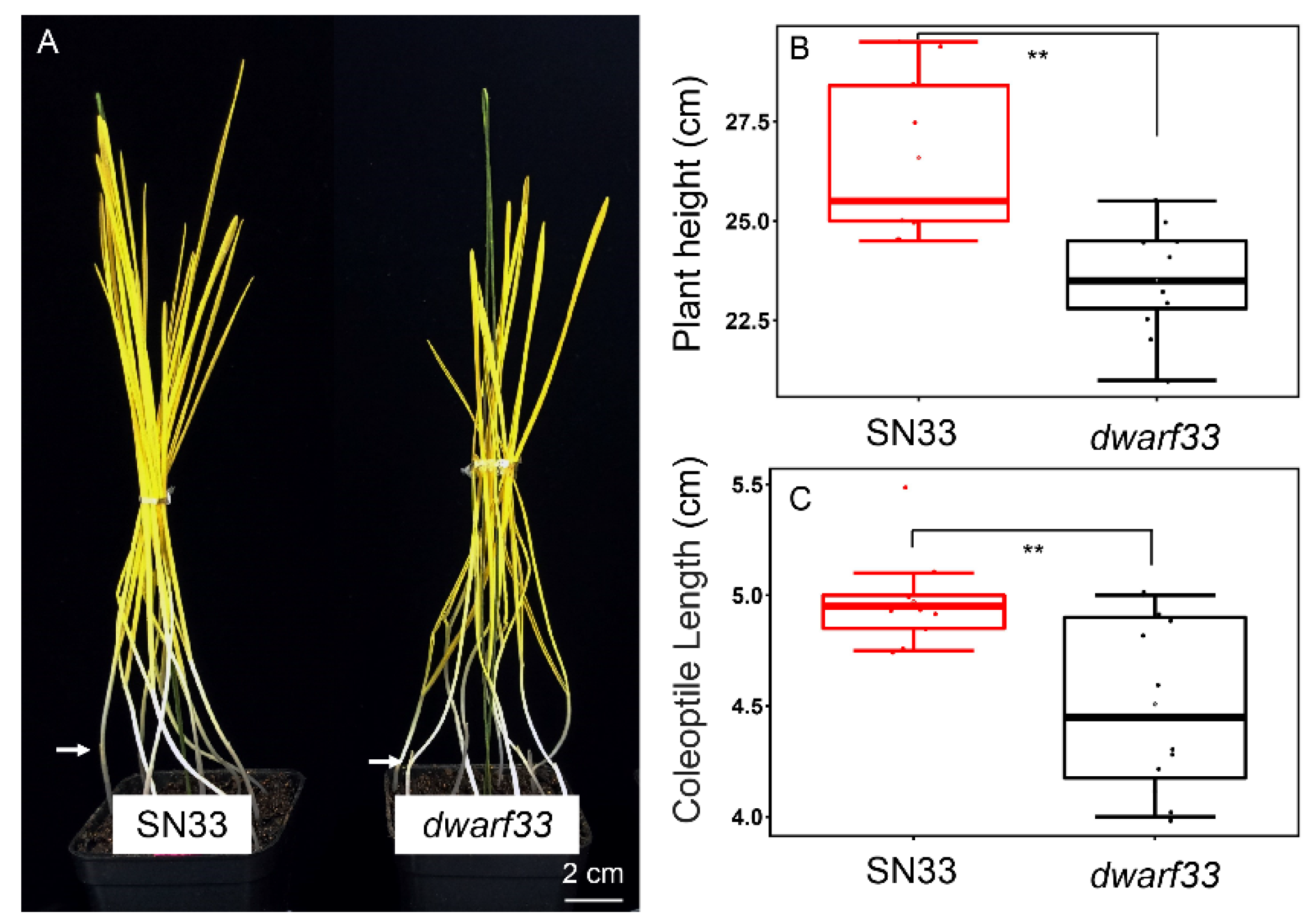
2.3. Coleoptile Length Measurement
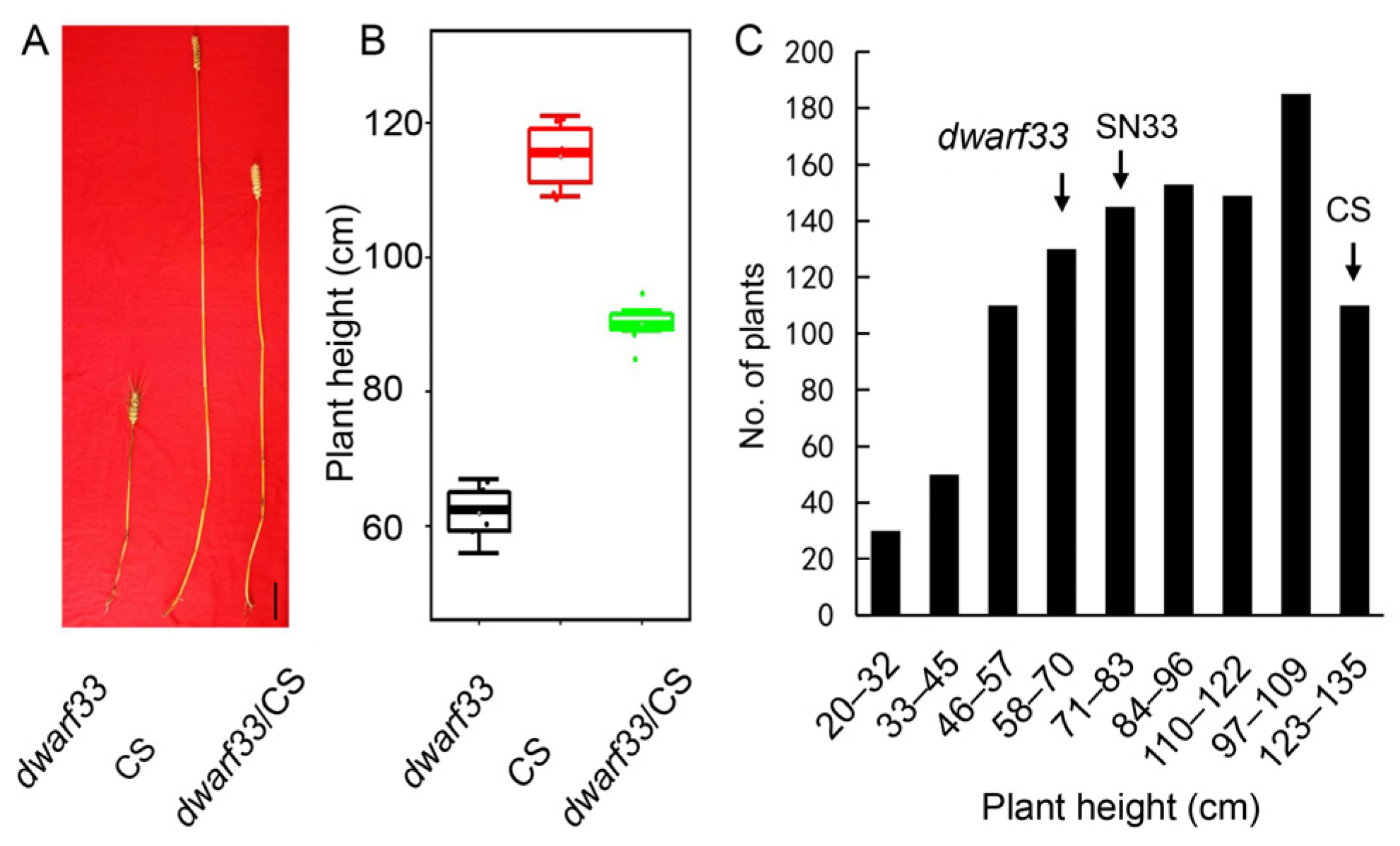
2.4. Dwarf Phenotype of dwarf33 Is Controlled by a Recessive Gene
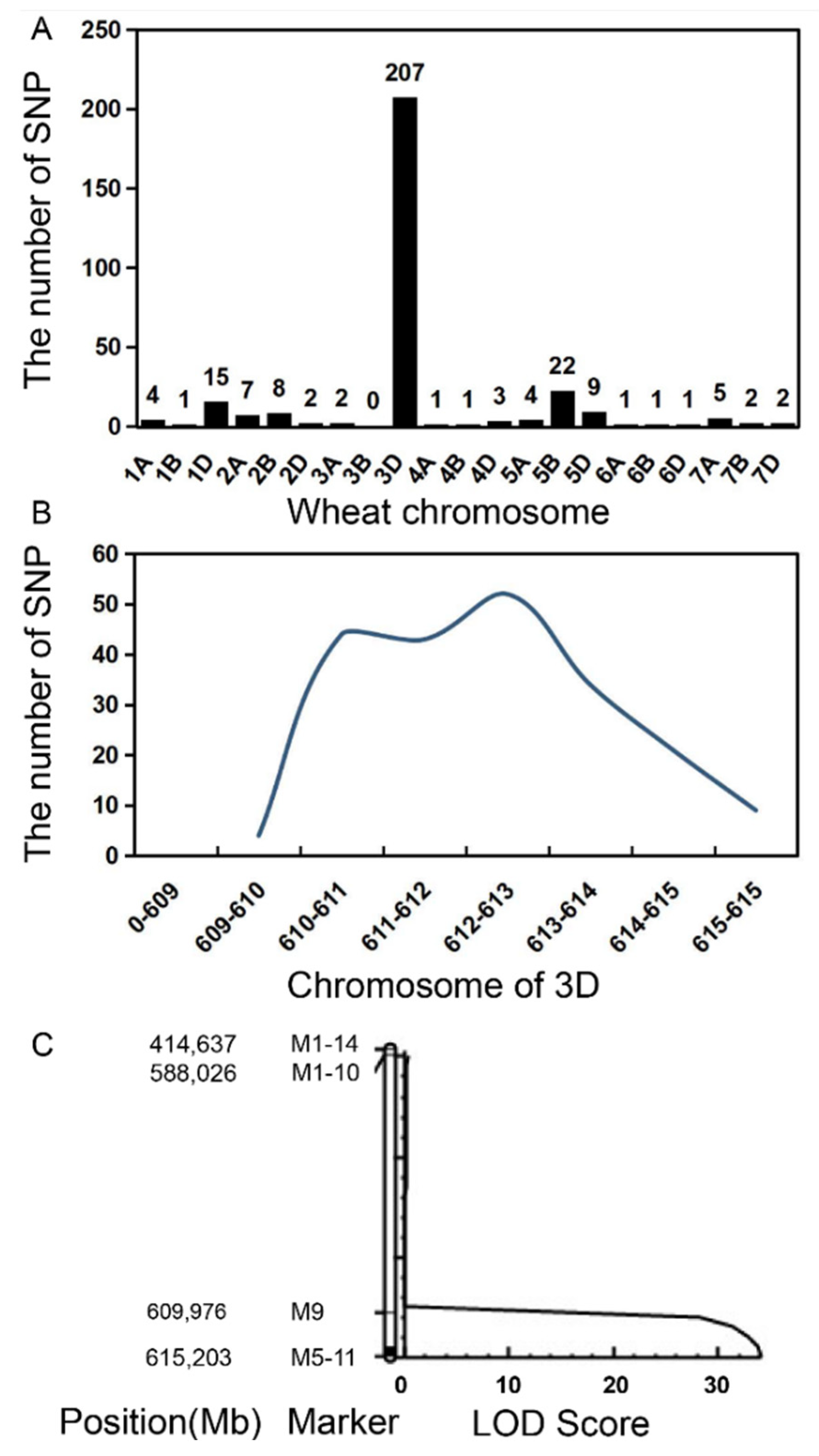
2.5. Dwarf Gene Mapping Based on BSA and the Wheat SNP Chip
2.6. Candidate Gene Prediction
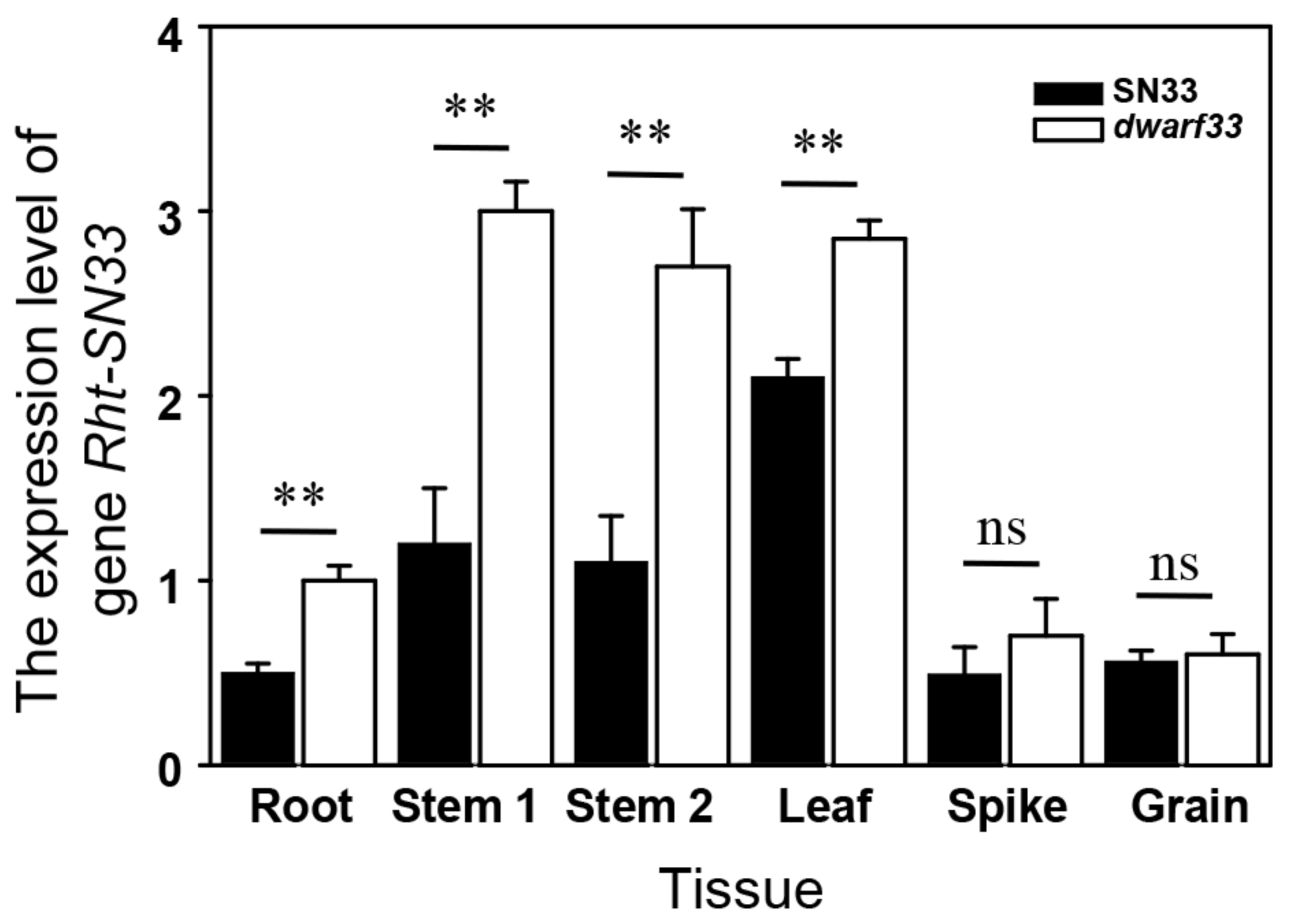
2.7. Expression Pattern of Rht-SN33d
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Phenotypic Assessment
4.3. DNA and RNA Isolation and RT-PCR and RT-qPCR
4.4. Microscopic Observation of Stem Tissue
4.5. Marker Design and Gene Mapping
4.6. Gene Prediction and Cloning
4.7. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, M.; Wang, H.; Yi, Y.; Ding, J.; Zhu, M.; Li, C.; Guo, W.; Feng, C.; Zhu, X. Effect of nitrogen levels and nitrogen ratios on lodging resistance and yield potential of winter wheat (Triticum aestivum L.). PLoS ONE 2017, 12, e187543. [Google Scholar] [CrossRef] [PubMed]
- Hedden, P. The genes of the Green Revolution. Trends Genet. 2003, 19, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Börner, A.; Plaschke, J.; Korzun, V.; Worland, A.J. The relationships between the dwarfing genes of wheat and rye. Euphytica 1996, 89, 69–75. [Google Scholar] [CrossRef]
- Flintham, J.E.; Gale, M.D. The Tom Thumb dwarfing gene Rht3 in wheat: 2. Effects on height, yield and grain quality. Theor. Appl. Genet. 1983, 66, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Ellis, M.H.; Rebetzke, G.; Azanza, F.; Richards, R.A.; Spielmeyer, W. Molecular mapping of gibberellin-responsive dwarfing genes in bread wheat. Theor. Appl. Genet. 2005, 111, 423–430. [Google Scholar] [CrossRef]
- Tian, X.; Xia, X.; Xu, D.; Liu, Y.; Xie, L.; Hassan, M.A.; Song, J.; Li, F.; Wang, D.; Zhang, Y.; et al. Rht24b, an ancient variation of TaGA2ox-A9, reduces plant height without yield penalty in wheat. New Phytol. 2022, 233, 738–750. [Google Scholar] [CrossRef]
- Xiong, H.; Zhou, C.; Fu, M.; Guo, H.; Xie, Y.; Zhao, L.; Gu, J.; Zhao, S.; Ding, Y.; Li, Y.; et al. Cloning and functional characterization of Rht8, a “Green Revolution” replacement gene in wheat. Mol. Plant 2022, 15, 373–376. [Google Scholar] [CrossRef]
- Chai, L.; Xin, M.; Dong, C.; Chen, Z.; Zhai, H.; Zhuang, J.; Cheng, X.; Wang, N.; Geng, J.; Wang, X.; et al. A natural variation in Ribonuclease H-like gene underlies Rht8 to confer “Green Revolution” trait in wheat. Mol. Plant 2022, 15, 377–380. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, S.; Zhou, Y.; He, Z.; Xia, X. Distribution of the Rht-B1b, Rht-D1b and Rht8 reduced height genes in autumn-sown Chinese wheats detected by molecular markers. Euphytica 2006, 152, 109–116. [Google Scholar] [CrossRef]
- Flintham, J.E.; Börner, A.; Worland, A.J.; Gale, M.D. Optimizing wheat grain yield: Effects of Rht (gibberellin-insensitive) dwarfing genes. J. Agric. Sci. 1997, 128, 11–25. [Google Scholar] [CrossRef]
- Peng, J.; Richards, D.E.; Hartley, N.M.; Murphy, G.P.; Devos, K.M.; Flintham, J.E.; Beales, J.; Fish, L.J.; Worland, A.J.; Pelica, F.; et al. ‘Green revolution’ genes encode mutant gibberellin response modulators. Nature 1999, 400, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Van De Velde, K.; Thomas, S.G.; Heyse, F.; Kaspar, R.; Van Der Straeten, D.; Rohde, A. N-terminal truncated RHT-1 proteins generated by translational reinitiation cause semi-dwarfing of wheat Green Revolution alleles. Mol. Plant 2021, 14, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Gasperini, D.; Greenland, A.; Hedden, P.; Dreos, R.; Harwood, W.; Griffiths, S. Genetic and physiological analysis of Rht8 in bread wheat: An alternative source of semi-dwarfism with a reduced sensitivity to brassinosteroids. J. Exp. Bot. 2012, 63, 6057. [Google Scholar] [CrossRef]
- Navarro, C.; Yang, Y.; Mohan, A.; Grant, N.; Gill, K.S.; Sandhu, D. Microsatellites Based Genetic Linkage Map of the Rht3 Locus in Bread Wheat. Mol. Plant Breed. 2014, 5, 43–46. [Google Scholar] [CrossRef]
- Wen, W.; Deng, Q.; Jia, H.; Wei, L.; Wei, J.; Wan, H.; Yang, L.; Cao, W.; Ma, Z. Sequence variations of the partially dominant DELLA gene Rht-B1c in wheat and their functional impacts. J. Exp. Bot. 2013, 64, 3299–3312. [Google Scholar] [CrossRef]
- Li, P.; Chen, J.; Wu, P.; Zhang, J.; Chu, C.; See, D.; Brown-Guedira, G.; Zemetra, R.; Souza, E. Quantitative Trait Loci Analysis for the Effect of Rht-B1 Dwarfing Gene on Coleoptile Length and Seedling Root Length and Number of Bread Wheat. Crop Sci. 2011, 51, 2561–2568. [Google Scholar] [CrossRef]
- Jatayev, S.; Sukhikh, I.; Vavilova, V.; Smolenskaya, S.E.; Goncharov, N.P.; Kurishbayev, A.; Zotova, L.; Absattarova, A.; Serikbay, D.; Hu, Y.-G.; et al. Green revolution ‘stumbles’ in a dry environment: Dwarf wheat with Rht genes fails to produce higher grain yield than taller plants under drought. Plant Cell Environ. 2020, 43, 2355–2364. [Google Scholar] [CrossRef]
- Khalid, M.; Afzal, F.; Gul, A.; Amir, R.; Subhani, A.; Ahmed, Z.; Mahmood, Z.; Xia, X.; Rasheed, A.; He, Z. Molecular Characterization of 87 Functional Genes in Wheat Diversity Panel and Their Association With Phenotypes Under Well-Watered and Water-Limited Conditions. Front. Plant Sci. 2019, 10, 717. [Google Scholar] [CrossRef]
- Rebetzke, G.; Richards, R.; Fischer, V.; Mickelson, B. Breeding long coleoptile, reduced height wheats. Euphytica Neth. J. Plant Breed. 1999, 106, 159–168. [Google Scholar] [CrossRef]
- Richards, R. The effect of dwarfing genes in spring wheat in dry environments. I. Agronomic characteristics. Aust. J. Agric. Res. 1992, 43, 517–527. [Google Scholar] [CrossRef]
- Tang, N.; Jiang, Y.; He, B.-R.; Hu, Y.-G. The Effects of Dwarfing Genes (Rht-B1b, Rht-D1b, and Rht8) with Different Sensitivity to GA3 on the Coleoptile Length and Plant Height of Wheat. Agric. Sci. China 2009, 8, 1028–1038. [Google Scholar] [CrossRef]
- Liu, Q.; Wu, K.; Song, W.; Zhong, N.; Wu, Y.; Fu, X. Improving Crop Nitrogen Use Efficiency Toward Sustainable Green Revolution. Annu. Rev. Plant Biol. 2022, 73, 523–551. [Google Scholar] [CrossRef]
- Mo, Y.; Vanzetti, L.S.; Hale, I.; Spagnolo, E.J.; Guidobaldi, F.; Al-Oboudi, J.; Odle, N.; Pearce, S.; Helguera, M.; Dubcovsky, J. Identification and characterization of Rht25, a locus on chromosome arm 6AS affecting wheat plant height, heading time, and spike development. Theor. Appl. Genet. 2018, 131, 2021–2035. [Google Scholar] [CrossRef]
- Schomburg, F.M.; Bizzell, C.M.; Lee, D.J.; Zeevaart, J.A.D.; Amasino, R.M. Overexpression of a Novel Class of Gibberellin 2-Oxidases Decreases Gibberellin Levels and Creates Dwarf Plants. Plant Cell 2003, 15, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Otani, M.; Meguro, S.; Gondaira, H.; Hayashi, M.; Saito, M.; Han, D.-S.; Inthima, P.; Supaibulwatana, K.; Mori, S.; Jikumaru, Y.; et al. Overexpression of the gibberellin 2-oxidase gene from Torenia fournieri induces dwarf phenotypes in the liliaceous monocotyledon Tricyrtis sp. J. Plant Physiol. 2013, 170, 1416–1423. [Google Scholar] [CrossRef] [PubMed]
- Wuddineh, W.A.; Mazarei, M.; Zhang, J.; Poovaiah, C.R.; Mann, D.G.J.; Ziebell, A.; Sykes, R.W.; Davis, M.F.; Udvardi, M.K.; Stewart, C.N. Identification and overexpression of gibberellin 2-oxidase (GA 2ox) in switchgrass (Panicum virgatum L.) for improved plant architecture and reduced biomass recalcitrance. Plant Biotechnol. J. 2015, 13, 636–647. [Google Scholar] [CrossRef]
- Li, S.; Lin, D.; Zhang, Y.; Deng, M.; Chen, Y.; Bin Lv, B.; Li, B.; Lei, Y.; Wang, Y.; Zhao, L.; et al. Genome-edited powdery mildew resistance in wheat without growth penalties. Nature 2022, 602, 455. [Google Scholar] [CrossRef]
- Talamè, V.; Bovina, R.; Sanguineti, M.C.; Tuberosa, R.; Lundqvist, U.; Salvi, S. TILLMore, a resource for the discovery of chemically induced mutants in barley. Plant Biotechnol. J. 2008, 6, 477–485. [Google Scholar] [CrossRef]
- Stewart, C.N.; Via, L.E. A rapid CTAB DNA isolation technique useful for RAPD fingerprinting and other PCR applications. BioTechniques 1993, 14, 748–750. [Google Scholar]
- Weigel, D.; Glazebrook, J. Fixation, Embedding, and Sectioning of Plant Tissues. CSH Protoc. 2008, 2008, t4941. [Google Scholar] [CrossRef]
- Meng, L.; Li, H.; Zhang, L.; Wang, J. QTL IciMapping: Integrated software for genetic linkage map construction and quantitative trait locus mapping in biparental populations. Crop J. 2015, 3, 269–283. [Google Scholar] [CrossRef]
- Dong, C.; Zhang, L.; Chen, Z.; Xia, C.; Gu, Y.; Wang, J.; Li, D.; Xie, Z.; Zhang, Q.; Zhang, X.; et al. Combining a New Exome Capture Panel with an Effective varBScore Algorithm Accelerates BSA-Based Gene Cloning in Wheat. Front. Plant Sci. 2020, 11, 1249. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Dong, C.; Chen, Z.; Gui, L.; Chen, C.; Li, D.; Xie, Z.; Zhang, Q.; Zhang, X.; Xia, C.; et al. WheatGmap: A comprehensive platform for wheat gene mapping and genomic studies. Mol. Plant 2021, 14, 187–190. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Wang, L.; Rimbert, H.; Rodriguez, J.C.; Deal, K.R.; De Oliveira, R.; Choulet, F.; Keeble-Gagnère, G.; Tibbits, J.; Rogers, J.; et al. Optical maps refine the bread wheat Triticum aestivum cv. Chinese Spring genome assembly. Plant J. 2021, 107, 303–314. [Google Scholar] [CrossRef] [PubMed]

| Name/Plant Condition | SN33 (cm) ± SD | dwarf33 (cm) ± SD | p-Value |
|---|---|---|---|
| Normal irrigation | 81.6 ± 2.3 | 63.3 ± 3.1 | 5.4 × 10−11 |
| Drought | 66.7 ± 4.3 | 46.1 ± 2.7 | 3.6 × 10−6 |
| Plant height decrease (%) | 18.3 | 27.2 |
| Population | Generation | No. Plants | No. Plants | Expected Ratio | χ2 | p-Value | |
|---|---|---|---|---|---|---|---|
| d | T | ||||||
| dwarf33 | P1 | 10 | 10 | 0 | |||
| SN33 | P2 | 10 | 0 | 10 | |||
| dwarf33/SN33 | F1 | 31 | 0 | 31 | |||
| F2 | 125 | 435 | 1:3 | 1.11 | 0.708 | ||
| SN33/dwarf33 | F1 | 30 | 30 | ||||
| F2 | 75 | 262 | 1:3 | 0.67 | 0.586 | ||
| Genotype | RhtD1a | RhtD1a/1b | RhtD1b | (RhtD1a) − (RhtD1b) |
|---|---|---|---|---|
| rht-SN33d | 119.4 | 108.6 | 86.2 | 33.2 |
| Rht-SN33d/rht-SN33d | 111.1 | 106.2 | 82.1 | 29.0 |
| Rht-SN33d | 83.4 | 76.9 | 59.8 | 23.6 |
| (rht-SN33d) − (Rht-SN33d) | 36.0 | 31.7 | 26.4 |
| Chr | Start | Stop | ID | Description |
|---|---|---|---|---|
| chr3D | 612311199 | 612312868 | TraesCS3D02G541100 | TOX high mobility group box protein |
| chr3D | 612312442 | 612318234 | TraesCS3D02G541200 | Vacuolar fusion protein MON1 |
| chr3D | 612604471 | 612605205 | TraesCS3D02G541300 | Enoyl-[acyl-carrier-protein] reductase |
| chr3D | 612763464 | 612764795 | TraesCS3D02G541400 | Regulator of chromosome condensation (RCC1) family with FYVE zinc finger domain-containing protein |
| chr3D | 612859406 | 612859915 | TraesCS3D02G541500 | Disease resistance protein RPP13 |
| chr3D | 612878354 | 612881039 | TraesCS3D02G541600 | Disease resistance protein (NBS-LRR class) family |
| chr3D | 612886487 | 612898293 | TraesCS3D02G541700 | ABC transporter B family protein |
| chr3D | 612899761 | 612906345 | TraesCS3D02G541800 | Chaperone protein |
| chr3D | 613057363 | 613058851 | TraesCS3D02G541900 | Plant/T31B5-30 protein |
| chr3D | 613076025 | 613076727 | TraesCS3D02G542000 | Pectinesterase inhibitor |
| chr3D | 613119248 | 613126869 | TraesCS3D02G542100 | SNARE-interacting protein KEULE |
| chr3D | 613357955 | 613358990 | TraesCS3D02G542200 | Extra-large guanine nucleotide binding family protein |
| chr3D | 613383728 | 613390403 | TraesCS3D02G542300 | Protein kinase-like protein |
| chr3D | 613480307 | 613481622 | TraesCS3D02G542400 | lectin-receptor kinase |
| chr3D | 613569028 | 613570372 | TraesCS3D02G542500 | Disease resistance protein (TIR-NBS-LRR class) family |
| chr3D | 613588995 | 613592124 | TraesCS3D02G542600 | Plant cadmium resistance protein |
| chr3D | 613594052 | 613594433 | TraesCS3D02G542700 | Dirigent protein |
| chr3D | 613597346 | 613599060 | TraesCS3D02G542800 | Gibberellin 2-beta-dioxygenase |
| chr3D | 613601793 | 613602401 | TraesCS3D02G542900 | Dirigent protein |
| chr3D | 613607577 | 613610693 | TraesCS3D02G543000 | transmembrane protein |
| chr3D | 613618086 | 613622258 | TraesCS3D02G543100 | Chaperone protein |
| chr3D | 613631318 | 613633402 | TraesCS3D02G543200 | SKP1-like protein |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Zhang, L.; Xie, Y.; Irshad, A.; Guo, H.; Gu, J.; Zhao, L.; Xiong, H.; Zhao, S.; Wang, C.; et al. Agronomic Trait Analysis and Genetic Mapping of a New Wheat Semidwarf Gene Rht-SN33d. Int. J. Mol. Sci. 2023, 24, 583. https://doi.org/10.3390/ijms24010583
Wang C, Zhang L, Xie Y, Irshad A, Guo H, Gu J, Zhao L, Xiong H, Zhao S, Wang C, et al. Agronomic Trait Analysis and Genetic Mapping of a New Wheat Semidwarf Gene Rht-SN33d. International Journal of Molecular Sciences. 2023; 24(1):583. https://doi.org/10.3390/ijms24010583
Chicago/Turabian StyleWang, Chaojie, Lili Zhang, Yongdun Xie, Ahsan Irshad, Huijun Guo, Jiayu Gu, Linshu Zhao, Hongchun Xiong, Shirong Zhao, Chengshe Wang, and et al. 2023. "Agronomic Trait Analysis and Genetic Mapping of a New Wheat Semidwarf Gene Rht-SN33d" International Journal of Molecular Sciences 24, no. 1: 583. https://doi.org/10.3390/ijms24010583
APA StyleWang, C., Zhang, L., Xie, Y., Irshad, A., Guo, H., Gu, J., Zhao, L., Xiong, H., Zhao, S., Wang, C., & Liu, L. (2023). Agronomic Trait Analysis and Genetic Mapping of a New Wheat Semidwarf Gene Rht-SN33d. International Journal of Molecular Sciences, 24(1), 583. https://doi.org/10.3390/ijms24010583







